0022-538X/87/092733-09$02.00/0
Copyright
© 1987, AmericanSociety
for MicrobiologyRabies Virus Infection of Cultured
Rat
Sensory Neurons
ERIKLYCKEt AND HENRITSIANG* Unite Rage, Institut Pasteur, Paris, France Received 10 February 1987/Accepted1 June 1987
The axonaltransportof rabies virus (challenge virus strain of fixed virus)was studied indifferentiatedrat embryonic dorsal root ganglion cells. In addition, we observed the attachment ofrabies virus to neuronal extensions and virus production by infected neurons. A compartmentalized cell culture system was used, allowing infectionandmanipulationofneuronalextensionswithoutexposing theneuralsomatothevirus.The cultures consisted of60% large neuronal cells whose extensions exhibited neurofilament structures. Rabies virus demonstrated highbinding affinitytounmyelinated neurites, assuggested byassaysof virus adsorption
andimmunofluorescence studies. Therateof axoplasmictransportofviruswas12to24mm/day,includingthe time required for internalization of the virus into neurites. The virus transport could be blocked by cytochalasin B, vinblastine, andcolchicine, noneof which negatively affected the production of virusin cells
oncetheinfectionwasestablished.Itwasconcluded that,for the retrograde transfer of rabies virusbyneurites from the peripherytothe neuronalsoma,the integrityof tubulin-andactin-containingstructuresis essential. Theratsensory neuronswerecharacterizedaspermissive, moderately susceptible, but low producers of rabies
virus. Theseneuronswerecapable of harboring rabiesvirusforlongperiodsof time and abletoreleasevirus intotheculturemedium without showingany morphological alterations. The involvement ofsensoryneurons
in rabies virus pathogenesis, bothinviral transportandas asiteforpersistent viral infection, is discussed.
The spread of rabies infection through theaxoplasmic flow in peripheral nerves and neurons in the central nervous
system(CNS)representsanimportant and significant patho-genetic characteristic. Interruption of the peripheral axoplasmic flow by ligature or sectioning (4, 15, 26) or by pharmacological impairment of the microtubulin function prevents rabies virus transport from the peripheral virus inoculation sitetothe CNS (8, 43). Peripheralnervesof both
motor and sensory neurons canbe used forpropagation of rabies virusinfection (10, 20), although sensory neurons are
mostprobably involved in the virus transfer from the site of the primary infection to the CNS (35). It is generally ob-served, by electron microscopy, that rabies virus buds from infected neurons and subsequently enters into uninfected cells(23, 24, 34, 39). However, the hypothesis ofanaxonal
transportof virus inthe CNS hasneverbeendiscarded (35). Virusinoculation into theeyehasresulted in virustransport into the CNS(31). Recently,experimental evidence has been provided foran axonal transport of rabies virus within the CNS. Stereotaxic inoculation of the virus into rat brains
gavepatterns ofvirus spread which areconsistentwith the hypothesis ofan axoplasmic flow-mediated transport(19).
Infection ofcultured nerve cells (44, 47) suggested that susceptibility to rabies virus is likely to vary markedly
depending on the types of neuronal cells. In the present study, we used rat dorsal root ganglia (DRG) neurons for evaluating axonal transport of rabies virus in vitro. In addition, westudiedvirusbindingtoneuritic extensions and itsreplication insensory neurons. Forthispurpose,wetook advantage ofa compartmentalized cell culture system (11) which allowed usto infect and manipulate neuronal exten-sions without exposingtheneuronal cell somatothe virus.
* Correspondingauthor.
t On leave of absencefrom theDepartmentofVirology,Institute
of MedicalMicrobiology, UniversityofGoteborg, Goteborg,
Swe-den.
MATERIALS AND METHODS
Virus.Challenge virus strain (CVS) fixed rabies viruswas obtained from supernatants of BHK-21 (baby hamster kid-ney)-infected cells. Cell monolayers were infected with a multiplicity of infection of 5 PFU/ml and incubated for 3 days at 37°C in Eagle minimal essential medium containing 2% fetal calf serum (Flow Laboratories Ltd., Ayrshire, United Kingdom), 2 mM glutamine, penicillin (100 U/ml), and streptomycin (100
[Lg/ml).
Infected supernatants were centrifugedtoremovecelldebris, divided into aliquots, and storedat -70° until used.Titration of infectious virus. The concentration of infec-tious viruswas determined on cultures of chicken embryo-relatedcellsasdescribedby Smithet al. (38). Briefly, these cellswere grownin amedium containing 1.2g ofNaHCO3
perliterandsupplementedwith10% fetalcalfserum,2 mM glutamine, and antibiotics.After6days of incubationina5% C02, humidity-saturated incubator at 37°C, the cells were
fixed withformaldehyde and stained withcrystal violet. Rabies virus antiserum. Anti-rabies virus antiserum was prepared by immunizing rabbits with beta-propiolactone-inactivated, purified rabies virus emulsified in Freund adju-vant, as described earlier (3). Rabbits were given four immunizing injections at 1-week intervals. One week after the last injection,ablood samplewascollectedfor titration
of the antibodies, using the plaque inhibition test. The antibody titerwasexpressed asinternationalunits, usingas a reference an international rabies antibody standard. The titerof the serumusedwas30 international units.
Nervecell cultures. DRGcellswereobtainedby dissecting
10 to 12 embryos of 15- to 17-day-pregnant Wistar rats. Ganglia were collected using forceps under a dissection microscope, and cells were dissociated by treatment with 0.25%trypsininCa2+-andMg2+-freeHanks bufferatpH7.2 for 30 min at 37°C. The cells were then separated from trypsin by low-speed centrifugationandsuspendedin culture 2733
on November 10, 2019 by guest
http://jvi.asm.org/
2734 LYCKE AND TSIANG
FIG. 1. The two-chamber cell culturesystem, acollagen-coated
petri dish with scratches made in the coat to direct neurites extending from differentiating neurons. In the centerof the dish, a
cloning cylinder is attached to its surface by silicon high-vacuum greaseandmethylcellulose. Neuronal cellsareseededinthecloning
cylinder (inner culturecompartment); neuritic extensionswillgrow
across the grease-cellulose seal and invade the outside of the cylinder (outerculture compartment).
medium. DRG cellswereseeded into theinnercompartment
ofatwo-chambercell culturesystemoriginally developed by Campenot (11). The system, which was adaptedfor studies
on nerve cell-virus interactions (32, 49), allows neuronal extensions which have outgrown in theouterculture cham-berto be infected with the virus, without exposure of the neuralcell somainthe innercompartment. I'hesystem(Fig. 1) consisted of a 35-mm collagen-coated (Vitrogen 100, Collagen Corp., Palo Alto, Calif.) plastic plate (Falcon; Becton Dickinson, Grenoble, France), scratched across its
centerwithstainless-steel needles placed inabrush 0.5mm
apart. An8-mm-wide glass cylinderwas putinthecenterof the plate and sealed with high-vacuum silicongrease. Inthe lower partof the glass cylinder, the trenches formed by the scratches in the collagen coat were sealed with 1%
methylcellulose (Methocel 400, Fluka AG, Buchs, Switzer-land) in Hanks buffer (pH 7.2). The glass cylinderserves as atight diffusion barrierinthe culture. After1 day of culture,
the cells were treated with 2.8 jig of cytosine arabinoside (SigmaChemical Co.,St. Louis, Mo.)permlofmediumfor
2 days to reduce the growth of cells exhibiting mitotic activity. On day 4, the cultures were rinsed and fresh
medium was added.
Wefurthermodified thetechniquedescribedabove forthe
present study.Theposition oftheglass cloning cylinderwas
identified on the external side of the plate to facilitate the microscope recognition of the areas observed, which are
eitherinsideoroutside the barrierevenafterremoval ofthe
cloning cylinder. We also used FIO cell culture medium (Boehringer, Mannheim, Federal Republic ofGermany)
sup-plemented with 10%, fetal calfserum because this medium
appeared torestrictgrowth ofcontaminatingfibroblasts. To stimulate differentiation of neuronal cells and outgrowth of neurites, UV light-sterilized GTlb ganglioside (thegenerous
gift of B. Hauttecoeur, Institut Pasteur) and nerve growth factor (Collaborative ResearchInc.,Lexington, Mass.)were added as suggested by Hauw et al. (21) to final concentra-tions of10 and 25 ng/ml, respectively.
It wasrepeatedlyobserved that the wall between the inner and outer culture compartments seemed a tight diffusion barrier, as (i) the fluid level inside the glass cylinder re-mained at a relatively higher level than outside fluid; (ii) radiolabeled low-molecular-weight substances did not dif-fuseacrossthe barrier; and (iii) the addition ofconcentrated
H2SO4 outside the glass cylinder to destroy peripheral neurites did not result in any morphological cell changes inside the barrier.
Destruction of neuritic extensions. Neuritic extensions pre-sent in the outer culture chamber were destroyed by the addition of200 to300,ul of concentrated H2S04. Aftera few secondsofcontactwith theacid, the culturewasrinsedwith medium until neutrality was restored.
Immunofluorescence. Rabies virus-infected cultures were stained witharabbit anti-rabies virus nucleocapsid immuno-globulin G conjugated with fluorescein isothiocyanate (FITC; kindly prepared byP.Versmisse)asdescribedearlier (46). Stainedcultureswerewashed with phosphate-buffered saline, mounted in Elvanol, and examined with an inverted Zeiss 1M32 microscope (Oberkochen, Federal Republic of Germany).
Staining of neurites with monoclonal antibodies against neurofilaments was performed with a mouse immunoglobu-linGl antibody (clone 2F11; Monosan, Uden, The Nether-lands).
RESULTS
Uninfected DRG cell culture. The DRG cells seeded into the inner culture compartment extended neurites which within 8 to 12 days grew across the seal into the areaoutside the barrier. About 2 x 105 to 3 x 105 cells were present in each cylinderascalculated from six cultures, of which 60% consisted of large neuronal cells. Neurons extending neurites outside the barrier hadno particular locationin the culture; they wereobserved with the samefrequency inthe centerof the culture as in the periphery. However, only a minor proportion of the neurons extended neurites capable
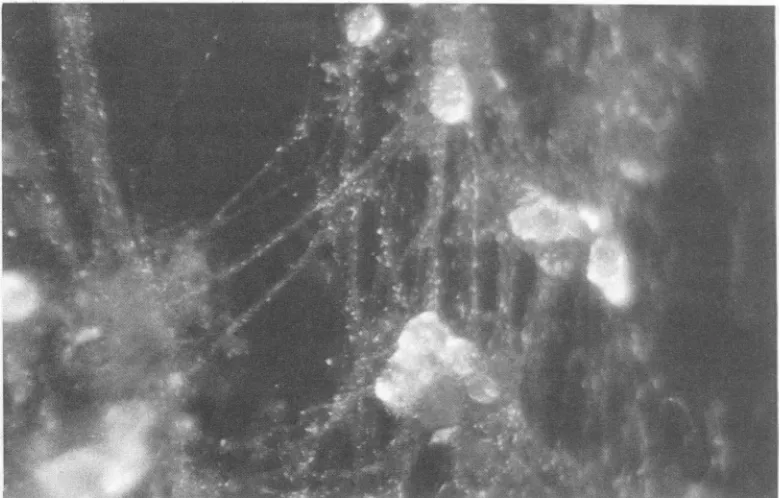
of reaching the outer culture compartment. No more than 236 neurites were counted in the outer compartment ofa culture(themeanvaluewasintherangeof68 to96;n = 18). However,since theneuronalextensions seemed mostoften to be able topenetrate thediffusion barrier onlyasfascicles of extensions twistedtogether, thetruenumberof neuritesin a culture might be greater. Most frequently, the neuritic outgrowth wasrestricted tothe areabetween twotrenches. However, someneurites wereable to traverse the trenches and formaneuritic network. Dependingon theefficiency of antimitotic treatment, neuritic extensions werefound to be associated or not with Schwann cells (Fig. 2). Staining the neuriteswith an FITC-labeled antineurofilament monoclonal antibody allowed us to demonstrate the presence of neuro-filaments in the extensions (Fig. 3). The neurites as well as the neurons maintained their morphological and physiologi-cal characteristics during the 3 or6 weeks of the culture.
Neuronal replication of CVS. Infection of DRG cultures directly in the cloning cylinder showed that the main target cells ofthe CVS virus werethe large sensory neurons (Fig. 4). However, production of virus and its release into the culture fluid over a24- to30-h postinfection period required amultiplicity of infectionintherangeof20PFU/ml. Kinetics J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:2.612.63.300.70.300.2]JI
FIG. 2. Neuritic extensions. Neurites in the outer culture chamber 10 to 12 days after DRG cells were seeded into the inner culture compartment. Dependinguponrelative efficiencyoftreatmentwithcytosinearabinoside,the drug used to reducegrowthofcells exhibiting mitotic activity, theoutgrowing neurites were or were notassociatedwith Schwanncells. Magnification, x2,000.
ofreplication atthismultiplicity ofinfection, asdetermined by assaying the PFU in the culture fluid at various time intervals, isillustrated in Fig. 5. At48 hpostinfection (p.i.),
104-5
PFU/ml (n = 5) was determined in the culture fluid, whereas 104-0 PFU/ml was detectable as cell-bound virus.FIG. 3. Neurofilaments in neuritic extensions. Immunofluores-cencestaining usinganFITC-labeledantineurofilament monoclonal antibody.Magnification, x3,200.
The intracellular virus was set free by freeze-thawing the infectedcells in suspension. In this way 1 log PFU was lost (notshown),and it was estimated that approximately75%of thetotal amountof detectable infectious virusremainedcell associated. The CVS-infected DRG neurons seemed to be low producers of virus since only 1 PFU of virus was produced per cell over a 48-h period. On the other hand, the gross morphology of most neurons in infected cultures
appearedtobe rather wellpreserved over 2 to 4 weeks p.i.
One week after infection, the neurites became slender and
appearedto "float" in theculturemedium, sometimesbeing attached atbothendsonly. Infectedneurons in the cultures
survivedforat least4weeks,overwhich interval they were
continuouslyproducing infectious viralparticles.
Rabies virus
attachment
to neuronal extensions. CVS was inoculated into the outer compartment of a number ofcultures to a final concentration of
107.85
PFU/ml. Thecultures were incubated at 37°C, samples were drawn at
various times between 1 and 4 h p.i., and residual virus
activity was assayed. The virus concentration obtained
under these conditions was compared with the concentra-tions of virus remaining on plates containing no cells but
otherwisetreated similarlyand in parallelwith thecultures. Within 1 h after inoculation, 90% ofthe virus had disap-pearedfrom the culturefluid and apparentlybeenadsorbed
to neurites (Fig. 6). To visualize the virus attachment, neurites exposedto virusfor4h at
37°C
were examinedby
immunofluorescence. CVS virus showed a high adsorption affinity for the neurites of DRG cells, since viralantigens
were found to be adsorbed all along the neuritic plasma
membrane (Fig. 7).
Finally,although themajority of infected cells were neu-rons, viral antigens were also found to be associated with cells which by their
morphology
and appearance in the cultures were classified as Schwann cells. Thisfinding,
whichpointstothepossible infection ofSchwann cellswith
;NK'
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.612.53.295.441.698.2]2736 LYCKE AND TSIANG
.I._1Es.--<.:.n .. i...:
I.~~~~~~~~~~W
FI oio luoecn DR cll at172 hp withrabies viu (CS Imnfursecsting (A.a wth an FITlaele
anti-rabies virus nucleoproteinconjugate. (B) Phase-contrast microscope picture. Magnification, x3,200.
CVS virus, will not be discussed in the present paperbutwill be dealt with elsewhere.
Transportofrabies virus in neuronal extensions. Neurites were infected by adding the virus (1066 PFU in a 0.5-ml volume) to the outer compartment of DRG cell cultures. At 1 h p.i., nonadsorbed virus was removed and the medium was replaced with fresh medium. The cloning cylinder was
filled with medium containing 8 international units of anti-rabies virus serum per mltoexcludenonspecific extraneural spread ofvirus. Incubation of the culturesat37°Cresultedin
propagation of the infection from the neurites to the cell soma. The presence of neutralizing antibodies in the cell cultures did not significantly modify the infection; most of the neuronsin thecylinderwerefoundtobeinfectedasthey were inuntreated control cultures. At 24to 30 hp.i., afew
5r
41 E
U. IL
0)
0
-i
single neurons in the culture inside the diffusion barrier
showed the presenceof rabies antigenasrevealedby immu-nofluorescence (Fig. 8). The infection spread from these primarily infected neurons to the neuritic network. Foci of infectednervecellswereobserved whichinvadedthewhole culture (Fig. 9).
The rateofuptake and transport of viruswassubsequently
estimated from a series of experiments in which the time necessaryforestablishing infection inside thecloning
cylin-der was determined after the virus
(107-85
PFU/ml)
wasinoculated outside the diffusion barrier. The cultures were incubated at 37°C for 1 h, and after various times
ranging
from 1 to 24 h, neurites outside the barrierwere destroyed.
8.or
7.51
ILA U.
6
7.0
06.5 3
F
1 24 48 72 96
HOURS FIG. 5. Rabies virus(CVS)replication in rat sensory neurons of dorsalrootganglia. Amounts of virus recovered from cultureliquids
at various timesp.i.
---'A
I
I@
I ***
. . . . .
2 4 6
HOURS
FIG. 6. Attachment rate of CVS to to neurites of the outer culturechamber. Dotted line, Concentration ofresidual infectious virus,plotted against time. Solidline. Controldisplaying reduction of virus ofacell-fiee culture system.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.612.69.562.75.265.2] [image:4.612.69.304.478.694.2]FIG. 7. Rabies virusantigenattached to neurites. Immunofluo-rescence using FITC-labeled rabbit anti-rabies virus serum for demonstration of viral antigens on neuriticextensions.Theneurites wereexposed to virus for 4 h.Magnification. x4.O00.
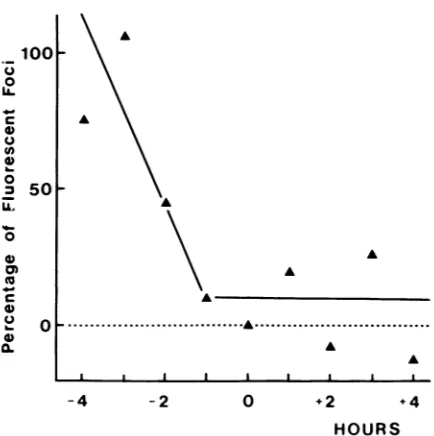
Thecultures were furtherincubated at 37°C in the presence of anti-rabies virus serum for a total period of 72 h, after which time the number of fluorescent foci was recorded. About 3.5 h was required for internalization and neuritic transport of the virus across the diffusion barrier (Fig. 10). Therefore a somatopetal transport rate of approximately 12 mm/day wasestimated. However, it cannot be excluded that the virus may also be transported at a higher rate. In
particular,single fluorescent neuronswereobservedassoon as 2or 3 h after neurite exposure to virus.
Drug-induced blockade of virus uptake and transport by neurites. The uptake, internalization, and neuritic transport of CVS virus wereexamined in the presence of two drugs,
cytochalasin B and vinblastine, which are known to affect plasma membrane andcytoskeleton functions. Rabies virus was alsoconsidered as a biological tracer for the inhibitory effectof colchicineonaxonal transport in cultured neurons.
Cytochalasin B and vinblastine were added to the outer chamber of cultures either simultaneously with or4 or24 h before CVS inoculation (0.5 ml ofa suspension containing
107.85
PFU/ml). The drugs were tested either separately at 0.1 mM and 0.01 mM or in combination at the sameconcentrations. Infected and treated cultureswereincubated at 37°C for a total period of 72 h. Fluorescent foci were
counted,andproduction ofinfectious viruswasdetermined. Atthe concentrations used, the drugs exhibited no cyto-toxic effects, since morphological changes in the neurites were not seen and CVS replication in DRG cells was not
inhibited. On theotherhand, bothdrugs suppressed uptake
andneuritic transport ofvirus,asindicatedbythereduction of the number of fluorescent foci and of the quantities of virus produced in the presence of the drugs (Table 1). The drugs were still effective in blocking virus transport by the neurites when added 4h p.i.
Colchicineataconcentrationof0.1 mM wasadded(0.5ml perculture) to the outer compartments of cultures showing many neuronal extensions. Colchicine treatment was per-formed either1 to 4hbefore, or atthe same timeas. or 1 to 4 hafter virusinoculation (0.5ml ofasuspensionwith
107.54
PFU/ml). It should be mentioned that colchicine did not inhibit replication of CVS virus in DRG cultures at the concentration used and didnotcauseanygrossmorhological
changes inexposed neurites throughoutthe
experiments.
Figure 11 shows the colchicine added 1 h p.i. or before
inoculation blocked infection spread across the culture bar-rier. In addition. at 2 h p.i. only about
50%
of the final number offluorescent foci, as related to untreated cultures, wasdetected, suggesting a partial impairment of the neuritic transport. Virus transport could not becompletely inhibitedby colchicine treatment, and residual transport was ob-served in different experiments.
DISCUSSION
In the present study, we have investigated on cultured neurons of rat DRG the involvement of sensory neurons in the pathogenesisof rabies virus. Immature neuronal cellsin culture are induced by nerve growth factor to differentiate
with theformation of neurite extensions. Within a few days, youngneurites develop into what appears to be axons (16), and theirunordered somallike cytoplasmgraduallyacquires the highly ordered, adult neuritic cytoskeleton structure (25). Taking advantage of the nerve growth factor-induced
differentiation of neuronal cells, we have studied the inter-actionofrat sensory neurons withrabies virus (CVS strain), using a compartmentalized cell culture system. We have focusedourinterestonthe axonal transport of rabies virus. Fixed rabies virus (CVS strain) was capable of readily infecting DRG neurons withoutrequiring prioradaptationby serial passages, which points to the natural susceptibility of these cells to the virus. Rabies virus exhibited a very high binding affinity for theunmyelinated neurites of rat sensory neurons. Some
90%
of the added virus was taken up from the culture fluid within1 h, and viralantigens were presentinthe neuronal extensions as shown by immunofluorescence. In-terestingly, accumulation ofrabies virus antigens of experi-mentally infected animals occurred in the muscular stretch propioceptors displaying unmyelinated threads of sensory nerve endings (35).FIG. 8. Axoplasmic transport ofCVS nucleocapsids. Immuno-fluorescence using FITC-labeled rabbit anti-rabies virus nucleo-capsid serum for
demonstr-ation
of CVS nucleocapsids in neuritesfromaneuron24 h p.i.
Magnification.
x3,200.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.612.51.291.71.247.2] [image:5.612.313.553.439.688.2]2738 LYCKE AND TSIANG
FIG. 9. The neurons and neurites which constitute the neuronal network in the inner chamber of the cylinder were infected and subsequently incubated (in the presence ofanti-rabies virus antibodies). The cultures were stained with anti-rabies virus nucleoprotein fluorescent conjugate 72 h after virus inoculation. Magnification, x3,200.
At present it is difficult to evaluate to what extent this binding involves virus-specific receptors. The rabies virus
receptorhasnotbeen identifiedasyet.Arole forglycolipids
in rabies virus binding (42), which would be mediated by
Q 100
o
A.,
CL
4,,.
0
X~~~~~~:
A:
_
0 2 4 6 8 24
HOURS FIG. 10. Rateofaxoplasmictransportof rabiesvirus in neurites. NeuritesintheouterculturecompartmentwereexposedtoCVS.At various intervals ranging from 1 min to 24 h p.i., all neuritic extensions outside the diffusion barrier of the culture were
elimi-natedby treating theouterculture compartmentwith H2S04. The
culturesweresubsequently incubatedat37°C foratotal period of72
h, after which time the number of fluorescent foci in the inner
culture compartment was recorded. The results are the mean of
three experiments. The number offluorescent foci isexpressedas
the percentageof the resultsobtained withanuntreatedbutinfected
control.
their sialic acid residues
(40),
appearslikely.
Consequently,
cellular
gangliosides might
be anintegral
part of the rabies virus receptor(41).
It is worth mentioningthat thenatureofgangliosides
variesaccording
tonerve celltypes (2).Although
neurons werepredominantly infected,
infection also affected other cell types (23, 34, 39). In aprevious
paper, we have reported that infection ofdissociated cells from the mouseDRG concerned 10% of nonneuronalcells,
some of which were identified as Schwann cells (47). It appears to us that rat Schwann cells can also beinfected,
although
their relative susceptibility to rabies virus is dif-ficult to evaluate(unpublished data).
The possible involve-ment ofSchwann cells in rabies pathogenesis is still tobe established.Itis welldocumented that theaxonaltransportmachinery
is also capable ofdirectingmovement offoreign
organelles
and particles such as injected synaptic vesicles and inert beads with charged surfaces (1, 6, 37). The rate of axonal transport of rabies virus in neuritic extensionswasestimated tobecloseto12mm/day,as2mm wastheminimaldistance TABLE 1. Neuritic transfer of rabiesvirus after treatment of
neurites with cytochalasinB andvinblastine"
Treatment" Time p.i. No.sc f LogPFU/ml
(h) fluorescent foci LgPUm
None 0 205(100) 2.54
Cyt 0 9(4) 0
Vin 0 12(6) 0
Cyt + Vin 4 22(16) 0
Cyt + Vin 24 190 (93) 2.17
"Rabies virus was inoculated into the outer culture compartment. The
presenceofviral antigens (fluorescent foci)orinfectious virus in the inner
culturecompartment at72 hp.i.wasconsideredasanindication ofneuritic
transfer of virus across thebarrierbetween thecompartments.
'Finalconcentrations ofcytochalasin B(Cyt) and vinblastine (Vin)were
0.1and 0.01mM, respectively.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.612.125.515.76.325.2] [image:6.612.328.568.606.678.2]FIG. 11.
Neurites in
colchicine (( after(-)ino
innercultur4
centfoci der Theresults;
percentages
tobe runf
barrier of t hwasthet
isconsider simplex vii (11). The r
acetylcholi mm/dayin direction, c axonaltrar
axonal trar
since the ti events, i.e
port. In the tion by ne
fusion ofe arapidrea
is likelyto transport.
not yet kr
microscope
shortly aft enveloped
may be me
E. Lyke, a pits were infection o
cervical ga
involve co, some time
rateofvirn
Recently ro-9-3-(2-hl intracellula
various wa
axons
werefound
to block the transport of the lectin wheat A germagglutinin
and of herpessimplex virus
in neurites of DRGcells(30). Thiseffect wasascribed to the action of the drugs on microtubular transport. Consistent with the prob-able importance of plasma membrane and cytoskeletonA \ activities for neuritic uptake and transport of viruses, we
found that both cytochalasin B and vinblastine efficiently blocked the neuritic transferofrabies virusinthe DRGcell cultures. Cytochalasin B inhibits microfilament formation by
v preventing actin from assembling and consequently blocks
endocytosis (9, 33). Cytochalasin B could also block axonal transport by abolishing microtubule formation (22).
Micro-A tubule inactivation resulting in transport dysfunction can
A also be caused by vinblastine, which is supposed to induce
A microtubule aggregation (7). In addition,
colchicine,
which is... most commonly used for
blocking
axonal transport,exer-A cises a
depolymerizing
effect on microtubules. We verifiedA that colchicine inhibited neuritic transfer of rabies virus
l I when it was administered either
before,
or at the same time-4 -2 0 +2 +4 as, orup to 1 hafter virusinoculation. At 10FM
concentra-HOURS tion. maximal inhibition of microtubule
assembly
inHOURS neuropils required 1h(36). Weconclude from these data that Effect ofcolchicineonneuritictransport of rabiesvirus. both tubulin- andactin-containing structures appeared to be the outer culture compartment were treated with involved in the neuronal transport of rabies virus.
However,
D.1 mM)eitherbefore (+).oratthesame timeas(0),
or therelative
participation
of each structure is not known at culation of CVS.The amountof virus transportedto theecompartment was recorded as the number of fluores- present.
nonstrable after atotalincubationperiod of 72 h at37C. The rat DRG sensory neuron can be described as being arethe mean ofthreeexperiments and areexpressed as permissive but only moderately susceptible torabies virus, of the results obtained with untreatedcontrol. which is suggested by the large multiplicity of infection necessary for establishing a productive infection. This con-trastswith thehigh susceptibility usually found with primary ortransferring infection across the diffusion-tight cultures ofneurons derived from the mouse and rat CNS (47; he compartmentalized culture system and 3.5to 4 unpublished data). Furthermore, the rat sensory neuron ime necessary forthistransferto occur.Thisrate appears capable ofproducing only relatively small amounts ably less thanthatpreviously reported forherpes of virus. We estimated that there was about 1 PFU of rus (50 mm/day), using the same culture system infectious virus produced per DRG neuron over a 48-h rapid phase of the axonal transport of molecular period, provided that no additional DRG cells in the culture ine esterase hasbeen estimated to be close to 150 became productively infected. The observation of a rela-theretrograde and410mm/day in the anterograde tively low virus production by the neuronal cells correlates and the corresponding slow phase of anterograde well with the scarcity of virus when infected neurons were
isport is estimated as 2.9 to 5.1 mm/day (13). The examined by electron microscopy (M. Derer, unpublished nsport rates are difficult to evaluate for viruses, data). Again, it is interesting to compare these results with me measured isusually theresult of asequence of the production of high yields of infectious virions by mouse ., attachment, internalization, and axonal trans- and rat CNSneurons (10- to 100-fold) (47; unpublished data)
ecase ofherpes simplex virus, whoseinternaliza- and to note the incapacity of superior cervical ganglia urites and nonneuronal cells seems to occur by neurons to replicate infectious rabies virions (45). Taken all nvelope and plasma membrane (18, 32), which is together. these results are in favor of the existence of various ction, the experimentally estimated transport rate levels in neurotropism. To what extent the low production of reflect more accurately the actual rate of axonal virus isrelated to the stability of neurons ininfected cultures The neuritic uptakemechanism for rabies virus is and to the prolonged production of virus remains to be iown. However, from our preliminary electron specified. The finding that rabies virus-infected DRG
neu-e observations, the virus found in the nerve cells rons can be maintained in culture for over 4 weeks demon-ter inoculation of the cultures appeared to be strates the capacity of this neuronal cell type to harbor the and internalized by coated pits, and thus uptake virus for long periods of time. Consequently, DRG neurons diated by an endocytosislike process (M. Derer, aregood candidates as sites for the protracted harboring of nd H. Tsiang, unpublished data). In fact, coated virus in naturally occurring cases of rabies exhibiting pro-observed in the early phases after rabies virus longed incubation times.
ofchicken embryo-related cells (40) and superior The observation that isolated neurons or isolated groups Lnglia neurons (45) If virus internalization were to of neurons can be infected through the neuritic network ated pits, virus uptake by neurites could require despite the presence of
neutralizing
antibodies deserves to and thus be responsible for the seemingly slow be discussed. This finding is tobe related tothe differential is axonal transport. cell-to-cell virus transmission ofpathogenic
andapathogenic
, threesubstances [taxol, nocodazole, and eryth- rabies virus strains (17). In ourstudy,
we observed the ydroxynonyl)adenine] which are known to inhibit infection of neuronspresumably
connected to the neuritestr transport of endosomes and to interfere in extending in the external compartment, followed
by
the tys with the retrograde transfer of organelles in progressiveinfection ofall theneurons in the inner compart-1oo0501F
U.)
-c
0 n
0 0
U)
C.) 0
0
on November 10, 2019 by guest
http://jvi.asm.org/
2740 LYCKE AND TSIANG
ment. Since this infection occurred in the presence of
neutralizing antibody even when the neurons were not contiguous,wepostulate that viral spreadoccursby neurite-to-cellcontact. This clearly shows that once rabies virus is
hidden inaneuronalcircuitry, itescapestothe host defenses andcanbe transported fromone neurontothe next. In this respect, theculture device described in thispapermimicsa brain model of rabies virus infection in which there is an
entrypathway and a neuronal network.
The relatively low susceptibility of DRGneurons,coupled
with alow capacity for producing viral particles,can bean advantage for this celltype, enablingitto sustainlong-term infection. DRGneurons have already been shown to repre-sent an excellent environment for persistent infection with other viruses, such as herpesvirus (29). It is tempting to
assume that rabies virus preferentially infects central
neu-rons(47) andmotorneurons(44), resultingin theproduction ofhigh yields of rabies virus, whereas DRG neurons should rather be considered as sites for virus entry in a persistent infection cycle. We have already shown that myotubes, which allow an abortive rabies virus infection to develop, could be a site where rabies virus canpersist extraneurally (44) andthat thesuperiorcervicalganglianeurons,whichare relatively resistant to rabies virus infection, could also harbor the virus forlong periodsof time withoutexcretingit (45). Thusavariety of celltypeswhicharenaturally
suscep-tible to rabies virus infection could harbor the virus and protect it from the host's natural defenses until an event triggers viral replication.
There is increasing interest in the investigation of virus circulation in the nervous system. As for rabies virus, herpesvirus transport has been reported in the brain (5, 12, 29)as wellasin culturedneurons (32, 49). The involvement of the neuralpathwaysinthetransportofmanyother viruses has been invoked, including the scrapie agent(28),
poliovi-rus (14), reovirus (48), and Semliki forest virus (27). The compartmentalized cell culture system described here for rabies virus is a useful tool for the investigation of neurotropic virustransport incultured neurons.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of Janine Cadinuand Simone Guillemer, and we thank Bernard Bizzini for reviewingthemanuscript.
Thestudywassupported bygrants from the Institut National de LaSantdetdelaRecherche Medicale (CRE 846019)and from the
Swedish Medical Research Council(4514).
LITERATURE CITED
1. Adams, R. J., and P. Bray. 1983. Rapid transport offoreign particles microinjected into crab axon. Nature (London) 303: 718-720.
2. Ando, S. 1983. Review. Gangliosides in the nervous system. Neurochem. Intern. 5:507-537.
3. Atanasiu, P.,H. Tsiang, and A. Gamet. 1974. Nouveau vaccin antirabique humain de culture cellulaire primaire. Ann.
Micro-biol.(Paris) 125B:419-432.
4. Baer,G. M. 1975. Pathogenesistothecentral nervoussystem,
p. 181-198. InG. M. Baer(ed.), The natural history of rabies.
AcademicPress, Inc., New York.
5. Bak,I.J., C. H. Markham, M. L. Cook, and J. Stevens. 1977.
Intraaxonal transportofherpes simplex virus in theratcentral
nervoussystem. Brain Res. 136:415-429.
6. Beckerle, M. C. 1984. Microinjected polystyrene beads exhibit saltatory motion in tissue culture cells. J. Cell Biol. 98: 2126-2132.
7. Bensch,K.G.,and S. E. Malawista. 1969. Microtubularcrystals
inmammaliancells. J.Cell Biol. 40:95-106.
8. Bijlenga, G., and T. Heaney. 1978. Post-exposure local treat-ment of mice infected with rabies with twoaxonalflow inhibi-tors,colchicineand vinblastine. J.Gen.Virol.39:381-385. 9. Brown, S., and J. Spundich. 1981. Mechanism of action of
cytochalasin: evidence that it bindsto actin filament ends. J. Cell Biol. 88:487-491.
10. Burrage, T. C., G. H. Tignor, and A. L. Smith. 1985. Rabies virusbindingatneuromuscularjunctions.Virus Res.2:273-279. 11. Campenot,R.B.1977.Local control of neuritedevelopmentby
nervegrowthfactor.Proc.Natl. Acad.Sci. USA 74:4516-4518.
12. Cook, M. L., and J. Stevens. 1973. Pathogenesis of
herpetic
neuritisandganglionitisin mice: evidence for intraaxonal trans-portofinfection. Infect. Immun. 7:272-288.13. Couraud,J.-Y.,and L.Giamberardino.1982. Axonaltransport of the molecular forms of AChE: its reversal at a nerve transection,p. 144-152. In D.G. Weiss(ed.),Axonaltransport. Springer-Verlag, Berlin.
14. DalCanto, M. C., R. L. Barbano, and B.Jubelt. 1986. Ultra-structural immunochemical localization of poliovirus during
virulent infection of mice. J. Neuropathol. Exp. Neurol. 45: 613-618.
15. Dean,D.J.,W.M.Evans,and R.C. McClure. 1963.
Pathogen-esis ofrabies. Bull. WHO29:803-811.
16. Dichter, M.A., A. S. Tischler,and L. A.Greene. 1977. Nerve growthfactor-inducedchangeinelectricalexcitabilityand
ace-tylcholine sensitivity of a rat pheochromocytoma cell line. Nature(London)258:501-504.
17. Dietzschold, B.,T.J.Wiktor,J. Q.Trojanowski,R. I. Macfar-Ian, W. H. Wunner, M. J. Torres-Anjel, and H, Koprowski.
1985. Differences in cell-to-cell spread of pathogenic and apathogenicrabiesvirus in vivo and invitro.J. Virol.56:12-18. 18. Fuller,A.O.,and P.G.Spear.1985.Specificitiesof monoclonal and polyclonal antibodies that inhibit adsorption of herpes
simplex virus to cells and lackof inhibitionbypotent neutral-izingantibodies. J. Virol. 55:475-482
19. Gillet,J.P.,P.Derer,and H.Tsiang. 1986.Axonaltransport of rabies virus in thecentralnervoussystemoftherat. J. Neuro-pathol. Exp. Neurol.45:619-634.
20. Harrisson,A.K.,and F. A.Murphy.1978. Lyssavirusinfection of muscle spindlesand motorend-platesinstriated muscles of hamsters. Arch. Virol. 57:167-175.
21. Hauw, J. J., S. Fenelon, J. M. Boutry, Y. Nagai, and R. Escourolle. 1981. Effect of braingangliosidesonneuritegrowth
inguineapig spinal gangliatissue cultures andonfibroblastcell cultures, p. 171-175. In M. M. Rapport and A. Gorio (ed.),
Gangliosides in neurological and neuromuscularfunction, de-velopmentand repair. Raven Press,NewYork.
22. Isenberg, G., P. Schubert, and G. Kreutzberg. 1982. Actin, a
neuroplasmic T constituent requisite for axonal transport, p. 314-321. In D. G. Weiss (ed.), Axonal transport.
Springer-Verlag,Berlin.
23. Iwasaki, Y.,andH. F. Clark. 1975.Celltocelltransmission of virusin thecentral nervoussystem. II. Experimentalrabies in
mouse.Lab. Invest. 33:391-399.
24. Iwasaki, Y., D.S. Liu, T.,T.Yamamoto,and H. Konno. 1985. On the replication and spread of rabies virus in the human central nervous system. J. Neuropathol. Exp. Neurol. 44: 185-195.
25. Jacobs,R.J.,andJ.K. Stevens.1986.Changesin the organiza-tion of the neuritic cytoskeleton during nerve growth factor-activated differentiation ofPC12 cells: a serial electron micro-scopic studyof thedevelopmentand controlofneuriteshape.J. Cell Biol. 103:895-906.
26. Johnson, R. T. 1965. Experimental rabies. Studies of vulnera-bility and pathogenesis using fluorescent antibody staining. J. Neuropathol. Exp. Neurol. 24:662-675.
27. Kaluza, G.,G.Lell,M.Reinacher,L.Stitz,and W. R.Willems. 1987.Neurogenic spread of Semliki forestvirus in mice. Arch. Virol. 93:97-110.
28. Kimberlin, R. H., and C. A. Walker. 1986. Pathogenesis of scrapie (strain 263K)inhamsters infectedcerebrally, intraperi-toneally orintraocularly.J. Gen. Virol.67:255-263.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
29. Klein, R. J. 1982. Thepathogenesis of acute, latent and recur-rentherpes simplex virus infection. Brief review. Arch. Virol. 72:143-168.
30. Kristensson, K., E. Lycke, M. Ryotta,B. Svennerholm, andA. Vahlne. 1986. Neuritic transport of herpes simplex virus in rat sensory neurons invitro. Effects of substances interacting with microtubular function and axonal flow (nocodazole, taxol and erythro-9-3 (2-hydoxynonyl) adenine). J. Gen. Virol. 67:2023-2028.
31. Kucera, P., M. Dolivo, P. Coulon, and A. Flamand. 1985. Pathway of the early progression of virulent andavirulentrabies strains from the eye to the brain. J. Virol. 55:158-162. 32. Lycke, E., K. Kristensson, B.Svennerholm, A.Vahlne,and R. J.
Ziegler. 1984. Uptake and transport of herpes simplex virus in neurites of rat dorsal root ganglia cells in culture. J.Gen. Virol. 65:55-64.
33. MacLean-Fletcher, S., and T. D. Pollard. 1980. Mechanisms of actionofcytochalasin B on actin. Cell 20:329-341.
34. Matsumoto, S. 1975. Electron microscopy of central nervous system, p. 217-235. In G. M. Baer (ed.), The natural history of rabies. Academic Press, Inc., New York.
35. Murphy, F. A. 1977. Rabies pathogenesis. Brief review. Arch. Virol. 54:279-297.
36. Reibman, J., K. A. Haines, A. M. Rich, P. Cristello, K. N. Giedd, and G. Weissmann. 1986.Colchicine inhibits ionophore-induced formationof leukotriene B4 by human neutrophils. The role ofmicrotubules. J. Immunol. 136:1027-1032.
37. Schroer, T. A., S. T. Brady, and R. B. Kelly. 1985. Fastaxonal transportofforeign synapticvesiclesinsquid axoplasm. J. Cell Biol. 101:568-572.
38. Smith, A. L., G. H. Tignor, K. Mifune, and T. Motohashi. 1977. Isolation and assay of rabies serogroup viruses in CER cells. Intervirology 8:92-99.
39. Sung, J. H.,M.Hayano,A.R.Mastri,and T.Okagaki.1976. A case of human rabies and ultrastructureof the Negri body. J. Neuropathol. Exp. Neurol. 35:541-559.
40. Superti, F.,M.Derer,and H.Tsiang.1984.Mechanism of rabies virusentryintoCER cells. J. Gen. Virol. 65:781-789. 41. Superti, F., B. Hauttecoeur, M. J. Morelec, P. Goldoni, B.
Bizzini, and H. Tsiang. 1986. Involvement ofgangliosides in rabiesvirus infection. J. Gen. Virol. 67:47-56.
42. Superti, F., L.Seganti, H. Tsiang,and N. Orsi. 1984. Role of phospholipids in rhabdovirus attachment to CER cells. Arch. Virol. 81:377-382.
43. Tsiang,H. 1979. Evidence foranintraaxonal transport of fixed
andstreet rabies virus. J. Neuropathol. Exp. Neurol.
38:286-296.
44. Tsiang, H., S. DeLaporte, D. J. Ambroise, M. Derer, and J. Koenig. 1986. Infection of cultured ratmyotubes and neurons from the spinal cord by rabies virus. J. Neuropathol. Exp. Neurol. 45:28-42.
45. Tsiang, H.,M.Derer,andJ. Taxi. 1983. An in vivo and in vitro study of rabies virus infection of the rat superior cervical ganglia.Arch. Virol. 76:231-243.
46. Tsiang, H., and J. C. Guillon.1981.Presence of specificantigens in neuronal cells infected with fixed and street rabies virus strains. Acta Neuropathol. 55:263-267.
47. Tsiang, H., A. Koulakoff, B. Bizzini, and Y. Berwald-Netter. 1983. Neurotropismof rabies virus. An in vitro study. J. Neu-ropathol. Exp. Neurol. 42:439-452.
48. Tyler, K. L., D. A. McPhee, and B. N. Fields. 1987. Distinct pathways of viral spread in the host determined by reovirusS1 gene segment. Science 233:770-774.
49. Ziegler, R. J., and R. E. Herman. 1980. Peripheral infection in culture ofratsensory neurons by herpes simplex virus. Infect. Immun. 28:620-623.