Vol. 65, No. 12 JOURNALOFVIROLOGY, Dec. 1991,p. 6782-6789
0022-538X/91/126782-08$02.00/0
CopyrightC) 1991, American Society for Microbiology
Translational Control of
Human
Cytomegalovirus
gp48
Expression
MARKR. SCHLEISS,t CATHERINER. DEGNIN,AND ADAM P. GEBALLE* Departmentof Molecular Medicine, Fred Hutchinson CancerResearch Center,
1124Columbia
Street,
Seattle,
Washington
98104Received 10 June 1991/Accepted 29 August 1991
Posttranscriptional controls modulate the expression of several human cytomegalovirus genes. Previous studies have shown that one cytomegalovirus gene transcript leader contains AUG codons which inhibit translation ofadownstream reading frame. However, twoothercytomegalovirus genetranscript leaders of similarstructure donot inhibit translation. We have extended these studiestothe analysisof the structural glycoproteingp48, whose predominant transcript contains threeupstreamAUG codons. The 5' leaderof this transcript strongly inhibits downstream translation in fibroblasts. Analysesof deletions and point mutations identify the second upstream AUG codon as an essential componentof the inhibitory signal. Other leader sequences,butneither the firstnorthethird AUG codon,arealsorequired.Intriguingly, the inhibitory signal appearsalso todependonthe amino acid coding information of the short readingframe associated with the second AUG codon. Insights derived from these studies are germane to understanding the translational regulation of other viral and cellulargenesof similarstructure.
Human cytomegalovirus (CMV) is a medically important herpesvirus responsible for severe infections in newborns andimmunocompromisedpatients (reviewed in reference 1). The 230-kb CMV genome encodes approximately 200 genes (7) whose expression is temporally and coordinately regu-lated (9, 22, 35, 36) during infection of human fibroblasts (HF) in cell culture. Although much research has concen-trated on the transcriptional events responsible for the
differential expression of a (or immediate-early), C (or ear-ly), and -y (or late) genes, posttranscriptional events also
influence the expression of CMV genes (reviewed in refer-ence 24). The expression of the majorot genes (33, 34) and
several 3 and y genes (12-15, 32, 38) is regulated in part posttranscriptionally, although the detailed mechanisms and
significanceof these controls during the viral life cycle have not been well characterized.
Previous studies of CMV posttranscriptional controls fo-cusedon a I gene
(2.7p)
encodinga2.7-kbtranscript whose 5' leadercontains cis-acting inhibitory signals (14). Either oftwoupstreamAUG codonswithin this leaderis required for inhibition of downstream translation (13). The presence of AUG codons in the 5' transcript leaders of several other CMV genes suggested that upstream AUG codons might be common signals mediating translational control of CMV gene expression. However, further studies demonstrated that the transcript leaders of two of these genes, the CMV DNApolymerase andpplS0,permittedefficient downstream
expression (3). Thesestudiesdemonstrated that the transla-tionalimpactof upstream AUG codons is unpredictable and maydepend on other sequences within the transcript leader
(3).
Wenowreport resultsof similar investigations of another CMV gene, the glycoprotein gp48 (UL4), whose protein product is synthesized as a
P
protein that is present in virions(6).Thegp48 open reading frame (ORF) is contained in two
p
(El
andE2)
andone-y(L)
transcript
withdifferent 5' endsandidentical3' ends (5). The 5' leaderof the most abundant
* Correspondingauthor.
tPresentaddress: Department ofPediatrics, ChildrensHospital ResearchFoundation, Cincinnati, OH45229.
of these transcripts (El) contains three upstream AUG codons with associated short reading frames and inhibits downstream translation in cell extracts (6). Our studies demonstrate that thegp48 leader inhibitstranslationin intact cells. In contrast totheconclusions drawn from the studies using cellextracts(6),wedemonstrate that the second of the three upstream AUG codons is an essential component of the inhibitory signal in cells. Surprisingly, downstream
se-quences includingtheauthentic amino acidcoding
informa-tion of the short ORF associated with this second AUG codon arealsorequiredfor translational inhibition.
MATERIALS ANDMETHODS
Cells, virus, and plasmids. Human CMV(Towne) was
grown in HF in Dulbecco's minimum essential medium
supplementedwith 10%Nuserum(Collaborative Research,
Inc., Bedford, Mass.) as described previously (30). CMV
genomic DNAwas purified overNaI gradients (31).
Thetransfectioncontrolplasmid pEQ134has been previ-ouslydescribed(3). pEQ3 (Fig. 1)isapromoterless
P-galac-tosidase
(P-gal)
expression plasmid derived frompON1 (30) byfirstinsertinga514-bpRsaIfragmentfrompEMBL8 (10), containingthefloriginofreplication,intoaPstl site(withinsimian virus 40-derived sequences) that had been blunted with T4 DNA polymerase. The fl origin in this plasmid
directspackaging of singlestrandscorrespondingtothe
plus
senseofp-gal.
ThepBR322originofreplication,removedas a ScaI/SmaI digestion of this plasmid, was replaced withpUC19 sequencesfrom position 805 to Scal containingthe
pUC19 plasmid origin of replication. Addition of a SmaI linkertothepUC19 fragmentendatposition805
unexpect-edly resulted in insertion of nine C's, including three C's which restored the SmaI site. The polylinker of pON1
between SmaI and Hindlll was replaced with a Small HindlIl pUC18 polylinkerthathad been
digested
withSphI,
blunted with mung bean nuclease, andreligated. The
oligo-nucleotide AGCTTACGTAGATCTCGAGCCATGGTAC was used to replace the sequences from HindIII to KpnI, including part of the pON1 polylinker and 5'
p-gal
leader(including all three in-frame AUG codons [25]) with new
6782
on November 10, 2019 by guest
http://jvi.asm.org/
A Eq I n gp48
+1 ,pE2
+12 rr pEE2!
+64 +86 n
+122
+198
39 51 pEO296
1-. pE0290
-_ pEO256
!-. pEE258
B 3,000
SmalXbal Sall PstlHlndiliSnaBIBgIllii Asp718
-.. I...
r
J pEQ176 FIG. 1. 1-Gal plasmids. The promoterless 13-gal plasmid pEQ3 wasconstructed as described in Materials and Methods. In addition tothe indicated unique restriction sites, the AUGcodon (boxed) thatinitiates translation of,3-galiscontainedin the multiple cloning site(MCS)atthe5'endof theE.coli lacZgene.pEQ176contains theCMV(Towne) majoraenhancer/promoter(Enh-Pr)derivedfrom pON249 (14), corresponding to bp 174873 through 173749 ofthe CMV(AD169) genome (GenBank accession number X17403) in-serted into thePstIIHindIII sitesofpEQ3. SV40, simian virus 40; ori, origin ofreplication.
polylinkersequences andasingle 1-gal translationinitiation codon.
A1.1-kb
PstIIHindIII
fragment from pON249 (14)contain-ing the CMV major aenhancer/promoter was inserted into thePstIIHindIII sites of pEQ3, generating pEQ176 (Fig. 1). The228-bpgp48 transcript leader sequence wasamplified by the polymerase chain reaction (PCR) from CMV(Towne)
genomic DNA, using the oligonucleotides GATCAAGCTT
AATCAGATGCCGGCCTTGTGATGCAG and GATCGG
TACCATCATAACGATACTCTTTCAGCCTTAC (gp48.3),
digested with Hindlll and Asp 718, and inserted into the
HindIIIlAsp 718 sites of pEQ176, resulting in pEQ239. pEQ359 was constructed in the same way but using CMV
(AD169) DNAinsteadof CMV(Towne) DNA as thetemplate
for PCR amplification.
The 5' deletion mutants (Fig. 2A) pEQ251, pEQ296,
pEQ290, pEQ256, andpEQ258wereconstructed by digest-ingtheCMV(Towne) gp48 leaderfragmentwithNaeI,FokI, AflII, RsaI, and SspI, respectively, and blunting the 5'
overhanging ends with DNA polymerase I, Klenow. After
beingcutwith Asp718,theresulting fragments wereligated into the BglII site (which had been blunted with DNA
polymeraseI,Klenow) and Asp 718 site ofpEQ176.Because
these 5' deletion fragments were inserted into the blunted
BglII site, they were expected to express transcripts with ninepolylinker-derived nucleotides,notpresentonpEQ239, attheir 5' ends (seeFig.4). The 3' deletionmutants(seeFig. 6A) pEQ323, pEQ312, pEQ330, and pEQ313 were
con-structed by digesting the CMV(Towne) gp48 leader
se-quenceswithSspI, RsaI,
AfllI,
andFokI,respectively, andblunting the 5' overhanging ends with DNA polymerase I, Klenow. After being cut with HindIII, the resulting
frag-Z
r_
a)
0 c
4)
0 U)
LD
0 .2 z:n
2,000
1,000
c
X U) 0 , _ ED 0 (0(O pl. C' U 0)0 U) U
N N N N N N
-,: 28s
f<,. .O.-.- ..% ad 0-gal
18S
a1 I -, -; * - pEO134
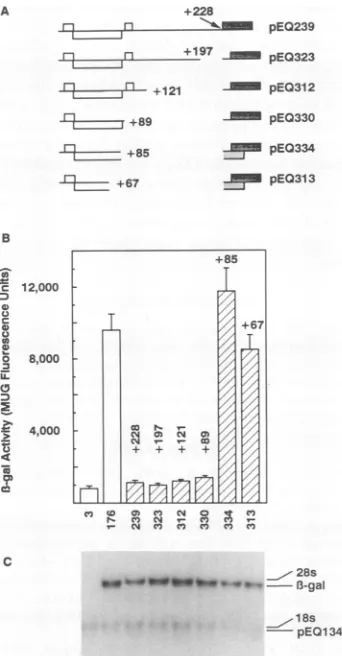
FIG. 2. Inhibitionby gp48leader and 5'deletions.(A)The 5' end ofthe gp48 El transcript (top) contains three short ORFs (white
rectangles)upstreamofthegp48protein coding ORF(cross-hatched
rectangle). pEQ239 containing the entire 228-nt gp48 leader up-streamofthe
13-gal
ORF(grayrectangle), and derivatives deletedto the indicated 5'positionswereconstructedasdetailed inMaterials andMethods. (B)13-Gal
activity expressedasarbitrary fluorescence units(meansand standard deviationsoftriplicate60-mmdishes)wasassayed by fluorescence measurements of medium of cells
trans-fected with the indicated control (openbars) orgp48test (striped
bars)plasmidsand then infectedwith CMV andfed withmedium containing MUGasdescribedinMaterialsand Methods.Numbers above thebarsrefertothe 5'end ofthegp48 leader in eachplasmid;
numbers belowthebarsrefertothepEQ number ofthetransfected plasmid. (C) Whole cellRNAharvested fromthesamecells usedto measure
1-gal
activity wasdetected on aNorthernblothybridizedwith a32P-labeledpEQ3 probe. The positions of 18S and 28SrRNAs and transcripts derived from test plasmids
(1-gal)
and from the enzymatically inactive transfection control plasmid pEQ134 are indicated.ments were
ligated
into pEQ176attheHindlll site andtheBglII site that had been blunted with DNA polymerase I,
Klenow. pEQ334 was derived from pEQ330 by
digestion
with
AflII,
blunting of the ends with mung bean nuclease,andreligation.
CMV(Towne)genomicDNAwasPCR
amplified
with thegp48.3
oligonucleotide
and theoligonucleotide
GATCAAGCTTAATCAGATGCCGGCCTTGTGAAGCAGCCG
tomu-tatethesecond AUG codon in thegp48leadertoAAG. The
amplified fragment was
digested
with HindIII andAsp 718 and inserted into the HindIIIlAsp 718 sites ofpEQ176
toon November 10, 2019 by guest
http://jvi.asm.org/
[image:2.612.356.528.79.411.2] [image:2.612.91.274.82.300.2]6784 SCHLEISS ET AL.
generate pEQ325. TheNaeIlKpnI gp48 leader fragment from pEQ325 was inserted into the BglII (blunted with DNA polymerase I, Klenow) and KpnI sites of pEQ176, resulting inpEQ332. TheHindIII/Asp 718 fragment derived from PCR amplification of pEQ313 with the mutantoligonucleotide and the ,B-gal2 primer (3) was inserted into theHindIII/Asp 718 sites of pEQ176, yielding pEQ333. The double-frameshift mutant pEQ370 was constructed by PCR amplification of CMV(Towne) genomic DNA with the oligonucleotides GA
TCAAGCTTAATCAGATGCCGGCCTTGTGATGCCAGC
CGC and CACTTAAGGCGCCGGATGTA. The amplified fragment was cut with HindIll and Aflll and inserted into
HindIII/AflIl
sites of pEQ239. All plasmid constructionswere verified by sequencing, using the Sequenase sequenc-ingkit (U.S. Biochemical Corp.) asdescribed by the manu-facturer.
Transfection assays. Plasmid DNA was transfected into HF, using DEAE-dextran as previously described (14, 30). Twenty-four hours later, cells were infected with CMV (multiplicity of infection = 10) and then fed with medium containing 0.44 mM
methylumbelliferyl-13-D-galactoside
(MUG; Sigma Chemical Co.) and 1% dimethyl sulfoxide.
13-Galactivity was measured in the cell culturemediumat27 hpostinfection as previously described (3). Thefold inhibi-tion attributed to a gp48 leader plasmid was calculated as the ratio of
3-gal
expression by the leaderlesscontrol pEQ176 to that of the gp48 leader plasmid after subtracting the 1-galactivity from cells transfected with the promoterless control pEQ3 from each value. Transfection data shown are mean (and standard deviation) ,-gal activities from triplicate 60-mm dishes.
RNA and polyribosome analysis. Whole cell RNA from transfected cells was solubilized inguanidinium isothiocya-nate, pelleted through CsCl, and analyzed on Northern (RNA) blots as previously described (12). Primerextension assays were performed as described elsewhere (21).
Polysomes were preparedfromtransfectedfibroblaststhat were infected with CMV for 24 h and thentrypsinizedin the presence ofcycloheximide (100 ,ug/ml). After resuspension andswelling of cells from two 150-mmplates in 0.75 mlof 20 mMTris-HCl (pH7.5)-10 mMNaCl-3 mMMgCl2(LSB) at 4°Cfor 3 min, 0.25 ml of LSB containing 1.2% Tritonn-101 and 0.2 M sucrose wasadded. ThesuspensionswereDounce homogenized and centrifuged briefly at 12,000 x g, 4°C.
Supernatants containing cytoplasmic extracts were loaded onto 15 to 50% sucrose gradients in LSB and centrifuged (222,000 x g, 110 min, 4°C). Fractions were collected with an ISCO model 185 collection system and, following
diges-tion with 20 ,ug of proteinase K per ml in1%sodiumdodecyl
sulfate, were phenol-chloroform extracted, ethanol
precip-itated, and analyzed on Northernblots.
RESULTS
The gp48 transcript leader inhibits expression of a down-stream reading frame. Using the same approach previously taken tocharacterize translational signals within other CMV transcript leaders (3, 13, 14), we analyzed reporter gene expression after transient transfection of plasmids that ex-pressed chimeric transcripts containing gp48 transcript leader sequences linked to the Escherichia coli lacZ gene. The control plasmid pEQ176 was constructed by inserting the CMV major ca enhancer/promoter into a promoterless
13-gal
plasmid, pEQ3 (Fig. 1). Transfection of pEQ176fol-lowed by infection with CMV resulted in activation of
transcriptionof
1-gal
transcripts initiatingwithintheHindlIlrecognition site (Fig. 1) and containing no CMV-derived sequences(14).Posttranscriptionalsignals within CMV tran-scriptleader sequences wereidentifiedbythealterations in 1-galactivity resultingfrom insertion of these sequences into
pEQ176.
Using synthetic oligonucleotides and PCR, a plasmid
(pEQ239) containing the precise 228-nucleotide (nt) tran-scriptleaderof the mostabundant CMV(Towne) gp48 tran-script,El(5),wasconstructedasdescribed in Materials and
Methods(Fig. 2A). Whenanalyzed inatransientexpression assay,pEQ239expressed approximately eightfold less 1-gal activity (calculated asdescribed in Materials andMethods) than did the control plasmid, pEQ176 (Fig. 2B). Thus, similar to results using cell translation extracts (6), the gp48 leaderinhibited expression of a downstream reading frame in cells.
We next constructed a series ofplasmids that expressed truncated transcripts (Fig. 2A) in order to delineate the 5' boundary of the inhibitory signal. The plasmid with dele-tion of sequences to +12 (pEQ251) expressed low levels of 1-gal comparable to those expressed by pEQ239, while plasmids with deletion to +64 (pEQ296), +86 (pEQ290), +122 (pEQ256), and +198 (pEQ258) expressed
13-gal
atlevels similar to that of the control, pEQ176 (Fig. 2B). Thus, inhibition of 1-gal expression depends on the sequences between +12 and +64.
Todetermine the level at which the gp48 leader inhibited 1-galexpression, RNA waspurified from thesamecells used to measure 1-gal activity. Northern analysis of these RNAs revealed that similarquantities of13-galRNAwereexpressed by thedifferent constructs(Fig. 2C). PlasmidpEQ134,which expresses truncated, enzymatically inactive 1-gal, was co-transfected with each test plasmid as a control for transfec-tion efficiencyand RNA recovery(3). Theslightlyincreased quantity of 1-gal transcripts seen in cells transfected with pEQ258 was notconsistently present in other experiments. These results demonstrated that the gp48 leader inhibits downstream expression by acting at some posttranscrip-tional step that does not affect steady-state RNA levels.
The secondupstream AUG codon is an essential component of the inhibitory signal. The gp48 El transcript leader
con-tains three upstream AUGcodons (6), referred to asAUG1, AUG2, andAUG3according to theirproximitytothe El cap site. The presence of AUG2 at nt +21 to +23, within the region shown to be required for inhibition in Fig. 2, sug-gested that this AUG codon might be a component of the inhibitory signal. Wetherefore constructed mutantplasmids
in which AUG2 was changed to AAG. In transfection analyses,plasmidscontaining this mutation in thefull-length
leader(pEQ325) or in the leader truncated to +12 (pEQ332)
expressed high levels of1-gal similartothe level expressed
by the control, pEQ176 (Fig. 3A and B). As in Fig. 2, the full-length leader construct pEQ239 and the truncated
con-struct with deletion of AUG1, pEQ251, expressed 10- to
20-fold lower levels of13-gal than did pEQ176. Steady-state RNAlevels were similar between thewild-type and mutated pairs ofconstructs (Fig. 3C). Thus, AUG2 is one element within the gp48 leader required forposttranscriptional inhi-bition ofdownstream expression.
The inhibitory signal acts at the level of translation. Al-though the 1-gal transcripts derived from the plasmids with or without AUG2 appeared similar by Northern analysis, it was possible that differences in posttranscriptional process-ing of transcripts could have altered the RNA primary
structure and thereby accounted for the different levels of
13-galexpression. Wetherefore analyzedthe structure ofthe
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
CMV TRANSLATIONAL CONTROL 6785 A
x
Plasmid
pEO239
- pEO325
--m' pEO251
-' pEO332
rx
x
nt 271 268 J
pEO313 X pEO333
-= pEO370
B
= 3,000
0
0
c
0 U1 0 C
0 2,000
U-u 1,000
C,
'C
C
114-3 176239 325251 332 313 333 370359
/28s
-pEQ134
FIG. 3. Inhibitoryrole of AUG2 and ORF2. (A) The 5' ends of pEQ239, pEQ251, and pEQ313 (Fig.2and6) and the corresponding
mutantplasmidspEQ325, pEQ332, and pEQ333 containing AAG in place of AUG2. The ,8-gal ORF (gray rectangles) and theupstream
ORFsconsisting of gp48 leadersequences(white rectangles) alone orfusedtopolylinker andp-galsequences(stippled rectangles) are shown. AUG2 mutations are indicated (x). The mutations in pEQ370 shift ORF2outof frame after the initiationcodon and back into frameonecodonbefore the last codon of the wild-type ORF2, resulting inanalmostcompletely altered amino acid codingcontent
(black rectangle) of ORF2. (B)p-Galactivity (seelegendtoFig. 2B) of cellsinfectedwith CMV aftertransfection withcontrolplasmids (white bars), with wild-type and AUG2 mutant pairs of plasmids (striped bars), with the double-frameshift (fs)mutantpEQ370(black bar), or with CMV(AD169) gp48 leader plasmid pEQ359 (cross-hatched bar). (C)Accumulated RNA from the indicatedtest plas-mids (P-gal) and the transfection control pEQ134, determined by Northernblotasdescribed inthe legendtoFig. 2.
transcripts by primer extension to determine whether the
wild-type and AUG2 mutant constructs expressed
tran-scripts with the expected 5' ends. Thefull-length (pEQ239
andpEQ325) transcripts yielded primerextension products
ofapproximately 271 nt(Fig. 4), consistent with
transcrip-tionalinitiationoccurringwithin 2or3ntoftheauthenticEl 5' end (5). Similar analysis ofthe constructs truncated to +12 (pEQ251 and pEQ332) revealed transcripts approxi-mately268 nt, asexpected since thesetranscripts delete 11
pEQ239
I C CO 4 _ Lo)0 1
AP
AT
T 3
-s
:-
§
~T
A_S c
A
_ _,]\
_-~ C
_~o]3G
.::]
~T
G
~~~T
_
Z Tj2C
FIG. 4. Primer extensionanalysis. Anend-labeled P-gal2 oligo-nucleotide (3) was annealed to 40 ,ug ofwholecellRNAobtained from cells transfectedwith theindicated plasmidsandinfectedwith CMV.Followingreversetranscription,the extendedproductswere
analyzed on a 4% sequencing gel. The approximate sizes of the extended products (left) were determined by alignment with the sequence of pEQ239 (right four lanes). Portions of the pEQ239 sequenceincludingthegp48Elcap site(*)and the three upstream AUG codonsareindicatedontheright.
ntofgp48 sequences, butcontainanadditional 9ntderived from thepolylinkerfusedtotheir 5'ends,comparedwith the
full-length leader constructs. The band migrating slightly
below the 268-nt products represents nonspecific back-ground, as it is present in all lanes, including the lane
containingtranscriptionallyinactive controlpEQ3.Thus,all of thetranscripts did indeed contain AUG2orthe mutated derivative AAG codon. As well, the lengths of the
tran-scripts within eachpairwereidentical.Therefore, the
differ-ences in P-gal expression of wild-type and AUG2 mutant constructs werenotcausedbydifferential 5' end
processing
of the RNA.
Another consideration that could confound these experi-ments was the potential presence ofa promoterwithin the sequences encodingthe gp48
major
transcript (El) leader.The gp48 gene has been reported to encode a second I transcript, E2, whose 5' end is atnt +85relative to the El
transcript start site (5) and thus does not contain AUG2. However, in our primer extension analysis, we did not
identify aband corresponding to the E2 start sitewhich, if present,would appearnearAUG3 inFig. 4.Furthermore,in
cycloheximide reversal experiments (30) inwhich a ,B
pro-moter driving expression at the E2 start site would be
inactive, we stillfound an inhibitory signal
mapping
to thesame regionof thegp48 leader(datanot shown).
Thus,
we VOL.65, 1991on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.612.341.543.83.375.2] [image:4.612.96.282.85.457.2]6786 SCHLEISS ET AL.
A
1 2 3 4 5 6 7 8 9 10
top boKtom
Fraction
B
1 2 3 top
4 5 6 7 8 9 10
bottom Fraction
FIG. 5. Polysome analysis oftransfected cell RNA. Polysome fractions of cells transfected with plasmids containing noCMV leader (pEQ176), the wild-type gp48 leader (pEQ239), the gp48 leader with AUG2 mutated(pEQ325),orthegp48 leader deleted from the5'endto+86 (pEQ290;seeFig. 1, 2A, and 3A) and then infected with CMVwereseparatedonsucrosegradientsasdescribed in Materials and Methods. P-Galtranscripts (A)weredetected by Northern blot analysis of equal proportions of RNA purified from each fraction. Thesameblotwas stripped and rehybridized withanactin probe (B). Absorbance profiles (datanotshown)of the gradients showed that ribosomal subunits and
monosomessedimented in fractions 2 and3while polysomes containing approximately 7to10ribosomes sedimented in fraction 7.
couldidentifyno crypticpromoterelements orsubtle
post-transcriptionalRNAprocessing eventsdependentonAUG2
that couldaccountfor differentialinhibition ofp-galactivity. To directly corroborate that the gp48 signal acted by inhibiting translation, weevaluated the polysomal distribu-tionofp-galtranscripts. HFweretransfectedwithplasmids (Fig. 2 and 3) containing no CMV leader (pEQ176), the
wild-type gp48 leader (pEQ239), the gp48 leader with AUG2 mutated (pEQ325),or a5'truncated gp48 leader with AUG2
deleted (pEQ290). Following infection with CMV,
poly-somes wereseparatedover sucrosegradientsasdescribedin Materials and Methods. RNA from the gradient fractions
wasanalyzed by Northern blot forp-galRNA (Fig. 5A).The
wild-type leader markedly inhibited loading of p-gal RNA ontothelarger polysomes comparedwiththe distribution of
p-gal transcripts lacking the inhibitory signal. The
absorb-anceprofiles of the gradients (datanotshown) showed that
the peak pEQ239 p-gal RNA comigrated with the 80S
monosome fraction (fraction 3), while the peak p-galRNA
expressed from pEQ176, pEQ325, and pEQ290 sedimented
withpolysomes containing approximately 7to10ribosomes (fraction 7). Althoughwe are uncertain why the total
quan-tity of pEQ239 RNA on polysomes appeared greater than
thatof the otherconstructs, it ispossible that transcripts that
were being translated more efficiently were also degraded more rapidly, as has been reported in other systems
(re-viewed in references 4 and 8). The similar abundance of pEQ239 RNA and other p-gal RNAs (Fig. 2, 3, and 6) in whole cell RNA samples coupled with the different total
quantities in polysome-associated RNAs (Fig. 5) might be
explained by thepresenceof nuclearorother nonpolysomal
p-gal transcripts. Regardless, the increased quantity of pEQ239 RNAon polysomes cannot explain the consistent reduction inp-galactivity translated from thisRNA(Fig.2, 3, and6).
To verify thatthe differences in p-gal RNA distribution
were notdueto differences in theintegrity ofthe polysome
preparations, the Northern blotswerestrippedand rehybrid-ized withanactinprobe. Thedistribution of actinRNAwas
indeed similar in all of the gradients (Fig. SB). Thus, the
pattern ofaccumulation ofp-galtranscripts ontopolysomes verified that the gp48leader signalinhibits geneexpression
at the level oftranslation.
Leader sequences downstream from AUG2 are also
re-quired for inhibition. Previous studies suggested that in addition to an upstream AUG codon, sequences further
downstreambutstill within the CMVtranscriptleaderswere
required for translational inhibition (3). To investigate the role ofgp48leader sequencesdownstream fromAUG2, we constructedplasmidswith deletions ofsequencesfrom
var-ious sites within the leaderto the3' end of the leader(Fig. 6A). Transfectionanalyses of these plasmids demonstrated that deletion of sequences from +89 to the 3' end of the leader(+228), includingdeletion ofAUG3,hadnoimpacton
p-gal expression (pEQ323, pEQ312, and pEQ330; Fig. 6B) compared with expression from the complete gp48 leader
plasmid, pEQ239. However, the additional deletion of nt +89 to +85 (pEQ334) or to +67 (pEQ313) very clearly
inactivated theinhibitory signal.
The resultspresentedabove revealed that increased p-gal
expressionresulted either from mutation of AUG2(Fig.3)or from deletion ofsequencesbeyond+67(or +85)of thegp48
leader. Totesttheeffect of mutations of bothelements, we constructed plasmid pEQ333 (Fig. 3A). If the gp48 leader containedtwoindependent inhibitory signals,then this dou-ble mutant would be expected to express higher levels of
p-galthan does eitherof theplasmids (pEQ313andpEQ325) with only one ofthe inhibitory elements. However, p-gal
activityexpressed bythedoublemutantpEQ333wassimilar to that expressed by either pEQ313 or pEQ325 (Fig. 3B). The levelofp-galRNAexpressed by pEQ333wassimilarto that from the other twoplasmids (Fig. 3C). Aswell, the 5' end of the pEQ333 RNA was at exactly the expected positionandwasidenticaltothat ofpEQ313 (Fig. 4). These data indicate that AUG2 and the leader sequences down-streamfrom +67areboth essentialcomponents ofthesame
inhibitorysignal.
Intriguingly, these two elements of the inhibitory signal
pEQ176
pEQ239
pEQ325
pEO290
- +- +- - va ii+
*.~
J. VIROL.
.1--
I.Ndmim-*I'Wd
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.612.142.465.83.258.2]+228
Q pEO239
r n ri +197 __n pEO323
Q I - +121
I +89
+85
+67
pEO312
-- pEO330
pEO334 _ pEQ313
ofwhich results in an amino acid coding change [Thr in
CMV(Towne) to Ala in CMV(AD169) at codon 16].
p-Gal
RNA levels in cells transfected with pEQ370 and pEQ359 were similar to those in cells transfected with the other constructs (Fig. 3C). Together, these data suggest that the intact translational inhibitory signal in the gp48 leader
re-quires the authenticORF2 coding information.
DISCUSSION
s
C 12,000
0 0 0)
o 8,000
0 4,000
41 U.
+85
+67
N Qn N ne
N C C OX
c
-I-28s " 0-0"we*am w-10*-n-Bgal
18s pEQl 34
FIG. 6. Mapping the inhibitory signal by using 3' deletional
mutants. (A) The5'leaders oftranscripts expressed from plasmids containing the gp48 sequences up to the indicated nucleotide. Upstream ORFs containing gp48 leader sequences (white
rectan-gles) alone orfused to polylinker and p-gal sequences (lightgray
rectangles) are shown upstream of the ,B-gal ORF (dark gray rectangles). (B) p-Galactivity from cells infected with CMV after transfection with the indicated control (open bars)orgp48
leader-containing(striped bars) plasmids. Numbers above the bars referto
the 3' endof the gp48 leader in each plasmid; numbers belowthe bars refer to the pEQ number of the transfected plasmid. (C) Northernblotanalysis (see legendto Fig.2C) of whole cell RNA harvestedfromthe cellstransfected and infected in panel B. contain the initiationand terminationcodons ofashort ORF
(ORF2) within the gp48 leader. To investigate whether the inhibitory signal required the authentic amino acid coding information of this ORF, as wellas its initiation and termi-nationcodons,weconstructedaplasmid (pEQ370) withaC
inserted betweennt +24 and +25 and GGinserted between
nt +81 and +82 of the gp48 leader(Fig. 3A). Theneteffect
of thesemutationswastoshifttranslationoutof frame after
the initiation codon and back into frame one codon before the termination codon. p-Gal was expressed athigh levels following transfection of pEQ370, similar to the level
ex-pressed from constructs with no gp48 leader (pEQ176) or with a mutated gp48 leader such as pEQ325 (Fig. 3B). In contrast to pEQ370, plasmid pEQ359 containing the gp48
leaderfromanotherviral strain, CMV(AD169), retainedthe
inhibitory signal (Fig. 3B). Within ORF2, the gp48 leader
sequencesofthetwoCMV strains (reference6andGenBank
accessionnumberX17403) differatsixnucleotides, onlyone
Previous studies of CMV translational control suggested
thatupstreamAUGcodonsintranscriptssuchasthatof the
2.7p
genemightmodulate the kinetics andmagnitudeofgeneexpression (13, 14). The actualregulatory role of upstream
AUG codons ofthe2.7, gene has remainedelusivebecause noprotein productof the 2.7, genehasbeenidentified (16). Further, generalizations about theimpactofupstreamAUG codons havenotbeenpossible because incontrast to
inhib-itory effects of the CMV 2.7p gene leader, the transcript
leadersoftwootherCMVgeneswithupstreamAUG codons
allowed efficient downstream translation (3). This report extends the analysis ofCMV translational control by
dem-onstratinganinhibitory ORFpresentinthetranscriptleader ofabona fideprotein-encoding gene, gp48.
Although the gp48 leader had been shown to inhibit translation in cell extracts(6),wecouldnotpredict whether a similar effect would occur in intact cells because of the
poor fidelity of cell-free translation systems when such systemsareusedtostudyregulation(19).Infact,incontrast totheconclusions from cell-free translation assays
suggest-ingthat theupstreamAUG codonswerenotacomponentof the inhibitory signal (6), our results unequivocally demon-strate that AUG2 is an essential component of the gp48 inhibitory signal (Fig. 3).
The inhibitory effectofthe AUG2 in the gp48 transcript
leader is consistent with the scanning model ofeukaryotic
translation (19) in that a scanning ribosome that initiates translationatanupstreamAUGcodonmaybeblocked from translatingadownstreamreadingframe. However the
scan-ning model does notfullyexplainprevious results of trans-lational inhibitionby other CMV leaders (3, 13), norinthe current studies does it explain why only AUG2, and not
AUG1orAUG3, inhibits translation. The contextof
nucle-otidessurroundingan AUGcodoncaninfluencerecognition of the AUGcodon, with(AL/)CCatgG beingoptimaland the nucleotides at -3 and +4 being most important (17).
Al-though the context of AUG3
(_jTGatgA)
is very similar to thatofAUG2(GTGatgC;reference 6 andFig. 4),deletion of AUG3 hadnoeffect(comparepEQ312withpEQ330; Fig. 6),whereas deletionormutationof AUG2 relievedthe transla-tional inhibition (Fig. 2and 3).
The inability of AUG codon context rules to predict
effects on downstream translation (3, 13; this report)
sug-geststhatother leadersequencesarerequiredfor inhibition.
Supportingthishypothesisisthe highlevelofp-gal
expres-sionresultingfrom deletion ofsequences65 nt downstream from AUG2, including the termination codon of ORF2
(comparepEQ330withpEQ334; Fig. 6). Sincedownstream RNA secondary structure can facilitate initiation at an upstream AUG codon (18, 20),it ispossiblethatnt +85 to +89 are critical components of a secondary structure ele-mentrequired forefficient translational initiation atAUG2.
Alternatively,itmaybethe termination codoninthe +85 to +89element that is essential for inhibition. Sequencesin the vicinity of the termination codon ofashort upstream ORFin A
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.612.102.273.82.410.2]6788 SCHLEISS ET AL.
the yeast GCN4 gene transcript leader are required for its
inhibitory
effectofthisgeneondownstream translation (23).Surprisingly, the double-frameshift mutant, pEQ370, ex-pressedhighlevelsof,B-gal(Fig.3). In contrast,changes that
primarilyalter the gp48leader nucleotide sequencebut only
minimallyalter thecoding information of ORF2 retained the
inhibitory signal (Fig. 3, pEQ359). Together, these results suggest that the inhibitory signal depends on the coding
content of ORF2 rather than secondary structure or other
features oftheleader. Therequirementfor the ORF2 termi-nation codonaswell(Fig.6) suggests that the actualpeptide product of ORF2 may mediate the inhibitory effect.
Simi-larly, a peptide translated from an upstream ORF may be
required fortranslationalregulationof the yeast CPA] gene
(37),
although
neitheritnorthegp48 ORF2 producthas beenidentified.
One puzzling observation was the high levels of
1-gal
expressedfrom constructs pEQ334 and pEQ313 (Fig. 6B). Since the deletion of the ORF2 stop codon in these con-structsresults inanORFthatterminates17 ntdownstreamfromthe
1-gal
initiation codon(Fig. 6A),ribosomesinitiatingtranslation at AUG2 would be expected to entirely bypass
the
1-gal
AUG codon. Expression of1-gal
from theseplasmids could result from initiation at AUG2followed by ribosomal frameshifting intothe
13-gal
readingframe. How-ever, high levels of ,8-gal were expressed by a plasmid similar to pEQ334 (Fig. 6A) but with one extra base pairadded 5' to the
13-gal
AUG codon (data not shown). As aresultofthe insertion, ribosomes initiatingat AUG2inthis
plasmidwould translateanotherreadingframewhich
termi-nates 43 nt 3' to the
13-gal
AUG codon. Frameshiftingfrom either oftwoframes into the 13-gal readingframe is highly improbable.Theseresults donotexcludethepossibilitythatribosomes initiating at AUG2 terminate downstream from the,B-gal AUGcodon, scanbackwards,andreinitiateatthe
,8-gal
AUG codon, as has beenreported
in another system(26, 27).
An alternative mechanism, similar to that proposed to explaintranslation of bicistronic simian virus 40transcripts (29), can account for both the inhibition of
13-gal
expres-sion by the authentic gp48 ORF2 and the high level of 3-gal expression by ORF2 mutants, including pEQ334 and
pEQ313. If initiation of translation at AUG2 is
inefficient,
perhapsbecause of thesuboptimal
AUG2flanking
sequencecontext(17)orbecauseofthe
proximity
ofAUG2tothe cap site (28),then mostribosomes would be expectedtobypass AUG2, scan downstream, and initiate at the13-gal
AUG codon(19). However, anoccasional ribosome will initiateatAUG2. If this ribosome encounters an elongation block in
ORF2,
it will obstruct subsequentribosomes fromscanning
tothe
1-gal
AUG codon(2, 11, 29). Thus, inconstructslikepEQ239
which preserve the ORF2inhibitory signal,
eveninefficient initiationatAUG2couldgradually result in
accu-mulation of transcripts in which translation of
13-gal
is occluded (29). In constructs lacking the ORF2signal,
theinfrequentribosome that initiatesatAUG2 willnotobstruct
subsequentribosomes and thus will haveonlyaslight impact
on
,3-gal
expression. Further studies are necessary to testthis model as well as to define the interactions of the authentic ORF2oritspeptideproduct with ribosomes which
are
responsible
forblocking downstream translation.The conservation of the inhibitory signal between two
CMV strains supports the likelihood that this signal is
important
viral regulatory element. However, thetransla-tional inhibition mediated by the gp48 ORF2 has thus far
been
investigated
only in transient transfection assays. It ispossible
thatduring
CMV infection the alternativegp48
1transcript,
E2, which does not contain AUG2 (5, 6), isactually
responsible
fortranslation of the gp48protein.
Wehave been unable to evaluate this
possibility
because E2 isnot
reproducibly
detectable inourexperiments.
Otheranal-yses,suchasconstructionofaviral strain withamutation in
AUG2, maybe
required
todetermine the regulatoryrole of AUG2 in its natural context within the viral genome. Theoccurrence of upstream ORFs in the
transcripts
of other viral and cellular genespromises
thatinvestigations
of theprecise signals
and mechanisms involved in translationalcontrol of CMV gene
expression
willprovide
valuableinsights
into translationalregulation
ofeukaryotic
geneexpression.
ACKNOWLEDGMENTS
We thank Ed Mocarski for providing pON plasmids, Bonita
Biegalkefor assistanceconstructing pEQ3,and JonCooperand M.
KymneGrayfor critical review of themanuscript.
This workwassupported byPublic Health ServicegrantA126672 fromtheNational Institutesof Health. M.R.S. wassupported bya
NIHAcademic PediatricTrainingGrantT32-HD07233. REFERENCES
1. Alford, C. A., and W. J. Britt. 1990. Cytomegalovirus, p. 1981-2010. In B. N. Fields and D. M. Knipe (ed.), Virology.
RavenPress,NewYork.
2. Barkan, A.,andJ.E. Mertz.1984. Thenumber of ribosomeson
simian virus 40 late 16S mRNA is determined in part by nucleotidesequenceof itsleader. Mol. Cell. Biol. 4:813-816. 3. Biegalke,B.J.,andA. P.Geballe. 1990.Translational inhibition
bycytomegalovirus transcriptleaders.Virology 177:657-667. 4. Brawerman,G. 1989. mRNA decay: findingthe right targets.
Cell57:9-10.
5. Chang, C.-P.,C. L.Malone,and M. F. Stinski.1989. A human
cytomegalovirusearlygene hasthree induciblepromotersthat are regulated differentially at various times after infection. J. Virol.63:281-290.
6. Chang,C.-P.,D. H.Vesole,J.Nelson,M. B. A.Oldstone, and M. F. Stinski. 1989. Identification andexpression ofahuman
cytomegalovirus earlyglycoprotein. J. Virol. 63:3330-3337. 7. Chee,M.S.,A. T.Bankier,S.Beck,R.Bohni,C. M.Brown,R.
Cerny, T. Horsnell, C. A. HutchisonIII, T. Kouzarides,J. A.
Martignetti,E. Preddie, S. C. Satchweli, P. Tomlinson, K. M.
Weston,andB.G. Barrell. 1990.Analysisoftheprotein-coding
content of the sequence of human cytomegalovirus strain
AD169, p. 125-169. In J. K. McDougall (ed.),
Cytomegalovi-ruses. Springer-Verlag,NewYork.
8. Cleveland, D. W. 1989. Multiple determinants of eukaryotic
mRNAstability. New Biol. 1:121-126.
9. Demarchi, J.M. 1981. Humancytomegalovirus DNA: restric-tion enzyme cleavage maps andmap locations for immediate
early, early,and late RNAs. Virology114:23-38.
10. Dente, L., G. Cesareni, and R. Cortese. 1983. pEMBL: a new
familyofsinglestrandedplasmids.Nucleic AcidsRes. 11:1645-1655.
11. Fajardo, J. E.,andA. Shatkin. 1990.Translation of bicistronic viral mRNA in transfected cells: regulation at the level of
elongation. Proc.Natl. Acad. Sci. USA87:328-332.
12. Geballe,A.P.,F.S.Leach,and E.S. Mocarski.1986.Regulation
ofcytomegaloviruslate geneexpression: ygenesarecontrolled
byposttranscriptionalevents. J.Virol. 57:864-874.
13. Geballe,A. P.,and E.S. Mocarski. 1988.Translationalcontrol ofcytomegalovirus gene expression is mediated by upstream AUGcodons. J. Virol.62:3334-3340.
14. Geballe, A. P., R. R. Spaete, and E. S. Mocarski. 1986. A
cis-actingelementwithin the 5' leader ofacytomegalovirus ,B
transcriptdetermines kineticclass.Cell46:865-872.
15. Goins,W.F., and M. F.Stinski. 1986. Expression ofahuman
cytomegaloviruslate gene isposttranscriptionallyregulated bya
3'-end-processing eventoccurring exclusively late after infec-J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
tion. Mol.Cell. Biol. 6:4202-4213.
16. Greenaway, P. J., and G. W. G. Wilkinson. 1987. Nucleotide sequence of the most abundantly transcribed early gene of human cytomegalovirusstrain AD169. Virus Res. 7:17-31. 17. Kozak, M. 1986.Point mutations defineasequenceflanking the
AUG initiator codon thatmodulates translation by eukaryotic ribosomes. Cell44:283-292.
18. Kozak, M. 1989. Context effects and inefficient initiation at non-AUG codons in eukaryotic cell-free translation systems. Mol. Cell. Biol. 9:5073-5080.
19. Kozak, M. 1989. Thescanning model for translation: an update. J.Cell Biol. 108:229-241.
20. Kozak, M. 1990. Downstream secondary structure facilitates recognition ofinitiator codons by eukaryotic ribosomes. Proc. Natl.Acad. Sci. USA 87:8301-8305.
21. Linial, M., N. Gunderson, and M. Groudine. 1985. Enhanced transcription of c-myc in bursal lymphoma cells requires con-tinuous protein synthesis. Science 230:1126-1132.
22. McDonough, S. H., and D. H. Spector. 1983. Transcription in humanfibroblasts permissively infected by human cytomegalo-virusstrain AD169. Virology 125:31-46.
23. Miller, P. F., and A. G. Hinnebusch. 1989. Sequences that surround the stop codons of upstream open reading frames in GCN4 mRNA determine their distinct functions in translational control. GenesDev. 3:1217-1225.
24. Mocarski, E. S. 1991. Evidence for posttranscriptional modula-tion of cytomegalovirus gene expression, p. 287-299. In E. Wagner (ed.),Herpesvirus transcription and its regulation. CRC Press, Boca Raton,Fla.
25. Mulligan, R. C., and P. Berg. 1981. Factors governing the expression of a bacterial gene in mammalian cells. Mol. Cell. Biol. 1:449-459.
26. Peabody, D. S., and P. Berg. 1986. Termination-reinitiation occursin the translationofmammaliancell mRNAs.Mol. Cell. Biol. 6:2695-2703.
27. Peabody, D. S., S. Subramani, and P. Berg. 1986. Effect of upstreamreading frames on translation efficiency in simian virus 40recombinants. Mol. Cell. Biol. 6:2704-2711.
28. Sedman, S. A., G. W. Gelembiuk, and J. E. Mertz. 1990. Translation initiation at a downstream AUG occurs with
in-creasedefficiency when the upstream AUG is located very close to the 5' cap. J.Virol. 64:453-457.
29. Sedman, S. A., P. J. Good, and J. E. Mertz. 1989. Leader-encoded openreading frames modulateboththeabsolute and relative rates ofsynthesis ofthevirionproteins of simian virus 40. J.Virol. 63:3884-3893.
30. Spaete,R.R.,and E.S.Mocarski. 1985.Regulation of cytomeg-alovirusgeneexpression: a and 1 promotersaretrans-activated by viral functions in permissive human fibroblasts. J. Virol. 56:135-143.
31. Spaete, R. R.,and E. S. Mocarski. 1985.The a sequence of the cytomegalovirus genome functionsas acleavage/packaging sig-nalfor herpes simplex virus defective genomes. J. Virol. 54: 817-824.
32. Spaete,R.R.,R. M.Thayer,W.S.Probert,F. R.Masiarz,S. H. Chamberlain,L.Rasmussen,T.C.Merigan,andC. Pachl. 1988. Human cytomegalovirus strainTowneglycoproteinB is proc-essedbyproteolytic cleavage. Virology 167:207-225.
33. Stamminger,T., E.Puchtler,andB. Fleckenstein.1991. Discor-dantexpression of the immediate-early1and2generegions of humancytomegalovirusatearly times after infection involves posttranscriptionalprocessingevents. J. Virol. 65:2273-2282. 34. Stenberg,R.M., A.S. Depto, J. Fortney,andJ.A. Nelson.1989.
Regulatedexpression of early and lateRNAsandproteins from the human cytomegalovirus immediate-early gene region. J. Virol.63:2699-2708.
35. Stinski, M. F. 1978. Sequence of protein synthesis in cells infected by human cytomegalovirus: early and late virus-in-ducedpolypeptides.J. Virol. 26:686-701.
36. Wathen,M.W., and M. F. Stinski. 1982.Temporal patterns of humancytomegalovirus transcription: mapping theviral RNAs synthesized at immediate early, early, and late times after infection.J. Virol. 41:462-477.
37. Werner, M.,A.Feller,F.Messenguy,and A. Pierard.1987. The leader peptide of yeast gene cpal is essential for the transla-tionalrepression of itsexpression. Cell49:805-813.
38. Wright, D. A., and D. H. Spector. 1989. Posttranscriptional regulation ofaclassofhumancytomegalovirusphosphoproteins encodedbyanearlytranscription unit.J. Virol.63:3117-3127.