Electrodeposited Platinum Nanoparticles on the Multi-Walled Carbon Nanotubes and its Electrocatalytic for Nitric Oxide
Full text
Figure

Related documents
In our previous work, we have demonstrated that the use of multi-walled carbon nanotubes modified electrode can improve the sensitivity for electrochemical
The AuPdCu nanoparticles presented high electrocatalytic activity to the oxidation of hydrazine, and at the resulting AuPdCu- MWCNT/GCE electrode, hydrazine could
The effect of solution pH on the electrocatalytic activity of the nano-CoOx modified electrodes towards the OER was examined by measuring the LSVs at the unmodified and
A convectional three-electrode system was employed, Ag/AgCl (3M KCl) as reference electrode, platinum electrode as a counter electrode and glassy carbon electrode (GC)
As compared with bare and PBCB modified electrodes, the PBCB-MWCNT modified electrode shows lower over-potential and higher current response to persulfate. This
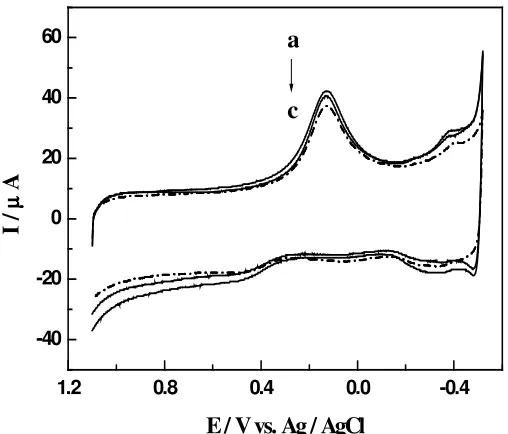
Carbon paste electrode modified with copper hexacyanoferrate film was tested on 0.1 M phosphate buffer so- lution pH 7.5 for its response to nitric (II) oxide ( Figure 5 ).. After
The modified electrode (DBT – CNT/GCE) was used to investi- gate electrocatalytic behavior of isoprenaline in phosphate buffer solution (pH = 7.0) using cyclic voltammetry,
A plot of the peak current against peak potential of paracetamol at the MWCNT/TiO 2 /GC composite film electrode in phosphate buffer solution using different scan rates of 5-600