JOURNALOFVIROLOGY, JUIY 1972, p. 67-72
Copyright ( 1972 American Society for Microbiology Printed in U.S.A.Vol. 10,No. I
Properties of Somatic
Cell
Hybrids Between
Mouse
Cells and
Simian Virus 40-Transformed
Rat
Cells
J. VAN DER NOORDAA, ANNE VAN HAAGEN, J. M. M. WALBOOMERS
AND H. VAN SOMEREN'
LaboratoriumvoordeGezonidheidsleer, University of Amsterdam, Mauritskade57, Amsterdam, The Netherlands
Received for publication6 March1972
Hybrids between mouse cells and simian virus 40 (SV40)-transformed rat cells weremade, and their properties and chromosome constitutionwereinvestigatedover
manygenerations. Their hybrid naturewas confirmed byenzymestudies. Duringa
period of 1 year a loss of 10 to 20% of the total number of chromosomes was
observed. The SV40tumorantigenwaspresentand remainedpresentin the hybrids.
Theparental andhybrid cells were studiedfor agglutination with concanavalin A,
forgrowth in softagar,and forserumrequirement. These growth and surface
char-acteristics of the transformed cells appeared in the hybrids.
Several hybrid cell lines
originating
from thefusion of normal and virus-transformed cells
have been described. Defendi et al.
(4)
havestudiedthepropertiesofhybridsbetween normal
mouse cells and mouse cells transformed
by
polyoma virus. The
hybrid
cells contained thepolyoma
virus-specific
tumorandtransplantation
antigens,
indicating
thepersistence
of the viralgenome inthe
hybrid
cell.Hybrids between mouse cells and
polyoma-transformed hamstercellswereshown
by
Basilicoand Wang
(1)
to contain the viral genome andappeared to havea number of
properties
ofthetransformed cells. Hybrids between mouse cells
and simian virus 40
(SV40)-transformed
humancells were described
by
Weiss(13).
TheSV40-specific tumor
(T) antigen
was present in thenuclei of thehybridcells. Thistypeof
interspecific
hybridundergoesa
rapid
lossofhumanchromo-somes andtherefore was considered suited to a
study of the effect of chromosome loss on the
presence of the SV40 viral genome. A
positive
correlationwasfound between the loss ofhuman
chromosomesand the loss ofT
antigen
from thehybrid cells, suggesting
integration
of the SV40genome intothechromosomes of thetransformed cell.
In our study we report on the
properties
ofhybrid cells between SV40-transformed rat cells
andnormal mousecells.
According
toWeiss and Ephrussi (14), rat-mouse hybridspreferentially
loserat chromosomes, and therefore this type of
IPresent address: Medical Biological Laboratory T.N.O.,
Rijswijk, andLaboratoryfor MolecularGenetics, University of Leiden.
hybrid was chosen to study the effect of chromo-some loss on theproperties ofthehybrid cell.
MATERIALS AND METHODS
Ratcells.Cultures of primary rat cells derived from
the brains of newborn BN/BI rats were inoculated
with SV40 (strain VA 45-54) at a multiplicity of
infection of about 10. A line of transformedcellswas
initiatedfrom a focus oftransformed cells. This line
will be referred to as rat-SV40cells. All cells of this
linecontained the SV40 T antigen. No infectious virus
could be recovered from the transformed cells after
either co-cultivation or fusion with monkeykidney
cells (BSC-1 andCVl).
Mousecells. Athymidinekinase-deficient derivative
from 3T3 cells was kindly provided by D. Bootsma
(Dept. of CellBiologyandGenetics,MedicalFaculty,
Rotterdam) andwillbereferred to as 3T3 TK-.
Hybrid cells. Theisolation of hybrid cells was
per-formed by the half-selective system of Davidson and
Ephrussi (3). The rat-SV40 (at passagelevel 13) and
3T3 TK-cells were mixed in aratio of 1:1,000 and
fused by Sendai virus (10) inactivated with
beta-propiolactone. After 24hr ofincubation at 37 C, the
mediumwasreplacedbyLittlefield'sselective medium
(7). This medium consists of standard medium
sup-plementedwith1 X 104 Mhypoxanthine,4 X 107M
aminopterin, and 1.6 X 105 M thynmidine (HAT medium). Aftertwoweeksof furtherincubation,two
types of colonies were observed: colonies with the
morphology of rat-SV40 cells and colonies with a
morphology different fromthe rat-SV40 cells. Three
of thelatter colonies wereisolated with the aid ofa
Pasteur capillary pipette, and one of these three
identical-looking colonies (H) was used for further study.
No macroscopically visible colonies of 3T3
TK-cellswerepresentatthattime,althoughsmall groups
67
on November 10, 2019 by guest
http://jvi.asm.org/
orsingle cells with themorphologyof3T3cells could
befound.
From colony H, four subclones were derived for
further experiments; these lines will bereferred to as
H2, H3, H8, andH1O.
Cultureconditions. Allcultures weremaintained in
Eagle basal medium supplemented with
10%O
calfserum. Parental and hybrid lines were subcultured
twiceaweek.
Enzyme studies. Enzyme electrophoresis was
per-formed oncelluloseacetate gel (9) with celllysates of
parentalandhybridlines. Thefollowing enzymeswere
tested: lactate dehydrogenase Aand B (LDH Aand
B), 6-phosphogluconate dehydrogenase (6PGD), and
indophenol oxidase (IPO).
SV40Tantigen. The SV40 T antigen wasdetected
by the indirect immunofluorescence test (12). For all
tests, serumfrom the same pool was used. Theserum
was obtained from hamsters bearing SV40-induced
tumors. Fluorescein-labeledantihamster globulinwas
purchased from Nordic
Phiarmaceuticals
andDiag-nostics.
Agglutination by concanavalin A. Cultures were
washed two times with Ca-and Mg-free
phosphate-buffered saline (PBS) and dispersed with 0.02%
ethylenediaminetetraacetic
acid at 37 C. Thedispersedcells werethenwashed twice with PBS free fromCa2+
and Mg2+. To study the effect of trypsin on
ag-glutination, cells were treated for 2 min with 0.05%c
trypsin,centrifuged, washed two times with PBS, and
resuspended. The agglutination reaction was
per-formed in 25-mm petri dishes (Falcon) with 0.5 ml of
cellsuspension (106 to 3 X 101cells1/ml)and 0.5 ml of
asolutioncontaining5,25, 50, or 125pg of
concanava-lin A. Concanavalin A (Serva, Heidelberg) was
dis-solved in saline, pH 7. After gently shaking for 15 min
at 25 C,thedegree ofagglutinationwasscored by two
investigatorsas thepercentageofagglutinatedcells.
Serum requirement. To determine the serum
re-quirement, cultures were fed with medium supple-mented with 1, 5, and 101'" serum. An inoculum of
2 X 104 cells was seeded in 60-mm petri dishes
(Falcon), and the cellswerecountedafter 1, 2, and3
days.
Ability to grow in soft agar. Appropriate dilutions
of cells in 2ml of0.34%/ agar medium were planted
on abase layer of5ml of0.5%" agarmediuminsmall
flasks (Falcon); the number ofcolonies wascounted
after 2weeks.
:Nmmi-n
mmA
_immim
-_
RESULTS
Enzyme and chromosome studies. The
zy-mogram patterns of the four hybrid lines for
LDH, 6PGD, and IPO) showed
intermediate
bands; Fig. 1 represents theresults for one of the
hybrids. These findings showed the hybrid nature
FIG. 1. Zymograms oflactate dehydrogenase (A),
6-phosphogltaconiate dehydrogenlase (B), and
indophe-nol oxidase (C)showing rat-SV40 (1), hybridH3 (2),
artificial mixture ofrat-SV40 and 3T3 TK- (3), and
3T3 TK- (4) patte(rtis.
... ..:....
...
.~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~...
*.:.:.:..
*~~~~~~~~~~~~~~~~~~~~~~
...:..:... .. .. .. ... ... ... ;.68
on November 10, 2019 by guest
http://jvi.asm.org/
[image:2.493.262.451.44.660.2]PROPERTIES OF SOMATIC CELL HYBRIDS
of the lines H2, H3, H8, and H10 at passage 14. Starting at passage 20, most of our studies were performed on cells cultured in standard medium and on cells cultured in HAT medium. At passage 45the hybrid cells were tested again; their hybrid naturehad been retained for the tested enzymes
in bothmedia used.
Figures 2 and 3 show the results of the chro-mosomal studies of the parental mouse 3T3 TK-cells and rat-SV40 TK-cells. The modal number of chromosomes of the 3T3 TK- cells is 63; they are all telocentric or acrocentric. The rat-SV40 cells had a modal number of 42 chromosomes, of which about 24 were telocentric or acrocentric and about 18 were metacentric or submetacentric. The number and distribution of the four hybrid
cell lines are shown in Table 1. When tested at
about the 12th subculture after cloning, thecells
ofthe four hybrid lines contained 70 to 80 telo-centric or acrotelo-centric chromosomes and 11 to 16 metacentrics. Upon further subculturing, a small
cL
44 X.LLS LL
Zo 10-_
,
r rr;1f
m40 50 60 70 >70
CHROMOSOMES/METAPHASE
FIG. 2. Histogram of clhromosomes/metaphase
againistiiumberofmetaphasesof3T3TK- cells.
e-1
,
2:e
co
Y-94 -tLLS
n 1;
F
ri
loss of telocentric or acrocentric chromosomes
andaminorchangeinthenumber ofmetacentric
orsubmetacentric chromosomes wasobserved. It
remains open to question whether rat or mouse chromosomes werelost.
Infectious virus and SV40 T antigen. The rat-SV40 and hybrid cells did not shed infectious
SV40, nor wasitpossible toinduce the production of SV40by means of fusionwith monkeykidney cells. The hybrid cells cultured in standard medium and HAT medium were first tested for the presence of the SV40 T antigen at about the 15th subculture. All cells from the four hybrid
lines contained the SV40 T antigen.The type of fluorescence staining was similar to that of the rat-SV40 cells. Everyfifth subculture of thehybrid
cells was investigated forTantigen; they remained
positive as faras tested, which includesthe 90th
subculture ofthelines H2 and H8 inbothmedia. The lines H3 and H10 have not been
systemati-cally investigatedfor T antigen beyond the 40th
subculture.
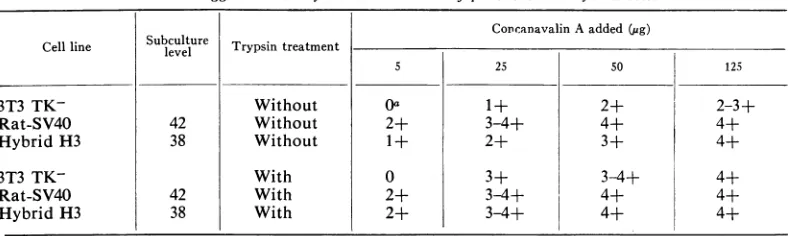
Agglutination. The results of agglutination by
concanavalin A with and without trypsin
treat-ment ofthe parental andhybiidcells areshown
inTable2.Without trypsin treatment,
agglutina-tion of 3T3 TK-cells occurred after addition of
25, 50,and 125 ,g of cancanavalin A. With 5
Ag,
noagglutination of 3T3 TK- cellswas observed;
at that concentration, 50% ofthe rat-SV40 and
25%ofthehybridcells hadagglutinatedafter 15
min.Withtrypsintreatment,anincreased
aggluti-nation of 3T3 TK- and hybrid cells was found. The degree ofagglutination of therat-SV40 cells
had not changed.
Growth in soft agar. Parental and
hybrid
cellswere tested for their ability to form colonies in
soft agar; the results are shownin Table 3. The
3T3 TK- cells formed no colonies at all; about
20%70
ofthetransformedcells gave risetocoloniesasdid 1 to 4% of the
hybrid
cells.The reduction of theefficiencyofplating (EOP)
in soft agarseems specificsince the EOP in fluid medium of both rat-SV40 and hybrid cells was
approximately80c.
Serum requirement. The serum
requirement
ofthehybridlineswas
compared
tothatofthetwoparental lines. Figure 4 contrasts the effect of
serum concentration on the growth rate of
rat-SV40 and 3T3 TK- cells. The transformed cells
grew at nearly the same rate in 10, 5, and
c,%o
serum concentration. No increase inthenumber
of 3T3 TK-cells was observed atthe1
%0
serumconcentration, and with a concentration of 5%
serum thegrowth rate was still lowerthan with
10%
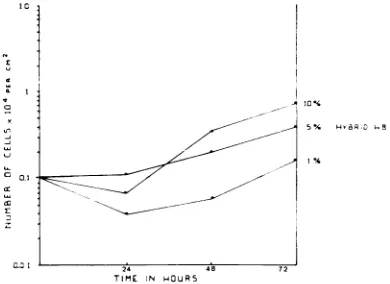
serum. Figure 5 shows the response of thehybridcellstoserumconcentration. Since all four
30 40 50 60 7r
CHROMOSOMES/METAPHASC
FIG. 3. Histogram of chromosomes/metaphase
againistnumberofmetaplhasesofrat-SV40cells.
VOL. 10, 1972 69
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.493.45.239.493.634.2]TABLE 1. Number anddistribution ofchromosomes ofthe hybridcellsa Cell line Hybrid H2 Hybrid H3 Hybrid H8 Hybrid HIO Sub-culture level 12 24 62 87 11 23 39 72 11 26 70 89 12 19 26 42 Standard medium
Totalno.of chromosomes 96.4 (89-98) 88.9 (84-97) 80.4 (52-110) 83.9 (64-88) 85.3 (65-96) 75.3 (59-102) 69.6 (54-83) 74.3 (60-85) 95.8 (82-105) 87.0 (78-94) 73.9 (60-83) 78.0 (68-86) 92.2 (88-103) 90.4 (83-96) 89.4 (83-98) 75.3 (51-89) Telo-centric/ acro-centric 80.6 73.3 65.7 69.4 69.1 58.7 53.8 62.5 80.3 71.2 59.8 60.9 77.4 75.6 75.3 61.1 Meta-centric/ submeta-centric 15.7 13.6 12.7 13.8 15.6 15.5 14.5 10.3 15.3 14.2 13.8 16.3 14.4 13.5 12.9 13.3 HATmediumb
Totalno.of chromosomes NTc 90.5 (77-100) 83.0 (71-94) 82.3 (53-134) NT 78.0 (55-92) 68.8 (52-85) NT NT 88.2 (79-94) 77.0 (59-89) 74.7 (62-138) NT 88.5 (82-94) 86.9 (73-94) 78.1 (68-92) Telocentric! acrocentric NT 74.9 66.8 66.3 NT 60.1 51.8 NT NT 71.9 58.5 55.0 NT 74.6 74.2 64.3 Meta-centric,' submeta-centric NT 13.8 14.6 15.2 NT 16.7 16.0 NT NT 14.9 17.2 19.2 NT 12.9 11.6 12.9
aThenumber of chromosomesrepresents themeanvalue of 20metaphases.Thefiguresinparentheses
denote the rangeofchromosomal numbers. The difference between the sumoftelocentric/acrocentric
andmetacentric/submetacentric chromosomes and the total number is caused by chromosomes that
cannot be identified.
bSeeMaterials and Methods.
[image:4.493.57.451.407.525.2]cNT = nottested.
TABLE 2. Agglutinationbyconcanavalin A ofparenital andhybridcells
Subculture
~~~~~~~~CoDcanavalin
Aadded(pug)Cellline cl Trypsintreatment
5 25 50 125
3T3 TK- Without Oa 1+ 2+ 2-3±
Rat-S V40 42 Without 2+ 3-4+ 4+ 4+
HybridH3 38 Without 1+ 2± 3+ 4+
3T3 TK- With 0 3+ 3-4+ 4+
Rat-S V40 42 With 2+ 3-4+ 4+ 4+
Hybrid H3 38 With 2+ 3-4+ 4+ 4+
aU = N~o
agglutination;
agglutinated.
1+=
LY70/
01 the ceiis agglutinateul; 2-j- =JUu/0;
35f = i;'4-1 =IVUIJy-hybrid lines had about the same
dependence
onserum,the results withonlyoneofthemaregiven.
Although the hybrid cells had a reduced growth
capacityin 1%serum,therestillwas anincrease in
the number of cells.
DISCUSSION
The results of the chromosomal studies ofthe
hybrid
cells show that over aperiod
of about ayear
only
10 to 20% of the chromosomes hasbeen lost. The continuous presence of the three
enzymemarkers alsoindicates the
stability
of thehybrids.
During thisperiod
of about a year, the cellsweresubculturedtwiceaweek.Thesmallloss of chromosomes isinagreement with thefindingsof Weiss and
Ephrussi (14).
In thestudy
ofrat-mouse
hybrids,
5to10%l
ofthechromosomeswaslostover aperiodof 8months;thepercentloss of
rat marker (metacentric) chromosomes was
greater than thatofthe total number of
chromo-J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
PROPERTIES OF SOMATIC CELL HYBRIDS
TABLE 3. Efficiencyof colony formation in agar of
parental and hybrid cells
Cell lineCellline Subculture
~level
Coloncells-forming(%a3T3 TK- 0
Rat-SV40 78 20.0
HybridH2 33 1.0
78 1.2
Hybrid H8 35 3.2
80 4.0
a Percentageis calculated from cell inocula of
2,000.
24 48 72
TIME IN HOUR5
FIG.4. The growth of3T3TK-and rat-S V40 cellsas
afinctionof theserumconcentration.
24 48
TIME 1N HOUR5
FIG. 5. Growth of hybridcells as afunction ofthe
serumconcentration.
somes, suggesting a preferential loss of rat
chromosomes. In our studies, no preferential
loss of metacentrics was observed, and most of
the chromosomal loss occurred among the
telo-centric and acrotelo-centric chromosomes. Further
subculturingof thehybridcellsisnecessaryto ob
tain further loss of chromosomes; this will per-haps be enhanced by changing the experimental conditions.
Because identification of chromosomes re-mains difficult, the use of more enzyme markers might be valuable for studying the loss of parental genomesfrom the hybridcells.
Allproperties of the SV40-transformed rat cells appear in the hybrid cells. The SV40 T antigen has been studied over manycell generations, and no loss of T antigen from the hybrid cells was observed. This is not surprising in view of the findings of Weiss (13) who studied the loss of Tantigen fromhybrids between mouse and SV40-transformed human cells. Cells became negative
for T antigen only after most of the human
chromosomeswerelost.Since inourstudy onlya
very limited loss of chromosomes has occurred,
the continuouspresenceofTantigen in the hybrid
cellsisnot unexpected.
During further subculturing, the hybrid cells
willbestudied for the presenceofTantigenand
thenumberofratchromosomes. The presence of
Tantigen indicates that the hybrid cellscontain
at least an early function of the viral genome.
Since no infectious virus couldbe recovered it is
not known whether the whole virus genome is
present.
Thefact that theSV40-transformed parentcells
did not yield infectious virus was considered
advantageous.The presence of infectiousvirusin
oursystemmight, after
superinfection
ofnegativecells, lead to the induction of T
antigen
or totransformation of themousecomponentof
hybrid
cells.
The altered structural organization of the
sur-facemembraneasdemonstrated by agglutination
with concanavalin A was found in
SV40-trans-formed cells (6). The degree of
agglutination
ofourhybrid cellssuggests that thesurface
proper-ties of the transformedcells are atleastpartially
present in the hybrid cells. Trypsin treatmentof
thehybridcells resulted inanenhanced
agglutina-tion by concanavalin A. Ithas been shown that
trypsin treatmentofnormal cells leads to an
in-creased agglutinationbyglycoproteins (2, 6, 11).
Fromthis it seemsreasonabletosupposethat the
hybrid cells also have surface
properties
ofnormal cells. It is not clear whether the surface
propertiesof thehybridcellsresult from
suppres-sion of the transformedphenotype
by
the normalmousegenome.
In addition, wehavestudied the
growth
char-acteristics (5)ofthe
hybrid
cells incomparison
tothose of the parental cells. All
growth
character-istics of the transformed cells wereexpressed
inthe hybridcells,
although
toalesserdegree.
Thismightenableustodeterminewhethertheeventual
VOL. 10, 1972 71
-1 I
xz
I I
---l<
I --I
i
---4
I. II
C:
r
.9
t
9
"I
-1 --l
u 0 0.1 (t x
:1r-10% 5 %
I %
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.493.45.240.149.374.2] [image:5.493.46.241.418.560.2]changes of the transformed phenotype of the hybrid are related to loss of chromosomes and
enzymemarkers.Ithas been shownby Marinand
Littlefield (8) that one of the growth
character-istics of polyoma-transformed hamster cells, growth in soft agar, was reduced after loss of chromosomes. As far as studied, no changes in
growthcharacteristics have occurredinourhybrid
cells, which isnotunexpectedinview of the small
loss of chromosomes. Future studies will be
directed towardrelatingloss ofchromosomesand enzymemarkersto propertiesof thehybridcells.
ACKNOWLEDGMENTS
WeareindebtedtoIneHassink for excellent technical
assist-anceandtoMeeraKhan(Departmentof HumanGenetics,
Uni-versity ofLeiden) for his advice.
LITERATURE CITED
1. Basilico,C., and R.Wang.1971.Susceptibilityof superinfec-tion of hybridsbetweenpolyoma"transformed" BHK and
"normal" 3T3 cells. NatureN. Biol. 230:105-108.
2. Burger, M.M. 1969. Adifference in thearchitectureofthe
surface membraneofnormalandvirallytransformedcells.
Proc. Nat. Acad. Sci.U.S.A.62:944-1002.
3. Davidson, R. J., andB.Ephrussi. 1965.Aselectivesystemfor theisolationofhybridsbetween L cells and normal cells.
Nature(London) 205:1170-1171.
4. Defendi, V., B. Ephrussi, H. Koprowski,andM.C.Yoshida. 1967. Properties of hybrids between polyoma-transformed
and normalmousecells.Proc.Nat. Acad. Sci. U.S.A. 57: 299-306.
5. Eagle, H., G. E. Foley, H. Koprowski, H. Lazarus,E. M. Levine, and R. A. Adams. 1970. Growth characteristics of virus-transformed cells. J. Exp. Med.131:863-879. 6. Inbar,M.,andL. Sachs. 1969. Interaction of the
carbohydrate-binding protein concanavalin A with normal and
trans-formedcells. Proc. Nat. Acad. Sci.U.S.A. 63:1418-1426. 7. Littlefield,J. W. 1964.Selection of hybrids from matings of
fibroblasts in vitro and their presumed recombinants. Science145:709-711.
8. Marin, G., and J. W. Littlefield. 1968.Selectionof
morpho-logically normal cell lines from pclyoma-transformed BHK21/13 hamster fibroblasts. J. Virol. 2:69-78. 9. Meera Khan, P. 1971. Enzymeelectrophoresis in cellulose
acetate gel:zymogram patternsin man-mouseand
man-chinese hamster somatic cell hybrids. Arch. Biochem. Biophys. 145:470-483.
10. Neff, J.M., and J. F. Enders. 1968. Poliovirus replication and cytopathogenicity in monolayer hamster cell cultures fused with betapropiolactone-inactivated 'Sendai virus. Proc. Soc. Exp. Biol. Med. 127:260-268.
11. Ozanne, B., and J. Sambrook. 1971. Binding of radioactively labelled concanavalin A and wheat germ agglutinin to normal and virus-transformedcells. Nature N. Biol. 232: 156-161.
12. Pope, J. H., and W. P. Rowe. 1964. Detection of specific anti-geninSV40-transformed cells by immunofluorescence. J. Exp. Med. 120:121-123.
13. Weiss, M. C. 1970.Further studiesonloss ofT-antigen from somatic hybridsbetweenmousecellsandSV40-transformed human cells. Proc. Nat.Acad. Sci. U.S.A. 66:79-86. 14. Weiss, M. C., and B. Ephrussi. 1966. Studies of interspecific
(ratxmouse) somatic hybrids.I.Isolation,growth and
evo-lution ofthekaryotype. Genetics 54:1095-1109.