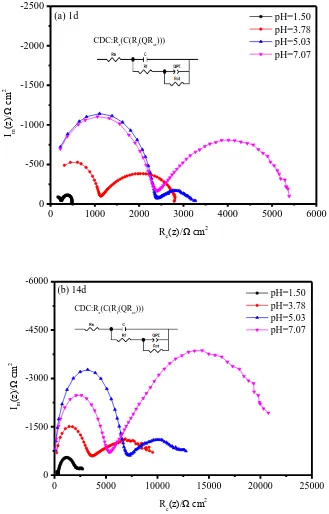
Study of the Electrochemical Corrosion Behaviour of X70 Steel in H2SO4 Contaminated Silty Soil
Full text
Figure



Related documents
The sample surface formed a single layer of corrosion products after exposure in the soil for 2 years, and some cracks spread along to the matrix, then, the corrosion product
The corrosion rates of the stainless steel in free and inhibited acidic media increased as the temperature increased, although the addition of the extract reduced the
The corrosion behavior of X70 high strength pipeline steel under stray AC with various current densities in Dagang simulated marine soil solution was investigated... (1) The AC
Average corrosion rate and corrosion images of Q235 steel, buried in indoor loamy soils with various moisture contents and different time, were investigated via mass loss,
1: the lowest Icorr value was for the steel cooled in icy water, whereas the highest corrosion rate was for specimen cooled in hot water, as can be seen on Fig.6, which shows
Keywords: Martensitic stainless steel, corrosion, polarization, sulphuric acid, potassium dichromate, inhibition.. Selection of materials for good performance in service and in
The plant extracts leaves, stems and flowers can be used as excellent corrosion inhibitors for mild steel in acidic medium. The performance of this extract as corrosion inhibitor
This study used 304 stainless steel manufactured by laser marking to discuss the surface morphology before and after corrosion by laser confocal and scanning