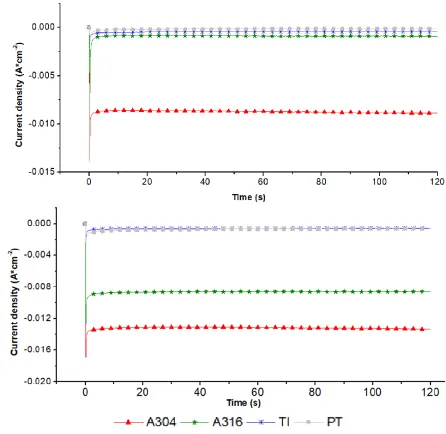
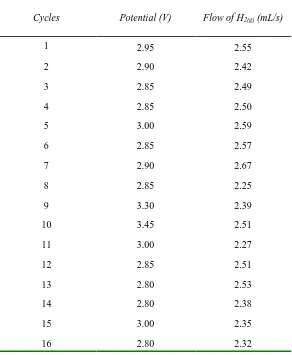
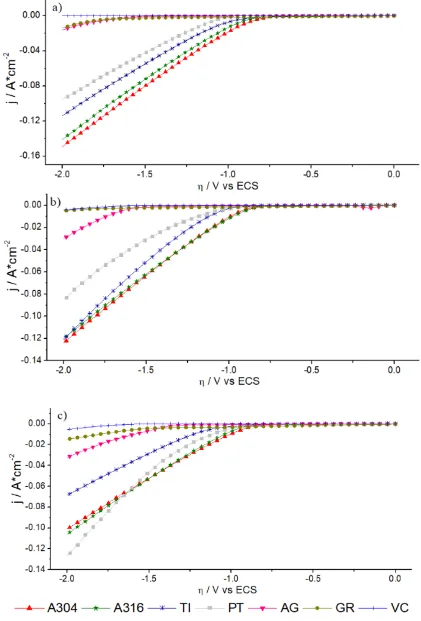
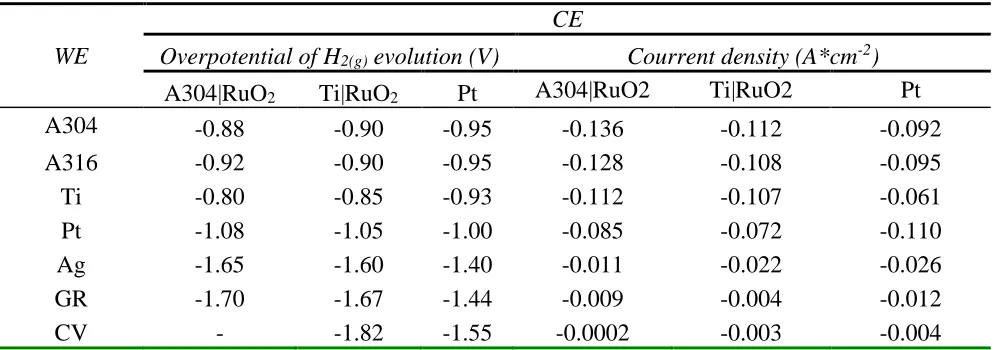
Effect of DSA electrode (A304|RuO2) on the electrochemical production of H2(g)
11
0
0
Full text
Figure
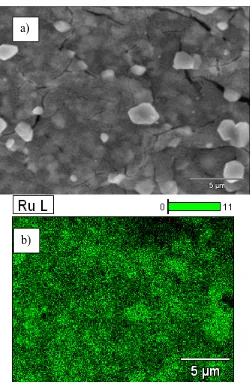
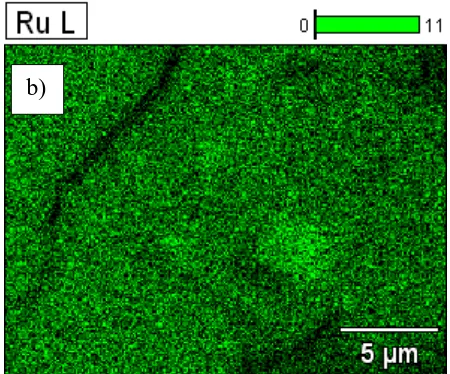
+3
Related documents