0022-538X/07/$08.00⫹0 doi:10.1128/JVI.00681-07
Copyright © 2007, American Society for Microbiology. All Rights Reserved.
The ORF3 Protein of Porcine Circovirus Type 2 Interacts with
Porcine Ubiquitin E3 Ligase Pirh2 and Facilitates p53
Expression in Viral Infection
䌤
Jue Liu,
1† Yu Zhu,
1Isabelle Chen,
1Jennifer Lau,
1Fang He,
1Adeline Lau,
1Zhilong Wang,
1Anbu K. Karuppannan,
1and Jimmy Kwang
1,2*
Animal Health Biotechnology Group, Temasek Life Sciences Laboratory, National University of Singapore, 1 Research Link,
Singapore 117604,1and Department of Microbiology, Faculty of Medicine, National University of Singapore,
Block MD4, 5 Science Drive 2, Singapore 1175972
Received 30 March 2007/Accepted 11 June 2007
Porcine circovirus type 2 (PCV2) is the primary causative agent of an emerging swine disease, postweaning multisystemic wasting syndrome. We previously showed that a newly identified protein, ORF3, plays a major role in virus-induced apoptosis and is involved in viral pathogenesis in vitro and in vivo. To characterize the role of the ORF3 protein in modulation of cellular function, a yeast two-hybrid system was used to screen a porcine cDNA library to find its interacting partner. We have isolated and characterized pPirh2 (for “porcine p53-induced RING-H2”), an E3 ubiquitin ligase, which specifically interacts with the ORF3 protein of PCV2. This interaction was further confirmed when the ORF3 protein coimmunoprecipitated with and colocalized to pPirh2 in PK15 cells. The ORF3 protein has been found to interact with the p53 binding domain of pPirh2 in yeast cells. Expression of the protein results in less pPirh2 expression in PCV2-infected cells. Furthermore, increases in p53 expression were observed in PCV2-infected and ORF3 (alone)-transfected cells. Phosphory-lation of p53 at Ser-46, which is related to p53-induced apoptosis, was also time-dependently activated in PCV-infected and ORF3-transfected cells. Taken together, our results show that the PCV2 ORF3 protein specifically interacts with pPirh2 and inhibits its stabilization; this may lead to increasing p53 expression, resulting in apoptosis.
Porcine circovirus(PCV) is classified in the genusCircovirus,
of the familyCircoviridae(29). PCV was originally identified as a contaminant of porcine kidney cell cultures (PK15; ATCC CCL-13) (34). The PCV virion is icosahedral, nonenveloped, and 17 nm in diameter. The genome of PCV is a single-stranded circular DNA of about 1.76 kb. Two serotypes have been recognized for this virus. The PK15 cell-derived PCV has been considered nonpathogenic to pigs and is designated PCV type 1 (PCV1). On the other hand, infection by PCV2 has been associated with postweaning multisystemic wasting syndrome in young weaned pigs. The disease was first recognized in Canada in 1991 and has since been described in virtually all regions of the world that produce pigs (1, 9, 11, 23). Two major open reading frames (ORFs) have been recognized for PCV: ORF1, called the repgene, which encodes a protein of 35.7 kDa that is involved in virus replication (24), and ORF2, called the capgene, which encodes the major immunogenic capsid protein of 27.8 kDa (4, 27). In addition to the replicase en-coded by ORF1 and the capsid protein enen-coded by ORF2, a novel protein, encoded by ORF3, has been detected in PCV2 productive infection. This protein is not essential for PCV2
replication in cultured cells but plays a major role in virus-induced apoptosis and is involved in viral pathogenesis in vitro and in vivo (19, 20). However, the role of the ORF3 protein in modulation of cellular function is still not clear.
The tumor suppressor p53 is a sequence-specific transcrip-tion factor that plays a pivotal role in the cellular response to DNA damage, as it controls DNA repair, cell cycle arrest, and apoptosis (18). Under normal conditions, p53 is maintained at a low level by Mdm2, Pirh2, or COP1 interaction and subse-quent ubiquitin-dependent degradation (5, 25). During cellular responses to a variety of genotoxic stresses, including UV or gamma irradiation, exposure to extreme heat, hypoxia, or star-vation, and after viral infection, p53 is stabilized and activated. For many viruses, replication depends on the induction of S phase via stimulating the expression of several proteins during that stage, which often leads to increased levels of p53. Viruses have been shown to manipulate p53 for their purposes by using specific viral proteins (8, 14, 33, 36). Furthermore, it is well documented that phosphorylation at Ser-46 of p53 plays a key role in apoptotic signaling by p53 through regulating the tran-scriptional activation of an apoptosis-inducing gene (28). Thus, the modulation of p53 seems to be an important event for the replication of many viruses. PCV genomic DNA replication depends on cellular enzymes expressed during S-phase growth of cultured cells (35). However, whether PCV2 replication can stabilize and increase p53 expression and whether ORF3 ex-pression-induced host cell apoptosis is associated with activa-tion of p53 levels are still not clear.
In this study we show for the first time, by yeast two-hybrid assay, that the PCV2 ORF3 protein interacts with the pPirh2 * Corresponding author. Mailing address: Animal Health
Biotech-nology Group, Temasek Life Sciences Laboratory, National University of Singapore, 1 Research Link, Singapore 117604. Phone: (65) 68727473. Fax: (65) 68727007. E-mail: kwang@tll.org.sg.
† Present address: Institute of Animal Husbandry and Veterinary Medicine, Beijing Municipal Academy of Agriculture and Forestry Sciences, No. 9, Shuguang Garden Central Road, Haidian District, Beijing 100097, People’s Republic of China.
䌤Published ahead of print on 20 June 2007.
9560
on November 8, 2019 by guest
http://jvi.asm.org/
(for “porcine p53-induced RING-H2”) protein, the homo-logues of which are androgen receptor N terminus-interacting protein (ARNIP) in mice and hPirh2 in humans (2, 17, 21). pPirh2 shows high homology to hPirh2, suggesting a role in modulation of p53-induced apoptosis. The results show that the PCV2 ORF3 protein is able to specifically interact with the ubiquitin E3 ligase pPirh2 and to inhibit its stabilization, thus increasing p53 expression and resulting in apoptosis.
Interaction of pPirh2 with the PCV2 ORF3 protein.In order to identify porcine proteins possibly interacting with the PCV2 ORF3 protein, the ORF3 protein was used as a bait protein for screening the porcine cDNA library in a yeast two-hybrid assay (Matchmaker GAL4 Two-Hybrid System 3; Clontech). The cell line PK15 was used to prepare a porcine cDNA library according to standard protocols. The constructed library con-tains approximately 9⫻105independent clones. Inserts were
found in 97% of the tested colonies. The bait gene was ampli-fied from the PQE-ORF3 plasmid (19) and subcloned into the GAL4 DNA binding fusion vector pGBKT7. The DNA bind-ing construct (pGBK-ORF3) and activation library plasmids (pGAD-library) were cotransformed into yeast strain AH109, and the transformants were selected on synthetic defined (SD)/ His⫺/Leu⫺/Trp⫺medium. Positive colonies were further se-lected on SD/Ade⫺/His⫺/Leu⫺/Trp⫺ ␣ -5-bromo-4-chloro-3-indolyl--D-galactopyranoside medium. Blue colonies were cultured in SD/Ade⫺/His⫺/Leu⫺/Trp⫺ broth and lysed with glass beads (Sigma) for plasmid isolation. A total of 10 positive colonies grew on the high-stringency plate, and five colonies corresponded to a cDNA with a high degree of homology to the published sequence of hPirh2 (2, 17). The sequence ob-tained by the yeast two-hybrid assay from the porcine cDNA library was shorter than that of Pirh2 and apparently lacked the N-terminal region. The complete sequence with a Kozak con-sensus start codon was determined from porcine cDNA by 5⬘ rapid amplification of cDNA ends and was named pPirh2. The coding region of pPirh2 is predicted to be 786 bp, which en-codes 261 amino acids, and the encoded protein shows a high degree of homology to Pirh2 proteins from other species. The pPirh2 protein has more than 95% and 89% homology with those of the human and mouse Pirh2 proteins, respectively.
To confirm the interaction between pPirh2 and ORF3, a pGAD-pPirh2 plasmid (with full-length pPirh2) was cotrans-formed into AH109 yeast cells with the bait plasmid pGBK-ORF3, while empty vectors served as controls. The controls were able to grow only on medium supplemented with adenine and histidine, while yeast cells transformed with pGADT7-pPirh2 and pGBK-ORF3 efficiently grew on the high-strin-gency selection plate and showed dark blue colonies (data not shown). The interaction was further confirmed by an in vitro glutathione S-transferase (GST) pull-down experiment of ORF3 with GST-fused full-length pPirh2 expressed in bacteria (data not shown).
Interaction between pPirh2 and ORF3 was next studied in porcine cells by coimmunoprecipitation in vivo. The ORF3 and pPirh2 full-length genes were inserted into the eukaryotic ex-pression vector pCMV-HA or pCMV-myc (Clontech), which allows the production of Myc or HA fusion protein. PK15 cells grown in T25 flasks were transfected or cotransfected with HA-pPirh2 or/and myc-ORF3 (2 g of plasmid per flask), using Lipofectamine Plus (GIBCO-BRL), as described in the
manufacturer’s protocol. The cells were washed once, at 24 h posttransfection, with ice-cold phosphate-buffered saline, lysed in 1 ml of lysis buffer (50 mM Tris [pH 8.0], 5 mM EDTA, 150 mM NaCl, and 0.5% NP-40) on ice, and then further dis-rupted. Cell debris was pelleted by centrifugation, and the supernatants were incubated with Myc or HA antibody tag beads (Clontech) for 3 h at 4°C with rotation. The beads were collected and washed five times in washing buffer (5% sucrose, 5.0 mM Tris-HCl [pH 7.4], 5 mM EDTA, 0.5 M NaCl, and 1% NP-40). Bound proteins were eluted and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) before Western blotting. After transfection of HA-pPirh2 in PK15 cells, the expression level of HA-HA-pPirh2 was very low when detected by anti-HA antibody, suggesting that pPirh2 may have a short half-life, as previously reported for hPirh2 (3, 21). The addition of a 5M concentration of the proteasome inhibitor MG132 (Calbiochem, San Diego, CA) after transfection greatly increased the expression level of HA-pPirh2. Therefore, MG132 was used to increase the level of pPirh2 expression for coimmunoprecipitation experiments. Whole-cell extracts from PK15 cells transfected with HA-pPirh2 and/ormyc-ORF3 plasmids were subjected to immu-noprecipitation. A reciprocal specific coimmunoprecipitation of HA-pPirh2 andmyc-ORF3 was observed with either anti-Myc antibody (Fig. 1A) or anti-HA antibody (Fig. 1B) (Clon-tech). Taken together, the results showed that ORF3 and pPirh2 physically and specifically interact in vivo.
Cellular localization of ORF3 and pPirh2.To validate the pPirh2-ORF3 interaction in the physiological environment, we decided to study the colocalization patterns of the two pro-teins. The cellular localizations of different proteins were tested by fluorescence confocal microscopy. PK15 cells were cotransfected with myc-ORF3 and HA-pPirh2 plasmids. At 24 h after transfection, the cells were fixed in 4% paraformal-dehyde. The cells were incubated with anti-Myc monoclonal and rabbit anti-HA polyclonal antibodies (Clontech) followed by incubation with fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (IgG) and rhodamine-conjugated anti-rabbit IgG (DAKO). Individual expression of HA-pPirh2 protein exhibited a diffuse cytoplasmic and nuclear localiza-tion, while Myc-ORF3 protein displayed predominantly a dif-fuse cytoplasmic distribution and a lesser degree of distribution in the nucleus of the transfected cells. Coexpression of HA-pPirh2 and Myc-ORF3 proteins showed that HA-HA-pPirh2 colo-calized with the ORF3 protein in the cytoplasm and to a lesser degree in the nucleus, as indicated by the yellow color in merged images (Fig. 1C). This result confirmed that the two proteins colocalize in the cellular environment of the trans-fected cells.
p53 binding domain of pPirh2 (amino acids 120 to 137) is required for binding to ORF3. Computational analysis of pPirh2 resulted in the identification of three regions in the protein: a zinc finger domain, p53 binding domain, and RING finger domain spanning amino acids 21 to 94, 120 to 137, and 145 to 186, respectively. The presence of a RING domain in several proteins has identified their role as an E3 enzyme that covalently attaches ubiquitin peptides to substrates (15). pPirh2 is also an E3 ubiquitin ligase, as observed in a ubiquiti-nation assay of purified GST-pPirh2 (data not shown).
To determine whether these domains are required for the
VOL. 81, 2007 NOTES 9561
on November 8, 2019 by guest
http://jvi.asm.org/
interaction of pPirh2 with the ORF3 protein, we generated three pPirh2 deletion mutants that have a deletion of the zinc finger domain, p53 binding domain, or RING finger domain. The desired pPirh2 coding region was amplified by PCR from the pGAD-pPirh2 plasmid with deoxyoligonucleotide primers (Table 1). The fragment pPirh2 and its mutants were cloned in frame with the GAL4-AD domain in the yeast two-hybrid expression vector pGADT7 (Fig. 2). In our preliminary exper-iment, the N-terminal 30 amino acids of pPirh2 did not affect pPirh2 interaction with the ORF3 protein; thus, the partial
[image:3.585.112.472.69.358.2]pPirh2 protein, which lacks the N-terminal 30 amino acids, was used to construct these three pPirh2 deletion mutants. These constructs were then cotransformed with pGBK-ORF3 into the yeast strain AH109 to assess the binding of pPirh2 and its mutants to the ORF3 protein. The results obtained showed that two of the deletion mutants were capable of binding to the ORF3 protein compared to the full-length construct (Table 2). Only pPirh2 deletion mutant 2 (with the p53 binding domain deleted) failed to interact with the ORF3 protein. Thus, we concluded that the stretch containing amino acid residues 120 FIG. 1. In vivo Interaction of pPirh2 with the PCV2 ORF3 protein. (A and B) Coimmunoprecipitation of Myc-ORF3 protein with HA-pPirh2 in PK15 cells using Myc (A)- or HA (B)-specific antibodies. Antibody specificity is shown by the lack of immunoprecipitated materials (lanes 1). The left-hand panels show protein expression in whole-cell extracts of input samples. Proteins were expressed by transfection with the corre-sponding vectors, and the proteasome inhibitor MG132 was added for 8 h before cell harvest. WB, Western blotting; IP, immunoprecipitation;␣, anti. (C) Localization of ORF3 and pPirh2 in PK15 cells. Cells were cotransfected with recombinantmyc-ORF3 and HA-pPirh2 plasmids. At 24 h after transfection, the cells were fixed and doubly labeled with polyclonal anti-HA and monoclonal anti-Myc antibodies followed by fluorescein isothiocyanate-conjugated anti-mouse IgG or rhodamine-conjugated anti-rabbit IgG antibodies. Separate images showing ORF3 distribution (a and d), pPirh2 distribution (b and e), and a merger of both (c and f) were acquired. Colocalizations are shown in yellow in the merged images. Bars, 10m.
TABLE 1. Oligonucleotide primers used for amplifying pPirh2 and its deletion mutants
Primer used to create
deletion mutant Sequence
a pPirh2 fragment
amplified (bp)
pPirh2(5) 5⬘-CAGTGAATTCATGGCGGCCTCGGCG-3⬘ Full length
pPirh2(3) 5⬘-CGATGGATCCTCACTGCTGATCTAA-3⬘
pPirh2-91(5) 5⬘-CAGTGAATTCGCACCTTGCTGTGAC-3⬘ Partial
pPirh2-283(5) 5⬘-CAGTGATTCAAAGATAAGAAACAGTATCA-3⬘ 283–786
pPirh2-357(3) 5⬘-CCGGGAAACATTTTCACAGTGGAAAAAATC-3⬘ 91–357
pPirh2-412(5) 5⬘-GATTTTTTCCACTGTGAAAATGTTTCCCGG-3⬘ 412–786
pPirh2-432(3) 5⬘-TAAAGCAGAGTGCATATTCTGCCGGGAAAC-3⬘ 91–432
pPirh2-559(5) 5⬘-GTTTCCCGGCAGAATATGCACTCTGCTTTA-3⬘ 559–786
aUnderlined portions of sequences are restriction sites.
on November 8, 2019 by guest
http://jvi.asm.org/
[image:3.585.44.551.612.716.2]to 137 of pPirh2, which is believed to associate with p53, is required for its binding to the ORF3 protein.
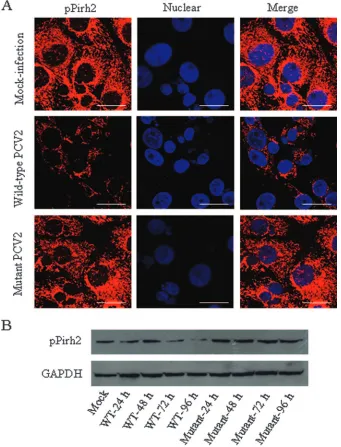
ORF3 expression reduces the amount of pPirh2 protein.To determine whether the interaction of the ORF3 protein with pPirh2 can have an effect on the expression of pPirh2 in vivo, we first used fluorescence confocal microscopy to detect pPirh2 in PCV2-infected PK15 cells. Overnight cultures of PK-15 cells were infected with wild-type PCV2 strain BJW or its mutant strain (19) at a multiplicity of infection of 1. Cells were addi-tionally treated with 300 mM D-glucosamine at 24 h after infection, as described previously (35). Following the incuba-tion, cells at 24, 48, 72, and 96 h postinfection were washed with phosphate-buffered saline and fixed in 4% paraformalde-hyde. The cells were incubated with guinea pig anti-pPirh2 polyclonal antibody, followed by incubation with
rhodamine-conjugated anti-guinea pig IgG (DAKO), and were then incu-bated with DAPI (2,4-diamidino-2-phenylindole) at a concen-tration of 1g/ml for 30 min at 37°C. In mock-infected PK15 cells, the pPirh2 protein displayed a broad distribution in the cytoplasm, with some speckles in the nucleus (Fig. 3A). When tested at 48 h postinfection with wild-type PCV2, the amount of pPirh2 in PK15 cells decreased markedly, in both cytoplasm and nucleus, compared with mock-infected cells. Expression of pPirh2 in the nucleus was not detected after wild-type PCV2 infection (Fig. 3A). However, the mutant PCV2, which lacks ORF3 protein expression, did not change the expression of pPirh2 in PK15 cells (Fig. 3A).
In further experiments, whole-cell lysates of the wild-type or mutant PCV2-infected PK15 cells were harvested at different time points after infection, and pPirh2 was then detected by Western blotting. As expected, the pPirh2 level was reduced in cell extracts of wild-type PCV2-infected cells but not of mutant PCV2-infected cells (Fig. 3B). Mock-infected cells were used as controls. Thus, it is possible that the observed decrease of pPirh2 upon ORF3 expression may be due to direct interaction between the two proteins.
[image:4.585.145.461.67.273.2]Expression of p53 was increased in PCV2-infected as well as in ORF3 (alone)-transfected cells.It has been shown that murine Pirh2 physically interacts with p53 and promotes ubiquitination of p53 independently of Mdm2 (17). Activa-tion of p53 induced by virus infecActiva-tion can lead either to cell cycle arrest, presumably to allow repair of damaged DNA, or to apoptosis. To investigate the role of p53 in apoptosis induced by ORF3, we first detected the transcription level of p53 after PCV2 infection. Total cell RNAs from PCV2-infected PK15 cells at 24, 48, 72, and 96 h postinfection were prepared for reverse transcription (RT)-PCR by using TRIzol RNA extract reagent (Invitrogen). The following primers were used: p53(5) (5⬘-CCTCACCATCATCACACTGG-3⬘) and p53(3) (5⬘-GGCTTCTTCTTTTGCACTGG-3⬘) for porcine p53 and sense (5⬘-CATCACTGCCACCCAGAAGA-3⬘) and antisense (5⬘-GCTG TAGCCAAATTCGTTGT-3⬘) for GAPDH (glyceraldehyde-FIG. 2. Deletion mutants of pPirh2. The deletion mutants (numbers correspond to amino acid positions) were fused to pGADT7 (AD) and used to bind protein expressed from pGBKT7 (BD)-ORF3 in yeast two-hybrid assays. White indicates deleted portions.
TABLE 2. ORF3 interactions with deletion mutants of pPirh2 as determined by yeast two-hybrid assaysa
Construct
Growth onb:
SD/Leu⫺/ Trp⫺
SD/His⫺/ Leu⫺/
Trp⫺
SD/Ade⫺/ His⫺/Leu⫺/
Trp⫺
AD⫹BD-ORF3 ⫹ ⫺ ⫺
AD-pPirh2 (full length)⫹
BD-ORF3 ⫹ ⫹ ⫹
AD-pPirh2 (partial)⫹BD-ORF3 ⫹ ⫹ ⫹
AD-pPirh2 (deletion mutant 1)⫹
BD-ORF3 ⫹ ⫹ ⫹
AD-pPirh2 (deletion mutant 2)⫹
BD-ORF3 ⫹ ⫺ ⫺
AD-pPirh2 (deletion mutant 3)⫹
BD-ORF3 ⫹ ⫹ ⫹
AD-pPirh2 (full length)⫹BD ⫹ ⫺ ⫺
aPlasmids encoding different mutants of pPirh2 were cotransformed with
BD-ORF3 and selected on media with increasing stringencies, as indicated. After 7 days, clones were assessed for growth (AD, pGADT7 empty vector; BD, pGBKT7 empty vector). Constructs AD ⫹BD-ORF3 and AD-pPirh2 (full length)⫹BD were used as negative controls.
b⫹, clone growth;⫺, no growth.
VOL. 81, 2007 NOTES 9563
on November 8, 2019 by guest
http://jvi.asm.org/
[image:4.585.42.285.523.675.2]3-phosphate dehydrogenase). cDNAs were reverse transcribed from total RNAs by the use of antisense primers and the First-Strand synthesis system (Avian Myeloblastosis Virus Re-verse Transcriptase kit; Roche). Quantitative real-time PCR was performed on a LightCycler (Roche) instrument according to the instructions of the LightCycler Fast Start DNA MasterPlus
SYBR green I kit (Roche). The results revealed that p53 tran-script accumulation was significantly increased over time in PCV2-infected cells (Fig. 4A, panel a), with the total amount from 24 to 96 h postinfection higher than in the mock-infected cells, indicating that p53 was progressively transcribed. We then examined p53 transcription in ORF3-transfected PK15
cells at 24 and 48 h posttransfection, and the level of p53 transcription was increased in a time-dependent manner in transfected cells (Fig. 4A, panel b).
To further determine whether the increased p53 mRNA levels resulted in increased p53 levels, extracts of PCV2-in-fected or mock-inPCV2-in-fected PK15 cells were prepared at various time points after infection and 30g from each sample was separated by SDS-PAGE and blotted with antibody specific to p53 (Cell Signal). As shown in Fig. 4B (panel a), a basal amount of p53 was observed in mock-infected cells but in-creased over time after infection. In contrast to p53, the level of GAPDH did not change at any time after infection with FIG. 3. (A) Expression of pPirh2 in wild-type and mutant PCV2-infected cells. PK15 cells were fixed at 48 h postinfection and stained with antibody raised against the pPirh2 protein and rhodamine-conjugated secondary antibodies. Nuclei were stained with DAPI. Merged signals are shown in the right-hand panels. Bars, 10m. (B) Western blot analysis of pPirh2 in wild-type (WT) and mutant PCV2-infected cells. Whole extracts of PK15 cells were electrophoresed by 12% SDS-PAGE, transferred onto nitrocellulose membranes, and detected by anti-pPirh2 antibody. GAPDH was used as a protein loading control.
on November 8, 2019 by guest
http://jvi.asm.org/
[image:5.585.123.464.71.518.2]respect to those detected in mock-infected cells. Consistent with the results observed with the PCV2-infected cells, the level of p53 increased over time in ORF3 plasmid-transfected PK15 cells (Fig. 4B, panel c).
It has been shown that p53-dependent apoptosis is mainly related to phosphorylation of p53 at Ser-46 (28). To determine whether phosphorylation of p53 at Ser-46 is associated with ORF3-induced apoptosis, whole-cell extracts of PCV2-in-fected, as well as ORF3-transPCV2-in-fected, PK15 cells were separated by SDS-PAGE and blotted with antibody specific to phosphor-ylated p53 at Ser-46 (Cell Signal). As shown in Fig. 4B (panel a), p53 is phosphorylated at Ser-46 after PCV2 infection in a time-dependent manner. In contrast, p53 phosphorylation was not activated in the PCV2 mutant-infected cells regardless of the duration after infection (Fig. 4B, panel b). As expected, p53 phosphorylation at Ser-46 also occurred in the ORF3-transfected PK15 cells as determined by using antibody to phosphorylated p53 at Ser-46 (Fig. 4B, panel c). Therefore, Ser-46 phosphorylation of p53 and induction of apoptosis are closely correlated in ORF3-expressing cells.
In the present study, we have identified a new protein, an E3
ligase, pPirh2, as a porcine cellular partner of the PCV2 ORF3 protein. This protein belongs to the class of E3 ubiquitin li-gases with a RING-H2 motif (17), which causes the ubiquiti-nation and degradation of p53 and impairs the growth-sup-pressing activity of p53. p53 is the main target of many cellular E3 ligases, such as Mdm2, Pirh2, and COP1, which mediate the downregulation of the protein (5, 10). Pirh2 could potentially affect p53-mediated apoptosis by both abrogating p53-medi-ated transcription and preventing its translocation to mito-chondria (5, 37). It has been shown that a region adjacent to the cysteine-rich RING motif of Pirh2 spanning amino acids 120 to 137 associates with the p53 central DNA binding do-main between amino acids 82 and 292 (17). Our yeast two-hybrid assay (Table 2) shows that the ORF3 protein interacts with the p53 binding domain of pPirh2 (residues 120 to 137). Further, the other domains in pPirh2, including the zinc finger domain (residues 1 to 94) and the RING domain (residues 143 to 186), are not essential for the interaction of the ORF3 protein with pPirh2 (Table 2). Considering our findings (Table 2) with published data (17), the binding of the ORF3 protein to the domain of pPirh2 involved in FIG. 4. (A) Accumulations of p53 transcripts in PCV2-infected (a) and ORF3-transfected (b) PK15 cells detected by real-time RT-PCR. The relative amount of p53 mRNA was normalized to that of GAPDH mRNA and is expressed as multiples of the normalized value for PCV2-infected or ORF3-transfected cells in mock-infected or -transfected cells (controls) in the same sample. Error bars represent the standard error of the mean from three independent experiments. (B) Expression of p53 and phosphorylation of p53 at Ser-46 in ORF3-expressing cells. Whole-cell extracts from PCV2-infected (a) or mutant PCV2-infected (b), as well as ORF3-transfected (c), PK15 cells were subjected to Western blot analysis using an anti-p53 (␣p53) antibody or an anti-p53P antibody that recognizes phosphorylated p53 at Ser-46. GAPDH was used as a protein loading control.
VOL. 81, 2007 NOTES 9565
on November 8, 2019 by guest
http://jvi.asm.org/
[image:6.585.40.535.71.409.2]binding with p53 may competitively block the interaction between pPirh2 and p53.
The compact RING finger structure of Pirh2 interacts with E2 ubiquitin-conjugating enzymes (2) and facilitates ubiquiti-nation of bound substrates. However, when mutations occur within the RING motif of the hPirh2 protein, the substrate may escape proteasome-mediated destruction (3). The E3 en-zymes can also be autoubiquitinated in cells, targeting them-selves for proteasomal destruction through their RING motif (10, 15). In the case of the pPirh2 protein, there is 89% and 95% homology with those of mice and humans, but the cys-teine-rich RING motif spanning amino acids 120 to 137 of pPirh2 possesses 100% identity to that of mPirh2 and hPirh2. In our study, we found that ORF3 expression reduces the pPirh2 level in PK15 cells (Fig. 3). Like Pirh2 in mice and humans (17, 21), pPirh2 is also an E3 enzyme. This suggests that the interaction between the ORF3 protein and pPirh2 may facilitate some degree of proteasome-mediated self-degrada-tion of pPirh2. Measles virus phosphoprotein has been shown to specifically interact with hPirh2 and stabilize the ubiquitin E3 ligase hPirh2 by preventing its ubiquitination (3). However, the ORF3 protein’s interaction with pPirh2 may degrade the ubiquitin E3 ligase pPirh2 via inhibiting its stability (Fig. 3). Thus, there are two possible mechanisms for the unavailability of pPirh2 to decrease p53 levels in ORF3-overexpressing cells: (i) the interaction of the ORF3 protein with pPirh2 may facil-itate self-mediated degradation of pPirh2, subsequently de-creasing its ability to ubiquitinate p53, and (ii) by binding to the p53-interacting domain on pPirh2 (residues 120 to 137), the ORF3 protein may block the interaction of p53 and pPirh2 and the subsequent ubiquitination and degradation of p53. Therefore, when the ORF3 protein interacts with pPirh2 in cultured cells, the ability of pPirh2 to ubiquitinate and to degrade p53 is inhibited and thus leads to an increase in p53 expression.
Activation of p53 by various forms of stress leads to tran-scription of genes that block the proliferation of the affected cells by inducing apoptosis. Many viruses, such as polyomavirus (7), adenovirus (31), herpesvirus 1 (6), African swine fever virus (13), and human cytomegalovirus (14, 16, 22, 26), induce a rapid increase in the level of p53 and its activation as a transcription factor, which is related to the extent of DNA damage. These viruses replicate in the nucleus of the infected cells or have an early nuclear stage of replication. Like these viruses, PCV2 also replicates in the nucleus of infected cells, indicating that DNA damage occurring in the nucleus could play a role in triggering the activation of p53. It has been shown that phosphorylation and acetylation play important roles in regulating the biological activities of p53 (12, 30, 32). Phos-phorylation of Ser-46 has been demonstrated to be important in regulating the ability of p53 to induce apoptosis (28). In this study, we first demonstrated that the marked increase in p53 protein in PCV2-infected as well as ORF3-transfected cells was due to transcriptional and translational events. This was shown by real-time RT-PCR in an analysis of the transcription of p53 mRNA (Fig. 4A) and by using anti-p53 antibody to detect its translation (Fig. 4B, panels a and c), respectively. The levels of p53 transcripts and protein increased after ORF3 protein expression, suggesting that p53 plays a role in ORF3 protein-induced apoptosis. By using antibodies specific for
phosphorylated p53, we further demonstrated that p53 is acti-vated at Ser-46 in ORF3-expressing cells (Fig. 4B, panels a and c), a result which is in agreement with the idea of p53-mediated apoptosis (28). Data from this study combined with our previ-ous reports (19, 20) suggest that ORF3 protein-induced apop-tosis might be related to phosphorylation of p53 at Ser-46. The effect of this activation in relation other cellular processes during PCV2 infection is yet to be investigated.
In conclusion, we have constructed a porcine cDNA library and used the PCV2 ORF3 protein as bait in a yeast two-hybrid system to demonstrate that a physical interaction between the ORF3 protein and the pPirh2 E3 ligase occurs. The interaction of the ORF3 protein with pPirh2 interferes with the function of the porcine E3 ubiquitin ligase, resulting in an increase in p53 expression levels in ORF3-expressing cells and thus suggesting that the ORF3 protein-induced apoptosis in cultured cells may be p53 mediated. This is further supported by the activating phosphorylation of p53 at Ser-46. Therefore, we propose a model whereby binding of PCV2 ORF3 to the E3 ubiquitin ligase (pPirh2) interferes with its function of ubiquitination of p53, leading to increased p53 levels and ultimately leading to apoptosis.
Nucleotide sequence accession number. The complete se-quence of the pPirh2 gene has been deposited in the GenBank database under accession number EF043261.
We thank Wong Lin Sheng (Genomax, Singapore) for technical assistance in preparing the porcine cDNA library.
This work was supported by a grant from the Temasek Life Sciences Laboratory, Singapore.
REFERENCES
1.Allan, G. M., F. McNeilly, S. Kennedy, B. Daft, E. G. Clarke, J. A. Ellis, D. M. Haines, B. M. Meehan, and B. M. Adair.1998. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the USA and Europe. J. Vet. Diagn. Investig.10:3–10.
2.Beitel, L. K., Y. A. Elhaji, R. Lumbroso, S. S. Wing, V. Panet-Raymond, B. Gottlieb, L. Pinsky, and M. A. Trifiro.2002. Cloning and characterization of an androgen receptor N-terminal-interacting protein with ubiquitin-protein ligase activity. J. Mol. Endocrinol.29:41–60.
3.Chen, M., J. C. Cortay, I. R. Logan, V. Sapountzi, C. N. Robson, and D. Gerlier.2005. Inhibition of ubiquitination and stabilization of human ubiq-uitin E3 ligase PIRH2 by measles virus phosphoprotein. J. Virol.79:11824– 11836.
4.Cheung, A. K.2003. Transcriptional analysis of porcine circovirus type 2. Virology305:168–180.
5.Corcoran, C. A., Y. Huang, and M. S. Sheikh.2004. The p53 paddy wagon: COP1, Pirh2 and MDM2 are found resisting apoptosis and growth arrest. Cancer Biol. Ther.3:721–725.
6.Devireddy, L. R., and C. J. Jones.1999. Activation of caspases and p53 by bovine herpesvirus 1 infection results in programmed cell death and efficient virus release. J. Virol.73:3778–3788.
7.Dey, D., J. Dahl, S. Cho, and T. L. Benjamin.2002. Induction and bypass of p53 during productive infection by polyomavirus. J. Virol.76:9526–9532. 8.Dobner, T., N. Horikoshi, S. Rubenwolf, and T. Shenk.1996. Blockage by
adenovirus E4orf6 of transcriptional activation by the p53 tumor suppressor. Science272:1470–1473.
9.Edwards, S., and J. J. Sands.1994. Evidence of circovirus infection in British pigs. Vet. Rec.134:680–681.
10.Fang, S., J. P. Jensen, R. L. Ludwig, K. H. Vousden, and A. M. Weissman.
2000. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J. Biol. Chem.275:8945–8951.
11.Fenaux, M., P. G. Halbur, M. Gill, T. E. Toth, and X. J. Meng.2000. Genetic characterization of type 2 porcine circovirus (PCV-2) from pigs with post-weaning multisystemic wasting syndrome in different geographic regions of North America and development of a differential PCR-restriction fragment length polymorphism assay to detect and differentiate between infections with PCV-1 and PCV-2. J. Clin. Microbiol.38:2494–2503.
12.Giaccia, A. J., and M. B. Kastan.1998. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev.12:2973–2983. 13.Granja, A. G., M. L. Nogal, C. Hurtado, J. Salas, M. L. Salas, A. L. Carrascosa,
on November 8, 2019 by guest
http://jvi.asm.org/
and Y. Revilla.2004. Modulation of p53 cellular function and cell death by African swine fever disease. J. Virol.78:7165–7174.
14.Jault, F. M., J. M. Jault, F. Ruchti, E. A. Fortunato, C. Clark, J. Corbeil, D. D. Richman, and D. H. Spector.1995. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J. Virol.69:6697–6704.
15.Joazeiro, C. A., and A. M. Weissman.2000. Ring finger proteins: mediators of ubiquitin ligase activity. Cell102:549–552.
16.Kovacs, A., M. L. Weber, L. J. Burns, H. S. Jacob, and G. M. Vercellotti.
1996. Cytoplasmic sequestration of p53 in cytomegalovirus-infected human endothelial cells. Am. J. Pathol.149:1531–1539.
17.Leng, R. P., Y. Lin, W. Ma, H. Wu, B. Lemmers, S. Chung, J. M. Parant, G. Lozano, R. Hakem, and S. Benchimol.2003. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell112:779–791.
18.Levine, A. J.1997. p53, the cellular gatekeeper for growth and division. Cell
88:320–331.
19.Liu, J., I. Chen, and J. Kwang.2005. Characterization of a previously un-identified viral protein in porcine circovirus type 2-infected cells and its role in virus-induced apoptosis. J. Virol.79:8262–8274.
20.Liu, J., I. Chen, Q. Du, H. Chua, and J. Kwang.2006. The ORF3 protein of porcine circovirus type 2 is involved in viral pathogenesis in vivo. J. Virol.
80:5065–5073.
21.Logan, I. R., V. Sapountzi, L. Gaughan, D. E. Neal, and C. N. Robson.2004. Control of human PIRH2 protein stability: involvement of TIP60 and the proteasome. J. Biol. Chem.279:11696–11704.
22.Lokensgard, J. R., M. C. Cheeran, G. Gekker, S. Hu, C. C. Chao, and P. K. Peterson.1999. Human cytomegalovirus replication and modulation of ap-optosis in astrocytes. J. Hum. Virol.2:91–101.
23.Mankertz, A., M. Domingo, J. M. Folch, P. LeCann, A. Jestin, J. Segale´s, B. Chmielewicz, J. Plana-Dura´n, and D. Soike.2000. Characterization of PCV2 isolates from Spain, Germany and France. Virus Res.66:65–77.
24.Mankertz, A., J. Mankertz, K. Wolf, and H. J. Buhk.1998. Identification of a protein essential for replication of porcine circovirus. J. Gen. Virol.79:
381–383.
25.Mayo, L. D., and D. B. Donner.2002. The PTEN, Mdm2, p53 tumor sup-pressor-oncoprotein network. Trends Biochem. Sci.27:462–467.
26.Muganda, P., O. Mendoza, J. Hernandez, and Q. Qian.1994. Human cyto-megalovirus elevates levels of the cellular protein p53 in infected fibroblasts. J. Virol.68:8028–8034.
27.Nawagitgul, P., I. Morozov, S. R. Bolin, P. A. Harms, and S. D. Sorden.2000. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J. Gen. Virol.81:2281–2287.
28.Oda, K., H. Arakawa, T. Tanaka, K. Matsuda, C. Tanikawa, T. Mori, H. Nishimori, K. Tamai, T. Tokino, Y. Nakamura, and Y. Taya.2000. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell102:849–862.
29.Pringle, C. R.1999. Virus taxonomy at the XIth International Congress of Virology, Sydney, Australia. Arch. Virol.144:2065–2070.
30.Prives, C.1998. Signaling to p53: breaking the MDM-p53 circuit. Cell95:5–8. 31.Querido, E., J. G. Teodoro, and P. E. Branton.1997. Accumulation of p53 induced by the adenovirus E1A protein requires regions involved in the stimulation of DNA synthesis. J. Virol.71:3526–3533.
32.Shieh, S. Y., J. Ahn, K. Tamai, Y. Taya, and C. Prives.2000. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev.14:289–300.
33.Takada, S., N. Kaneniwa, N. Tsuchida, and K. Koike.1997. Cytoplasmic retention of the p53 tumor suppressor gene product is observed in the hepatitis B virus X gene-transfected cells. Oncogene15:1895–1901. 34.Tischer, I., H. Gelderblom, W. Vettermann, and M. A. Koch.1982. A very
small porcine virus with circular single-stranded DNA. Nature295:64–66. 35.Tischer, I., D. Peters, R. Rasch, and S. Pociuli.1987. Replication of porcine
circovirus: induction by glucosamine and cell cycle dependence. Arch. Virol.
96:39–57.
36.Wang, X. W., K. Forrester, H. Yeh, M. A. Feitelson, J. R. Gu, and C. C. Harris.1994. Hepatitis B virus X protein inhibits p53 sequence-specific DNA binding, transcription activity, and association with transcription factor ERCC3. Proc. Natl. Acad. Sci. USA91:2230–2234.
37.Yang, Y., C. C. Li, and A. M. Weissman.2004. Regulating the p53 system through ubiquitination. Oncogene23:2097–2106.
VOL. 81, 2007 NOTES 9567