0022-538X/79/03-1131/11$02.00/0
Cell-Free Synthesis of
Mouse Mammary
Tumor
from
Virion
and Intracellular
mRNA
Virus Pr77
HANS-HENRIK M. DAHLt AND CLIVE DICKSON*
TranslationandViralOncology Laboratories, Imperial Cancer Research Fund, Lincoln's Inn Fields, London, WC2A3PX, United
Kingdom
Received for publication10October 1978
Mousemammarytumorvirus (MuMTV)waspurifiedfromtwo celllines (GR
andMm5MT/cl), and the genomic RNAwasisolatedandtranslatedin vitro in
cell-freesystemsderived frommouseLcellsandrabbitreticulocytes.The major
translation product in both systems was a protein with the molecular weight
77,000. Several other products werealso detected,amongthema 110,000-dalton
and in minor amounts a 160,000-dalton protein. All three polypeptides were
specifically immunoprecipitated by antiserum raised against the major core
protein of MuMTV (p27), but theywere notprecipitated byantiserum against
the virionglycoprotein gp52. Analysis of the in vitro products by tryptic peptide
mapping established their relationship tothe virion non-glycosylated structural
proteins. The 77,000-dalton polypeptidewasfoundtobesimilar, ifnotidentic.al,
toananalogous precursorisolated from MuMTV-producing cells. Peptide
map-ping of the 110,000-dalton protein shows that it contains all of the
methionine-labeledpeptides found in the 77,000-dalton protein plussomeadditional peptides.
We conclude thatthe products synthesized in vitro from the genomic MuMTV
RNAarerelatedtothenon-glycosylated virion structural proteins. Polyadenylic
acid-containing RNA from MuMTV-producing cells also directed the synthesis
of the 77,000-dalton polypeptide in the L-cellsystem. If this RNA preparation
wasfirstfractionated bysucrosegradient centrifugation, the 77,000-dalton protein
appearedtobesynthesized from mRNA withasedimentationcoefficient between
25and35S.
Mousemammarytumorvirus (MuMTV) isa typeB oncovirus of thefamilyRetroviridae. The
typeB oncovirusesarecharacterizedby having
a subviral form called the intracytoplasmic
A-typeparticle,detectablebythin-section electron
microscopy, andbythemorphologyof the virion
particle which shows an eccentrically located
electron-dense nucleoid (3). In contrast to the
type C oncoviruses that are usuallyassociated
with leukemias andsarcomas, the MuMTV
in-duces mammary carcinomas in infected mice
(48). In cellcultures which shedMuMTV,
tran-scriptionof theintegratedviralgenomeappears
tobe underglucocorticoid hormone control(29,
38, 39, 43). The virions contain a 70S RNA
complex which is probably composed of two
subunitsofsingle-stranded genomicRNA which
sedimentatapproximately 35Sandanumber of
tRNA's of which the predominant species is
tRNALYS (9, 11, 53). The 70S RNA complex,
tPresent address: Section for Medical Enzymology and
Molecular Biology, University of Amsterdam, Amsterdam,
The Netherlands.
together with several non-glycosylated
low-mo-lecular-weight proteins,composesthecore
struc-tureof thevirion(5, 47).
The nondefective avian type C oncoviruses
have beenmore thoroughly characterized than
have thetype Boncoviruses and also shown to
containa70SRNAcomplex composed of similar
subunits. Type C oncoviruses contain the
ge-netic informationtocode forthe internal
struc-turalproteins (gag),theRNA-dependentDNA
polymerase (pol), the envelope glycoproteins
(env), andinthecaseof thesarcomaviruses the
src geneproduct. These genes are arranged in
the order5'-gag-pol-env-src-p(A)-3' (18, 19, 52).
Cell-freetranslation of the intactgenomicRNA
of severaltype C virusesprimarily gives rise to
apolyproteinthat is similartotheprecursorfor
thelow-molecular-weight proteinsthatcompose
thecorestructureof thevirion(gag) (13, 24-26, 33, 41, 50, 51). In some instances a protein of
180,000 daltons has beendetected, and this
ap-pearstocontain thegaggeneand thepolgene
products (25, 30, 35).Theenvelope proteinsand
1131
on November 10, 2019 by guest
http://jvi.asm.org/
the src gene product appear to be synthesized
onintracellular subgenomic mRNA's (4, 12, 15,
32, 46,54).
Severalrecent studies havedemonstrated that
the internal structural proteinsofMuMTV are
synthesized in cells as part of a polyprotein
precursor of77,000to 75,000 daltons (Pr77/75)
(6, 36, 42). In this studywehave used in vitro
translationto investigate the genomic RNA of
MuMTV obtained from two mouse cell lines
continuously producing the virus. The products werecharacterizedbyimmunoprecipitation and tryptic peptide mapping. We found that the
major product synthesized was a 77,000-dalton
protein (p77) thatresembled the Pr77/75 found
in cultured cells. Inaddition, atranslation
prod-uctof110,000daltons (plIO) and aminor
prod-uct of 160,000 daltons (p160) were detected
which also have their counterparts in infected
cells (6). The in vitro-synthesized plO was
shown to contain all ofthe methionine-labeled
trypticpeptidesfound inthep77plus additional
peptides.
When cellular polyadenylic acid
[poly(A)]-containing mRNA from virus-producing cells
wasexamined,asimilarprotein of 77,000 daltons
could beimmunoprecipitated. This mRNA
giv-ing rise to p77appeared tosediment in a sucrose
densitygradientatabout 25 to35S.
MATERIALS AND METHODS
Cell cultures. Two cell lines producingMuMTV wereusedtoprepareviralgenomicRNA.One,the GR line, was grown in Eagle minimal essential medium withantibiotics, 10% horse serum, and 10jgof dexa-methasone per ml (37). The other cell line,
Mm5MT/cl,was aclone(10)fromamammarytumor
cell line derived from C3H mouse mammary tumor
(28). Thislatter cell line wasgrown ina similar
me-dium, butwith fetal calfserumsubstituted for horse serum.Theproductionof MuMTVbyboth celllines was routinelymonitored witha reverse-transcriptase
assayusingeitherMg2+orMn2'asthe divalent cation to distinguish between type B and type C viruses as
previously described (56).
Preparation of RNA. The cells were grown in roller cultures (480-cm2Corning flasks) and continu-ously labeled with 10 1iCi of [3H]uridine (Amersham/Searle) per ml. Culture mediumwas har-vestedat 5- or 16-hperiods andclarifiedby centrifu-gation at 12,000 x g for 10 min, and the virus was
pelletedby centrifugation at 140,000 xg for40 min. The viruswassuspendedin buffer(0.01M Tris-hydro-chloride, pH 7.2, 0.1 M NaCl, 0.001 M EDTA), dis-ruptedwith 0.5% sodiumdodecyl sulfate (SDS), and extracted three times with phenol-chloroform-iso-amyl alcohol at 24:24:1 and twice with chloro-form-isoamyl alcohol at 24:1. The RNAcontained in theaqueousphasewasprecipitated with 2.5 volumes of alcohol in 0.2 M sodiumacetate.The 70S RNA was fractionated on a 3 to 30%sucrose density gradient
containing0.05MTris-hydrochloride (pH7.5),0.05M EDTA, and 0.2%SDSbycentrifugationat280,000x g for 45min. The fractions composing the 70S peak werepooled, ethanolprecipitated,heatdissociatedat
100°C for 45 s, andrun on asimilargradient for100 min. The subunit RNAsedimenting ataround 28 to 35Swaspooled and ethanolprecipitated. Before
trans-lation, the RNAwasreprecipitatedto remove traces ofSDS, dissolved in water at 1 mg/ml, and stored at -700C.
Cytoplasmic RNA from MuMTV-producing cells was isolated, and poly(A)-containing RNA was se-lected on polyuridylic acid [poly(U)]-Sepharose 4B columns as previously described (32). 32P-labeled rRNAwaskindlysupplied byG. Peters.
Cell-free protein-synthesizing systems. The rabbitreticulocyte lysate was prepared as described byPelham and Jackson (34),except the amino acids (minus methionine)were added to aconcentration of 0.2 mMeach, and2-aminopurinewasaddedto afinal concentration of6mM. The 25-1lreactions were ini-tiatedbythe addition of15 to251iCi of
[35S]methio-nine (Amersham/Searle; 600 to 1,100 Ci/mmol) and 0.1 to1
tig
ofexogenous RNA andincubatedfor 1.5h at34°C. To determine theefficiencyofincorporation of[35S]methionine,2-pl
portionswerewithdrawn and treated with 10 Ml of 30%hydrogen peroxideand 100Al
of 0.1 M KOHat370C for 15minbeforeprecipita-tion with cold 10% trichloroacetic acid. The precipi-tates werecollectedon0.45-ammembrane filters (Mil-lipore Corp.), washed,andcounted inaliquid scintil-lation spectrophotometer. Samples for analysis on SDS-polyacrylamide gelswere prepared by mixing4
[Lof thefinal reaction mixture with 10Mlofwaterand 15 Mul of electrophoresis sample buffer, followed by heatingat100°Cfor 2 min.
TheL-cellS30wasprepared as previously described (31).To 1ml oftheL-cellS30were added all of the components necessary for protein synthesis, except methionine butincluding CaCl2(1.0 mMfinal concen-tration),togiveatotalvolumeof 1.6 ml. The S30 was then preheated to 20°C for 2 min, and micrococcal nuclease (P-LBiochemicals) was added to a concen-tration of 10Mg/ml. After a further 10min at 20°C, the mixturewascooledto0WC,and 200 mM ethylene-glycol-bis(,B-aminoethyl ether)-N,N-tetraacetic acid (pH7.0) wasaddedto afinal concentration of 2.0 mM. Portions of this nuclease-treated L-cell S30 were stored inliquidN2.Thereaction mixtureconsisted of 16
Al
ofthe nuclease-treated S30supplemented with 5 Mgof ascitestRNA,aminoacids(minus methionine)at a concentration of 0.2 mM, 15 to 25 MCiof [s5S]-methionine, and 0.1 to 1 Mg ofexogenous RNA in a tinalvolume of 25Ml. The Mg" andKCl concentra-tions which gave optimum incorporation of
[35S]me-thionine,usingcellmRNA, were determined for each lysate preparation. Incubations were performed at 37°C for1 h. Portions of 2ML were taken for trichlo-roaceticacidprecipitation, and samples were prepared forelectrophoresisasdescribedpreviously (31).
Antisera. The preparation and characterization of antiserumtodisruptedMuMTVand to electrophoret-icallypurified p27have been described previously (6, 7). Theanti-gp52serum(lot8/10/25/77) was obtained
as a gift fromMeloy Laboratories Inc., through the
on November 10, 2019 by guest
http://jvi.asm.org/
Office of Program Resources and Logistics, National Cancer Institute.
Immunoprecipitation. Samples of the cell-free protein-synthesizing systems were immunoprecipi-tated as described by Paucha et al. (31). Briefly, 15 to 20 ,ul of the reactionmixture was diluted with 50AI of NET buffer (0.05 MTris-hydrochloride [pH 7.4], 0.15 MNaCl, 0.005 M EDTA, 0.05% Nonidet P-40, 1 mg of bovineserumalbumin per ml) and incubated with 5
Al
of antiserum at 22°C for 1 h. Immune complexes were absorbed to 15p1
of a 10% Formalin-treated suspension of Staphylococcus aureus (22). The bac-terial suspension was washed twice with NET buffer (minus the bovine serum albumin), and the immune complexesweredissociated by the addition of electro-phoresis sample buffer followed by heating at 100°C for2min. The bacteria were removed by centrifuga-tion before application of the samples onto the poly-acrylamide gel.Electrophoresis. The samples were separated on 15% discontinuous SDS-polyacrylamide gels as de-scribed previously (23). Marker proteins of known molecular weights were labeled with ['4C]iodoacetic
acid as described by Smith et al. (45) and run in a
parallel track. The proteins were ,B-galactosidase
(120,000), phosphorylase a (94,000), fructose-6-phos-phatase (81,000), catalase (60,000), glutamate dehy-drogenase (53,000), creatine kinase (40,000), and lyso-zyme (14,000).
Tryptic peptide mapping. The appropriate bands were cut outfrom dried gels, rehydrated with 75 ,ul of a 1-mg/ml solution ofbovine serum albumin, frag-mented, and extracted with 3 ml of 0.1% SDS. The eluted proteinswere concentratedby trichloroacetic acidprecipitation, washed with diethyl ether, oxidized, andtrypsinized aspreviously described (6). The sep-aration of the tryptic peptideswasperformedon cel-lulosethin-layer platesbyelectrophoresisatpH4.6in the first dimension andchromatographyinthe second
(7).
RESULTS
In vitro translation of MuMTV virion
RNA. MuMTV virion RNA isolated from the
GR cellline asdescribed above sedimented on
sucrose gradientsas abroadpeak of 25to35S.
Harvesting atshorter intervals did not signifi-cantly change this size distribution. Since the
virion RNA containspoly(A) atits 3'terminus,
theRNA wasfurther purified by affinity
chro-matography on a
poly(U)-Sepharose
column. Theelutedpoly(A)-containing
RNA(1.5,Lg)wasfractionated on a 3to30%sucrose
density
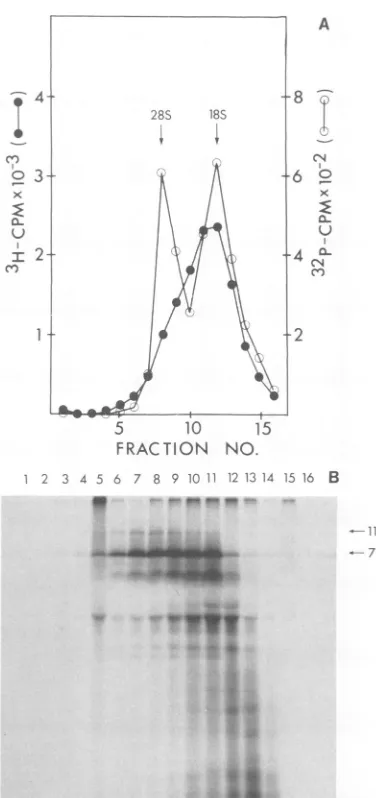
gra-dientwith 32P-labeled28and18SrRNA assize markers (Fig. 1A). The RNA species stillsedi-ments as a broad peak of25 to 35S. An equal
portionof eachgradientfractionwastranslated
in themessenger-dependent rabbit
reticulocyte
lysate, and the products were analyzed by SDS-polyacrylamide gel electrophoresis (Fig. 1B). The major translation
product
was apro-tein ofmolecularweight 77,000whichwas
effi-ciently translated off RNA sedimentingbetween 35and 25S.Aprotein of110,000daltonswasalso synthesized in the same fractions, and in frac-tions6 to 8 a product of160,000daltons (p160)
could be detected. Several otherpolypeptides of
lower molecular weightwerealso synthesized in
response to thefractionatedvirionRNA. When
these translationproductswere immunoprecip-itated withananti-p27serum, themajor product
p77andpllOandp160 werespecifically
precip-itated (Fig. 1C).
To comparethe efficacyof the cell-free
sys-tems torespond to 70S RNAcomplexorsubunit
RNA, MuMTVvirion RNA wasalso translated
in both the messenger-dependent mouse L-cell
S30 and rabbitreticulocyte systems.
MuMTV-relatedproducts synthesizedintheL-cellsystem appeared to be similar to those made in the rabbit reticulocyte lysate. When undissociated 70S virion RNAwasaddedtoeitherof the
cell-freesystems,thesamestimulationof aminoacid
incorporation andasimilarpolypeptide pattern were obtained aswith the subunit virionRNA
(Fig. 2),although the amount ofthe
lower-mo-lecular-weight productswasgreater with the 70S
RNA.Theseresultsdemonstratethatthe rabbit
reticulocyte lysatewas asefficientintranslating
MuMTV virion RNA as was the homologous
mouseL-cell system, and the products
synthe-sizedwereofsimilar molecularweight.
VirionRNA was also isolated from virus
pro-duced bythe Mm5MT/cl mouse cell line and
translated inbothcell-freesystems. This virion RNA,asexpected, coded for similar
products
tothe GR virion RNA; the main product was a polypeptide of about 77,000 daltons (Fig. 2). However, p77 and pllO from the Mm5MT/cl cells hadslightlylower molecularweights than the corresponding products from the GR cell
virus RNA. The ratio of pllO to p77 appeared
tobe similar with both strains of MuMTV.
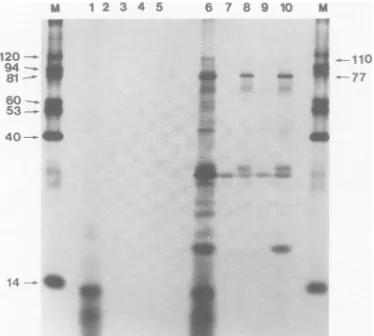
Characterizationof theinvitroproducts using
specific
antisera. Theproteinssynthe-sized in response to the virion RNA werefurther
analyzed by
immunoprecipitation
and SDS-polyacrylamidegel electrophoresis.
The resultsof such an
immunoprecipitation
of theproductssynthesized in the L-cell system are shown in
Fig. 3. The rabbit antiserum raised
against
themajorvirionstructural
protein
p27precipitated
p77 andp110,aswellasseveral
lower-molecular-weight proteins (Fig. 3, track 8). When goat
antiserum raisedagainstthevirion
glycoprotein
gp52 was used, no specific
polypeptides
wereimmunoprecipitated
(Fig.
3, track9), although
a small amount of p77 was detected. The
im-munoprecipitationofp77results fromalow anti-p27 activityin the anti-gp52 serum. We cannot
on November 10, 2019 by guest
http://jvi.asm.org/
1134 DAHL AND DICKSON
exclude the possibility that peptides related to
theglycoproteinweremade inthecell-free
sys-tem, but not
immunoprecipitated
withtheanti-gp52 serum. For example, the antiserum may
not react with a
non-glycosylated
glycoproteinprecursorsynthesized in vitro. When the in vitro
products wereanalyzedwithantiserumraisedin
rabbits against whole detergent-disrupted
MuMTVvirions,thesame
polypeptides
and oneadditional polypeptide of molecular weight
17,000 were
immunoprecipitated
as with theanti-p27serum(Fig. 3,track10). Thisantiserum is known to recognize the virion
antigens
plO,p14, and p27 and the
glycoproteins
gp36 andgp52. The controland immunerabbitsera
non-specifically precipitate a 28,000-dalton poly-peptide seen in all tracks (Fig. 3, track 7). We conclude from the
immunoprecipitation
datathat the
majority
of theproducts
synthesizedinresponse to the MuMTVvirionRNAin the
cell-free systems are related to theinternalstructural
proteinsof the virion.
Tryptic peptide analysis of the MuMTV products.Tofurtherestablish the
relationship
of the invitro-synthesized productstothe virion
proteins, two-dimensional tryptic peptide maps were prepared. The in
vitro-synthesized
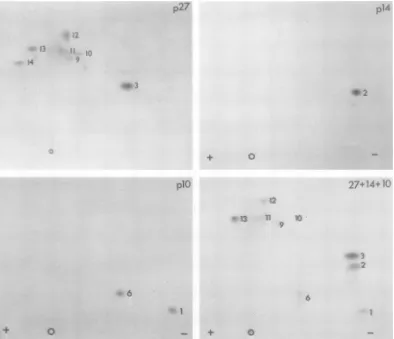
77,000-and110,000-daltonproteinswerecompared with thePr77/75 isolated fromGR cells (6) (Fig. 4).From the maps we conclude that p77 made in
vitro and the Pr77/75 isolated from cells are
very similar,with only one spot (12A in Fig. 4) appearing in a
slightly
different position. Fur-thermore,the invitro-synthesized p110 containsall of the methionine-labeled peptides foundin
p77 plus threeadditional peptides (spots 15, 16, and 17). The methionine-labeled peptides
con-tained in p77 can also be allocated to specific
viral structural proteins (Fig. 5). p27 contains peptides 3 and 9 to 14 as
previously
described(6). In some peptide maps of p27, spot 14 is
absent and spot 13 doubles in
intensity;
thereason for the variation in unknown. p14 and
plO of the MuMTVvirion are alsorepresented
by spot 2 and spots 1 and 6, respectively. A
mixingexperiment confirmed the relative
posi-tions of these methionine-labeledpeptides (Fig.
5). We therefore conclude that the internal
structuralproteins p27,p14,andplO of the virion
arecontained in the invitro-synthesizedp77 and
p110.
Translation of cellular mRNA from GR
cells. Total cell RNA was isolated from the
MuMTV-producing GR cell line and poly(A)-containing RNA selected by poly(U)-Sepharosse column chromatography. When
translated in the messenger-dependent L-cell
S30,
the mRNA gave rise to alarge
number ofpolypeptides.
Immunoprecipitation yielded a77,000-dalton protein
which could bespecifically
precipitated
with the antiserum against either p27ordisrupted
whole virions.To furtherana-lyze
the cellular RNA coding for the77,000-dalton
protein,
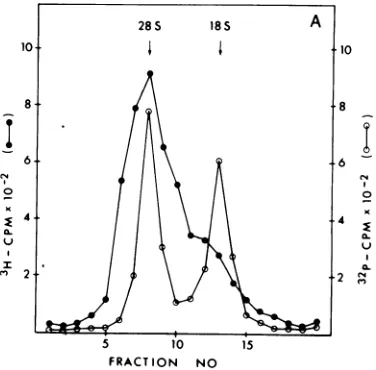
thepoly(A)-containing RNAwasfractionated on a 3to 30% sucrose density gra-dient.32P-labeled28and18S rRNA'swereadded
assize markers.ThemRNA'sfrom thegradient
fractions were translated in the L-cell system, immunoprecipitated with antiserum
against
MuMTV virions, and analyzed by
polyacryl-amide
gel
electrophoresis. As Fig. 6 shows, the77,000-dalton
proteinwassynthesizedonmRNA sedimenting between25 and 35S relativeto the28S rRNA marker.Thisisabroaderdistribution
than expected ifa single class of mRNA gives
riseto p77in the GR cells. However, the
sedi-mentationprofileofvirus-related mRNAis very
1
I
x
I1
10
0
I
n
x)
FRACTION NO
FIG. 1. Analysis of MuMTVvirion RNA by sedi-mentation on a sucrose density gradient, in vitro translation of thefractionatedRNA, and immuno-precipitation of the translation products. VirionRNA was extracted from [3H]uridine-labeled MuMTV shedbytheGR cell lineasdescribed inthetext. (A)
Profile ofthepoly(A)-containing virion RNA frac-tionatedon a 3 to30%osucrosedensity gradient. 32P-labeled28and 18Sribosomal markerswereincluded.
(B) Autoradiogram of the products synthesized in response tothefractionated RNA in the messenger-dependentrabbitreticulocyte lysate. Portions (4
[Ll)
wereanalyzed byelectrophoresison a15% SDS-poly-acrylamide gel. (C) Autoradiogram of the anti-p27 serumimmunoprecipitates separatedon a15% SDS-polyacrylamide gel. Tracks1 to 20referto the gra-dientfractions,track Mcontains"4C-labeledmarker proteins,and in track b no RNA wasaddedto the in vitroincubation mixture.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.503.267.455.281.466.2]similar to that of the genomic RNA shown in Fig. 1.
DISCUSSION
Poly(A)-selected virion RNA from MuMTV sedimenting between 25 and 35S wasefficiently translated in the cell-free systems. The results
show that themajor product synthesizedwas a
77,000-dalton protein (Fig. 1 and2). The maxi-mal synthesis of viral proteinsseen inFig. 1 is detected at 28S. The 28S virion RNA could resultfromdegradationorpossibly bythe prod-uctofa defective MuMTV provirus. However,
whenvirionRNA sedimentingat28Sinsucrose
density gradients is subjectedtoelectrophoresis on methylmurcury agarose gels (1), the RNA migrates with a molecular weight of approxi-mately 3 x 106, in agreement with other
esti-matesof thegenomesize (D. Robertson and H.
Varmus,personal communication).
Immunopre-cipitation analysis demonstratedthat the
77,000-dalton protein has antigenic determinants in
common with the major core protein of
MuMTV, p27, and tryptic peptide mapping of the [35S]methionine-labeled proteins confirmed
thatp27 and p77sharecommontryptic peptides.
Theresults also suggest that p77 shares peptides
in common with virion proteins plO and pl4.
The structural protein p21 appears to contain
little methionine, and, consequently,wecannot concludefrom theseexperiments whether p21is containedwithin p77.However,inanotherseries of experiments using
[14C]lysine
and["4C]argi-nine tolabelthecell-associated Pr77/75 and the
virion proteins, all four small virion proteins
werefoundtobe present in thePr77/75
(unpub-lished data). The data shown in Fig. 4
demon-stratethat cell-derivedPr77/75and thecell-free
synthesized p77 were very similar.The doublet
natureof Pr77/75appears toresult froma
phos-M b 1 2 3 4 5
120-- so
94-8
81--?
60-
$
5
3-40--
Al
6 7 8 9 10 11
7-_
_-4ALio
12 13 14 15 16 17 18 19 20M B
160
- ---110
- 77
S
Up
M b 1 2 3 4 5 6 7 8 910 11 12 1314 15 16 17 18 1920 M C
120--94
-
81-60-_
53-
0
40_
ranmO
__ mmq
_"
-160
-110
77
p.
14- 0
29,1979
m-W
0
on November 10, 2019 by guest
http://jvi.asm.org/
DAHL AND DICKSON
4
f.)I
.AV
I
[image:6.503.124.411.61.262.2]4Wv
FIG. 2. Autoradiogram of polyacrylamide gel analysis of products synthesized in vitro in response to MuMTVvirion RNA.MuMTVvirion RNA wastranslated ineither themessenger-dependentL-cellS30(A) or themessenger-dependent reticulocyte lysate (B)andanalyzedbyelectrophoresis on 15%
SDS-polyacryl-amidegelsasdescribed in thetext.The[3Slmethionine-labeledproductsweredetectedbyfluorography. The in vitro systemswereincubated with(1)noRNA, (2)0.5pgofMuMTV70SvirionRNAfromthe GR cellline, (3) 0.5 ,ugof 30S MuMTV virion RNAfromtheGR cellline,and(4)0.5jigof30S MuMTV virion RNAfromthe Mm5MT/cl cell line.Track M is"C-labeledmarkers.
s
-..,,p
-~
FIG. 3. Autoradiogram showing the
immunopre-cipitation analysis of the in vitro-synthesized
MuMTVproducts.Noadded RNA (tracks to5)or
0.5 pLgofMuMTVvirion RNA (tracks 6 to 10) was translated in the messenger-dependentL-cellS30as described in the text. A 4-jil portion was taken for
directanalysis(tracks1and6). Theremainderofthe reactionmixturewasdivided intofour equal portions
and immunoprecipitated, as described in the text, withcontrol rabbit serum (tracks2and 7), anti-p27
serum(tracks3and8),anti-gp52serum (tracks4and
9),oranti-MuMTVserum(tracks5and10). TrackM is '4C-labeled markerproteins. The products were
analyzed by electrophoresis on 15%
SDS-polyacryl-amide slabgels, and labeledproducts weredetected
byfluorography.
phorylation ofp77 (R. Nusse, F. A. M.
Assel-bergs, M. H. L. Salden, R. J. A. M. Michalides,
andH. Bloemendal, Virology,inpress).
The MuMTV genomic RNA is in many
re-spects analogous to the type C virion RNAs
from Roussarcomavirusorthemurineleukemia
viruses insynthesizinga polyprotein that gives
rise tothe smallnon-glycosylated structural
pro-teins oftheir respective virions (20, 25, 26, 33,
49-51). We are not aware of any examples of
eucaryotic mRNA's which have more than one
active initiation site and, although the mRNA
might potentially possess internal initiation
sites,these appeartobenonfunctional (44).
Con-sequently, from an analogy with the type C
viruses, we might expect that the gene coding
for p77 is located near the 5' terminus of the
MuMTV genomic RNA.
A comparison ofthemajor translation
prod-ucts synthesized in vitro, using viral RNA
ob-tained from both GR and Mm5MT/cl cells,
showed that the77,000-dalton andthe
110,000-dalton proteins obtained with the latter virus
areslightly smallerand seem torepresent avirus
straindifference.
The cell-free translations also gave rise to
products of 110,000 and 160,000 daltons, the
latter inverysmall amounts. Both of these
pro-teins are immunoprecipitable with anti-p27
se-rum(Fig. 1C), and, furthermore,pl 10 was shown
to contain the methionine-labeled peptides of
p77 as a subset. It is not clear at present what
J. VIROL.
B ..
4C.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.503.71.258.342.510.2]CELL-FREE 1137
.% 12
w13 *, 11
0
12A
13
+ 0
12A
13 11
17
+ 0
FIG. 4. Autoradiogram
sionalseparation ofthe/'
ticpeptides obtained wit}
p110,p77,andPr77/75ob
latterwaspreviouslypubl
electrophoresis was carrie
origin (0) and is shown
Chromatographywasfrom
additional information
ofsimilarmolecularwei,
inGRcellculturesbyirr
PR77/75 the
anti-p27
serum(6).
High-molecular-weight
polypeptides containing the gag-pol geneprod-ucthavebeenfound in Rous sarcoma virus- and
MuMLV-infected cells and in
cell-free
transla-10 tionsofthese virionRNAs
(17,
20,
25, 27, 30,
35).
9 5 MuMTV p160 might represent a
similar
poly-4 protein. A
protein equivalent
topllO
has not 8 4w3 been described for the translation products ofd3
2 other nondefective oncoviruses. This protein7 may be synthesized on the same mRNA as p77,
6 and a low level of suppression of the p77
termi-nation site would lead to a read-through product. Alternatively,initiation at a site to the 5' side of the p77 initiation site and direct read-through to the normal termination codon would give a sim-ilar product. However, this would imply from
the molecular weightestimatesthat this second
p77 initiation site is approximately 1,000 bases to the
5' side of the p77 initiation site. Synthesis on a
separate species of RNA present in minor
10 amounts in the virion preparations is also a
9 4 possibility. This minor species of RNA might
8 have the termination
condon(s) spliced
out or403 deleted, as seems to be the case for several of
7 2 the defective oncoviruses (2, 14, 55). The
110,000-dalton polypeptide could also be the
6 normal gene product of another MuMTV (16,
56). However, the observation that the
molecu-lar weights of pllO and p77 are both slightly
lower in the Mm5MT/cl viral RNA translates
suggests that the messenger(s) coding for
pllO
and p77 is derived from the same provirus. The reticulocyte lysate and the messenger-P110 dependent L-cell
S30
produce several proteins of lower molecular weight that byimmunopre-cipitation arerelated to p77. The origin of these
lower-molecular-weight proteins is unclear,
al-10 5 though premature termination, internal
initia-4 16 tion, and proteolytic cleavage of p77 are
plausi-8 ble
explanations.
Authenticproteolytic
process-- 3 ing does not seem to occur, since the size of these
15 7 2 proteinsdoes not agree with those of the known
intracellular
intermediates detected invirus-6 producingcells(6).
The 70Svirion RNA complex from MuMTV
was as efficient a messenger as the heat-dena-tured virion RNA in the cell-free systems tested, similartothe findingof Naso et al. (26), for type
showing the two-dimen- C 70S viralRNA.
Nevertheless,
thiswasunex-S]methionine-labeledtryp- pected
since
thesubunit
RNA is probably thei the in vitro-synthesized naturalmessengerin vivo. Wewerenot able to
5tained from GR cells (the determine whether the 70S RNA
complex
was'ishedinreference 6). The dissociated during the incubation period or
sd out at pH 4.6 from an whether the complex itself functioned as a
mes-in the horizontal plane. senger. If the latter case applies, it might mean
bottomtotop. that the initiation codon and probably the
ribo-some-binding site are positioned to the 3' side
pllO contains. Proteins with respect to the primer tRNA-binding site.
ghts have been detected The70SRNA of MuMTV has also been shown
imunoprecipitation
with to act as an efficient messenger whenmicroin-VOL. 29,
4HIlI
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.503.50.236.72.559.2]1138 DAHL AND
DICKSONJ.VO.
+ 0
12
13 11 10
9
3
2
6
+ 0
FIG. 5. Autoradiogram showing the f0Slmethionine-labeled trypticpeptide mapsofthe isolated virion
proteins p27,p14,andp10 and the mixturep27plusp14plusp10.
jected intoXenopus oocytes. Under these
con-ditions Pr77 isprocessed to yieldseveral of the virionproteins (Nusseetal.,inpress).
The poly(A)-selected mRNA from GR cells
also contained mRNAspecies whichtranslated
togive p77and sedimentedat35 to25S. This is consistent with the observations for both the avian and themurinetypeC viruses thatappear to produce a genomicsize mRNA in cells, and
this yields the analogous viral structural poly-proteinupontranslation in vitro. Both thevirion
and thecellular RNAs whichgiverisetop'77are
ofsimilar size, but more heterogeneous in size thanexpected whencomparedwith the
equiva-lenttypeC virussystems (unpublisheddata; 13, 32). At higher sedimentation values more pll0
is produced compared with p77, whereas at a
sedimentationvalue well belowgenomicsize the
p77alone isefficiently produced,indicatingthat p110couldbesynthesizedoffaseparatemRNA. Theseresultsarecomparabletothefindingthat
35Sandnot30Savianmyeloblastosisvirus RNA
hasthecapacitytosynthesize gag-pol(30). The
heterogeneityof RNAsizemightbedueto deg-radation, splicing ofRNAs, the products of dif-ferent proviruses, or an artifact of the sucrose
gradient centrifugation (seeabove).
In the experiments with poly(A)-selected RNA, an anti-MuMTV serum was used to
im-munoprecipitate the virus-specific proteins. However, the presence of a candidate for the
non-glycosylatedglycoproteinprecursorwasnot detected. The results obtained with the avian
type C virus mRNA's would indicate that this
polyproteinprecursorismoredifficulttodetect
(32).Alternatively, thenon-glycosylatedformof theprecursor mayhaveno, oramuch reduced,
specif'icity for the antibodies which wereraised
against the fully glycosylated proteins. An an-swerto this problem could rely upon the addi-tion ofa microsomal fraction, which results in
glycosylation in vitro (40), or the use of the
oocytes to translate a putative glycoprotein
mRNA.
p27
12
13 10
9 14
-~3
*2
p10
27+14+
IC:
6
+ 0
J. VIROL.
I
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.503.62.456.67.406.2]285 I8S
i-o
34-A
-8
-1CN
-6 °
x
0E
u .4
0E
N
-2
5 10 15
FRACTION NO.
1 2 3 4 5 6 7 8 9 10 11 12 13 .4 15 16 B
---110
- _ +--- ~~~~~~77
'W,,
FIG. 6. Autoradiogram of polyacrylamide gel analysis of immunoprecipitated MuMTV products synthesized in the micrococcal nuclease-treated L-cellS30in responsetofractionated cellularmRNA. Cytoplasmic RNAs from virus-producing GR cells
werepoly(A) selectedon apoly(U)-Sepharose column,
and 25,ug was fractionated on a 3to 30%Q sucrose
gradient. mRNA in the 16 fractions collected was
concentratedbyethanolprecipitationinthepresence
of 10,ug of calf liver tRNA, and one-fourth of the
RNA in each fraction was added to the cell-free
system. After the incubation, the products were
im-munoprecipitatedasdescribed in thetextwith4,ul of anti-MuMTV. The immunoprecipitates were
ana-lyzed by electrophoresison 15%SDS-polyacrylamide
gels, andlabeledpeptidesweredetected by
fluorog-raphy. Tracks 1 to 16 are the immunoprecipitated gradient fractions.
ACKNOWLEDGMENTS
We thank M.A. Atterwill for excellent technicalassistance,
M. J.Hayman,H. F.Lodish,G.G.Peters,A. E.Smith,andR.
A. Weiss forhelpful discussions and critical reading of the manuscript, and A. Horwich for running the methylmercury gels.
LITERATURE CITED
1. Bailey, J. M., and N. Davidson. 1976. Methylmercury
asa reversible denaturing agent for agarose gel
electro-phoresis.Anal. Biochem. 70:75-85.
2. Barbacid, M., J. R. Stephenson, and S. A. Aaronson.
1976.gag gene of mammalian type-C RNA tumor virus.
Nature (London) 262:554-559.
3. Bernhard, W. 1958. Electron microscopy of tumor cells and tumor viruses: a review. Cancer Res. 18:491-509. 4. Brugge,J.S.,A. F.Purchio,and R. L.Erikson. 1977. Virus-specific RNA species present in the cytoplasm of
Roussarcomavirus-infected chicken cells.Virology83:
16-26.
5. Cardiff, R. D., M. J. Puentes, L. J. T.Young,G. H. Smith, Y. A. Teramoto, B. W. Altrock, and T. S.
Pratt. 1978.Serological and biochemical
characteriza-tion ofthemousemammary tumorvirus with
localiza-tion ofplO.Virology85:157-167.
6. Dickson,C., and M. Atterwill. 1978. Polyproteins
re-lated to the major core protein of mouse mammary
tumorvirus. J. Virol. 26:660-672.
7. Dickson,C., J.P.Puma, and S. Nandi.1976.
Identifi-cationofaprecursorproteintothemajorglycoproteins
ofmousemammary tumor virus. J.Virol. 17:275-282.
8. Duesberg, P., and P. B. Blair. 1966. Isolation ofthe nucleic acid of mouse mammary tumor virus. Proc. Natl. Acad. Sci. U.S.A.55:1490-1497.
9. Duesberg, P.H., and R. D. Cardiff. 1968.Structural relationships between the RNA ofmammary tumor virus and those ofother RNA tumor viruses.Virology 36:696-700.
10. Fine, D. L., L. 0. Arthur, J. K. Plowman, E. A. Hillman, and F. Klein.1974.Invitrosystem for
pro-duction of mouse mammary tumor virus.Appl.
Micro-biol.28:1040-1046.
11. Friedrich, R., V. L. Morris, H. M. Goodman, J. M. Bishop, and H.E.Varmus.1976. Differences between genomes of two strains of mouse mammary tumor virus
asshownby partialRNA sequence analysis. Virology
72:330-340.
12. Gielkins, A. L. J., M. Salden,and H. Bloemendal.
1974.Virus-specificmessenger RNA on free and
mem-brane-bound polyribosomes from cells infected with Rauscher leukemiavirus. Proc. Natl. Acad. Sci. U.S.A. 71:1093-1097.
13. Gielkins, A. L. J.,D. vanZaane,H. P. J.Bloemers,
andH. Bloemendal. 1976.Synthesisof Rauscher
mu-rineleukemiavirusspecific polypeptidesin vitro. Proc. Natl.Acad.Sci. U.S.A.73:356-360.
14. Hayman,M.J.,K.Bister,P. K.Vogt,B. Roger-Po-kova, and T.Graf.1978.Viralpolyprotein synthesis
incells infected withavian sarcoma-leukemiaviruses,
p. 214-226. In S. BarlatiandC.deGiuli-Morghan (ed.),
AvianRNA tumor viruses. E.Piccin,Padova.
15. Hayward,W. S. 1977.Sizeandgenetic contentof viral
RNAs in avian oncovirus-infected cells. J. Virol. 24:
47-63.
16. Hilgers, J., and P. Bentvelzen. 1978. Interaction
be-tween viral and genetic factors in murine mammary
cancer, p. 143-189. In G. Klein and S. Weinhouse(ed.),
Advances incancerresearch,vol. 26. Academic Press
Inc.,London.
17. Jamjoom, G.A.,R. B.Naso, and R. B.Arlinghaus.
1977.Furthercharacterization of intracellular precursor
polyproteinsofRauscher leukemia virus.Virology78: 11-34.
18. Joho,R.H.,M. A.Billeter,and C. Weissmann. 1975. MappingofbiologicalfunctionsonRNA of aviantumor
viruses:location ofregionsrequiredfor transformation
on November 10, 2019 by guest
http://jvi.asm.org/
[image:9.503.47.235.66.465.2]1140 DAHL AND DICKSON
anddetermination of hostrange.Proc.Natl. Acad. Sci.
U.S.A. 72:4772-4776.
19. Joho, R.H., E. Stoll, R. R. Friis, M. A. Billeter, and
C. Weissmann. 1976. Apartial geneticmapof Rous
sarcomavirus RNA: location of polymerase, envelope
and transformation markers,p. 127-145. In D.
Balti-more,A.S. Huang, and C. F. Fox (ed.), Animal virology. ICN-UCLASymposiumonMolecularand Cellular
Bi-ology. Academic Press Inc., New York.
20. Kerr,I. M., U.Olshevsky,H.Lodish, and D.
Balti-more.1976. Translation of murine leukemia virus RNA
in cell-free systems from animal cells. J. Virol. 18:
627-635.
21. Kessler, S. W. 1975.Rapid isolation of antigens from cells
with a staphylococcal protein A-antibody adsorbent:
parametersof theinteraction ofantibody-antigen
com-plexes with protein A. J. Immunol. 115:1617-1624. 22. Laemmli, U. K. 1970. Cleavage of structural proteins
during the assembly of the head of bacteriophage T4. Nature (London) 227:650-655.
23. Laskey, R. A., and A. D. Mills. 1975. Quantitative film
detectionof:Hand"4Cinpolyacrylamidegels by fluo-rography.Eur. J. Biochem. 56:335-341.
24. Martin, H. L., M. Salden, and H. Bloemendal. 1976.
Translation of avian myeloblastosis virus RNA in a
reticulocyte cell-free system.Biochem. Biophys. Res.
Commun.68:249-255.
25. Murphy,E.C.,J. J.Kopchich,K. F.Watson, and R.
B.Arlinghaus. 1978. Cell-freesynthesisofa precursor
polyproteincontaining bothgagandpolgeneproducts
by Rauscher murine leukemia virus35SRNA. Cell 13:
359-369.
26. Naso, R. B., L. J. Arcement, T. G. Wood, and R.
Arlinghaus. 1975. The cell-free translation of
Rauscher leukemia viral RNA into high molecular
weight polypeptides. Biochim. Biophys. Acta 383: 195-206.
27. Oppermann, H., J. M. Bishop, H. E. Varmus, and L.
Levintow. 1977. Ajoint productofthegenesgagand
pol of avian sarcoma virus: a possible precursor of
reversetranscriptase. Cell 12:993-1005.
28. Owens, R. B., and A. J. Hackett. 1972. Tissue culture
studiesofmousemammarytumorcells and associated
viruses.J. Natl. Cancer Inst. 49:1321-1332.
29. Parks, W.P., J. C. Ransom, H. A. Young, and E. M.
Scolnick. 1975. Mammary tumorvirusinduction by
glucocorticoids.J.Biol. Chem. 250:3330-3336.
30. Paterson, B.M., D. J. Marciani, and T. S. Papas. 1977.
Cell-free synthesis of the precursor polypeptide for
avian myeloblastosis virus DNA polymerase. Proc.
Natl.Acad. Sci. U.S.A. 74:4951-4954.
31. Paucha,E., R. Harvey, and A. E. Smith. 1978. Cell-free
synthesis of simian virus 40T-antigens. J. Virol. 28: 154-170.
32. Pawson, T., R. Harvey, and A. E. Smith. 1977. The
size ofRoussarcomavirus mRNAsactivein cell-free
translation. Nature(London)268:416-420.
33. Pawson,T., G. S. Martin, and A. E. Smith. 1976.
Cell-freetranslation of virion RNA from nondefective and
transformation-defective Roussarcomaviruses. J.
Vi-rol. 19:950-967.
34. Pelham, H. R.B., and R. J. Jackson. 1976. An efficient
mRNA-dependent translationsystemfrom reticulocyte
lysates. Eur. J. Biochem. 67:247-256.
35. Philipson, L., P. Andersson, U.Olshevsky, R.
Wein-berg, D. Baltimore, and R. Gesteland. 1978.
Trans-lationof murine leukemiaandsarcomavirus RNAs in
nuclease-treatedreticulocyteextracts:enhancement of
thegag-polpolypeptidewithyeastsuppressortRNA.
Cell 13:189-199.
36. Racevskis, J., and N. H. Sarkar. 1978. Synthesis and
processingofprecursorpolypeptidestomurine
mam-mary tumor virus structural proteins. J. Virol. 25: 374-383.
37. Ringold, G., E. Y. Lasfargues, M. J. Bishop, and H.
E. Varmus. 1975. Production ofmousemammary
tu-morvirusby cultured cells in the absence andpresence
of hormones:assayby molecularhybridization.Virology
65:135-147.
38. Ringold, G. M., K. R. Yamamoto, M. J. Bishop, and
H. E. Varmus. 1977. Glucocorticoid-stimulated
accu-mulation ofmouse mammarytumor virus RNA:
in-creasedrateofsynthesis of viral RNA. Proc. Natl. Acad.
Sci. U.S.A. 74:2879-2883.
39. Ringold, G. M., K. R. Yamamoto, G. M. Tomkins, J.
M.Bishop, and H. E. Varmus.1975.
Dexamethasone-mediated induction ofmouse mammary tumor virus
RNA:asystemforstudyingglucocorticoidaction. Cell 6:299-305.
40. Rothman, J. E., and H. E. Lodish. 1977. Synchronized
transmembrane insertion andglycosylation ofanascent
membraneprotein. Nature (London) 269:775-780.
41. Salden, M., H. L. Selten-Versteegen, and H.
Bloe-mendal. 1976. Translation of Rauscher murine
leuke-miaviral RNA:amodelfor the function ofvirus-specific
messenger. Biochem. Biophys. Res. Commun. 72: 610-618.
42. Schochetman,G., C. W. Long, S. Oroszlan,L.Arthur,
andD.L.Fine. 1978. Isolation ofseparateprecursor
polypeptidesformouse mammarytumorvirus
glyco-proteins andnonglycoproteins. Virology 85:168-174.
43. Scolnick, E.M., H. A. Young, and W. P. Parks.1976.
Biochemical andphysiologicalmechanisms in
glucocor-ticoid hormone induction ofmouse mammarytumor
virus. Virology 69:148-156.
44. Smith, A. E. 1977.Crypticinitiation sitesineukaryotic
virusmRNAs,p.37-46. In B. F. C. Clarketal.(ed.),
Geneexpression. FEBS Symposium, vol. 43. Pergamon
Press, Oxford.
45. Smith, A. E., R. Smith, andE. Paucha.1978.Extraction
andfingerprint analysis of simianvirus40large and
smallT-antigens. J. Virol. 28:140-153.
46. Stacey, D. W., V. G. Allfrey, andH.Hanafusa.1977.
Microinjection analysis ofenvelope-glycoprotein
activ-ities of avian leukosis viral RNAs. Proc. Natl. Acad.Sci.
U.S.A.74:1614-1618.
47. Teramoto, Y.A., R. D. Cardiff, and J. K. Lund. 1977.
The structure of the mouse mammary tumor virus:
isolation and characterization of thecore.Virology 77:
135-148.
48. Tooze, J. (ed.). 1973. The molecular biology oftumour
viruses. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
49. VanZaane, D., A. L. J. Gielkens, W. G. Hesselink,
and H. P. J. Bloemers. 1977. Identification of
Rauscher leukemiavirus-specific mRNAs for the
syn-thesis of thegag- and ent-geneproducts. Proc. Natl.
Acad. Sci. U.S.A. 74:1855-1859.
50. Vogt, V. M., R. Eisenman, and H. Digglemann. 1975.
Generation of avianmyeloblastosis virus structural
pro-teinsbyproteolytic cleavageofaprecursorpolypeptide.
J. Mol.Biol.96:471-493.
51. vonderHelm, K., and P. H. Duesberg. 1975.
Transla-tion of Rous sarcomavirus RNA incell-freesystems
fromascites Krebs IIcells. Proc. Natl. Acad.Sci. U.S.A.
72:614-618.
52. Wang, L.-H., D. Gatehouse, P. Mellon, P. Duesberg,
W.S.Mason, and P.K.Vogt. 1976. Mapping
oligo-nucleotides of Roussarcomavirus RNA thatsegregate
withpolymerase and group-specific antigen markers in
recombinants. Proc. Natl. Acad. Sci. U.S.A. 73:
3952-3956.
53. Waters, L. C. 1978. Lysine tRNA is the predominant
tRNAinmurinemammarytumorvirus. Biochem.
on November 10, 2019 by guest
http://jvi.asm.org/
phys.Res. Commun.81:822-827.
54. Weiss, S. R., H. E.Varmus, and J. M. Bishop.1977. The sizeand geneticcomposition of virus-specific RNAs in thecytoplasmof cellsproducing avian
sarcoma-leu-kosis viruses.Cell12:983-992.
55. Witte, 0.N.,N.Rosenberg,M.Paskind,A.Shields,
andD.Baltimore. 1978. Identification ofanAbelson
murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc.Natl. Acad. Sci. U.S.A.75:2488-2492.
56. Zavada, J., C. Dickson, and R.Weiss. 1977.
Pseudo-typesof vesicular stomatitis virus with envelope
anti-gensprovided bymurinemammarytumorvirus.
Virol-ogy82:221-231.
![FIG. out at pH 4.6 from anin the horizontal plane.bottom in5tained'ished from insdshowingob to theS]methionine-labeledi the two-dimen- vitro-synthesized GR cells reference tryp-tic 6)](https://thumb-us.123doks.com/thumbv2/123dok_us/1517427.104348/7.503.50.236.72.559/horizontal-tained-insdshowingob-methionine-labeledi-vitro-synthesized-reference.webp)