Copyright01974 AmericanSocietyforMicrobiology Printed in U.S.A.
Replication
of
a
Nuclear
Polyhedrosis
Virus in
a
Continuous
Cell
Culture of
Spodoptera
frugiperda:
Purification, Assay
of
Infectivity,
and
Growth Characteristics
of
the
Virus
D. L. KNUDSON AND T. W. TINSLEY
N.E.R.C. Unit ofInvertebrate Virology, Oxford OXI 3RB, England
Received forpublication22April 1974
Nonoccluded virus, polyhedra, and occluded virus were purified from a
continuous cell culture ofSpodoperafrugiperda infected with nuclear polyhedro-sis virus. The
optimal
temperatureforthe replication and lateral transmission of infectivity for the nuclear polyhedrosis viruses (NPV) in cell culture was 27 C. End-point dilution and plaque assay procedures for the measurement of infectivity aredescribed andcompared. Dose-response data demonstrated thatasingle particle could initiatean infection, and the validity of therelationship of
0.7 PFU per mean tissue culture infective dose (TCID50) further substantiated
theaccuracyof theseinfectivityassays.
Particle-infectious
unit calculationsgave aratioof 62to310nonoccluded virusparticlesTCID,0.
Growth cycle and lateral transmission experiments indicated that infectious material was released fromcells 12 h
postinfection
(p.i.) andapproached
a maximal titer 4 days p.i. Thenumber ofpolyhedra, nonoccluded virions, and TCID5O producedpercellwasalso
presented. Typical yields of NPV producedper liter flask suggested that insect
cell culture systems representafeasible meansby which the replication of these
viruses could beinvestigated.
Nuclear
polyhedrosis viruses (NPV)
aremembers
ofthe
Baculovirus
genus(18)
and
have
been isolated from insects
inthe orders
Diptera, Hymenoptera, Lepidoptera, and
Or-thoptera
(6). The virion is bacilliform (40
to70by
250 to 400nm) and contains
covalently
closed,
supercoiled,
double-stranded DNA with
a
molecular
weight
ofapproximately
108(13-15). The virus replicates
inthe nucleus of
the insect
cell, and,
asthe infection
proceeds,
asubstantial
proportion
of
the
progeny areen-veloped and
subsequently
occluded
by
protein
into
polyhedral,
crystalline
matrixes
(0.1
to 10,um
indiameter).
The
inclusions
orpolyhedra
are
readily detectable
inthe
nuclei of
infected
insectcells
and present acytopathology that
ischaracteristic
ofinfections initiated
by
viruses of this genus(3-5).
Polyhedra
representthe
primary "vector"
by
which
virusinfections
aretransmitted
innature to insects.When larvae
ingest
foliage
that
iscontaminated with
polyhedra,
the alkaline
en-vironmentand
enzymatic
activity associated
with the larval
gutsolubilized the
protein
matrix ofthe
polyhedron, releasing
infectious virions (6).Polyhedra
are not infectious for insectcell
cultures (11), but cultures
dosupportthe
replication
ofthese
viruseswhen alternative
sources of
inoculum
areused. For example,
cultures have been infected with hemolymph
ofNPV-infected insects
(1, 2, 12),with purified
NPV DNA
(11),
with NPV-infected cell culture
extracts
(1,
11,16),
and with cell-free
extracts ofNPV-infected
larvae (16). There
is,however,
only
one reportwhere the
virus waspurified
from
infected cell cultures and shown
tobe
infectious
(8).Infectivity
assays ofpolyhedra
have relied
primarily
on a meanlethal dose fed
tolarvae.
An
end-point
dilution.
method using
aprimary
insect cell culture
(17) and
aquantal
responsemethod
using
acontinuous
cell culture (1) have
also
been
used
toassayinfectious
material other
than
polyhedra.
Morerecently, the first plaquemethod using
0.6%methylcellulose
as anover-lay has been reported
(10).In
this
paperdata
concerning
aspects ofthe
replication
ofthe
NPV ofSpodoptera frugiperda
in a continuous cell culture derived fromS.
frugiperda
ispresented.
Purificationprocedures
aredescribed, and the
temperatureoptimum
for
the
replication
ofthe
virus inthe cell
culture
is
established.
Infectivity assayprocedures
for anend-point dilution method and
aplaque
method
arealso
described
andcompared with
particle
countdata,
and theparticle-infectious
9:34
on November 10, 2019 by guest
http://jvi.asm.org/
NUCLEAR POLYHEDROSIS VIRUS IN S. FRUGIPERDA
unit
ratio is
calculated. The
growth
characteris-tics
of
the virus in cell
culture
arealso
investi-gated.
MATERIALS AND METHODS
Cells. The continuous cell culture was originally established from pupal ovaries of S.frugiperda, the fall armyworm(J.L.Vaughn, unpublisheddata), and it has been adapted to a modified medium, BML/ TC10 (TC100) (G. R. Gardiner and H. Stockdale, Proc. VIth Annu. Meet. Soc. Invertebr. Pathol., Oxford, England, Abstr., 1973). The cells were
re-ceived at the 57th passage level from H. Stockdale andhavebeenpassagedover 50times inthis labora-tory. The cells were polyploid, grew attachedtothe surface ofthe culture vessel, andproliferatedwith a populationdoublingtime ofapproximately24h. The cellswereroutinely subculturedatweeklyintervalsby removingthemfromthesurface of the culture vessel using arubberscraper andvigorously pipettinguntila
uniform suspensionwasobtained. Viable cell counts were made by using trypan blue and a hemocytome-ter. Glass flasks (32 oz. [ca. 7 g]) were seeded with 106cellsandincubatedat27 C(the optimal tempera-ture for growth of the cells) until confluency was reached (approximately2.5 x 107cells).
TC100 was prepared complete, sterilized by posi-tive pressure filtration through 142-mm membrane filters(Millipore Corp.), and stored at 4 C. Before it wasused, penicillin-streptomycin (100 to 200 U/ml) and kanamycin(100 to 200
Ag/ml)
were added.Virus. The virus was received from two sources:
onefrom H. Stockdaleas infectedcell cultures and the other from J. L. Vaughn as polyhedra of S. frugiperda NPV. The infected cell cultures were initiated byDr. Vaughn, and the viruses, therefore, were considered equivalent, even though they were received from different sources. Further, no differ-ences have been detected in this laboratory that wouldsuggestthe contrary. The NPV was the multi-ply embedded type, i.e., virions are occluded by the polyhedral protein matrix asgroups or bundles with more than one virion per group. Since it is well established that serial passage of virus in cell cultures frequently results in aberrant virus forms, the NPV produced in cell culture was always kept within 10 passages of apassagethrough the insect. The methods for the production and purification of NPV from insectshave been describedelsewhere (K. A. Harrap and J. F.Longworth,J.Invertebr.Pathol., in press).
The NPV was routinely produced in liter flasks which were seeded with 2.5 x 101cells,incubated for 24 h, and infected during the logarithmic growth phase of the cells at amultiplicity of infection (MOI) of 0.01 mean tissueculture infective dose
(TCID.0)/
cell. Theuninfectedcellsgrew under theseconditions and formed a confluent monolayer. They were har-vested after 7 to 10 days of incubation, when the monolayer degenerated and a proportion of the cells haddetached from thesurface of the culture vessel.Incorporation of radioactive precursors. Radio-active NPVwasproduced by seeding75-cm2Falcon
Flaskswith2.5 x 106cells/10ml,incubatingfor 24h, and infecting with an MOI of 0.1 TCIDSO per cell. Twenty-four hours later, 10
MCi
of[methyl-3HIthymi-dineper ml(15,000 to 30,000mCi/mmol; Radiochemi-cal Centre, Amersham) was added to each flask, which was incubated for 7 to 10 days before the virus was harvested. All radiochemical data presented in this paper are expressed astrichloroacetic acid pre-cipitable counts/min. Thelabeled samples were pre-pared for counting by adding a carrier, bovine serum albumin fraction V, to give a final concentration of 50
Ag/sample
whendiluted with an equal volume of 15% (wt/vol)trichloroacetic acid. The samples were left at 4C for at least 1h,and theprecipitateswerecollected bysuction filtration through25-mmglassfiberdisks (GF81, Whatman)andwashedwith 10 ml of cold 7.5% trichloroaceticacid andethanol.Thedisksweredried andplaced intoglassvials, and 10 ml ofscintillant was added (4 g of 2,5-diphenyloxazole and 0.1 g of 1,4-di[2-(5-phenyloxazolyl) ]-benzeneperliter of tolu-ene). Thesamples were counted in a Phillips liquid scintillationanalyzerPW4510/00with a 40%counting efficiency for 3H.Purification. Infected cultures were harvested, andthe suspension was pelleted at10,000 x g for20
min, removing cellular debris and polyhedra and leaving virus that had not been incorporated into polyhedra (nonoccluded virus) in the supernatant. The 10,000 g pellet wasresuspended in asmall volume of water and sonicated at 4 C for 30 s at 5 A usinga
Dawe Soniprobe type 1130A fitted with a soniprobe converter type
1130/1A.
The samplewas repelleted, andthe 10,000 gsupernatantswerepooled.Thispellet representedthe crudepolyhedrafraction.The10,000 gsupernatant waspelletedat30,000 x g for1 h,and the supernatant was discarded. The 30,000g pellet representedthe crudenonoccludedvirusfraction.The 10,000 and 30,000 gpelletswereresuspendedin water andlayered onto15-ml lineargradientsof 1.0 to 2.25 M sucrose in water. The gradients were cen-trifuged at 60,000 x g for 1.5 h, and the visible polyhedra and virus bands were collected and repel-leted, respectively. Thepolyhedrawererecentrifuged ongradients toobtain a fraction of polyhedra devoid ofcellulardebris. Thegradient-purifiednonoccluded virus was resuspended and pelleted three times at 2,000 x g for 15 min aseptically, and the supernatant was stored frozen at-20 C.The polyhedra were either stored frozen or subjected to an alkali dissolution procedure (Harrap and Longworth, in press) to yield gradient-purifiedoccluded virus.
Thegradientswerecentrifuged at 4 C and fraction-atedby displacementwithconstant volumes of liquid paraffin.The percent(wt/wt)sucrose wasdetermined by using an
Abbe
"60" refractometer that was at-tached to acirculating Haake FE constant-tempera-turewaterbathsetat 25 C.Temperature optimum. Leighton tubes (16 by 120 mm) containing 9 by 35 mm glass cover slips were seededwith5 x 105 cells/ml, and the virusinoculum (0.2 ml) was adjusted toyield approximately 20% of the cells infectedafter 24 h of incubation at 27 C. The cultures wereinoculated, incubated at the appropri-atetemperature
(-1
C), and scored daily for 6 daysVOL.14,1974
935
on November 10, 2019 by guest
http://jvi.asm.org/
KNUDSON
for the percentage of cells exhibiting a cytopathic effect(CPE).
End-point dilution method for the assay of infectivity.Leighton tubes were seeded with5 x 104 cells/ml and incubated for 24 h. The virus to be assayed wasserially diluted inTC100, and 0.2 ml of eachdilution was added per tube using five tubes per dilution. Where necessary the medium was decanted before inoculation and, once inoculated, the tubes were left atambient temperature for 1 h. They were washed three times with 1 ml of TC100 and a final milliliter of medium was added. The cultures were incubated for 5 to 7 days, or until confluency was reached, and prepared for scoring. The cells were fixed to the cover slips by two 5-min treatments of cold acetone (-20C) which was evaporated overnight. The cells wererehydrated in 2 ml of water for 15 min, and the cover slips were removed from the Leighton tubes, rinsedinwater, andmounted on a slide in 90% glycerol in 0.01 M phosphate buffer, pH 7.5. Those coverslips showing a group or groups of infected cells when examined with a lightmicroscope were scored as positive, andthe
TCID,0
titer wascalculated.Plaque method for the assay of infectivity. Leightontubes were seeded with 2 x 105cells/mland incubated for 24 h. The cells were inoculated and fixed3 dayspostinfection (p.i.) when confluency was reached. The cover slips were examined, plaques were counted, and PFU were calculated. The procedure was essentially the same for the 5-day assay except thatthe initial seeding density was 5 x 104cells/ml.
Particle-infectious unit ratio. The samples were preparedby mixing titrated nonoccluded virus, poly-styrene latex spheres (2.5 x 1011spheres/ml), uranyl
acetate(1% wt/vol), and bovineserum albumin
frac-tion V(1%wt/vol) inaratio of2:2:2:1.Asingledrop wasaddedto aFormvar-coated 200-mesh coppergrid, and the excesswasdrained after2 min. Afterabrief rinse in wateranddrying,thegridwasexamined in an AEI EM6B electron microscope at an accelerating voltage of 60 kV. Several grids were examined per sample, and photographsweretaken ofselected fields
at amagnification of x10,000. Thepolystyrene latex spheres had a mean diameter of88 + 0.8 nm(Dow ChemicalCo.).
Growth cycle and lateral transmission of infectivity. One 25-cm2 Falcon flask was seeded for each experiment with 2.5 x 105
cells/ml
and incu-batedfor24h. The cellswereinfectedatthe appropri-ateMOIwithanadsorptionperiodof 1h.Theculture was washed three times with 5 ml of medium and incubated with a final5 ml. Ateach interval tested, the mediumwasremovedandpelletedat1,000xg for 10min tobring down anyfloatingcells. Thesuperna-tant wasstoredfrozen fortitration, and5mloffresh
mediumwasaddedtothecentrifugetube, mixed,and returned tothe culture vessel.
RESULTS
Purification.
Consistent
profiles
wereob-served
when
fractions fromthe
purification
procedure
wereanalyzed
by
quasi-equilibrium
centrifugation
through
linear
sucrosegradients.
A reproducible pattern was seen when
the
30,000
gpellet
of thenonoccludedvirus
fraction wasanalyzed (Fig. 1A). The virus banded
asareasonably homogeneous peak
at 47 to 49%(wt/wt)
sucrose, and there waslittle
radioactive
material left at the top of the gradient, suggest-ing that the purification procedure had
ade-quately
separatedthe virus from cellulardebris.
A
homogeneous peak
wasfoundat a54 to 55%(wt/wt)
sucrose level when the 10,000 gpellet
of the
polyhedra
fraction wasanalyzed.
Consid-erable cellular
debris, however, also entered the
gradient, and as a result the
polyhedra
werecol-lected
andrecentrifuged. The
profile of the sec-ond cycle of centrifugation clearlyindicated
that thepolyhedra were well
separated
from thecontaminating
material that was left at the top of thegradient (Fig.
1B).
If the
purified polyhedra
weresubjected toan alkali dissolution procedure (Harrap andLong-worth, inpress) and the 30,000 g
pellet that
was obtained wassimilarly analyzed,
a characteris-tic profile was observed(Fig. 1C). The occluded virus appeared to be quiteheterogeneous
when compared with the profile of nonoccluded virus (Fig. 1A).Moreover,
theoccluded
virus
profile was oneof
multiple
bands
over a 35 to 52%(wt/wt) sucrose
range,and
substantial
radioac-tivity
was also detected at the top ofthe
gradient, indicating,
perhaps, that degradation
of the virus had
occurred
as aresult
of the dissolution procedure.Temperature optimum. Cultures were in-fected with NPVand
incubated
atsix different temperatures(17,
21, 23,27,
30, and
37 C). They were scoreddaily
for 6days
for thepercentage
of infected cells withpolyhedra
in their nuclei. Since theinput
virusinoculum wasadjusted
toyield
20%infection when incubated at 27C,
it waspossible
to determine the tem-perature optimum for thereplication
of the NPV and for the lateral transmission of theinfectious
material
fromcell
to cell.The cultures incubated at 27 C had the
highest percentage
oftheir cells
infected
when scored oneday
p.i., and,
therefore,
27 Cwastheoptimal
temperature forthe
replication
of NPV(Fig. 2).
The 27 Ccultures reached
a100% level ofinfection within 3days p.i.,
and,
hence,
the temperature was alsooptimal
for the lateral transmission ofinfectivity.
The results of the cultures incubated at 30 C resembled those of the 27C
cultures,
although
the latter
weremarginally
moreefficient.
The cultures
incu-bated
at 17and
37C
gavesimilar
results,
but 37 C was deleterioustothecells,
asthey rarely
remained
viable for more than 3days.
Never-theless, polyhedra
weredetected
incells
incu-J.VIROL.on November 10, 2019 by guest
http://jvi.asm.org/
VOL. 14,1974 NUCLEAR POLYHEDROSIS VIRUS IN S. FRUGIPERDA
937
0A 60
bated
at37
C, suggesting
that the virus could
0'
60
replicate
atthe
higher
temperature.
'os
tI
t
Assay of
infectivity.
The distinct CPE
ob-50 0
served in
the
nuclei
of
NPV-infected
cells
was
used
asthe basis for the scoring of the end-point
C
4dilution titrations of
the virus.
Cultures
were°
t
'°
40seeded with a cell
density which formed
ax
I
0t
°' C confluentmonolayer
of cells on the cover slipE .
t
~
°)
after
5 to 7days
of
incubation
at 27C,
thus
0
facilitating
the
scoring
of infected cultures
as1 2
.
several cycles of virus replication had occurred.*
'1 . Leighton tubes were used, instead of the
com-.
.
S
montube
method,
because
the
improved optics
20 40 60 100
.m
FRACTION NUMBER
B
0l
o 60 L 80
"0
3:lDY
OS
NETO
2 \
>
0"O
I FI.2 eprtr pimmsuyfrterpi\0
0 o0 NP nishmlgu,S fuied otnoscl\0
0*
hi
\0
~40*
CA
0. \O l cutr.Tecluej er netdwt ncl
0 0 ox
(J1
~~
IS
~
0eeiouae
Il C,eaue,+IC)adsoe al frtepretg
Ist!
Il
~~~~~mu
f C;CAm Lo,U323 C; *, 27400
7
20-.Itt
I AIG.1. Sucrose equilibriumdensity0OA
20 40 60
FRACTION NUMBER
2 4 6
6
01,10
~
DAYS
POST
INFECTION
1.
00
FIG. 2.Temperature optimum study
fortherepli-\0
50~~~~~~cation
and lateral transmissionof
infectivity of
the0 NPV in itshomologous, S.frugiperdacontinuous cell
culture. The cultures were
infected
with inocula o fj ~~~~~~ 40~
where 20% of the cells exhibited the characteristic ~~~,Po~~~ * CPE when incubatedfor24hat27C. The cultures 0 0,~~~~C
wereinoculated and incubatedat theindicatedtem-I.)05/
~ ~ ~~~;0
peratures(±+
1 C) and scoreddaily forthepercentage ~~~ 7~~~0
ofcellsexhibitingaCPE.Symbols:@0,
17C; 0,21 C;cn
~
.1m 0,23 C;E,
27C; A,30C; andA,37C.FIG. 1. Sucrose equilibrium density gradients
I.
~~~~~~~~~representing
thecharacteristicprofilesobserved when the nonoccluded virus (A), polyhedra (B), andoc-_____________________cluded
virus(C)fractionswereanalyzed.Sedimenta-20 40 60 tion is right to left. Symbols:
*,
trichloroacetic acid-precipitable 3Hcounts/mmn
and 0, percent FRACTION NUMBER wt/wtsucrose.on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.502.263.456.228.519.2]allowed the CPE to be
readily distinguished.
The cover slips, of course, could
be fixed and
stored as a permanent
record of the virus
titration.
Cultures that
were
infected
with a
high MOI did not become
confluent, and the
number of cells present at inoculation
was
unchanged, suggesting that cell division did not
occur after infection.
Cultures that were
in-fected with a low
MOI, however, were confluent
with groups or plaques of infected cells
ran-domly
distributed in the monolayer. Single cells
exhibiting CPE
were
also
found,
but plaques
were
always seen when the entire monolayer
was examined.
Cultures, therefore, were scored
positive
when a group of three
ormore
cells with
CPE
was
observed.
The
appearance of plaques in cultures
in-fected with a low
MOI suggested
that
the
end-point dilution
method for the assay of
infectivity also presented a
possible plaque
assay system
when the
plaques
werecounted,
rather than the culture simply scored as
in-fected or uninin-fected.
Figure 3A represents
the
LI)
w
a
0-0.
LLJ
o
z
4030
20
10
0-5 1 0
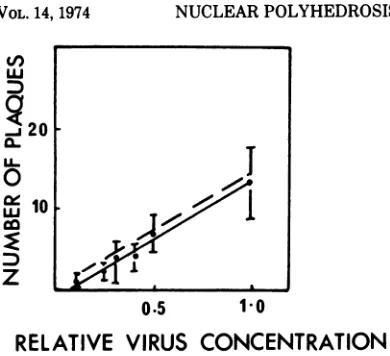
dose-response data when the total number of
plaques was compared with the virus dilution 5
days p.i. A comparison was made between the
observed data and the expected data which was
calculated from the known TCID5O titer of the
inoculum assuming 0.7
PFU/TCID,0.
A
com-parison of the slopes of the two regression
lines
revealed that the slope of the observed data was
3.5
times
greater than
the slope of the expected
data.
Two
plaque types, however, were
distinguish-able when the monolayers were reexamined. A
small plaque was a group of three or more
infected cells with a plaque diameter less
than
100
,m
(Fig. 4), and those plaques greater than
100
um
were designated as large plaques
(Fig.
5).
Figures 3B and C, respectively, represent the
dose-response
data for the small and large
plaques compared with the expected data.
The
slopes of the regression lines through the
ob-served and expected data points revealed
that
the small plaque data were twice that of the
expected slope,
whereas the large plaque and
(I)
wi
B
oIt
i..0C10
-z
i-0~~~0
1.00
RELTIE
IRS
IONCNRTO
LI)
_ 20
co
111 10
z
0-5 1 0
RELATIVE
VIRUS CONCENTRATION
RELATIVE VIRUS CONCENTRATION
FIG. 3. Dose-response curveexperiments
scored5days p.i. (A)
represents the totalplaques
seen versusthe relative virusdilution. When thecoverslips
wererescoredfor
small(B)
andlarge plaques (C), only
thelarge plaquedatacorrespondedwith theexpected
result based upon theTCID5O titerof
the inoculum.Symbols:
*, datapointswith standard deviationsof
thefive
scoredcultures;
,calculatedregression
linethrough
data; and- - --,expectedregression
based upon the TCID,0 titer, assuming0.7PFU/TCID5,.on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.502.59.453.334.632.2]NUCLEARPOLYHEDROSIS VIRUS IN S.FRUGIPERDA
FIG. 4. Photomicrograph ofacharacteristic smallplaque that isseenwhen culturesareinfected with the NPV at a low MOI and scored 5days p.i. Polyhedraappearasrefractile inclusions inthe nucleusof infected cells, resultingin areadily distinguishable CPE. (Magnification x2,350).
the
expected slopes
wereessentially equivalent.
The expression
ofthe
twoplaque
typesimplied
that
twophenotypes
were present inthe
virusinoculum.
Yet, the dose-response data (Fig. 3A,
B, and
C)
were contrary tothis
and suggestedthat the small plaques
were derived fromthe
large plaques
as aresult of asecondary,
lateraltransmission
of
infectivity.
No
distinction
ofplaque
size wasmade whenthe dose-response
experiment wasrepeated,but
scored
3days instead
of 5days
p.i.,because the
plaques
were all approximately 100gm
indiameter
(Fig.
6).Particle-infectious
unit ratio.Table
1illus-939
VOL.14,1974
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.502.95.409.76.565.2]FIG. 5. Photomicrograph ofacharacteristic large plaquethat is seenwhen culturesare infectedwith the NPV at alowMOI and scored5days p.i.
tratesthecomplete particlecountdatainwhich three determinations of the ratio were made
from three separate preparations of titrated nonoccluded virus that was stored at 4 C. The means and their standard deviations are
pre-sented for each of the threesamples, andafinal calculation from the pooled data gave a mean
value of 186 + 124 particles/TCID50 with a
standard error of 21%. A
corresponding
value of266 177particles/PFU
wascalculated
usingthe
relationship
of 0.7PFU/TCID5O.
Growth
cycle and lateral
transmission ofinfectivity.
Figure
7representsthe
growth cycle
ofextracellular
infectivity that
wasproduced
when
acell culture
wasinfected with
NPV with anMOI
of4.5.The titerofthe released material J. VIROL.on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.502.105.409.80.560.2]NUCLEAR
POLYHEDROSIS
VIRUS INS. FRUGIPERDA941
were
isolated,
but the data is inconclusive and
there
may
be
some
envelope
material associated
with
the nonoccluded virus.
This material,
however, was used for the
calculation of the
particle-infectious unit ratio, and
the
prepara-tions
consistently revealed well-dispersed
unen-veloped nucleocapsids.
The profile
(Fig. 1C)
that was seen when purified
polyhedra
were
disrupted with alkali releasing occluded virus
(both enveloped and unenveloped
nucleocap-sids) is
similar
to
other
multiply
embedded
NPV of
this type (4, 6; Harrap and
Longworth,
0.5 1 0
RELATIVE VIRUS CONCENTRATION
FIG. 6. Dose-response curve experiment scored 3
days p.i. The plaques thatwereseenwereall
approxi-mately thesamesize, i.e., approximately 100um in
diameter.Symbols:asdescribed inFig.3.
was
determined
atintervals
and
anaccumu-lated titer
wascalculated. Infectious
material wasreleased from the infected cells before
24hp.i. and it approached
amaximal titer
4days
p.i.
The
growth cycle experiment
wasrepeated
overthe
initial 24-h
period
todetermine
whether elution of virus
adsorbed
tothe surface
of
the cells had
occurred and/or
todefine
moreprecisely when infectious material
wasreleased.
Figures 8A, B, and C,
respectively,
representthe
early
portion
of thegrowth cycle
whencultures
wereinfected
withMOI's
of0.2, 4.5,
and
25TCID5O percell. Elution
of the virus didnot appear to
significantly
contribute
tothe
observed titer.
Moreover, infectivity
wasfound
extracellularly
12hp.i. regardless
oftheMOI.
DISCUSSION
Virus rods
ornonoccluded virions
areformed
in
the cell nucleus during the cycle
ofthe NPV
replication
inthe insect. As the infection
pro-ceeds,
anouterenvelope surrounds
the virus or groupsof viruswhich then become
incorporated
into
amatrix
of the
polyhedral protein (5-7). A
similarpatternofviral
morphogenesis has
beenobserved in NPV-infected cell cultures
(unpub-lished
data). Polyhedra and nonoccluded virus
can be
found
intra- and
extracellularly
asthe
replicative
cycle progresses. In the resultsre-ported here
purification of thesetwo viralenti-ties has
been
effected, and there are severalobservations
which substantiate
this claim. Forexample, the
homogeneity of the profile ofnonoccluded
virus (Fig. 1A) suggested that apopulation
of unbundled virions was isolated.The binding
density ofthe nonoccluded virus(Fig. 1A)
suggests that nucleocapsids, in fact,TABLE 1. Particletoinfectious unit ratio
Spheres Particles Particles
Sample Particles x
TCIDso
1 1 2 3 Total 6.4 3.1 7.7 10.3 6.5 6.7 9.1 6.3 6.0 4.8 26.5 11.0 40.0 12.3 22.0 9.3 16.6 27.0 15.3 7.0 37.3 9.8 15.3 9.8 21.7 16.3 9.2 11.7 21.3 10.5 15.6 28.0 16.5 20.3 15.0 19.2 186 392.6 813.2 328.0 246.8 386.5 376.1 276.7 403.3 421.7 520.5 278.4 576.7 232.6 175.0 274.1 266.7 196.2 286.0 299.1 369.2 295 113 95.5 124.6 230.0 300.3 63.3 82.6 206.5 269.6 115.0 150.1 271.1 353.9 152.4 199.0 93.7 122.3 165.0 215.4 361.4 471.8 67.9 88.7
216+ 123
257.3 134.0 165.9 86.4 258.2 134.5 116.8 60.8 154.9 80.7 276.0 143.8 216.9 113.0 118.6 61.8 241.0 125.5 162.2 84.5 90.4 47.1 153.3 79.9 124.4 64.8 168.7 87.9 132.0 68.8 92 31 124Particles/TCID50 VOL. 14, 1974
w
ui0c
co
Dz
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.502.55.250.59.237.2] [image:8.502.263.451.232.667.2]6
E
U
5
4
3
24 72 120
HOURS
POST
INFECTION
FIG. 7. Growth curve of the nuclearpolyhedrosis
virus incell culture. A culturewasinfectedatanMOI
of 4.5
TCID,
Jcell, and the extracellular infectivitywasmonitoredattheindicated intervals. Symbols: *, log TCID5Jml measured at each interval and 0,
accumulatedlogTCID5dml calculated for each inter-val.
in press). The alkali treatment must have disruptedorremoved the envelopes fromsome
oftheoccluded virus, sinceacorrelationofpeak fractions was observed between nonoccluded
virusandoccluded virus. Moreover, radioactive material wasfound at the top of the gradient,
indicating that the procedure had affected the integrity of the enveloped nucleocapsids.
Thenonoccluded viruswasusedasthesource
of inoculum for several reasons. For example,
the alkali dissolutionprocedurewaseliminated as a purification step and the yield of
nonoc-cluded virus approached ten times that of
occluded virus. There is also evidence suggest-ing that the dissolution procedure reduces the infectivityofthe virus(unpublished data).
The end-point dilution and plaque methods for the assayofinfectivityoffer distinct advan-tages over preexisting methods. The end-point dilution method using primary cultures as the
assay system (17) has the obvious drawback of
the difficulty inherent in the establishment of
such cultures. Thequantalresponsemethod, in
which the
percentage of uninfected cells werescored
24h p.i. (1),
gaveconservative
estimatesof
the
virus titer sincethe
populationdoubling
time of
the cells used
was 16h
(9).The
percentage of
uninfected cells, therefore,
was increasing.The
plaque assay method,employ-ing
a 0.6%methylcellulose overlay
(10), raisesseveral questions when compared with the
datapresented in
this
paper.For example,
the
au-thors
reported (10) that
twoplaque
types,the
"many
polyhedra" plaque (MP) and the
"fewpolyhedra" plaque (FP),
werefound.
The MP
plaques
werelarger
when
compared withthe
FPplaques, and
there
were 4 to 12 times more FPthan MP plaques when the
inputinoculum
gave40
to60
total plaques
per assay. When the FPand MP plaques
werecloned and
grQwn
in theinsect,
purified, and reinoculated
forplaque
assay,
both FP and MP
wererecovered from
each
clone. Moreover,
a dose-response experi-mentis
presented where only
two pointswith
a10-fold difference
wereused toindicate
that the
response was
linear. The reported cloning
dataimplied that
twoplaque
types were notisolated.
Either
the
passagethrough the
insecthad
someunexplained
effect, the clones
werecross-con-taminated,
orpexhaps the FP and MP plaque
types were not true
phenotypes
ofthe
possible
heterogeneity
ofthe
virus inoculum. Perhapsthe
twoplaque
typesexist,
but
their
plaque
assay
method does
not preventsecondary,
lat-eral transmission of infectivity from the MP
plaque, and
difficulty, therefore, would be
en-countered when attempting
toisolate
orpick
a"true" FP plaque
fromthe "false"
FPplaques
that
mayalso
be
present.The analogous
situa-tion of
twoplaque
typesexisted in the
S.
frugiperda plaque
assay systemwhen
assays werescored
5days p.i. However, evidence has
been
presented which demonstrates that the
slopes of the plaque and expected data
areequivalent when the
assayis
scored
3days p.i.,
suggesting that
asecondary,
lateral
transmis-sion of
infectivity had
occurred
in
the
5-day
assay. The smallplaques,
therefore, werede-rived
fromthe large plaques. The 3-day
assay suggeststhat
asingle
virusparticle
caninitiatethe infection
ofthe cell. It also confirms the
relationship
of 0.7PFU/TCID,0
and
further
substantiates the
accuracy ofthese
infectivity
measurements?
The
growth
cycle data demonstrates that
infectious material
wasreleased
frominfected
cells
12 hp.i.,
reaching
amaximal
titer4days
p.i.Cytolysis did
occur to alimited
extent2 to3days p.i.,
andperhaps
the
adsorption
of virustonewly exposed
receptor sites accounts forthe
on November 10, 2019 by guest
http://jvi.asm.org/
[image:9.502.54.245.66.360.2]NUCLEAR
POLYHEDROSIS VIRUS IN S. FRUGIPERDA
0
to
ur
0
0
12 24
0
I-12 24 12 24
HOURS
POST INFECTIONHOURS POST INFECTION
HOURS POST
INFECTIONFIG. 8. Growthcurvesof the nuclearpolyhedrosisvirus in cell cultureovera24-h interval. Cultures were
infectedatanMOIof 0.2 (A),4.5(B),and 25(C)TCID,Iml,andtheextracellularinfectivitywasmonitoredat
the indicated intervals. Symbols:asdescribedfor Fig. 7.
drop in the interval titer. It is interestingto note that lateral transmission ofinfectivityoccurred
1to2days before anylysiswasobserved. Virus,
however, has been detected in the nuclei of infected cells 12 h p.i. and has been observedin the process of budding from the cell surfaces (unpublished data).
Several conditions were established for the
optimal replication of NPV inacontinuous cell
culture
of S.frugiperda.
The temperatureop-timum for the growth ofthe cells of 27 C was
also theoptimaltemperature forthereplication ofthe virus. In preliminary experiments where cultures were maintained atconfluencyon
me-dium containing2% fetal bovineserum instead of 10% serum and infected, no CPE was ob-served(unpublished data). The virusmay repli-cateinthese cultures withoutproducingaCPE,
due to the altered conditions of the medium. Yet, the observation that once the cell is
in-fected, cell division is halted may suggestthat the cells must be actively dividing or in their
logarithmic growth phase for infection to
pro-ceed. Moreover, this phenomenon may explain
why plaques (Fig. 4 and 5) are found without
the use of an overlay that would normally
impede the lateral transmission of infectivity. The seeding density of the cells, therefore, is important in this plaque assay method. The
cultures must be seeded to allow the develop-ment of a plaque, and, at the same time, the
uninfected cells must grow and approach
con-fluency which then, apparently, renders them
incapable of producing
aCPE.
The number
ofTCID5O
produced
percell
canbe calculated from the
growth
cycle experiment.
It
mustbe
noted, however, that the titers of
virus
stored
at 4C
ascompared
with those
stored
at -20C
maydiffer
by
half
alogarithm
(unpublished
data), and the total TCID5O
pro-duced, therefore,
was 1.6 to 5 x 106TCID,dml.
Since
5 x 105cells/ml
wereused,
avalue
of 3 to 10TCID,dcell
canbe calculated. Table
2 repre-sentsthe
typical yields
ofvirus
that
wereproduced under the described conditions. The
calculations
areapproximations,
but they
prob-ably
represent areasonable
estimate of
the
yields
of NPV
that
canbe expected from
infected cell cultures. Nevertheless, it becomes
[image:10.502.55.450.63.286.2]readily
apparentthat insect cell
systemswill
TABLE 2. VirusproductionYield Values
Nonoccluded
Polyhedra
virus
TCID,OPer liter flask" 7 x losb 9 x 109c 108-5x 108
Percelld 12-40 150-500 3-10
aConditions:seed,5 x 106cells; MOI, 0.01 (5 x104
TCID,0); harvest,7 to 10days.
bPolyhedral protein: 0.7 mg. cNonoccluded virus protein: 0.3 mg.
dCalculation based on the observations that the
liter flask was confluent (5 x 107 cells), a ratio of 4 polyhedra:50virions:TCID,o was produced, and 3 to
10TCID,Owereproduced per cell (growth cycle data). VOL. 14, 1974
A
51
E 4
0
to
J 3
-943
on November 10, 2019 by guest
http://jvi.asm.org/
[image:10.502.260.451.515.620.2]provide
afeasible
meansby
which the replica-tion ofthese viruses can be investigated.
LITERATURE CITED
1. Faulkner, P., and J. F. Henderson. 1972. Serial passage of the nuclear polyhedrosis disease virus of the cabbage looper(Trichoplusia ni) in a continuous tissue culture cell line. Virology 50:920-924.
2. Goodwin, R. H., J. F. Vaughn, J. R. Adams, and J. J.
Louloudes. 1970. Replication of a nuclear polyhedrosis virus in anestablished insect cell line. J. Invertebr. Pathol. 16:284-188.
3. Harrap, K. A. 1972.The structure of nuclear polyhedrosis viruses.I. Theinclusionbody.Virology 50:114-123.
4. Harrap,K. A. 1972. The structure of nuclearpolyhedrosis
viruses. II.The virus particle.Virology 50:124-132. 5. Harrap, K. A. 1972. The structure of nuclear polyhedrosis
viruses.III.Virus assembly.Virology50:133-139.
6. Harrap, K. A. 1973. Virusinfection ininvertebrates, p.
271-299. In A. J. Gibbs (ed.), Viruses and
inverte-brates.North HollandPublishing Co.,Amsterdam.
7. Harrap, K. A., and J. S. Robertson. 1968. A possible infection pathway in the development of a nuclear
polyhedrosisvirus.J. Gen. Virol.3:221-225.
8. Henderson, J. F., P. Faulkner, and E. A. MacKinnon. 1974.Somebiophysicalproperties of virus present in tissueculturesinfected with thenuclearpolyhedrosis
virus ofTrichoplusia ni. J.Gen.Virol.22:143-146.
9. Hink,W. F. 1970. Establishedinsectcell line from the
cabbage looper, Trichoplusia ni. Nature (London)
226:466-467.
10. Hink, W. F., and P. V. Vail. 1973. A plaque assay for titration of alfalfalooper nuclear polyhedrosis virus in a cabbagelooper (TN-368) cell line. J. Invertebr. Pathol. 22:168-174.
11. Ignoffo, C. M., M. Shapiro, and W. F. Hink. 1971. Replication and serial passage of infectious Heliothis nucleopolyhedrosis virus in an established line of Heliothis zea cells. J. Invertebr. Pathol. 18:131-134. 12. Sohi, S. S., and J.C. Cunningham.1972. Replication of a
nuclearpolyhedrosis virus in serially transferred insect
hemocyte cultures.J.Invertebr.Pathol. 19:51-61.
13. Summers, M. D., and D. L. Anderson. 1972. Character-ization of deoxyribonucleic acid isolated from the granulosis virus of the cabbagelooper, Trichoplusia ni andthefallarmyworm,Spodoptera frugiperda. Virol-ogy 50:459-471.
14. Summers,M.D., and D. L. Anderson. 1972. Granulosis
virusdeoxyribonucleic acid:aclosed, double-stranded
molecule. J. Virol. 9:710-713.
15. Summers,M.D.,and D. L. Anderson. 1973.
Character-ization of nuclearpolyhedrosis virusDNAs. J. Virol. 12: 1336-1346.
16. Vail, P. V., D. L.Jay, and W. F. Hink.1973.Replication
and infectivityofthe nuclearpolyhedrosisvirus ofthe
alfalfa looper, Autographa califomica, produced in
cellsgrown in vitro. J. Invertebr.Pathol. 22:231-237.
17. Vaughn,J.L., and M.S.Stanley. 1970. Amicromethod
fortheassay of insect viruses in primary culturesof insect tissue.J. Invertebr. Pathol. 16:357-362.
18. Wildy, P. 1971. Classification andnomenclature of
vi-ruses.InMonographsinvirologyno. 5.S. KargerAG,
Basel.