0022-538X/92/074551-05$02.00/0
Copyright © 1992, American Society for Microbiology
Phosphorylation of the Budgerigar Fledgling
Disease Virus
Major
Capsid Protein VP1t
JOHNI. HAYNES IIAND RICHARD A. CONSIGLI* Divisionof Biology, Sectionof Virology and Oncology, Ackert Hall,
KansasState University, Manhattan, Kansas 66506-4901
Received 19February 1992/Accepted 30 March1992
The structural proteins of the budgerigar fledglingdiseasevirus, the first known nonmammalian polyoma-virus, were analyzed by isoelectric focusing and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The major capsid protein VP1wasfoundtobe composedofatleastfive distinct species having isoelectric points ranging from pH 6.45 to 5.85. By analogy with the murine polyomavirus, these species
apparentlyresult fromdifferent modifications ofaninitial translation product. Primary chicken embryo cells
were infected in the presence of32p; todetermine whether the virus structural proteins were modified by
phosphorylation. SDS-PAGE of the purified virus structural proteins demonstrated that VP1 (along with both minor capsid proteins) was phosphorylated. Two-dimensional analysis of the radiolabeled virus showed phosphorylation ofonly thetwo most acidic isoelectric species of VP1, indicating that this posttranslational modification contributestoVP1 species heterogeneity. Phosphoamino acid analysis of 32P-labeled VP1 revealed thatphosphoserine is theonlyphosphoamino acidpresentin the VP1 protein.
Budgerigar fledgling disease virus (BFDV) has been iso-lated from fledgling budgerigars (Melopsittacus undulatus) showing lesions in numerous tissues, including skin, feather,
follicle, kidney, uropygial gland, crop, liver, heart, bone marrow,spleen, and brain(3, 9, 10, 17, 33). BFDV, the first reported nonmammalian polyomavirus, can causemortality ratesof upto100% in budgerigar aviaries (3, 5, 9, 10, 18, 33). Its classification as a polyomavirus is based on electron
microscopic studies of its morphology, its resistance to
organic solvents, its ability to replicate in the nuclei of infected cells, the buoyant density of virions (1.34g/ml),and thecharacteristics of its DNA, which is supercoiled, with a molecular weight of 330,000 (5, 11, 22). In addition, Lehn and Muller (22)wereable to demonstrate transformation of nonpermissive primary hamster embryo fibroblasts by BFDV.
Elucidation of the complete nucleotide sequence (35) of BFDV revealedsimilarities with both simian virus 40(SV40)
and murinepolyomavirus. TheDNAcontains4,980bp and, like those of the relatedviruses, isdivided into early and late
coding regions. The early region codes for two proteins,
which have beendesignatedthelargeTand small Tantigens
byanalogywith otherpapovaviruses. However, thelarge T antigen ofBFDV is considerably smaller than the large T antigen of either SV40orpolyomavirus. Another distinction is that an open readingframe for middle Tantigenfound in rodentpolyomaviruses is notpresent in the BFDV genome. The late region of the genome codes for three structural
proteins, VP1, VP2, and VP3, with apparent molecular masses of42, 39, and 29 kDa, respectively. As with SV40 andmousepolyomavirus,VP3 appearstobeidenticaltothe carboxy terminus of VP2. Recently, a sequence in the carboxy terminus of VP2 hasbeenshown toconfer nuclear localizationonthisprotein (34). Acomparison ofthe struc-tural proteins between this avian polyomavirus and its
*Correspondingauthor.
t Contribution92-439-Jfromthe KansasAgricultural Experiment
Station, Kansas StateUniversity, Manhattan, KS 66506.
murine counterpart revealed 47% identity between theVP1 proteins and 26.5 and 29.5% identitybetween theVP2 and VP3proteins, respectively (35).
Previous studies (1, 2, 4, 15, 24-26, 32, 41) have demon-strated that themajorcapsidprotein of themouse
polyoma-virus is differentially modified after translation in several ways, includingphosphorylation (1, 2, 4, 15, 24, 32). These modificationsarebelievedtocontributetotheexistence ofat least six species of the protein, which can be separated by isoelectric focusing (4). In the murine polyomavirus, these species have been designated A (most basic) to F (most
acidic). Two-dimensional analysis of SV40 VP1 also re-vealed the presence of several different isoelectric forms of this protein (31). SV40 VP1, like polyomavirus VP1, was showntobedifferentially phosphorylated(32).It wasthus of interesttodetermine whetherVP1of the avianpolyomavirus BFDValsoundergoesmodification byphosphorylation.We alsowishedto investigatethepossibleexistence ofmultiple
isoelectricspecies ofBFDV VP1which may bedifferentially
phosphorylated.
For purification of BFDV, primary cultures of chicken embryo cells were prepared as described previously (29).
Infected cultures were maintained at 39°C in serum-free Dulbecco'smodifiedEagle'smedium (DMEM). BFDV was purified from infected-cell lysates as described previously
for polyomavirus (28). The CsCl gradients used in the purification were prepared by the method of Brunck and Leick(7)asdescribed in greater detail elsewhere(6).Priorto
CsCl purification, sucrose-pelletedviruswas treated with a
1:1 mixture of phosphate-buffered saline (PBS; 10 mM sodiumphosphate[pH7.2], 150 mMNaCl)and RIPA buffer (20 mM morpholinepropanesulfonic acid [MOPS, pH 7.0],
150 mM NaCl, 1%
[wt/vol]
desoxycholate, 1%[vol/vol]
Nonidet P-40, 0.1% sodium dodecyl sulfate[SDS],
2 mMEDTA) containing 10 ,ug each of the protease inhibitors
aprotinin, N-tosyl-L-lysinechloromethyl ketone, N-tosyl-L-phenylalaninechloromethyl ketone,and
phenylmethylsulfo-nyl fluoride perml.The lowerband,containing
intact BFDV virions,wascollected from theCsClgradients
andusedfor allsubsequentanalyses.4551
on November 9, 2019 by guest
http://jvi.asm.org/
A.
66- B.
:-;: A B C D E
245-45I
-VP,,
- vvv29-4444! -VP3
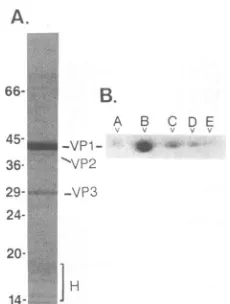
214-FIG. 1. Electrophoretic analysis of BFDV structural proteins. (A) The three BFDV structural proteinsand host-contributed
his-toneswereresolvedbySDS-PAGE(12.5%polyacrylamide) (21)and visualizedbyCoomassie blue staining. Sizes(in kilodaltons)were
obtained by electrophoresing Dalton Mark VII-L molecular size' markers (Sigma) in an adjacent lane, and the BFDV structural
proteins and host-contributed histones are indicated. (B) The five isoelectric species of BFDV VP1 (designatedA [most basic] to E
[most acidic]) were resolved by IEF in the first dimension and SDS-PAGE in the second dimension and visualized byCoomassie bluestaining.
SDS-polyacrylamidegel electrophoresis (SDS-PAGE) (21)
was performed on purified BFDV virions with a 12.5%
acrylamide gel and a0.33% bisacrylamide cross-linker. The three BFDVstructural proteins as well as the host-contrib-utedhistonesare shown inFig. 1A. TheVPlIVP2NVP3ratio appears to be quite similar to that found in the murine
polyomavirus andSV40 (40),with the maj'or capsid protein
VP1 constitutingat least75% of the totalprotein of all three viruses. In ordertodetermine whether isoelectric heteroge-neityexists in the BFDVmajor capsid protein, as it does in
murine polyomavirus and SV40, two-dimensional electro-phoresis wa's performed on purified virusby the method of
O'Farrell,, essentiallyasdescribed previously (2, 4, 12, 30). Prior to electrophoresis, 20 p.g of BFDV virions was
dis-ruptedin2% SDS-5%2-mercaptoethanolfor 2minat1000C andacetone precipitated.Theviral pelletswere then
resus-pended in a 25-pAl solution composed of 9.5 M urea, 5%
2-mercaptoethanol, 2% Nonidet P-40, and 2% ampholines.
The ampholine mixture used for isoelectric focusing (IEF)
consisted ofequalvolumes ofpH2.5-5 (Pharmacia), pH4-6
(Bio-Rad), pH 5-7 (Bio-Rad), pH 6-8 (Bio-Rad), and pH
3.5-10 (Pharmacia) ampholines. Figure lB shows the
pres-ence of at least five different isoelectric species of VP1,
which have been designated A through E. From the Coomassie blue staining reaction, there appear to be two
majorisoelectricspecies,designated BandC,and three less abundant species, designated A, D, and E. These five
specieswereconsistentlyseenuponCoomassie bluestaining
oftwo-dimensionalgels.The fact that BFDV VP1speciesB
anld C were predominant is consistent with observations
reported for the murinepolyomavirus VP1 species (4).
Toobtain the approximateisoelectricpoints (pls)of these five isoelectric species, the chloramine T method (14) was
usedto label purified BFDV with 1251 and 5 x 10~cpm of virions was included in a companion tube gel, which was
sliced into 2-mm segments after IEF. The locations of the viralproteinswereascertainedby countingtheradioactivity
[image:2.612.128.243.71.223.2]in eachgelslice inagammacounter, and thepHof each slice
TABLE 1. Characteristicsof BFDV virion VP1
isoelectric species
Species Apparentpla 3Pincorporated'
A 6.45
B 6.30
C 6.15
D 6.05 +
E 5.85 +
aApparentplsweredetermined byincubatingagel slice containingthe
respectivespeciesin 1 mlof distilledwaterandmeasuringthepHatroom
temperature.
bDetermined by autoradiography of in vivo 32P-labeled BFDVvirions electrophoresedon anO'Farrell(30) two-dimensionalgel.-,
nonphosphory-latedspecies; +,phosphorylated species.
was then measured in distilled water. The pIs of the five isoelectricspecies of VP1rangedfrom6.45to5.85,asshown in Table 1. The minor capsid proteinswere also shown to have pIs within this range (asshown inFig. 2B),so estima-tion of theirpIswasmadebycomparingtheirpositionsinthe IEFdimensionwith thepositions of the VP1species.
To studyprotein phosphorylation, BFDVwaslabeled in vivo with 3
Pi
by removing the serum-free DMEM from chickenembryo cellsat 18 hpostinfection andreplacing
the medium with serum-free Eagle'smediumcontaining 20%of the normalphosphate concentration and supplementedwith approximately 3.6 x 108 cpm of 32p; (carrier free; ICNRadiochemicals, Irvine, Calif.) per 100-mm2dish. Infection wasallowedtoproceed until the culturesshowedcytopathic
effect, atwhich time the cells were harvested and the virus waspurifiedasdescribed above.
Foranalysisof
phosphorylation
bySDS-PAGE,2.5 x 104cpmofpurified intact
32P-labeled
virions wasfirst immuno-precipitated with anti-BFDV VP1 monoclonal antibody4F10F4 (13) to eliminate possible contaminating cellular proteins. Fourvolumes of RIPA bufferwere added to the mixture, whichwasthenrockedat4°C for 2h,afterwhich50
plof a20% suspensionofStaphylococcusaureusPansorbin cells (Calbiochem, San Diego, Calif.) in RIPAbuffer was added. The mixture wasrocked at 4°C foran additional 45 min, and theparticulate material waspelleted by
centrifuga-tion and washed three times in RIPAbuffer, twice in PBS, andonceindistilledwater.Thefinalpelletwasresuspended in SDS-PAGE sample buffer (21),boiled for 2 min to elute theantigen-antibodycomplexes from thecells, and recentri-fuged. The supernatants were analyzed by SDS-PAGE on
12.5% polyacrylamide gels, stained with Coomassie blue,
andautoradiographed, asshown in lane 2 ofFig. 2A. These results demonstrate that three proteins from intact virus whichmigrate identically to VP1, VP2, andVP3 (compare
Fig. 2A, lanes 1 and 2) and which are immunoprecipitated withamonoclonal antibodyagainst VP1 arephosphorylated. Previous studies showed that VP2 and VP3 are
phosphory-latedtosome extentinbothmurinepolyomavirus(4, 32)and SV40 (32), but further information about the
phosphoryla-tion of theseminorcapsidproteins hasnot beenreported. Previous studies inourlaboratory(2,4) demonstrated that
only the three most acidic isoelectric species of murine
polyomavirusVP1 (D, E, andF) arephosphorylated. Two-ditnensional electrophoretic analysis and autoradiography were performed on purified in vivo
32P-labeled
BFDV to determine whichindividual VP1speciesarephosphorylated
andto investigate further the contention that VP2 and VP3 are also phosphorylated. Purified1251-labeled
BFDV wason November 9, 2019 by guest
http://jvi.asm.org/
A. B.
C.
14-P- SLH-:
e.1
P-THHl- '-... " Cr
0
-'J--VP2
VP3
FIG. 2. (A) SDS-PAGE analysis of 32P-labeled BFDV virions.
Lane1, BFDVstructural proteins separated by SDS-PAGE (12.5% polyacrylamide) and stained with Coomassie blue. Lane 2, autora-diography of purified in vivo 32P-labeled and immunoprecipitated BFDV structural proteins separated by SDS-PAGE. Positions of size markersare indicated(in kilodaltons). (B)
AutoradiograVhy
ofpurified BFDV proteins whichwere labeled in vitro with 12I (105 cpm), dissociated, and separated byIEF inthefirstdimension and SDS-PAGE in the second dimensionas described in the text. (C) Autoradiography of purified BFDV proteins labeled in vivo with 32p (105 cpm), immunoprecipitated, dissociated, and separated by IEF in thefirstdimension and SDS-PAGE in the second dimension.
alsosubjectedtotwo-dimensional gel analysis (asdescribed above) and autoradiography to illustrate the migration pat-ternsof allBFDVstructuralproteinsaswellastoserveasa
reference autoradiogram for identifying the BFDV phos-phoproteins, as will be discussed later. An agarose plug containing molecular weight markers (Sigma, St. Louis, Mo.)waselectrophoresed adjacenttothetubegelinorderto
facilitate estimation ofproteinmolecularweights. Autoradi-ographyofthetwo-dimensionalgelofiodinated virions(Fig. 2B)showed that in additiontothe five VP1 protein species,
twootherproteinswerepresent.By usingthemolecular size markers as aguide, it was demonstrated that one of these proteins had a molecular mass of approximately 39 kDa, while the othershowedamolecular massof29 kDa. These molecularmasses are in agreement with the predicted mo-lecularmasses of VP2 andVP3, respectively. In addition, bothproteinswereobserved intwo-dimensional gelswhena
monoclonal antibodymade againstVP1 was usedto immu-noprecipitate intact BFDV inRIPAbufferpriorto dissocia-tion (data not shown). An additional minor protein which migratedatabout40kDawasdetectednearthebasic end of some gels, but this protein was not coprecipitated by the monoclonal antibodyandmayrepresentacopurifying cellu-larprotein.Wetherefore believe that themoreacidic39-kDa protein,which hasapl (about 6.3) nearly equivalenttothat ofVP1species B,is VP2and that the 29-kDaprotein,which has aplof about6.45, isVP3.
For determination of which species of BFDV VP1 are phosphorylated, 32P-labeled virionswere first immunopre-cipitatedwithanti-BFDV VP1monoclonalantibody4F10F4 (as described above), and two-dimensional electrophoresis andautoradiographywereperformed (Fig.2C). BFDV phos-phoproteins were identified by alignment ofthis autoradio-gramwiththat of the125I-labeled virions(Fig. 2B).From this alignment, it appears that onlythe twomostacidic species (species D and E) of VP1 as well as VP2 and VP3 were phosphorylated. It is possible that the faint signal which
P-T1
[image:3.612.395.479.78.308.2]YR-1 2
FIG. 3. Phosphoamino acid analysis of BFDV VP1. Approxi-mately 2.5 X 104 cpm of 32P-labeled BFDV was used for
phos-phoamino acid analysis. Lane 1, Phosphos-phoamino acid standards (1
jig
eachofphosphotyrosine [P-TYR],phosphothreonine [P-THR], and phosphoserine [P-SER]) visualized following electrophoresis by spraying the plate with0.2%ninhydrin in acetone and heating briefly
to allow color development. Lane 2, 32P-labeled phosphoamino acids of BFDVVP1visualized by autoradiography of the thin-layer plate.
seems tomerge with the basic side ofspeciesD represented anadditional formof VP1whichwasphosphorylated. How-ever, thissignal was not observed consistently.
Studies by Tan and Sokol (38,39) revealed that phospho-serine is the onlyphosphoamino acid present in SV40VP1. Anders and Consigli (2) found phosphorylation of both threonine and serine residues ofVP1frommurine polyoma-virus, with phosphothreonine accounting for approximately
70% of the polyomavirus VP1 phosphoamino acids and
phosphoserine accounting for the other 30%. In order to determine which aminoacid(s) in BFDV VP1 serves as the phosphate acceptor,weperformedphosphoaminoacid
anal-ysis on in vivo
32P-labeled
VP1. BFDV structural proteinswere resolved by SDS-PAGE andelectrophoretically trans-ferredtoanImmobilon-P membrane(Millipore) accordingto the manufacturer'sinstructions.Theregionof themembrane containing VP1 wasvisualized by autoradiography and ex-cised. Acid hydrolysis ofVP1 was performed as described previously (19), and thin-layer electrophoresis was carried out atpH 3.5asdescribedbyCooperetal.(8).Figure3,lane 1, shows the thin-layer electrophoretic separation and
nin-hydrin staining of the phosphoamino acid standards. The relativepositionsof three discretespots,representing
phos-phoserine, phosphothreonine, and phosphotyrosine, were
equivalent to their expected positions under these buffer conditions (8). Lane 2shows the autoradiogram of labeled
phosphoamino acids found in the
32P-labeled
BFDV VP1. Phosphoserine was the only phosphoamino acid found in BFDV VP1. Overexposure of the autoradiogram failed to reveal the presence of either32P-labeled phosphothreonine
orphosphotyrosine.Bolen et al. (4) were able to ascribe several different
on November 9, 2019 by guest
http://jvi.asm.org/
[image:3.612.86.273.80.228.2]functions to VP1 of themousepolyomavirus. These include a structural role, a role in the virus'sability toadsorb to and agglutinate guinea pig erythrocytes, a role invirus attach-ment tocellularreceptors, resulting in productiveinfection,
and an unknown role suggested by thebindingofVP1 to the virion DNA-protein core. At least six isoelectric species of the mouse polyomavirus major capsid protein VP1 have been identified (2, 4, 15, 24, 27, 32), and allofthemappearto contain the identical aminoacid sequence (2). Inthemurine polyomavirus studies byBolen etal. (4),VP1species Awas the only species associated with the DNA-protein core, whereas species D and Fappeared to be involved in agglu-tination of guinea pig erythrocytes and species E was re-quired for virus adsorption tospecific cellular receptors. In addition, structural roles appear to be served by species B through F. Modifications whichresult inmurine polyomavi-rus VP1 species ofdifferent pIs include phosphorylation of serine and threonine (1, 2, 4, 15, 24, 32), acetylation (4), sulfuration of tyrosine (25), and hydroxylation of proline (26).
Within the papovaviruses, posttranslational modifications are not limited to themousepolyomavirus.Two-dimensional analysis ofSV40 VP1 demonstrated the presence of several different isoelectric forms (31), which are attributed to posttranslational modifications. Whether specific functions can be similarly assigned to the five isoelectric species of BFDV VP1 remains to be shown. The current finding that VP1 of BFDV exists as multiple isoelectric species lends furthersupport tothecontention thatposttranslational mod-ification of the majorcapsid protein is ageneral property of papovaviruses. A posttranslational modification of BFDV VP2 as well as ofVP2 of SV40 and murine polyomavirus, namely, myristoylation, has also been reported (20, 36, 37). ThefunctionalsignificanceofBFDV VP1 phosphorylation remains elusive. As it is a papovavirus, it is possible that phosphorylation ofBFDV VP1 is involved in the regulation ofvirus assembly, as has been suggested by studies of the murine polyomavirus and SV40 (15, 16, 31). Nontransform-ing host range (hr-t) mutants of polyomavirus, which are blocked in assembly when used to infect nonpermissive cells,wereshown to bedeficient in the phosphorylated VP1 species (15).Perhapsphosphorylation of VP1 plays a rolein theinteraction of this protein with the minor capsidproteins orwith the minichromosome. The elucidation of the SV40 crystallographic structure at high resolution (23) should lead to increased insight with regard to a structural role for papovavirus VP1 phosphorylation.
Phosphorylation of BFDV VP1 may also play a role in virus-cellinteractions. Phosphorylated murine polyomavirus VP1 species D and F were specifically recognized by anti-bodies whose only known function was to inhibit virus adsorption to and agglutination of guinea pig erythrocytes (4). This observation suggests that only these species are directly involved in binding to receptors on guinea pig erythrocytes. We have recently noted that BFDV is capable ofagglutinating chicken erythrocytes (13), and we postulate that, by analogy, one or both of the phosphorylated BFDV VP1 species could mediate this interaction. Furthermore, a distinct, phosphorylated VP1 species (species E) of murine polyomavirus was shown to be the only species that was
immunoprecipitated with neutralizing antibodies (4). This VP1 species isbelieved to be the virus proteinnecessary for the adsorption to specific mouse kidney cell or mouse embryo cellreceptors that leads to aproductive infection. It should be pointed out that phosphorylation may not be the sole modification of VP1 necessary for virus-cell
interac-tions,
but it isinteresting
that thespecies
of the murinepolyomavirus
involved in these interactions were allphos-phorylated. The presenceofat least five isoelectric
species
ofBFDVVP1,
twoof which arephosphorylated,
suggeststhat
charge
effectsproduced
by
phosphorylation may besuperimposed
oncharge
effects generatedby
other, asyetunidentified, modifications.
The function of
phosphorylation
of the minorcapsid
proteins
in the BFDV lifecycle
also remains to beeluci-dated. Further characterization of these
phosphoproteins
shouldprovide
avenuesforspeculationas totherole of theirphosphorylation
in the lifecycles
of BFDV and the otherpapovaviruses
and will be the focus ofa separatestudy.
Herewereportthe existence of fiveisoelectric species of
the
major capsid protein
ofanavian polyomavirus,BFDV,
andwe
identify phosphorylation
as aparticularmodificationwhich contributes to differences in pIs. Posttranslational modifications of
proteins
may be a means bywhich certain viruses can maximize the genetic economy of a genomehaving a limited coding capacity. In this scenario, single
translation productsare differentially modified tocarryout separate and specific functions. The discovery of this
phe-nomenon in BFDV, coupled with knowledge of its occur-rence in murine polyomavirus and SV40, may demonstrate how papovaviruses have circumvented the limitations
im-posed
onthemby
their smallgenome size.Thisinvestigation was supported byPublicHealth Servicegrant CA07319 fromthe National CancerInstitute, by NASA NAGWno. 1197 and 2328, and by the Wesley Foundation of Wichita, Kans. J.I.H. is also a predoctoral fellow under NationalCancer Institute training grant CA09418.
We thank Daryl Riley, Kevin Mapes, and LaDonna Grenz for technical assistance.
REFERENCES
1. Anders, D.G., and R. A. Consigli. 1983. Chemicalcleavage of polyomavirus major capsid structural protein VP1: identifica-tion ofcleavage products andevidence that thereceptormoiety resides in thecarboxy-terminal region. J. Virol. 48:197-205. 2. Anders, D. G., and R. A. Consigli. 1983. Comparison of
non-phosphorylated and non-phosphorylated species of polyomavirus major capsid protein VP1 and identification of the major phos-phorylation region. J. Virol. 48:206-217.
3. Bernier, G., M. Morin, and G. Marsolais. 1981. Ageneralized inclusion body disease in the budgerigar (Melopsittacus undu-latus) caused by a papovavirus-like agent. Avian Dis. 25:1083-1092.
4. Bolen, J. B., D. G. Anders, J.Trempy, and R. A.Consigli. 1981. Differences in the subpopulations of the structural proteins of polyoma virions and capsids:biological function of themultiple
VP,
species. J. Virol. 37:80-91.5. Bozeman, L. H., R. B. Davis, D. Gaudry, P. D. Lukert, 0. J. Fletcher, and M. J.Dykstra. 1981.Characterizationofa papova-virus isolated from fledgling budgerigars. Avian Dis. 25:972-980.
6. Brady, J. N., V. D.Winston, and R.A.Consigli. 1977. Dissoci-ation of polyoma virus by chelation of calcium ions found associated withpurified virions. J. Virol. 23:717-724.
7. Brunck, C. F.,and V. Leick. 1969. Rapid equilibrium isopycnic CsClgradients. Biochim. Biophys. Acta 179:136-144. 8. Cooper, J. A.,B.M. Sefton, and T.Hunter.1983. Detection and
quantification of phosphotyrosine in proteins. Methods En-zymol. 99:387-402.
9. Davis, R. B., L. H. Bozeman, D. Gaudry, 0. J. Fletcher, P. D. Lukert, and M. J. Dykstra. 1981. A viral disease of fledgling budgerigars. Avian Dis.25:179-183.
10. Dykstra, M. J., and L. H.Bozeman. 1982. Alight and electron
on November 9, 2019 by guest
http://jvi.asm.org/
microscopicexamination of budgerigar fledgling disease virus in tissue and in cell culture. Avian Pathol. 11:11-28.
11. Dykstra, M. J., C. C. Dykstra, P. D. Lukert, and L. H. Bozeman. 1984.Investigations of budgerigar fledgling disease virus. Am. J. Vet. Res.45:1883-1887.
12. Fattaey, A. R., and R. A. Consigli. 1989. Synthesis, posttrans-lational modifications, and nuclear transport of polyomavirus major capsid protein VP1. J. Virol. 63:3168-3175.
13. Fattaey, A. R., L. Lenz, and R. A. Consigli. Production and characterizationof monoclonal antibodies to budgerigar fledg-ling disease virus major capsid protein VP1. Avian Dis., in press.
14. Frost, E., and P. Bourgaux. 1975. Decapsidation of polyoma virus: identification of subviral species. Virology 68:245-255. 15. Garcea, R. L., K. Ballmer-Hofer, and T. L. Benjamin. 1985.
Virion assembly defect of polyomavirus hr-t mutants: under-phosphorylationof major capsid proteinVP1 before viral DNA encapsidation. J. Virol. 54:311-316.
16. Garcea, R. L., and T. L.Benjamin.1983.Host-range transform-ing geneof polyoma virus plays a role in virus assembly. Proc. Natl. Acad. Sci.USA 80:3613-3617.
17. Graham, D. L., and B. W.Calnek. 1986.Papovavirus infection in hand-fed parrots: virus isolation andpathology. Avian Dis. 31:398-410.
18. Kaleta, E. F., W. Herbst, F.-J. Kaup, R. Jank-Ludwig, H.-J. Marshall, W.Drommer, and M.Krautwald.1984. Untersuchun-gen zurVirusatiologie einermitHepatitisund Befiederungssto-rungeneinhergehenden Krankheit bei Wellensittichen (Melop-sittacusundulatus).Zentralbl. Veterinaermed. Reihe B 31:219-224.
19. Kamps, M. P., and B. M. Sefton.1989. Acid and basehydrolysis ofphosphoproteinsbound to Immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal. Bio-chem.176:22-27.
20. Krauzewicz, N.,C. H. Streuli, N. Stuart-Smith, M.D.Jones, S. Wallace, and B. E. Griffin. 1990. Myristylated polyomavirus VP2: role in the lifecycleof the virus. J. Virol.64:4414-4420. 21. Laemmli, U.K.1970.Cleavageofstructuralproteins duringthe
assembly of the head ofbacteriophage T4. Nature (London) 227:680-685.
22. Lehn, H., andH.Muller. 1986.Cloningandcharacterizationof budgerigar fledglingdiseasevirus, an avianpolyomavirus. Vi-rology151:362-370.
23. Liddington, R.C., Y. Yan, J. Moulai, R. Sahli, T. L.Benjamin,
andS. C.Harrison. 1991. Structureof simian virus 40at3.8-A
resolution. Nature(London)354:278-284.
24. Ludlow, J. W., and R. A.Consigli. 1987.Differences in biolog-ical activity and structural protein VP1 phosphorylation of polyomavirus progeny resulting from infection of primary
mousekidney and primary mouse embryo cell cultures. J. Virol. 61:509-517.
25. Ludlow, J. W., and R. A. Consigli. 1987. Polyomavirus major capsid protein VP1 is modified by tyrosine sulfuration. J. Virol. 61:1708-1711.
26. Ludlow, J. W., and R. A.Consigli. 1989.Hydroxyproline in the major capsid protein VP1ofpolyomavirus. J. Virol. 63:2881-2884.
27. Marriott, S. J., and R. A. Consigli. 1985. Production and characterizationof monoclonalantibodiestopolyomavirus ma-jor capsid proteinVP1. J. Virol. 56:365-372.
28. McMillen, J., M. Center, and R. A.Consigli.1976.Origin of the polyomavirus-associatedendonuclease. J. Virol. 17:127-131. 29. Minocha, H. C., R. A. Consigli, and A. Eisenstark. 1968.
ConcentrationandpurificationofNewcastledisease virus.Am.
J. Vet. Res. 29:877-882.
30. O'Farrell, P. H. 1975.High-resolutiontwo-dimensional electro-phoresisofproteins. J. Biol.Chem. 250:4007-4021.
31. O'Farrell, P. Z., and H. M. Goodman. 1976. Resolution of simian virus 40proteins inwhole cell extracts by two-dimen-sional electrophoresis: heterogeneity of the major capsid pro-tein. Cell 9:289-298.
32. Ponder, B. A., A. K. Robbins, and L. V. Crawford. 1977. Phosphorylation ofpolyoma and SV40 virusproteins. J. Gen. Virol. 37:75-83.
33. Randall, C. J., S. Lees, and D. M. Inglis. 1987.Papovavirus-like infection in budgerigars (Melopsittacus undulatus). Avian Pathol. 16:623-633.
34. Rihs, H.-P., R. Peters, and G. Hobom. 1991. Nuclear localiza-tionofbudgerigar fledglingdisease viruscapsidprotein VP2 is conferredby residues 308-317. FEBS Lett.291:6-8.
35. Rott, O., M. Kroger, H. Muller, and G. Hobom. 1988. The genomeofbudgerigarfledglingdiseasevirus,anavian polyoma-virus. Virology 165:74-86.
36. Schmidt, M., H.Muller,M. F.G. Schmidt,andR.Rott. 1989. Myristoylationofbudgerigar fledglingdisease viruscapsid pro-tein VP2. J. Virol. 63:429-431.
37. Streuli, C. H., andB.E.Griffin. 1987. Myristicacid iscoupled
to a structural protein of polyoma virus and SV40. Nature (London)326:619-622.
38. Tan, K. B., and F. Sokol. 1972. Structural proteins of simian virus 40: phosphoproteins. J.Virol. 10:985-994.
39. Tan, K. B.,and F.Sokol. 1973.Phosphorylationof simian virus proteins in acell-free system. J. Virol. 12:696-703.
40. Tooze, J. (ed.). 1980. DNAtumorviruses,2nded. ColdSpring HarborLaboratory, Cold Spring Harbor,N.Y.
41. Yuen, L. K. C., and R. A. Consigli. 1985. Identification and protein analysisofpolyomavirus assemblyintermediates from infectedprimary mouseembryocells.Virology 144:127-138.