Copyright © 2004, American Society for Microbiology. All Rights Reserved.
Apoptosis Induced by the Histone Deacetylase Inhibitor FR901228 in
Human T-Cell Leukemia Virus Type 1-Infected T-Cell Lines
and Primary Adult T-Cell Leukemia Cells
Naoki Mori,
1* Takehiro Matsuda,
1,2Masayuki Tadano,
1Takao Kinjo,
3Yasuaki Yamada,
4Kunihiro Tsukasaki,
5Shuichi Ikeda,
6Yoshihiro Yamasaki,
7Yuetsu Tanaka,
8Takao Ohta,
2Teruo Iwamasa,
3Masao Tomonaga,
5and Naoki Yamamoto
9Division of Molecular Virology and Oncology,1Graduate School of Medicine, Divisions of Child Health and Welfare,2Pathology
and Cell Biology,3and Immunology,8Faculty of Medicine, University of the Ryukyus, Nishihara, Okinawa 903-0215, Division
of Laboratory Medicine, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki 852-8501,4Department of
Hematology, Molecular Medicine Unit, Atomic Bomb Disease Institute, Nagasaki University School of Medicine,
Nagasaki 852-8523,5Department of Hematology, Sasebo City General Hospital, Sasebo 857-8511,6
Department of Internal Medicine, Kokura Memorial Hospital, Kitakyushu 802-8555,7and
Department of Molecular Virology, Bio-Response, Graduate School, Tokyo
Medical and Dental University, Tokyo 113-8519,9Japan
Received 21 July 2003/Accepted 28 January 2004
Inhibition of histone deacetylase (HDAC) activity induces growth arrest, differentiation, and, in certain cell types, apoptosis. FR901228, FK228, or depsipeptide, is an HDAC inhibitor effective in T-cell lymphomas. Adult T-cell leukemia (ATL) is caused by human T-cell leukemia virus type 1 (HTLV-1) and remains incurable. We examined whether FR901228 is effective for treatment of ATL by assessing its ability to induce apoptosis of HTLV-1-infected T-cell lines and primary leukemic cells from ATL patients. FR901228 induced apoptosis of Tax-expressing and -unexpressing HTLV-1-infected T-cell lines and selective apoptosis of primary ATL cells,
especially those of patients with acute ATL. FR901228 also efficiently reduced the DNA binding of NF-B and
AP-1 in HTLV-1-infected T-cell lines and primary ATL cells and down-regulated the expression of Bcl-xLand
cyclin D2, regulated by NF-B. Although the viral protein Tax is an activator of NF-B and AP-1,
FR901228-induced apoptosis was not associated with reduced expression of Tax. In vivo use of FR901228 partly inhibited the growth of tumors of HTLV-1-infected T cells transplanted subcutaneously in SCID mice. Our results
indicated that FR901228 could induce apoptosis of these cells and suppress the expression of NF-B and AP-1
and suggest that FR901228 could be therapeutically effective in ATL.
Adult T-cell leukemia (ATL) is an aggressive malignancy of mature activated CD4⫹T-cells associated with human T-cell
leukemia virus type 1 (HTLV-1) infection (18, 42, 58). It de-velops in 1 to 3% of infected individuals after more than 2 decades of viral persistence. HTLV-1-mediated T-cell trans-formation presumably arises from a multistep oncogenic pro-cess in which the virus induces chronic T-cell proliferation resulting in an accumulation of genetic defects and the dys-regulated growth of infected cells. HTLV-1 transforms primary human CD4⫹T cells via both interleukin-2 (IL-2)-dependent
and -independent manners in vitro. Although the mechanisms of transformation and leukemogenesis are not yet fully eluci-dated, several lines of evidence indicate that the viral protein Tax plays a crucial role in these processes and its expression is sufficient to immortalize primary human CD4⫹ T cells and
transform rat fibroblast cell lines in vitro (1, 57). Tax has pleiotropic effects; not only does Tax transactivate the viral promoter, but it can also activate or repress the expression or functions of a wide array of genes. For instance, Tax modulates
the gene expression of a variety of growth- and survival-related genes, such as those encoding proto-oncoproteins (c-fos, c-jun,
fra-1, and c-myc) (13, 14), cytokines (IL-2 and IL-15) (4, 49),
their receptors (the␣chain of 2 receptor [2R] and IL-15R) (5, 26, 49), G1cyclins (cyclin D2) (19), cyclin-dependent
kinase (CDK) inhibitors (p18) (51), apoptosis inhibitors (Bcl-xL) (31, 38), and proapoptotic proteins (Bax) (7). In
ad-dition, Tax directly interferes with the functions of cell cycle regulators. It inhibits the transactivating functions of the tumor suppressor p53 (36, 41) and binds to the CDK inhibitor p16 (50), cyclin D1/cyclin D3 (37), and CDK4 (17). Thus, Tax activates CDK4 and CDK6. These pleiotropic functions of Tax are thought to contribute to deregulated proliferation of HTLV-1-infected cells.
At present, there is no accepted curative therapy for ATL, and the patients progress to death with a median survival du-ration of 13 months in acute ATL (55). ATL remains of poor prognosis, mainly because of its resistance to conventional as well as high-dose chemotherapy. Therefore, the establishment of new therapeutic strategies for ATL is important. Tax is necessary for the transformation of HTLV-1-infected T-cells. Tax also activates multidrug resistance protein expression and subsequently affects the efflux of doxorubicin from nucleus to cytoplasm (45). However, the expression level of Tax in
leu-* Corresponding author. Mailing address: Division of Molecular Virology and Oncology, Graduate School of Medicine, University of the Ryukyus, 207 Uehara, Nishihara, Okinawa 903-0215, Japan. Phone: 81 (98) 895-1130. Fax: 81 (98) 895-1410. E-mail: n-mori@med.u -ryukyu.ac.jp.
4582
RETRACTED
on November 8, 2019 by guest
http://jvi.asm.org/
Downloaded from
on November 8, 2019 by guest
http://jvi.asm.org/
Downloaded from
on November 8, 2019 by guest
http://jvi.asm.org/
kemic cells of ATL patients is extremely low (i.e., the expres-sion can be detected only by reverse transcriptase-PCR) (16). Furthermore, leukemic cells from several ATL patients ex-press a mutated, truncated Tax protein, which is therefore functionally inactive (15, 39). Thus, Tax may not be essential in the maintenance of the leukemic phenotype in the last stage of leukemogenesis, indicating that Tax may not be a good thera-peutic target for ATL.
Histone deacetylase (HDAC) inhibitors are potent inducers of apoptosis and growth inhibition in a variety of transformed cells in vitro and in vivo, including malignancies originating from lymphoid cells (8, 27, 40). On the other hand, HDAC inhibitors are relatively nontoxic to normal cells, when mea-sured by viability (8), making pharmacological reagents with HDAC inhibitory activity good candidates for novel antitumor therapy. FR901228, isolated fromChromobacterium violaceum, is a member of the cyclic peptide class of HDAC inhibitors and is currently in clinical trials for evaluation of its anticancer efficacy (46). Recent results using FR901228 in patients with cutaneous T-cell lymphoma suggest significant activity in that disease (40).
In this study, we investigated the in vitro and in vivo cyto-toxic effects of FR901228 on T-cell lines infected with HTLV-1 and leukemic cells obtained from patients with ATL. The re-sults showed that FR901228 induced inhibition of proliferation and apoptotic cell death in all cell lines and primary patient leukemic cells tested, associated with decreased DNA binding of NF-B and AP-1 and decreased expression of Bcl-xLand
cyclin D2. FR901228-induced apoptosis did not result in de-creased Tax expression. These studies suggest that FR901228 might be a promising new agent in the treatment of ATL patients.
MATERIALS AND METHODS
Cell lines and human specimens.The T-cell leukemia cell line MOLT-4 and HTLV-1-infected T-cell lines MT-2 (30), MT-4 (56), SLB-1 (24), C5/MJ (43), HUT-102 (42), MT-1 (29), and ED-40515(⫺) (3) were maintained in culture with RPMI 1640, supplemented with 10% heat-inactivated fetal bovine serum (HyClone Laboratories, Inc., Logan, Utah), 50-U/ml penicillin, and 50-g/ml streptomycin (Gibco BRL, Grand Island, N.Y.) at 37°C in 5% CO2. MT-2, MT-4, SLB-1, and C5/MJ are HTLV-1-transformed T-cell lines. MT-1 and ED-40515(⫺) are T-cell lines of leukemic cell origin established from ATL patients. The clonal origin of HUT-102 is unclear. We also used leukemic cells from nine patients diagnosed with either the acute (patients 1 to 3, 5, 6, and 8) or chronic (patients 4, 7, and 9) type of ATL. The diagnosis of ATL was based on clinical features, hematological findings, and the presence of anti-HTLV-1 antibodies in the sera. Monoclonal HTLV-1 provirus integration into the DNA of leukemic cells was confirmed by Southern blot hybridization in all cases (data not shown). Peripheral blood mononuclear cells (PBMCs) from healthy volunteers and pa-tients with ATL were purified by Ficoll-Hypaque gradient centrifugation (Phar-macia LKB, Uppsala, Sweden), and washed with phosphate-buffered saline (PBS). Each patient sample contained more than 90% leukemic cells at the time of analysis. All samples were obtained after written, informed consent was received.
Growth inhibition assay.FR901228 was a gift from Fujisawa Pharmaceutical Co. (Osaka, Japan). A 5-mg/ml solution of FR901228 was prepared in ethanol and stored at⫺80°C. The effect of FR901228 on cell growth was assayed by the WST-8 method as described previously (20). The WST-8 cell counting kit was obtained from Wako Chemicals (Osaka, Japan). Briefly, 2⫻104(cell lines) or 2⫻105(PBMCs) cells were incubated in a 96-well microculture plate under the above conditions in the absence or presence of various concentrations of FR901228. After 72 h of culture, 10l of WST-8 solution was added and the cells were further incubated for another 2 h. The number of surviving cells was measured with a microplate reader (Bio-Rad, Richmond, Calif.) at a reference
wavelength of 655 nm and test wavelength of 450 nm. Cell viability was deter-mined as a percentage of that of the control (i.e., the absence of FR901228).
Cell cycle analysis.Cell cycle analysis was performed with the CycleTEST PLUS DNA reagent kit (Becton Dickinson, San Jose, Calif.). In brief, 106cells were washed with buffer solution containing sodium citrate, sucrose, and di-methyl sulfoxide, suspended in solution containing RNase A, and stained with 125-g/ml propidium iodide for 10 min. After passing the cells through a nylon mesh, cell suspensions were analyzed on a FACScalibur (Becton Dickinson) using CellQuest. The population of cells in each cell cycle phase was determined with ModFit software.
Apo2.7 immunostaining.Quantification of apoptosis was performed by immu-nostaining cells with Apo2.7, which specifically detects the 38-kDa mitochondrial membrane antigen 7A6 present only on the mitochondrial membrane of apo-ptotic cells and can be used as an early apoapo-ptotic marker in cells (48, 59). Cells cultured for 72 h with FR901228 or media were labeled with the Apo2.7-phycoerythrin-conjugated monoclonal antibody Coulter/Immuno-tech, Miami, Fla.) or mouse immunoglobulin G1 isotype control (Beckman-Coulter/Immunotech) and subsequently analyzed by flow cytometry.
EMSA.Cells were placed in culture at 1⫻106(cell lines) or 5⫻106(PBMCs) cells/ml and examined for inhibition of NF-B and AP-1 after exposure to FR901228 (5 ng/ml) for 24 h at 37°C. Nuclear proteins were extracted, and NF-B and AP-1 binding activities toB or AP-1 elements were examined by electrophoretic mobility shift assay (EMSA) as described previously (33, 34). In brief, 5g of nuclear extracts was preincubated in a binding buffer containing 1 g of poly(dI:dC) (Pharmacia, Piscataway, N.J.), followed by addition of␣-32 P-labeled oligonucleotide probes containing NF-B or AP-1 elements (approxi-mately 50,000 cpm). These mixtures were incubated for 15 min at room temper-ature. The DNA-protein complexes were separated on a 4% polyacrylamide gel and visualized by autoradiography. To examine the specificity of the NF-B or AP-1 element probe, unlabeled competitor oligonucleotides were preincubated with nuclear extracts for 15 min before incubation with probes. The probes or competitors used were prepared by annealing the following sense and antisense synthetic oligonucleotides: a typical NF-B element from the IL-2R␣chain gene (5⬘-gatcCGGCAGGGGAATCTCCCTCTC-3⬘), an NF-B mutant (5⬘-gatcCGG CAGatctATCTCCCTCTC-3⬘), an AP-1 element of the IL-8 gene (5⬘-gatcGTG ATGACTCAGGTT-3⬘), and an AP-1 mutant (5⬘ -gatcGTGATatCTCAGGTT-3⬘). Underlined sequences represent the NF-B or AP-1 binding sites, and mutations are indicated in lowercase. The oligonucleotide 5⬘-gatcTGTCGAAT GCAAATCACTAGAA-3⬘, containing the consensus sequence of the octamer binding motif (underlined), was used to identify specific binding of the transcrip-tion factor Oct-1. This transcriptranscrip-tion factor regulates transcriptranscrip-tion of a number of so-called housekeeping genes. To identify NF-B and AP-1 proteins in the DNA protein complex revealed by EMSA, we used antibodies specific for various NF-B family proteins, including p50, p65, c-Rel, and p52, and various AP-1 family proteins, including c-Fos, FosB, Fra-1, Fra-2, c-Jun, JunB, and JunD (Santa Cruz Biotechnology, Santa Cruz, Calif.), to elicit a supershift DNA pro-tein complex formation. These antibodies were incubated with the nuclear ex-tracts for 45 min at room temperature before incubation with radiolabeled probes.
Western blot analysis.Treated cells were solubilized at 4°C in lysis buffer (0.5% sodium deoxycholate, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate [SDS], 66-g/ml aprotinin, 100-g/ml phenylmethylsulfonyl fluoride, and 1 mM sodium orthovanadate). Cell lysates (50g) were resolved by electrophoresis on SDS-polyacrylamide (10 or 12%) gels and transferred to polyvinylidine difluoride membranes. After blocking of the membranes in 3% skim milk and 0.05% Tween 20 in Tris-buffered saline, the blots were incubated with the mouse monoclonal antibody to Tax, Lt-4 (52), Bcl-2 (InnoGenex, San Ramos, Calif.), XIAP (Med-ical & Biolog(Med-ical Laboratories Co., Nagoya, Japan), p53, p21, or Bax (NeoMar-kers, Fremont, Calif.), or the rabbit polyclonal antibody to cyclin D2, actin (Santa Cruz Biotechnology), or Bcl-xL(Transduction Laboratories, Lexington, Ky.). After several washes, the protein bands recognized by the antibodies were visu-alized with the enhanced chemiluminescence Western blotting detection system (Amersham, Arlington Heights, Ill.).
Plasmids and transfection.Reporter plasmidsB-LUC (kindly provided by J. Fujisawa, Kansai Medical University, Osaka, Japan) and 2⫻AP-1 site LUC (a kind gift from N. Mukaida, Kanazawa University, Kanazawa, Japan) are lucif-erase expression plasmids controlled by five tandem repeats of an NF-B binding site from the IL-2R␣chain gene and two copies of the AP-1 binding site from the IL-8 promoter, respectively. Transient transfections were performed in SLB-1 and HUT-102 cells by electroporation using 107cells and 10g of appropriate reporter plasmids. To normalize transfection efficiencies, a thymi-dine kinase (TK) promoter-drivenRenillaluciferase plasmid (pRL-TK, 1g; Promega, Madison, Wis.) was cotransfected as an internal control plasmid. Then,
RETRACTED
on November 8, 2019 by guest
http://jvi.asm.org/
16 h after transfection, FR901228 was added to the cultures at a concentration of 5 ng/ml, and the cells were further cultured for 24 h for assay of luciferase activity. Transfected cells were collected by centrifugation, washed with PBS, and lysed in reporter lysis buffer (Promega). Lysates were assayed for reporter gene activity with the dual-luciferase reporter assay system (Promega).
In vivo administration of FR901228 to SCID mice.Five-week-old female C.B-17/Icr-scid mice obtained from Ryukyu Biotec Co. (Urasoe, Japan) were maintained in containment level 2 cabinets, with all food and water autoclaved. Mice were engrafted with 107HUT-102 cells by subcutaneous injection in the postauricular region and were randomly placed into two cohorts of five mice each that received PBS and FR901228, respectively. Treatment was started on day 3 after the injection. FR901228 was dissolved in ethanol at a concentration of 5 mg/ml, and 0.5-g/g (body weight) FR901228 was injected intraperitoneally three times a week. Tumor size was monitored once a week. This experiment was performed according to the guidelines for Animal Experimentation University of the Ryukyus, and was approved by the Animal Care and Use Committee, Uni-versity of the Ryukyus.
Statistical analysis.The tumor volumes of HUT-102 (at days 12 and 19 after inoculation of HUT-102) were compared with those of the PBS-treated controls by the Mann-Whitney U test.
RESULTS
FR901228 induces apoptosis of HTLV-1-infected T-cell lines
and primary ATL cells from ATL patients.We first examined
the effects of FR901228 on proliferation and apoptosis of HTLV-1-infected T-cell lines as well as ATL cells from pa-tients. Tax protein was detected by immunoblot analysis in the five HTLV-1-infected T-cell lines (MT-2, MT-4, C5/MJ, SLB-1, and HUT-102) but not in the 2 ATL-derived T-cell lines [MT-1 and ED-40515(⫺)] and uninfected MOLT-4 cells
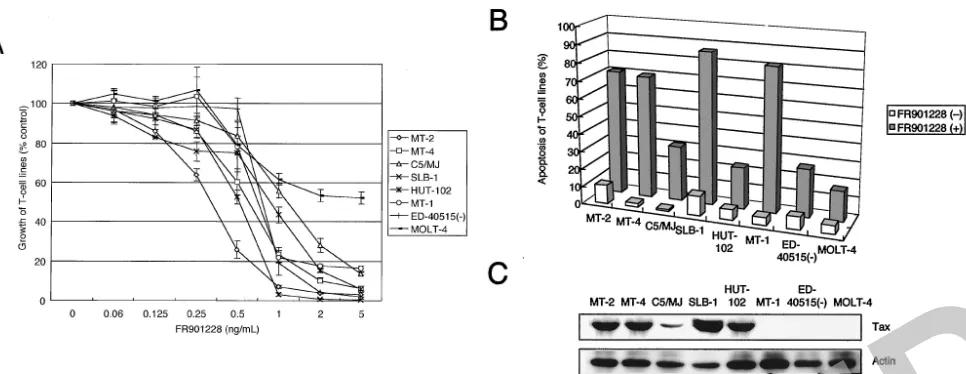
FIG. 1. FR901228 reduces cell growth and induces apoptosis of HTLV-1-infected T-cell lines. Various HTLV-1-infected T-cell lines were placed in culture at a density of 105cells/ml without or with FR901228 for 72 h. (A) Cell growth was assessed by the WST-8 method and is expressed
as a percentage of control (untreated cells) and represents the mean⫾standard deviation of three independent experiments. (B) Induction of apoptosis of HTLV-1-infected T-cell lines. A total of 106cells were labeled with phycoerythrin-conjugated Apo2.7 and analyzed by flow cytometry.
[image:3.603.58.541.68.255.2]Data represent the mean percentages of apoptotic cells from three independent experiments for both untreated (open bars) and 5-ng/ml FR901228-treated (solid bars) cells. (C) Expression of Tax protein in HTLV-1-infected T-cell lines determined by Western blotting.
FIG. 2. FR901228 reduces cell growth and induces apoptosis of primary ATL cells. ATL cells were cultured at 106cells/ml without or with
5-ng/ml FR901228 for 72 h. (A) ATL cell growth was assessed by the WST-8 method, is expressed as a percentage of that of the control (untreated cells), and represents the mean⫾standard deviation of three independent experiments. (B) Induction of apoptosis of ATL cells. Data represent the mean percentages of apoptotic cells from three independent experiments for both untreated (open bars) and FR901228-treated (5 ng/ml; solid bars) cells. A, acute type; C, chronic type.
RETRACTED
on November 8, 2019 by guest
http://jvi.asm.org/
(Fig. 1C). HTLV-1-infected T-cell lines were cultured with various concentrations (0 to 5 ng/ml) of FR901228 for 72 h. Cultivation with FR901228 suppressed the cell growth in a dose-dependent manner in seven of seven lines tested as as-sessed by the WST-8 assay (Fig. 1A). To examine whether the induction of apoptosis accounts for the cell growth inhibition observed in HTLV-1-infected T-cell lines, cells treated with FR901228 were stained by anti-7A6 antibody (Apo2.7; a mi-tochondrial membrane antigen expressed in early stage apo-ptosis) conjugated with phycoerythrin, and the stained cells were analyzed by flow cytometry (Fig. 1B). Significant apopto-sis of HTLV-1-infected T-cell lines was observed. In contrast, uninfected cell line MOLT-4 was less sensitive than HTLV-1-infected T-cell lines (Fig. 1A and B). We also evaluated the effect of FR901228 on freshly isolated ATL cells from nine patients. Tax protein was not detected by immunoblot analy-sis in all patients (data not shown). ATL cells treated with FR901228 significantly reduced cell survival, compared to nor-mal healthy controls (Fig. 2A). Apoptosis of ATL cells cul-tured for 72 h with FR901228 was also assessed by Apo2.7-phycoerythrin (Fig. 2B). Importantly, in FR901228-treated cultures we observed a greater reduction in cell survival in ATL cells from the patients with the acute type than in those from patients with the chronic type (Fig. 2A).
FR901228 induced a reduction in the number of cells in S-phase and subsequent apoptosis of HTLV-1-infected T-cells.
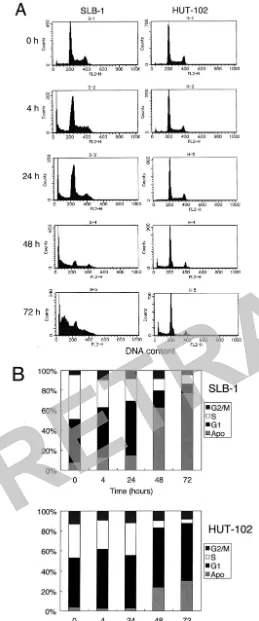
The effect of FR901228 on cell cycle progression was investi-gated in SLB-1 and HUT-102 cells. The cells were incubated with FR901228 (5 ng/ml) for various periods (0 to 72 h) and analyzed for cell cycle distribution by flow cytometry (Fig. 3). In SLB-1 cells, cultivation with FR901228 for 4 h slightly in-creased the population of the cells in the G1phase from 43 to
50%, with a marked reduction of the cells in the S phase from 45% to 28%. At 48 h after treatment, a strong induction of apoptosis was shown by the appearance of a hypodiploid DNA peak, with the proportion of apoptotic cells reaching 63% (Fig. 3). HUT-102 cells were relatively resistant to FR901228. At 48 h after treatment, HUT-102 cells in the S phase of the cell cycle markedly decreased (from 34% to 7%), with an increase in the percentage of apoptotic cells (from 4% to 24%). Al-though the proportion of apoptotic cells varied among the cell lines, FR901228 also induced a reduction in the number of cells in S phase in other HTLV-1-infected T-cell lines (data not shown). These results were consistent with the cellular prolif-eration assay performed after 72 h of treatment with FR901228, indicating that FR901228 led to reduction in the number of cells in S phase followed by apoptosis.
Expression of intracellular regulators of cell cycle and apo-ptosis in FR901228-treated HTLV-1-infected T-cell lines and
primary ATL cells from patients. To clarify the molecular
[image:4.603.33.292.69.690.2]mechanisms by which FR901228 induces inhibition of cell growth and apoptosis in HTLV-1-infected T-cell lines, we ex-amined the expression of viral Tax and several intracellular
FIG. 3. Cell cycle analysis of SLB-1 and HUT-102 cells cultured with FR901228. (A) Individual fluorescence-activated cell sorter plots. SLB-1 and HUT-102 cells were cultured in the absence or presence of
5-ng/ml FR901228 for 0, 4, 24, 48, and 72 h and then stained with propidium iodide. DNA content was analyzed by flow cytometry. (B) Quantitative analysis of the data shown in Fig. 3A. G1, S, and G2/M
indicate the cell phases. The percentage of cells in the sub-G1region
indicates significant FR901228-induced apoptosis. Apo, apoptotic cells.
RETRACTED
on November 8, 2019 by guest
http://jvi.asm.org/
regulators of cell cycle and apoptosis, including CDK inhibi-tors, cyclins, p53, Bcl-2, Bcl-xL, Bax, and XIAP, by Western
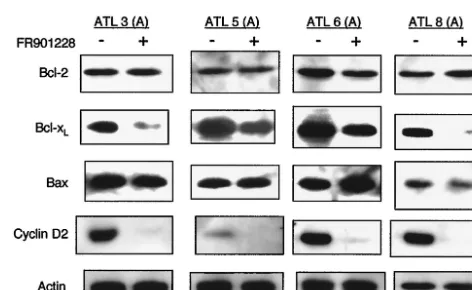
blot analysis. As shown in Fig. 4A, levels of Bcl-2, Bax, XIAP, p53, and p21 were not altered by FR901228. Interestingly, expression of cyclin D2 and Bcl-xLwas significantly decreased
after treatment with FR901228. Comparable loading of pro-tein was confirmed with a specific antibody for the housekeep-ing gene product actin (Fig. 4A). To assess the relevance of our findings in vitro to the ATL cells in vivo, primary ATL cells were treated with FR901228, and protein levels of the above
intracellular regulators of cell cycle and apoptosis were exam-ined. As shown in Fig. 5, among the proteins studied, cyclin D2 and Bcl-xLprotein expression was significantly decreased after
treatment with FR901228, as observed in HTLV-1-infected T-cell lines, suggesting that cyclin D2 and Bcl-xLare targets for
FR901228. Comparable loading of protein was confirmed with a specific antibody for the housekeeping gene product actin (Fig. 5). Because cyclin D2 and Bcl-xL are Tax-responsive
genes (19, 31, 38), we also examined the level of Tax expres-sion. Of note, increased expression of Tax protein was seen in SLB-1 cells after 4 and 24 h of exposure to FR901228. How-ever, FR901228 did not change the protein level of Tax in either Tax-positive cells (MT-2, MT-4, C5/MJ, and HUT-102) or Tax-negative cells [MT-1, and ED-40515(⫺), and primary ATL cells] (Fig. 4A and B) (data not shown). The altered expression levels of cyclin D2 and Bcl-xLprotein might,
there-fore, not result from Tax down-regulation.
Modulation of activated NF-B and AP-1 by FR901228.
Several reports have suggested that nuclear transcription fac-tors NF-B and AP-1 can act as survival factors and are re-quired for the proliferation of a variety of different tumor cell types (6, 10, 23, 28). Because NF-B and AP-1 are constitu-tively active in Tax-expressing and HTLV-1-infected T-cell lines as well as primary ATL cells (33, 34) and Tax stimulates expression of cyclin D2 and Bcl-xLthrough the NF-B pathway
(19, 31, 38), we examined whether FR901228 could inhibit the NF-B and AP-1 pathways. To study the DNA-binding activity of NF-B and AP-1, we performed EMSA with radiolabeled, double-stranded NF-B and AP-1 oligonucleotides and nu-clear extracts from untreated or FR901228-treated HTLV-1-infected T-cell lines. NF-B and AP-1 oligonucleotide probes with nuclear extracts from untreated HTLV-1-infected T-cell lines generated DNA-protein gel shift complexes irrespective of Tax expression (Fig. 6A). Previously, we showed that all of these complexes were due to specific bindings of nuclear pro-teins to the NF-B and AP-1 sequences (33, 34). We also showed that NF-B and AP-1 complexes contain p50, c-Rel,
[image:5.603.301.537.505.650.2]FIG. 4. Expression of viral Tax and the cell cycle- and apoptosis-associated proteins in HTLV-1-infected T-cell lines treated with FR901228. (A) SLB-1 and HUT-102 cells were treated with 5-ng/ml FR901228 for the indicated periods. Total cellular proteins (50g/ lane) were separated on SDS-polyacrylamide (10 or 12%) gels and transferred to the membrane. Protein levels were detected by Western blotting with antibodies directed against each protein. Comparable protein loading was verified with an antibody specific for the house-keeping gene product actin. (B) Effect of FR901228 on the expression of Tax in HTLV-1-infected T-cell lines. Cell lines were treated with 5-ng/ml FR901228 for the indicated periods, and then total cellular proteins were extracted and Western blot analysis was performed.
FIG. 5. Expression of Bcl-2, Bcl-xL, Bax, and cyclin D2 in primary
ATL cells treated with FR901228. Leukemic cells obtained from patients with ATL were treated with (⫹) or without (⫺) 5-ng/ml FR901228 for 24 h. Cell lysates (50g of protein per lane) were fractionated on SDS-polyacrylamide (10 or 12%) gels and analyzed by Western blotting with antibodies directed against each protein. Com-parable protein loading was verified with an antibody specific for the housekeeping gene product actin.
RETRACTED
on November 8, 2019 by guest
http://jvi.asm.org/
and JunD, respectively (Fig. 6C and D). As shown in Fig. 6A, nuclear extracts prepared from HTLV-1-infected T-cell lines treated with FR901228 for 24 h, exhibited a decrease in the intensity of the NF-B- and AP-1-containing gel shift com-plexes, which suggests that FR901228 down-regulates the DNA-binding activity of NF-B and AP-1. We next investi-gated whether treatment of primary ATL cells from patients with FR901228 similarly suppressed constitutive NF-B and AP-1. Treatment with FR901228 suppressed constitutive NF-B and AP-1 binding in fresh isolated ATL cells from three patients (Fig. 6B). Neither NF-B nor AP-1 binding was found in MOLT-4 and PBMC extracts from healthy volun-teer. Of note, no differences in the absence or presence of FR901228 in binding to the octamer motif on DNA were found (Fig. 6A and B). These results indicate that FR901228 sup-presses both NF-B and AP-1 binding, not only in HTLV-1-infected T-cell lines, but also in primary ATL cells.
FR901228 inhibits NF-B and AP-1 activation.Using SLB-1
and HUT-102 cells, we examined whether FR901228 inhibits NF-B and AP-1 activation functionally. Luciferase expression plasmids regulated by NF-B and AP-1 elements were trans-fected into cells, and the cells were then treated with 5-ng/ml FR901228. Activity of NF-B and AP-1 in cells was signifi-cantly inhibited by culture with FR901228 for 24 h (Fig. 7).
In vivo treatment of subcutaneous HUT-102 tumors with
FR901228.We finally examined whether FR901228 was active
against ATL in vivo by treatment with FR901228. HUT-102 was inoculated into 10 SCID mice. The mice inoculated with HUT-102 were divided into two groups: untreated mice (n⫽
5) and FR901228-treated mice (n⫽5). Treatment commenced on day 3. On day 19, the mean tumor volume of FR901228-treated mice was significantly lower than that of PBS-FR901228-treated mice (P⬍0.05 by the Mann-Whitney test) (Fig. 8).
DISCUSSION
In a preliminary study, FR901228 was reported to be effec-tive in T-cell lymphomas (40). Subsequent to these clinical observations, similar findings were also reported in laboratory models (25). Among hematopoietic malignancies, ATL is com-monly refractory to conventional chemotherapies. Our study investigated the effects of FR901228 on the ATL model. We demonstrated that HTLV-1-infected T-cell lines, including those that were derived from ATL patients, were sensitive to FR901228. FR901228 led to a reduction in the number of cells in S-phase and subsequently induced apoptotic cell death (as detected by expression of mitochondrial membrane antigen 7A6). Moreover, FR901228 induced apoptosis of primary ATL cells from nine of nine patients and had less effect on normal PBMCs.
Like other HDAC inhibitors, FR901228 has been shown to induce cell cycle arrest in both G1and G2/M phases and to
FIG. 6. Inhibition of constitutive NF-B and AP-1 activities in HTLV-1-infected T-cell lines and primary ATL cells treated with FR901228. HTLV-1-infected T-cell lines (A) and primary ATL cells (B) were treated with (⫹) or without (⫺) 5-ng/ml FR901228 and assessed for NF-B and AP-1 binding. After 24 h, nuclear proteins were extracted and EMSA was performed with NF-B-, AP-1-, or Oct-1-specific radiolabeled oligonucleotide probes. Specificity of NF-B and AP-1 binding was determined by using antibodies to the NF-B components p50, p65, c-Rel, and p52 (C) and AP-1 components c-Fos, FosB, Fra-1, Fra-2, c-Jun, JunB, and JunD (D), resulting in supershift.
RETRACTED
on November 8, 2019 by guest
http://jvi.asm.org/
induce apoptosis in several cell lines (54). FR901228 is re-ported to induce increased expression of p21 (47). However, immunoblot analysis in our study revealed that the expression of p21 protein was not affected by FR901228 in HTLV-1-infected T-cell lines. In agreement with others (2, 9, 11), we found that p21 was overexpressed in HTLV-1-infected T-cell lines. This phenomenon was observed irrespective of the func-tional status of p53 (9). Tax is responsible for the high expres-sion of p21 (9, 11). The constitutive high-level expresexpres-sion of p21 in HTLV-1-infected T-cell lines is paradoxical because these cells proliferate rapidly despite the presence of high levels of this protein. The antiproliferative effect of p21 might be directly suppressed or alternative pathways might stimulate cell cycle progression regardless of the presence of a functional p21 in HTLV-1-infected T-cell lines. In contrast, immunoblot analysis revealed that the expression of cyclin D2 or Bcl-xL
protein was inhibited by FR901228 in both HTLV-1-infected T-cell lines and primary ATL cells. Tax-mediated induction of cyclin D2 and Bcl-xLexpressions through NF-B was reported
to be associated with development of IL-2 independence and resistance to apoptosis in mouse T-cells (21, 32, 53). More importantly, FR901228 treatment did not inhibit the expres-sion of Tax. Furthermore, FR901228 induced apoptosis in Tax-negative HTLV-1-infected T-cell lines and primary ATL cells. Therefore, the growth inhibition of HTLV-1-infected T-cell lines and primary ATL cells induced by FR901228 may be mediated by a Tax-independent pathway, although we cannot exclude the possibility that FR901228 interferes with Tax func-tion. FR901228 treatment also inhibited cyclin D2 and Bcl-xL
expression in Tax-negative MT-1 and ED-40515(⫺) cells (data not shown). At least in our model, cyclin D2 and Bcl-xL
down-regulation may be responsible for FR901228-induced cell growth inhibition and cell death.
Our results also showed that FR901228 treatment decreased
the NF-B binding activity by EMSA. Recently, we reported that Bay 11-7082, an inhibitor of NF-B, induced apoptosis of HTLV-1-infected T-cell lines and primary ATL cells through down-regulation of cyclin D2 and Bcl-xL(35). These
results suggest that the decrease in the NF-B activity and the down-regulation of cyclin D2 and Bcl-xL may contribute to
FR901228-induced cell growth inhibition and apoptosis in both HTLV-1-infected T-cell lines and primary ATL cells and that blocking NF-B signaling may be associated, at least in part, with the down-regulation of cyclin D2 and Bcl-xL. We also
FIG. 7. FR901228 inhibits NF-B and AP-1 activation.B-LUC or AP-1-LUC was transfected into SLB-1 or HUT-102 cells. Then 16 h after transfection, cells were treated for 24 h with 5-ng/ml FR901228. To normalize variations, the construct containing the TK promoter-drivenRenillaluciferase (pRL-TK) was cotransfected and the activities of firefly andRenilla luciferases were measured sequentially from a single sample by means of a dual-luciferase reporter assay system. Relative luciferase activity is expressed relative to the basal level mea-sured in cells transfected with the reporter plasmid without further treatment, which was defined as 100. Data are the mean⫾standard deviation of three separate transfections.
FIG. 8. Effect of FR901228 on growth of HUT-102 cells in SCID mice. HUT-102 cells (107per mouse) were injected subcutaneously
into SCID mice. The mice (five per group) were treated with either PBS or FR901228 (0.5 g/g intraperitoneally three times a week). Treatment commenced 3 days later when subcutaneous tumors be-came palpable. The mice were monitored for tumor volumes at 5, 12, and 19 days after injection of cells. FR901228 suppressed the growth of HUT-102 cells, in contrast to the significant increase in the tumor burden generated in PBS-treated control mice. (A) Photographs of an untreated mouse (top left) and FR901228-treated mouse injected 19 days earlier with HUT-102 cells subcutaneously in the postauricular region (top right). Tumors were excised on day 19. The photographs show a representative tumor of an untreated mouse (bottom left) and that of FR901228-treated mouse (bottom right). (B) Serial changes in tumor volume in treated and untreated mice. Data are the mean⫾ standard deviation of five mice each.
RETRACTED
on November 8, 2019 by guest
http://jvi.asm.org/
found that FR901228 treatment suppressed constitutive AP-1 activity in both HTLV-1-infected T-cell lines and primary ATL cells. Because AP-1 mediates the proliferation of various tu-mor cells (12), this may also explain FR901228-induced growth inhibition and apoptosis. FR901228 has been reported to be effective in cutaneous T-cell lymphoma (40). Interestingly, cu-taneous T-cell lymphoma has been reported to constitutively express both NF-B and AP-1 (22, 44). These findings suggest that NF-B and AP-1 could be considered as a general target of FR901228 in T-cell malignancies. Further studies are nec-essary to clarify whether FR901228 inhibits NF-B and AP-1 transcription factors.
The potent and selective apoptotic effect of FR901228 against ATL patient cells and all HTLV-1-infected T-cell lines in vitro prompted us to evaluate its in vivo anti-ATL effect in SCID mice bearing an HTLV-1-infected T-cell line, HUT-102. In the subcutaneous model, HUT-102 cells did not reconstitute leukemia in peripheral blood or bone marrow. However, this system was useful for directly measuring in vivo antitumor effects. Importantly, FR901228 inhibited the growth of HUT-102 cells in our model.
In conclusion, we have evaluated the effects of FR901228 in a panel of HTLV-1-infected T-cell lines and primary ATL cells. Our results showed for the first time that FR901228 can induce a reduction in the number of cells in S phase and the subsequent apoptosis in these cells. We found that FR901228 suppressed the constitutive expression of NF-B and AP-1. The down-regulation of cyclin D2 might be responsible for the reduction in the number of cells in S phase, whereas the down-regulation of Bcl-xL might play an important role in
FR901228-induced apoptosis. Considering the two major ef-fects of FR901228 of suppressing both NF-B and AP-1 in ATL cells, we propose the use of FR901228 as a novel thera-peutic or chemopreventive agent in any new strategy for the treatment of ATL.
ACKNOWLEDGMENTS
We are deeply indebted to the many patients with ATL and the control subjects who donated blood for these studies. We thank J. Fujisawa and N. Mukaida for providing luciferase reporter constructs B-LUC and IL-8 AP-1-LUC. We are grateful to Fujisawa Pharma-ceutical Co., Ltd., for providing FR901228. We thank M. Maeda for providing ED-40515(⫺) and Fujisaki Cell Center, Hayashibara Bio-medical Laboratories (Okayama, Japan), for providing the MT-1, HUT-102, and C5/MJ cell lines. We also thank M. Yamamoto and M. Sasaki for excellent technical assistance.
This work was supported in part by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science.
REFERENCES
1. Akagi, T., H. Ono, and K. Shimotohno.1995. Characterization of T cells immortalized by Tax1 of human T-cell leukemia virus type 1. Blood86:4243– 4249.
2. Akagi, T., H. Ono, and K. Shimotohno.1996. Expression of cell-cycle regu-latory genes in HTLV-I infected T-cell lines: possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4and p21Waf1/Cip1/Sdi1. Oncogene
12:1645–1652.
3. Arima, N., M. Kamio, K. Imada, T. Hori, T. Hattori, M. Tsudo, M. Okuma, and T. Uchiyama.1992. Pseudo-high affinity interleukin 2 (IL-2) receptor lacks the third component that is essential for functional IL-2 binding and signaling. J. Exp. Med.176:1265–1272.
4.Azimi, N., K. Brown, R. N. Bamford, Y. Tagaya, U. Siebenlist, and T. A. Waldmann. 1998. Human T cell lymphotropic virus type I Tax protein trans-activates interleukin 15 gene transcription through an NF-B site. Proc. Natl. Acad. Sci. USA95:2452–2457.
5.Ballard, D. W., E. Bohnlein, J. W. Lowenthal, Y. Wano, B. R. Franza, and
W. C. Greene.1988. HTLV-I tax induces cellular proteins that activate the B element in the IL-2 receptor␣gene. Science241:1652–1655. 6.Bargou, R. C., F. Emmerich, D. Krappmann, K. Bommert, M. Y. Mapara, W.
Arnold, H. D. Royer, E. Grinstein, A. Greiner, C. Scheidereit, and B. Dorken.
1997. Constitutive nuclear factor-B-RelA activation is required for prolif-eration and survival of Hodgkin’s disease tumor cells. J. Clin. Investig.
100:2961–2969.
7.Brauweiler, A., J. E. Garrus, J. C. Reed, and J. K. Nyborg.1997. Repression ofbaxgene expression by the HTLV-I Tax protein: implications for sup-pression of apoptosis in virally infected cells. Virology231:135–140. 8.Byrd, J. C., C. Shinn, R. Ravi, C. R. Willis, J. K. Waselenko, I. W. Flinn, N. A.
Dawson, and M. R. Grever.1999. Depsipeptide (FR901228): a novel thera-peutic agent with selective, in vitro activity against human B-cell chronic lymphocytic leukemia cells. Blood94:1401–1408.
9.Cereseto, A., F. Diella, J. C. Mulloy, A. Cara, P. Michieli, R. Grassmann G. Franchini, and M. E. Klotman.1996. p53 functional impairment and high p21waf1/cip1expression in human T-cell lymphotropic/leukemia virus type I-transformed T cells. Blood88:1551–1560.
10. Colotta, F., N. Polentarutti, M. Sironi, and A. Mantovani.1992. Expression and involvement of c-fos and c-jun protooncogenes in programmed cell death induced by growth factor deprivation in lymphoid cell lines. J. Biol. Chem.267:18278–18283.
11. de La Fuente, C., F. Santiago, S. Y. Chong, L. Deng, T. Mayhood, P. Fu, D. Stein, T. Denny, F. Coffman, N. Azimi, R. Mahieux, and F. Kashanchi.2000. Overexpression of p21waf1 in human T-cell lymphotropic virus type 1-in-fected cells and its association with cyclin A/cdk2. J. Virol.74:7270–7283. 12. Dixit, V. M., R. M. Marks, V. Sarma, and E. V. Prochownik.1989. The
antimitogenic action of tumor necrosis factor is associated with increased AP-1/c-jun proto-oncogene transcription. J. Biol. Chem.264:16905–16909. 13. Duyao, M. P., D. J. Kessler, D. B. Spicer, C. Bartholomew, J. L. Cleveland,
M. Siekevitz, and G. E. Sonenshein.1992. Transactivation of the c-myc promoter by human T cell leukemia virus type 1taxis mediated by NF-B. J. Biol. Chem.267:16288–16291.
14. Fujii, M., T. Niki, T. Mori, T. Matsuda, M. Matsui, N. Nomura, and M. Seiki.1991. HTLV-1 Tax induces expression of various immediate early serum responsive genes. Oncogene6:1023–1029.
15. Furukawa, Y., R. Kubota, M. Tara, S. Izumo, and M. Osame.2001. Existence of escape mutant in HTLV-Itaxduring the development of adult T-cell leukemia. Blood97:987–993.
16. Furukawa, Y., M. Osame, R. Kubota, M. Tara, and M. Yoshida.1995. Human T-cell leukemia virus type-1 (HTLV-1) Tax is expressed at the same level in infected cells of HTLV-1-associated myelopathy or tropical spastic paraparesis patients as in asymptomatic carriers but at a lower level in adult T-cell leukemia cells. Blood85:1865–1870.
17. Haller, K., Y. Wu, E. Derow, I. Schmitt, K.-T. Jeang, and R. Grassmann.
2002. Physical interaction of human T-cell leukemia virus type 1 Tax with cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol. Cell. Biol.22:3327–3338.
18. Hinuma, Y., K. Nagata, M. Hanaoka, M. Nakai, T. Matsumoto, K. Kino-shita, S. Shirakawa, and I. Miyoshi.1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA78:6476–6480.
19. Huang, Y., K. Ohtani, R. Iwanaga, Y. Matsumura, and M. Nakamura.2001. Direct trans-activation of the human cyclin D2 gene by the oncogene product Tax of human T-cell leukemia virus type I. Oncogene20:1094–1102. 20. Ishiyama, M., M. Shiga, K. Sasamoto, M. Mizoguchi, and P. He.1993. A
new sulfonated tetrazolium salt that produces a highly water-soluble formazan dye. Chem. Pharm. Bull.41:1118–1122.
21. Iwanaga, Y., T. Tsukahara, T. Ohashi, Y. Tanaka, M. Arai, M. Nakamura, K. Ohtani, Y. Koya, M. Kannagi, N. Yamamoto, and M. Fujii.1999. Human T-cell leukemia virus type 1 Tax protein abrogates interleukin-2 dependence in a mouse T-cell line. J. Virol.73:1271–1277.
22. Izban, K. F., M. Ergin, J. Z. Qin, R. L. Martinez, R. J. Pooley, Jr., S. Saeed, and S. Alkan.2000. Constitutive expression of NF-B is a characteristic feature of mycosis fungoides: implications for apoptosis resistance and pathogenesis. Hum. Pathol.31:1482–1490.
23. Karin, M., Z. Liu, and E. Zandi.1997. AP-1 function and regulation. Curr. Opin. Cell Biol.9:240–246.
24. Koeffler, H. P., I. S. Y. Chen, and D. W. Golde.1984. Characterization of a novel HTLV-infected cell line. Blood64:482–490.
25. Koyama, Y., M. Adachi, M. Sekiya, M. Takekawa, and K. Imai.2000. His-tone deacetylase inhibitors suppress IL-2-mediated gene expression prior to induction of apoptosis. Blood96:1490–1495.
26. Mariner, J. M., V. Lantz, T. A. Waldmann, and N. Azimi.2001. Human T cell lymphotropic virus type I Tax activates IL-15R␣gene expression through an NF-B site. J. Immunol.166:2602–2609.
27. Marks, P. A., V. M. Richon, and R. A. Rifkind.2000. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J. Natl. Cancer Inst.92:1210–1216.
28. Miyamoto, S., and I. M. Verma.1995. Rel/NF-B/IB story. Adv. Cancer Res.66:255–292.
29. Miyoshi, I., I. Kubonishi, M. Sumida, S. Hiraki, T. Tsubota, I. Kimura, K.
RETRACTED
on November 8, 2019 by guest
http://jvi.asm.org/
Miyamoto, and J. Sato.1980. A novel T-cell line derived from adult T-cell leukemia. Jpn. J. Cancer Res.71:155–156.
30. Miyoshi, I., I. Kubonishi, S. Yoshimoto, T. Akagi, Y. Ohtsuki, Y. Shiraishi, K. Nagata, and Y. Hinuma.1981. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukae-mic T cells. Nature294:770–771.
31. Mori, N., M. Fujii, G. Cheng, S. Ikeda, Y. Yamasaki, Y. Yamada, M. To-monaga, and N. Yamamoto.2001. Human T-cell leukemia virus type I Tax protein induces the expression of anti-apoptotic gene Bcl-xLin human T-cells through nuclear factor-B and c-AMP responsive element binding protein pathways. Virus Genes22:279–287.
32. Mori, N., M. Fujii, M. Hinz, K. Nakayama, Y. Yamada, S. Ikeda, Y. Yama-saki, F. Kashanchi, Y. Tanaka, M. Tomonaga, and N. Yamamoto.2002. Activation of cyclin D1 and D2 promoters by human T-cell leukemia virus type 1 Tax protein is associated with IL-2-independent growth of T cells. Int. J. Cancer99:378–385.
33. Mori, N., M. Fujii, S. Ikeda, Y. Yamada, M. Tomonaga, D. W. Ballard, and N. Yamamoto.1999. Constitutive activation of NF-B in primary adult T-cell leukemia cells. Blood93:2360–2368.
34. Mori, N., M. Fujii, K. Iwai, S. Ikeda, Y. Yamasaki, T. Hata, Y. Yamada, Y. Tanaka, M. Tomonaga, and N. Yamamoto.2000. Constitutive activation of transcription factor AP-1 in primary adult T-cell leukemia cells. Blood95:
3915–3921.
35. Mori, N., Y. Yamada, S., Ikeda, Y. Yamasaki, K. Tsukasaki, Y. Tanaka, M. Tomonaga, N. Yamamoto, and M. Fujii.2002. Bay 11–7082 inhibits tran-scription factor NF-B and induces apoptosis of HTLV-I-infected T-cell lines and primary adult T-cell leukemia cells. Blood100:1828–1834. 36. Mulloy, J. C., T. Kislyakova, A. Cereseto, L. Casareto, A. LoMonico, J.
Fullen, M. V. Lorenzi, A. Cara, C. Nicot, and C.-Z. Giam, and G. Franchini.
1998. Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J. Virol.72:8852–8860.
37. Neuveut, C., K. G. Low, F. Maldarelli, I. Schmitt, F. Majone, R. Grassmann, and K.-T. Jeang.1998. Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin D-cdk and p110Rb. Mol. Cell. Biol.18:3620–3632. 38. Nicot, C., R. Mahieux, S. Takemoto, and G. Franchini.2000. Bcl-XLis up-regulated by HTLV-I and HTLV-II in vitro and in ex vivo ATLL samples. Blood96:275–281.
39. Okazaki, S., R. Moriuchi, N. Yoshizuka, K. Sugahara, T. Maeda, I. Jinnai, M. Tomonaga, S. Kamihira, and S. Katamine.2001. HTLV-1 proviruses encoding non-functional TAX in adult T-cell leukemia. Virus Genes23:123– 135.
40. Piekarz, R. L., R. Robey, V. Sandor, S. Bakke, W. H. Wilson, L. Dahmoush, D. M. Kingma, M. L. Turner, R. Altemus, and S. E. Bates.2001. Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of periph-eral and cutaneous T-cell lymphoma: a case report. Blood98:2865–2868. 41. Pise-Masison, C. A., R. Mahieux, H. Jiang, M. Ashcroft, M. Radonovich, J.
Duvall, C. Guillerm, and J. N. Brady.2000. Inactivation of p53 by human T-cell lymphotropic virus type 1 Tax requires activation of the NF-B path-way and is dependent on p53 phosphorylation. Mol. Cell. Biol.20:3377–3386. 42. Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo.1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA77:7415–7419.
43. Popovic, M., P. S. Sarin, M. Robert-Gurroff, V. S. Kalyanaraman, D. Mann, J. Minowada, and R. C. Gallo.1983. Isolation and transmission of human retrovirus (human T-cell leukemia virus). Science219:856–859.
44. Qin, J.-Z., R. Dummer, G. Burg, and U. Dobbeling.1999. Constitutive and interleukin-7/interleukin-15 stimulated DNA binding of Myc, Jun, and novel Myc-like proteins in cutaneous T-cell lymphoma cells. Blood93:260–267.
45. Sakaki, Y., K. Terashi, A. Yamaguchi, N. Kawamata, Y. Tokito, H. Mori, M. Umehara, T. Yoshiyama, H. Ohtsubo, K. Arimura, N. Arima, and C. Tei.
2002. Human T-cell lymphotropic virus type I Tax activates lung resistance-related protein expression in leukemic clones established from an adult T-cell leukemia patient. Exp. Hematol.30:340–345.
46. Sandor, V., S. Bakke, R. W. Robey, M. H. Kang, M. V. Blagosklonny, J. Bender, R. Brooks, R. L. Piekarz, E. Tucker, W. D. Figg, K. K. Chan, B. Goldspiel, A. T. Fojo, S. P. Balcerzak, and S. E. Bates.2002. Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin. Cancer Res.8:718–728. 47. Sandor, V., A. Senderowicz, S. Mertins, D. Sackett, E. Sausville, M. V.
Blagosklonny, and S. E. Bates.2000. P21-dependent G1 arrest with down-regulation of cyclin D1 and updown-regulation of cyclin E by the histone deacety-lase inhibitor FR901228. Br. J. Cancer83:817–825.
48. Seth, A., C. Zhang, N. L. Letvin, and S. F. Schlossman.1997. Detection of apoptotic cells from peripheral blood of HIV-infected individuals using a novel monoclonal antibody. AIDS11:1059–1061.
49. Siekevitz, M., M. B. Feinberg, N. Holbrook, F. Wong-Staal, and W. C. Greene.1987. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA84:5389–5393. 50. Suzuki, T., S. Kitao, H. Matsushime, and M. Yoshida.1996. HTLV-1 Tax
protein interacts with cyclin-dependent kinase inhibitor p16INK4Aand coun-teracts its inhibitory activity towards CDK4. EMBO J.15:1607–1614. 51. Suzuki, T., T. Narita, M. Uchida-Toita, and M. Yoshida.1999.
Down-regu-lation of the INK4 family of cyclin-dependent kinase inhibitors by Tax protein of HTLV-1 through two distinct mechanisms. Virology259:384–391. 52. Tanaka, Y., A. Yoshida, Y. Takayama, H. Tsujimoto, A. Tsujimoto, M. Hayami, and H. Tozawa.1990. Heterogeneity of antigen molecules recog-nized by anti-tax1monoclonal antibody Lt-4 in cell lines bearing human T cell leukemia virus type I and related retroviruses. Jpn. J. Cancer Res.
81:225–231.
53. Tsukahara, T., M. Kannagi, T. Ohashi, H. Kato, M. Arai, G. Nunez, Y. Iwa-naga, N. Yamamoto, K. Ohtani, M. Nakamura, and M. Fujii.1999. Induc-tion of Bcl-xLexpression by human T-cell leukemia virus type 1 Tax through NF-B in apoptosis-resistant T-cell transfectants with Tax. J. Virol.73:7981– 7987.
54. Ueda, H., H. Nakajima, Y. Hori, T. Goto, and M. Okuhara.1994. Action of FR901228, a novel antitumor bicyclic depsipeptide produced by Chromo-bacterium violaceumno. 968, on Ha-ras transformed NIH3T3 cells. Biosci. Biotechnol. Biochem.58:1579–1583.
55. Yamada, Y., M. Tomonaga, H. Fukuda, S. Hanada, A. Utsunomiya, M. Tara, M. Sano, S. Ikeda, K. Takatsuki, M. Kozuru, K. Araki, F. Kawano, M. Niimi, K. Tobinai, T. Hotta, M. Shimoyama et al.2001. A new G-CSF-supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma. Br. J. Haematol.113:375–382.
56. Yamamoto, N., M. Okada, Y. Koyanagi, M. Kannagi, and Y. Hinuma.1982. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science217:737–739.
57. Yamaoka, S., H. Inoue, M. Sakurai, T. Sugiyama, M. Hazama, T. Yamada, and M. Hatanaka.1996. Constitutive activation of NF-B is essential for transformation of rat fibroblasts by the human T-cell leukemia virus type I Tax protein. EMBO J.15:873–887.
58. Yoshida, M., I. Miyoshi, and Y. Hinuma.1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implica-tion in the disease. Proc. Natl. Acad. Sci. USA79:2031–2035.
59. Zhang, C., Z. Ao, A. Seth, and S. F. Schlossman.1996. A mitochondrial membrane protein defined by a novel monoclonal antibody is preferentially detected in apoptotic cells. J. Immunol.157:3980–3987.
RETRACTED
on November 8, 2019 by guest
http://jvi.asm.org/
Copyright © 2011, American Society for Microbiology. All Rights Reserved.
RETRACTION
Apoptosis Induced by the Histone Deacetylase Inhibitor FR901228 in Human
T-Cell Leukemia Virus Type 1-Infected T-Cell Lines and
Primary Adult T-Cell Leukemia Cells
Naoki Mori, Takehiro Matsuda, Masayuki Tadano, Takao Kinjo, Yasuaki Yamada,
Kunihiro Tsukasaki, Shuichi Ikeda, Yoshihiro Yamasaki, Yuetsu Tanaka,
Takao Ohta, Teruo Iwamasa, Masao Tomonaga, and Naoki Yamamoto
Division of Molecular Virology and Oncology, Graduate School of Medicine, Divisions of Child Health and Welfare, Pathology and Cell Biology, and Immunology, Faculty of Medicine, University of the Ryukyus, Nishihara, Okinawa 903-0215, Division of
Laboratory Medicine, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki 852-8501, Department of Hematology, Molecular Medicine Unit, Atomic Bomb Disease Institute, Nagasaki University School of Medicine,
Nagasaki 852-8523, Department of Hematology, Sasebo City General Hospital, Sasebo 857-8511, Department of Internal Medicine, Kokura Memorial Hospital, Kitakyushu 802-8555, and
Department of Molecular Virology, Bio-Response, Graduate School, Tokyo Medical and Dental University, Tokyo 113-8519, Japan
Volume 78, no. 9, p. 4582–4590, 2004. The publisher hereby retracts the above article due to evidence of data manipulation, a clear violation of ASM’s ethical standards.