“PREVALENCE OF HELICOBACTER PYLORI INFECTION IN PATIENTS WITH PERFORATED DUODENAL ULCER IN
COIMBATORE MEDICAL COLLEGE HOSPITAL
Partial fulfilment of the regulations
THE TAMILNADU Dr. M.G.R. MEDICAL UNIVERSITY
1
PREVALENCE OF HELICOBACTER PYLORI INFECTION IN PATIENTS WITH PERFORATED DUODENAL ULCER IN
COIMBATORE MEDICAL COLLEGE HOSPITAL
Dissertation Submitted in
fulfilment of the regulations requiredfor the award of M.S. DEGREE
In
General Surgery Branch - I
THE TAMILNADU Dr. M.G.R. MEDICAL UNIVERSITY CHENNAI - 600 032
APRIL 2015
PREVALENCE OF HELICOBACTER PYLORI INFECTION IN PATIENTS WITH PERFORATED DUODENAL ULCER IN
COIMBATORE MEDICAL COLLEGE HOSPITAL"
requiredfor the award of
2
CERTIFICATE
This is to certify that the dissertation titled “PREVALENCE OF HELICOBACTER PYLORI INFECTION IN PATIENTS WITH
PERFORATED DUODENAL ULCER IN COIMBATORE
MEDICAL COLLEGE HOSPITAL” submitted to the Tamil Nadu Dr. M.G.R Medical University, Chennai in partial fulfilment of the requirement for the award of M.S Degree Branch- I (General Surgery) is a Bonafide work done by Dr. K. THENMOZHI, post graduate student in General Surgery under my direct supervision and guidance during the period of September 2013 to September 2014.
Prof. Dr. S.Natarajan, M.S. Prof. Dr. V. Elango, M.S.
Professor of Surgery Professor and Head of the Department Dept. of General Surgery Dept. of General Surgery
Coimbatore Medical College Coimbatore Medical College
Hospital Hospital
Dr. S. Revwathy, M.D. The Dean
6
ACKNOWLEDGEMENT
I express my gratitude to Dr. S. Revwathy, M.D., D.G.O., D.N.B., Dean, Coimbatore Medical College Hospital for permitting me to use the clinical material for the study.
It gives me immense pleasure to express my deep sense of gratitude to my Unit Chief Prof. Dr. S. Natarajan, M.S., General Surgery, Department of General Surgery, Coimbatore Medical College Hospital, for his for his excellent guidance and valuable suggestions during the course of study and in preparation of this dissertation.
I am grateful to Prof. Dr. V. Elango, M.S., Professor and Head of the Department of General Surgery, Coimbatore Medical College Hospital, for his guidance throughout this study.
Ialso thank the former head of the department of surgery to Prof. Dr. P.V.Vasantha Kumar, M.S.
7
I am deeply indebted to my Assistant Professors, Dr. N. Narayana Moorthy M.S., Dr. S. Meenaa, M.S., for their help and guidance throughout this study.
I express my thanks to my friends and all others who have helped me in the preparation of this dissertation.
8
DECLARATION
I hereby declare that the dissertation entitled “PREVALENCE OF HELICOBACTER PYLORI INFECTION IN PATIENTS WITH
PERFORATED DUODENAL ULCER IN COIMBATORE
MEDICAL COLLEGE HOSPITAL”was done by me at Coimbatore Medical College Hospital Coimbatore - 641018 during the period of my post graduate study for M.S. Degree Branch-1 (General Surgery) from 2013 to 2014
This dissertation is submitted to the Tamil Nadu Dr. M.G.R. Medical University in partial fulfilment of the University regulations for award of M.S., Degree in General Surgery.
Dr. K.THENMOZHI Post Graduate Student M. S. General Surgery
9
TABLE OF CONTENTS
Sl.No TITLE Page.No
1. Introduction 1
2. Aim & objectives 3
3. Review of literature 4
4. Materials and methods 76
5. Results 83
6. Discussion 92
7. Conclusion 99
8. Summary 101
9. Bibliography 10. Annexures
i) Proforma ii) Abbreviations
iii) Key to master Chart ii) Master Chart
10
LIST OF CHARTS
Sl. No. Title Page No.
1. AGE DISTRIBUTION IN SELECTED CASES 83 2. GENDER DISTRIBUTION IN SELECTED CASES 84 3. H.PYLORI POSITIVE CASES IN ALCOHOL 85 4. H.PYLORI POSITIVE CASES IN NSAID’S 86 5. H.PYLORI POSITIVE CASES IN SMOKING 87 6. H.PYLORI POSITIVE CASES IN RU TEST 88 7. H.PYLORI POSITIVE CASES IN GIEMSA STAIN 89 8. H.PYLORI POSITIVE CASES IN CULTURE 90
9.
POSITIVE CASES OF H.PYLORI IN PERFORATED DUODENAL ULCER
11
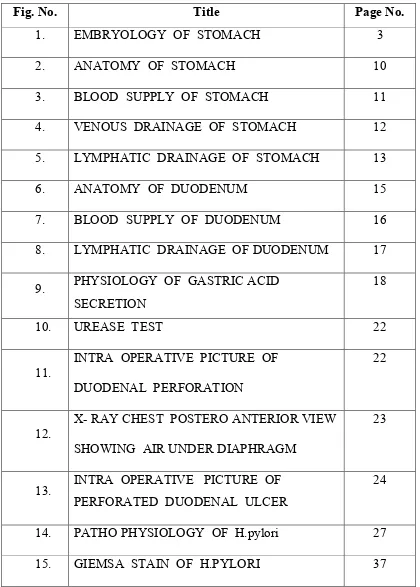
[image:11.612.121.541.111.698.2]LIST OF COLOUR PICTURES
Fig. No. Title Page No.
1. EMBRYOLOGY OF STOMACH 3
2. ANATOMY OF STOMACH 10
3. BLOOD SUPPLY OF STOMACH 11
4. VENOUS DRAINAGE OF STOMACH 12 5. LYMPHATIC DRAINAGE OF STOMACH 13
6. ANATOMY OF DUODENUM 15
7. BLOOD SUPPLY OF DUODENUM 16 8. LYMPHATIC DRAINAGE OF DUODENUM 17
9. PHYSIOLOGY OF GASTRIC ACID SECRETION
18
10. UREASE TEST 22
11.
INTRA OPERATIVE PICTURE OF DUODENAL PERFORATION
22
12.
X- RAY CHEST POSTERO ANTERIOR VIEW SHOWING AIR UNDER DIAPHRAGM
23
13. INTRA OPERATIVE PICTURE OF PERFORATED DUODENAL ULCER
24
12
INTRODUCTION
Perforative peritonitis is one of common acute abdominal condition commonly produces one of the most dramatic pictures of acute abdominal catastrophies. It occasionally confront the surgeon with some of the most difficult problem and decision making problems during the time of surgery and also during the preoperative period evaluation and supporting treatment.
Absence of air under diaphragm in 50% of cases is also one of the reasons for missing the diagnosis. In some of the cases it may confuse as whether to open the abdomen or not and by the time the decision to operate may be taken it may be too late. 52 cases of duodenal ulcer perforation were studied in this study. Majority of the patients presented with acute abdominal pain. If secondary peritonitis set , mortality of the patient increases from 10 to 40%. Due to duodenal ulcer perforation is 0 to 10%. Wittman has noted even delay of six hours prior to the treatment can increase the mortality of 10 to 30%. In this study 52 cases are evaluated for duodenal ulcer perforation biopsy for H. pylori. Recently H. pylori is the main cause for peptic ulcer disease.
13
patients with gastritis and peptic ulceration in the lancet. This flagellated organism produces urease initially referred as campylobacter pylori is now known as H. pylori. In 1994 NIH (National institute of health consensus) panel reviewed the data available on H.pylori ,mucosal colonisation, PUD. Perforation is serious and potentially fatal complication of peptic ulcer disease. The immediate simple closure of perforation remains single most effective form of treatment in emergency
14
AIM AND OBJECTIVES
The aim of the study is to assess the prevalence of H.pylori infection in patients undergoing laparotomy for repair of perforated duodenal ulcer.
OBJECTIVES:
1. To provide H.pylori eradication therapy based on biopsy report.
2. To reduce the ulcer recurrence by providing H.pylori eradication treatment.
15
REVIEW OF LITERATURE
DEVELOPMENT OF STOMACH
During embryogenesis stomach develops from the primitive foregut. At first it’s seen as a fusiform dilatation of the foregut just distal to the oesophagus. Fold of peritoneum which connects the dorsal border of stomach and posterior abdominal wall is called dorsal mesogastrium. Another fold of peritoneum which connects the ventral border of stomach and septum transversum is called ventral mesogastrium. The liver and diaphragm are formed subsequently in the substance of the septum transversum. The part of the ventral mesogastrium which lies between the liver and the stomach forms the lesser omentum. By the development of spleen dorsal mesogastrium divided into two, the part between stomach and spleen is called gastro splenic ligament and part between spleen and posterior abdominal wall is called lino-renal ligament. The stomach undergoes differential growth resulting in alteration in shape & orientation. The ventral border faces upwards and then left to form lesser curvature. The dorsal border points downwards and then left, to form greater curvature.
16
Acid and enzyme production first occur at the 4th month of foetal life and are well established at the time of birth. The new born stomach is fully developed and is similar to that of the adults
17
ANATOMY OF STOMACH
The stomach is a muscular bag. It is widest and most distensible pan of the digestive lube. It starts from the lower end of oesophagus to the duodenum Stomach occupies from the 11thoracic vertebra to first lumbar vertebra in the upper and left part of the abdomen.14 It has two surfaces (anterior and posterior), two curvatures (lesser and greater), and two orifices (cardiac and pyloric).
Anatomically it is divided into four parts.
• Cardia
Cardia is an indistinct small zone. It occupies just distal to the gastro- oesophageal junction and merges into the fundus distally. It lies between the end of the oesophagus and the body of the stomach.
• Fundus
Fundus is a part of body which rises above the cardiac end of the stomach.
• Body
18
• corpus Pylorus
Pyloric part is situated below the body and consists of pyloric antrum pyloric canal. The pyloric antrum forms the distal most part of stomach, which leads into the pyloric sphincter. In this region, helicobacter pylori is most commonly colonized.
HISTOLOGY
Stomach wall is composed of a mucosa, submucosa, muscularis, and serosa. The lining of the fundus and body has prominent folds, the gastric rugae. The mucosa consists of surface epithelium, lamina propria and muscularis mucosae.
MUCOSA
Gastric mucosa varies with the anatomic region. Surface mucus- secreting, columnar epithelium extends into numerous foveolae, or pits. These are the orifices of millions of branched, tubular glands.
There are three types of glands:
19
CARDIAC GLANDS
These are either simple tubular or tubulo-alveolar type glands confined to the cardiac region. They contain mainly mucus secreting cell
GASTRIC GLANDS
The gastric glands are the main secretory elements of the stomach. They are present in the fundus and body of the stomach. They are densely arranged perpendicular to the mucosa and enter the base of the foveola through a narrowed segment called the neck of the gland.
1. Zymogen or chief cells: These cells seen primarily in the lower half of gastric glands. They are pyramidal shaped, basophilic cells with zymogen granules. They secretes pepsinogen.
2. Parietal or oxyntic cells: These cells are seen in the upper half of the gastric gland. They are oval or pyramidal shape, eosinophilic cells. They secrete hydrochloric acid. They contain numerous mitochondria to provide energy for the ion transport needed for acid secretion. These cells also produce intrinsic factor, which is necessary for intestinal absorption of vitamin B12.
20
4. Endocrine cells: These cells are scattered in the gastric glands, mostly between the zymogen cells and the basement membrane. They are small, round, or pyramidal cells filled with granules. It contains biogenic amines such as serotonin and polypeptide hormones (e.g., gastrin and somatostatin). They are gastrin-secreting cells (G cells) and vasoactive intestinal peptide (VIP). These cells are best visualized by immunoperoxidase techniques.
5. Pyloric glands: They are branched, conspicuously coiled structures, emptying into foveolae. The glands are lined by pale cells similar in appearance to mucous neck cells and cells of Brunner glands in the duodenum. The endocrine cells include gastrin secreting G cells
LAMINA PROPRIA
The lamina propria, consists of connective tissue fibres. These give structural support to the overlying epithelium. It contains fibroblasts, histiocytes, plasma cells, lymphocytes, there are capillaries, arterioles and non-myelinated nerve fibres.
MUSCULARIS MUCOSA
21
SUBMUCOSA
It is located between the muscularis mucosa and lamina propria; it consists of loose connective tissue, autonomic nerve plexus, veins, arteries & lymphatics.
MUSCULAR LAYER
The muscularis propria has three layers outer longitudinal, inner circular and innermost oblique layer.
SEROSA
It is the outermost layer, consisting of connective tissue that covers the muscularis externa. It is lined by simple squamous mesothelium.
22
BLOOD SUPPLY OF STOMACH
CT – COELIAC TRUNK H- HEPATIC
LG- LEFT GASTRIC GD- GASTRODUODENAL
RG- RIGHT GASTRIC DP- PANCREATICODUODENAL
23
24
LYMPHATIC DRAINAGE OF STOMACH
25
DUODENUM
Develop partly from foregut and partly from midgut.
TERM
The term duodenum is a Latin corruption of the Greek word dudekudaktulos meaning twelve fingers
DEFINITION AND LOCATION
The duodenum is the shortest, widest, and most fixed part of the small intestine. It extends from the pylorus to the duodenojejunal flexure. It is curved around the head of pancreas in the form of letter C. the
duodenum lies above the level of umbilicus opposite to L1, L2, and L3.
FIRST PART
It begins at pylorus and passes backwards, upwards and to the right to meet the second part at the superior duodenoflexure.
Peritoneal relations
Proximal 2.5cms is movable. It is attached to the lesser omentum above and below to the greater omentum.
• VISCERAL RELATIONS:
• anteriorly to gall bladder, quadrate lobe of liver
• Posteriorly to portal vein, bile duct,gastroduodenal artery • Superiorly to epiploicae foramen
26
ANATOMY OF DUODENUM
Blood supply
Arterial
Superior pancreatico duodenal
inferior pancreatico duodenal right gastric
supraduodenal
27
Venous drainage Splenicvein
superior mesenteric vein
portal vein
28
Lymphatic drainage Pancraticoduodenal, Hepatic
coeliac
mesenteric nodes Nerve supply
Sympathetic- T9-T10
Parasympathetic- from vague nerve
29
30
PERFORATED DUODENAL ULCER
Incidence of perforated duodenal ulcer is 5%. The mean age is between 35-45 years. Anterior ulcer perforates commonly in 80% of cases with prior history of chronic duodenal ulcer. 20% of the duodenal ulcer is silent. Perforation is precipitated by steroids, analgesics, alcohol and antimalarial.
STAGES OF PERFORATION STAGE 1-Chemical peritonitis
STAGE 2-illusion
STAGE 3-Diffuse bacterial peritonitis
STAGE OFCHEMICAL PERITONITIS
Perforation leads to increased acid secretion which causes chemical peritonitis
STAGE OF ILLUSION
Peritoneal fluid neutralises the escaped contents that causes decreasing pain. This stage will last for six hours.
STAGE OF DIFFUSE PERITONITIS
31
SEQUENCE OF EVENTS IN PATHOPHYSIOLOGY OF DUODENAL ULCER:
1. Depletion of antral D-cells stomatostatin
2 . Increased gastrin release from G cells
3. Increased acid secretion
4. Increased acid load in duodenum leads to gastric metaplasia
5. Further inflammation and ulcer formation
H.pylori and NSAIDS are independent risk factor for ulcer formation but is not clear that the patients with NSAIDS should undergo eradication therapy.
Smoking confers an increase risk of gastric ulcer and duodenal ulcer to some extent.
NSAIDS are associated with peptic ulcer disease due to impaired mucosal defence mechanism.
32
CLINICAL FEATURES • Epigastric pain • Tenderness
• Abdominal tenderness • Guarding
• Rigidity
• Obliteration of liver dullness • Silent abdomen
INVESTIGATIONS
• Plain x-ray abdomen- shows air under diaphragm
33
UREASE TEST
34
35
36
HELICOBACTER PYLORI History
H. pylori was first discovered by Dr. Marshall and Dr. Robin Warren of Perth, Australia in 1982 in the stomachs of patients with gastritis and stomach ulcers. They concluded that most stomach ulcers and gastritis were due to this bacteria and not by spicy food or stress. For this invention, they were awarded the Nobel Prize in Physiology and Medicine in 2005.
Even before that in 1875, the German scientist discovered spiral shaped bacteria in the epithelium of human stomach. But they were not able to culture it and the results were forgotten. In l893, Giulio Bizzozero, Italian researcher described similar spiral shaped bacteria in the stomach of living dogs. Walev Jaworski, professor of the Jagiellonian University found bacteria with a characteristic spiral shape in the gastric washings from human in1899 and named that Vibrio rugula. He was the first person to explain the role of this organism in the pathogenesis of gastric diseases.
37
gastric ulcers were caused by H. pylori, and recommended antibiotics in the treatment regimen.
PATHOPHSIOLOGY OF H.pylori
In 1893 Bizzozero described that spiral bacilli colonized the mucus and glands of the stomach of healthy dogs.
38
PATHOPHSIOLOGY OF H.pylori
In 1893 Bizzozero described that spiral bacilli colonized the mucus and glands of the stomach of healthy dogs.
39
In 1906 Kremitz and Luger described 3 types of Spirochaetes in the stomach of a patient with carcinoma of lesser curvature of the stomach, one of which may have been Helicobacter pylori.
In 1938, Doenges described spiral bacteria is probably H. pylori in 43% of stomach sections taken at necropsy in a historical study of 242 cases, but was unable to correlate them with gastric disease .
In 1924, Luck and Seth described the presence of considerable urease activity in the stomach . Fitzgerald D. Murphy P studied the physiological chemistry and clinical significance of urease and urea with special reference to stomach
In 1940 Freedbeg and Barron reported similar organism in 37% gastrectomy specimen from patients particularly in association with malignant or benign ulcer
40
In 1955, an extensive review by Komberg and Davies concluded that gastric urease was located mainly in the corpus of the stomach and was of bacterial origin
In 1959, Leeberg and Lefevre suggested that urease may be bacterial in origin, no link was made between this urease activity and the presence of gastric spiral bacteria until 1984.
During 1970's the introduction of fibreoptic gastroscopy discovery of Helicobacter (formerly Campylobacter) pylori. For the first time it was possible to see the mucosa of the stomach and duodenum and take guided biopsy specimens of the gastric antrum.
In 1975, the role of gastric bacteria in the pathogenesis of Peptic ulcer disease was rekindled.
41
It 1979, Robin Warren, a pathologist in Perth, Australia, noticed curved bacteria in gastric biopsy specimens. These organisms were present in the mucous layer overlying the gastric epithelial cells.
In 1983, Warren and Marshall were the first to describe and isolate the organism and to associate it with gastritis, but even Rollinson et al in 1984 made a similar histological observation at about the same time.
Barry Marshall and Warren sought to isolate the organism from biopsy specimens. They used methods recommended for isolation of Campylobacter species.since most campylobacter grew within 48 hrs , plates without visible growth were discarded within three days. The initial cultures were negative but by chance growth was observed when one culture was incubated for five days. The organisms were characterized and originally called Campylobacter pyloridis. Soon many other reports confirmed the presence of these organisms m the gastric mucosa.
42
In 1991 reports were published showing an association between H.pylori infection and gastric carcinoma. In 1994 WHO declared that H.pyiori was a carcinogen of humans.H.pylori infection has also been associated with development of gastric nonhodgkins lymphoma and lympho-proliferative disorder,gastric mucosa associated lymphoid tissue lymphoma
Initially for the treatment of H.pylori monotherapy with bismuth preparation with a single antibiotic resulted in recrudescence of infection. A triple therapy including bismuth , metranidazole and either tetracycline or amoxicillin was used for eradication of infection. A substantial decrease in efficacy was observed due to primary resistance to metronidazole.
43
In 1995 consumer research by the American Digestive Health foundation finds that nearly 90% ulcer sufferers are unaware that H.pylori causes ulcers.
In 1996, the Food and Drug Administration approves the first antibiotic for treatment of ulcer disease.
44
NATURAL HISTORY OF H.pylori INFECTION:
High level of acid secretion acts on normal gastric mucosa
Acute H.pylori infection
Chronic H.pylori infection
Atrophic pangastritis and antral predominant gastritis
DUODENAL ULCER
EPIDEMIOLOGY:
45
infected with it at an early age are more likely to develop gastritis followed by atrophic gastritis with an increased risk of ulcer, gastric cancer or both. Infection at an older age, more likely leads to duodenal ulcer. In developing worlds the infection rate is high as compared with the developed countries. The prevalence of H.pylori infection has been steadily declining in industrialized and emerging countries. This is mainly due to good hygiene and widespread use of antibiotics. Despite declining rates of H. pylori infection in general, the prevalence rate of H. pylori in patients who undergoing endoscopy remains significant. Therefore, H.pylori should be considered in all gastric biopsy specimens examined, regardless of the patient’s age.
MODE OF TRANSMISSION
46
PHYSIOLOGY OF H.PYLORI
H.pylori does not utilises carbohydrates either fermentatively or oxidatively. They exhibit glucokinase activity associated with bacterial cell membrane enzymes for pentose phosphate pathway.
H.pylori appears to be capable of catabolising D-glucose. They also possess specific D-glucose transporters with unique characteristics. The Entner Doudoroff pathway for urease has been demonstrated in H.pylori. the urease enzyme has shown to be useful for colonisation.
Fumarate reduction is an essential component of metabolism of H.pylori and is a potential target for therapeutic intervention. H.pylori metabolises aminoacid by fermentative pathway similar to anaerobic bacteria
MICROBIOLOGY
H. pylori is a gram negative and helix-shaped bacterium. It measures about 0.5 to 1.0 micrometre in width and 2.5 to 4.0 micrometre in length. It has four to six unipolar sheathed flagellae with bulbous tips. It is microaerophilic; that requires oxygen at lower concentration than in the atmosphere.15
47
that the bacteria did not belong to the genus Campylobacter. Then it was placed into the genus, Helicobacter. This genus name is derived from the Greek ancient word. Helix means spiral or coil.
H. pylori consists of live major outer membrane protein (0MP) families. Among this, putative adhesions is the largest one. The other four families were porins, iron transporters, flagellum-associated proteins and proteins of unknown function. The outer membrane of H. pylori is made of phospholipids, cholesterol glucosides and lipopolysaccharide (LPS), The O antigen of LPS is fucosylated and it mimics Lewis blood group antigens which is found on the gastric epithelium. H. pylori has four to six lophotrichous flagella highly motile owing to flagella. The characteristic sheathed flagellar filaments of Helicobacter are composed of two copolymerized flagellins, FlaA and FlaB.
PATHOPHYSIOLOGY
Helicobacter pylori has unique features that adapt to the special gastric environment. These features allow t
mucus of the mucosal barrier, attach to the epithelium, eva responses, proliferate and colonize the bacteria.
After ingestion, H pylori must evade the bactericidal activity of the gastric lumen and enter the mucus layer. It enters the mucus by means of corkscrew like bacterial movement and enzyme producti
lipase). Gastric mucin digested by bacterial proteases enzyme facilitates its movement. Urease creates an alkali environment around the bacterium
48
GIEMSA STAIN OF H.PYLORI
PATHOPHYSIOLOGY
Helicobacter pylori has unique features that adapt to the special gastric environment. These features allow the organisms to enter the
the mucosal barrier, attach to the epithelium, eva responses, proliferate and colonize the bacteria.
After ingestion, H pylori must evade the bactericidal activity of the gastric lumen and enter the mucus layer. It enters the mucus by means of corkscrew like bacterial movement and enzyme production (urease and lipase). Gastric mucin digested by bacterial proteases enzyme facilitates its movement. Urease creates an alkali environment around the bacterium Helicobacter pylori has unique features that adapt to the special he organisms to enter the the mucosal barrier, attach to the epithelium, evade immune
49
and protects the H pylori from the luminal acid. The further outcome of infections depends on strain- specific, environmental and host related factors.
Bacterial adhesions, BabA is a 78-KD outer bacterial membrane protein. It binds to the fucosylated Lewis B blood group antigen.28 Terminal carbohydrate structure of Lewis blood group are present on the ends of MUC1 carbohydrate side chains as well as on secreted mucins.29 Major mucin produced by foveolar cell is MUC5AC, also binds with bacterial adhesins and facilitates epithelial colonization of bacteria.30 It attaches at or near intercellular junctions preferentially, moves down along the lateral cell membranes and penetrates the junctional complexes. This disruption allows the luminal contents (acid) to flow between the cells.
Strain specific Gag pathogenicity and inflammation
50
these genes facilitate translocation of the Cag A protein ‘into foveolar cells. Once in the cells, Cag A is phosphorylated and binds to the SHP-2 tyrosine phosphatase, leading to host growth factor like cellular responses, cytokine production and cell proliferation. These cytokines mobilize leukocytes to areas of immune challenge. CagA also plays a major role in disruption of the apical junctional complexes. CagA-positive strains associate with increased epithelial cell apoptosis.
The OipA (outer inflammatory protein) gene encodes one of the outer membrane proteins and is an inflammation-related gene near, but not in, the CagA PAI. OipA functional status correlates with clinical presentation, H.pylori density and gastric inflammation.36 OipA and the cag PAI are both necessary for full activation of the IL-8 promoter. Nitric oxidase synthase and cyclooxygenase-2 are induced by H.pylori infections; these enzymes modulate the inflammatory responses. Helicobacter cysteine-rich proteins (Hep), particularly llcpA (hp021 1), arc also known to trigger an immune response, causing inflammation.
51
Increases the number of parietal cells. The increased acidity damages the duodenum, which may eventually result in ulcers forming in the duodenum.
The inflammatory response recruited by H.pylori colonization can result in atrophy of stomach lining and eventually ulcers in the stomach. This also may increase the risk of stomach cancer.
Host responses to helicobacter pylori
H.pylori infections produce inflammation in the gastric mucosa (gastritis) in almost all infected persons but the severity of the changes varies among individuals. Gastritis results from both the infection and its associated inflammation. Bile reflux and dietary irritants further enhance the deleterious bacterial effects. In addition to that, anti-H.pylori antibodies that cross-react with the gastric mucosa induce further damage. Few individuals develop an autoantibody response directed to the H+ K+ ATPase pump in parietal cells leading to atrophy of the corpus.
52
significant cellular and humoral responses via antigenic stimulation of mucosal monocytes and T cells.
The inflammatory cells produce numerous cytokines TNF, interferon and interleukins 1, 6, and 8, prostaglandins, proteases, and reactive oxygen metabolites which produces epithelial necrosis and mucosal injury. IL-8, is a potent neutrophil-activating chemokine expressed by gastric epithelium and plays a central role in the inflammatory response. H. pylori bacteria containing the cag PAI causes a stronger IL-8 response than cag-negative strains. Few cytokines promote leukocyte adhesion to endothelial cells and others recruit additional leukocytes to the affected site. Local humoral response mediators, such as mucosal IgA, attract the eosinophils, which then degranulate. Induced B cells differentiate into IgM, IgA, and IgG antibody producing cells. Complement-dependent phagocytosis and H.pylori killingby polymorphonuclear neutrophils (PMNs) are promoted by IgA. Secretory IgA combines with IgG to promote antibody-dependent cell-mediated cytotoxicity, which induced by PMNs, monocytes, and lymphocytes. High level of anti- H.pylori IgG antibody is correlate with severe antral gastritis and dense H.pylori antral colonization.
involves proximally and destroys the oxyntic mucosa and results in hypochlorhydria Acid secretion increases via sev
Patients with an increased parietal cell mass and hyperchlorhydria restricts to antral gastritis. The high acid levels protect the corpus from bacterial adhesion and inflammation.
53
involves proximally and destroys the oxyntic mucosa and results in hypochlorhydria Acid secretion increases via several mechanisms. Patients with an increased parietal cell mass and hyperchlorhydria restricts to antral gastritis. The high acid levels protect the corpus from bacterial adhesion and inflammation.
55
CLINICAL FEATURES
Many infected patients are asymptomatic. Patients with H. pylon- associated gastritis may present with dyspepsia, epigastric pain, nausea, vomiting and gastrointestinal bleeding.
GENERAL HISTOPATHOLOGICAL CHANGES IN STOMACH INFECTION WITH HELICOBACTER PYLORI:
NEUTROPHIL INFILTRATION:
56
MONONUCLEAR INFILTRATION
Antral mucosa contains only few scattered mononuclear inflammatory such as lymphocytes and plasma cells were seen normally. But normal corpus mucosa contains virtually none. Gastritis is diagnosed when there are at least several clusters of five or more mononuclear cells present in the lamina propria or the infiltrates is diffuse. Presence of intraepithelial lymphocytes are diagnostic of gastritis. Infiltration of the mucosa with lymphocytes, plasma cells, and a variable number of eosinophils and mast cells is characteristic of chronic H. pylori gastritis. Autoimmune gastritis preferentially involves the fundus and corpus andainly consists of diffuse mononuclear infiltrate that extends into the deep portion of mucosa. The predominant cell being plasma cells and lymphocytes.
LYMPHOID FOLLICLES AND AGGREGATES
57
the endoscopic appearance of nodularity and often referred to as follicular gastritis.
EOSINOPHIL INFILTRATION
Prominent eosinophilic infiltration is usually seen in eosinophilic gastritis. Mild eosinophil infiltration often amidst with other inflammatory cells is seen in adult with H .pylori gastritis. But when compared with adult children have greater eosinophilic infiltration.
MUCOSAL EDEMA AND HYPEREMIA
Mucosal hyperaemia (congestion) and edema are often associated with chemical injury. However, congestion and edema also may be prominent features in H. pylori gastritis, which may be caused by an increase in the number of mast cells.
SURFACE EPITHELIAL DEGENERATION
Surface epithelium degeneration is a nonspecific response to injury seen in all forms of gastritis. It is most conspicuous in two forms of gastritis chemical gastritis and H. pylori gastritis.
58
of “buds” of cells at the surface of the mucosa, which is made up of multiple cells at the surface.
SURFACE EROSIONS
Epithelial injury and necrosis lead to the development of surface erosions. Endoscopically flat surface erosions are associated with chemical gastritis, elevated surface erosions are often caused by H.pylori gastritis. The erosion usually filled with fibrinoid necrosis containing neutrophils and cellular debris and surrounding margin show hyperplastic and regenerative changes of the epithelium.
FOVEOLAR HYPERPLASIA
59
INTESTINAL METAPLASIA
Intestinal metaplasia is defined as the replacement of gastric mucinous epithelial cells with small intestinal (goblet, enterocytes, etc.) cells. H. pylori usually does not adhere to intestinal-type epithelium and because the organism usually disappears in mucosa with extensive intestinal metaplasia and atrophy. Hence, intestinal metaplasia represents a host defense against H. pylori infection. It has been implicated in the development of both gastric and esophageal carcinoma. Usually it starts at junction between the antrum and corpus in a patchy distribution and multifocal fashion. Then it spreads both proximal and distal to involve the entire antrum and fundus of the stomach.
Goblet cells are easily seen on H&E-stained sections. However, an Alcian blue/PAS stain is commonly used to identify the goblet cells since it stains all acidic mucins blue-purple and neutral mucin magenta and is easy to perform and interpret.
ATROPHY
60
If the injured glands fail to undergo regeneration, thelamina propria will be replaced by extracellular matrix and fibroblast which leads to loss of function of the gastric epithelium and known as atrophy. The adjacent gastric glands will undergo pyloric metaplasia or intestinal metaplasia. Pyloric metaplasia is named because metaplastic glands resemble normal pyloric glands. Intestinal metaplasia is named because the glands have absorptive intestinal epithelial lining with goblet cells. In autoimmune gastritis immune mediated destruction occurs in the fundic gland epithelium which result in atrophy.
HELICOBACTER PYLORI RELATED DISEASES
The following gastric diseases are associated with H pylori infection. • Acute gastritis
• Chronic gastritis
61
• Intestinal metaplasia • G-cell hyperplasia • Gastric adenocarcinoma • Gastric Lymphoma • Menetrier disease.
H.PYLORI RELATED GASTRITIS
H.pylori bacteria especially colonize the antrum, but they may infect any part of the stomach where they cause gastritis. When treated, the bacteria migrate from the antrum to the corpus with decreasing activity of the antral gastritis. Infection of VacA-positive H.pylori strains result in acute gastritis.
Histopathological changes in acute gastritis
1. Cytoplasmic swelling 2. Vacuolization
3. Micro papillary changes 4. Mucin loss
5. Juxtaliminal cytoplasmic erosions 6. Desquamation of surface foveolar cells
62
The mucosa is elevated and expanded slightly due to the lymphoplasmacytic cell infiltrate in the superficial lamina propria. This is termed as chronic active gastritis or active chronic gastritis. Eosinophils may also be present.
Characteristic feature of regenerative pit bases arc:
• Loss of mucin
• Basophilic cytoplasm
• Increased mitotic activity and
• Nuclear hyperchromasia
63
and metabolically active. It is a morphologic indicators of genomic damage and repair.
H.pylori eradication causes rapid neutrophil disappearance. Eosinophils disappear more slowly. The surface changes reverse rapidly and the epithelial cells acquire their normal shape and spatial organization within a few days of H.pylori eradication. But atrophy that had developed remains, as do the lymphoid aggregates. These features become a permanent component of the once-infected gastric mucosa.
Quiescent superficial gastritis
In this acute inflammation, vascular congestion and edema disappear and the epithelium returns to normal. Only the lamina propria contains increased numbers of mononuclear cells.
Chronic superficial gastritis
Chronic superficial gastritis which progresses to the next stage, chronic atrophic gastritis. It takes about a period of 15 to 20 years. Chronic gastritis develops as a patchy process, but all stages of chronic gastritis often coexist in a single stomach which leads to multifocal atrophic gastritis.
64
mucosae. Lymphoid follicles with or without follicular centres is termed follicular gastritis. It represents an immune response to the bacterium. Antral lymphoid follicles can become quite prominent, sometimes causing mucosal nodularity, especially in children. Their presence provides a useful marker for H.pylori infections. Their number may decrease when the H.pylori infections are treated.
Granulomatous gastritis
The granulomas develop late in the disease, after the host becomes sensitized to the organisms. It occurs in 1% of H.pylori infected patients, usually in persons with small numbers of the organisms. Antibody-coated bacteria ingested by macrophages may stimulate a histiocytes response.
Diffuse antral gastritis (DAG)
65
Occasionally H.pylori infections lead to the development of enlarged gastric folds in the gastric body, creating an endoscopic pattern suggestive of a hypertrophic gastritis/ gastropathy. The H.pylori induced mucosal fold thickening is termed giant fold gastritis. This differs from Menetrier disease in that the mucosa is thinner in giant fold gastritis and there is less foveolar hyperplasia.
EXTRA DIGESTIVE DISORDERS ASSOCIATED WITH H.PYLORI
Ischaemic heart disease
Ischaemic cerebrovascular disease including carotid artery stenosis
Functional vascular disorders- Raynaud’s phenomenon
Immunological disease
- Henoch schonlein purpura
- Sjogren syndrome
- Autoimmune thrombocytopenic purpura
- Extra gastric malt lymphoma
- Siderophenic anaemia
- Hepatobiliary disease Cirrhosis
66
SYDNEY SYSTEM
In view of avoiding the diagnostic confusion in gastritis, in 1990 a workshop was conducted in World Congress of Gastroenterology in Sydney to establish guidelines for a new classification of gastritis based on a grading system called the "Sydney System". In 1994 it was modified at the workshopheld at the Houston, its importance lies to provide a universal language among the pathologist to give diagnosis. Sydney system has evolved a new classification of gastritis which is simple, comprehensive and easy to apply.
Sydney system consists of two divisions:
1. Histological
2. Endoscopic
The backbone of Histological division is topographical distribution of abnormalities of gastric antrum alone or gastric corpus alone or both pan gastritis.
UPDATED SYDNEY SYSTEM
67
and the findings summarized. This system classifies gastritis into three broad categories on the basis of topography, morphology and aetiology.
1. Acute
2. Chronic
3. Special (distinctive)
The morphologic component includes the five histologic features.
1. Chronic inflammation
2. Neutrophil activity
3. Glandular atrophy
4. Intestinal metaplasia and
5.H.pylori intensity.
Each of these histologic features were graded on mild, moderate and severe scale (None - 0, mild - 1, moderate - 2, severe - 3). Minor histopathological features were not graded, but simply assessed in case of their presence or absence.
68
Chronic inflammation: Increased mononuclear infiltrates such as lymphocytes and plasma cells in the lamina propria of the stomach.
Neutrophil activity: Neutrophil infiltration in the lamina propria, pits and surface epithelium. It is graded into mild, moderate and severe.
• Mild - infiltration in less than one third of pits and surface • Moderate - one third to two thirds.
• Severe - more than two thirds of pits and surface
Atrophy: Loss of specialized glands from either antrum or corpus. Graded as absent, mild, moderate and severe according to the visual analog scale.
Intestinal metaplasia: Replacement of gastric type epithelium by intestinal type epithelium. In Incomplete intestinal metaplasia the gastric glands shows the presence of colonic mucosa and goblet cells. Complete intestinal metaplasia which resembles small intestine and they shows presence of absorptive brush border cells and also Paneth and goblet cells. According to the visual analog scale it is graded into absent, mild moderate and severe.
69
• Mild - scattered organisms seen in less than one third of the surface.
• Moderate - organisms covers one third to two third of the surface. • Severe - more than two third of the surface and also seen in clumps
are aggregates.
NON ULCER DYSPEPSIA
Non ulcer dyspepsia is the most common gastrointestinal problem Encountered by clinician. Non ulcer dyspepsia is defined as complex Symptoms in the upper gastrointestinal tract which present for 4 weeks without any biochemical and structural alteration. Diagnostic criteria were first established by Rome consensus committee in 1991 and later it was updated in 1998 in Vienna. It usually present with multifactorial aetiology, thereby different mechanisms result in different subtypes of disease. Subgrouping is based on the predominant symptom type - ulcer like, reflux like and dysmotility like.
70
According to Rosen stock S et al (1997), studies symptoms of non-ulcer dyspepsia such as bloating and belching give a clue clinically for the presence of H.pylori in the gastric mucosa.
Tally NJ (1999), mention that 40-90 percent of the patient with non-ulcer dyspepsia bear H.pylori infection.
Varasa TA et al (2008) observed that early detection and eradication therapy of H.pylori gives more benefit for those patient with non-ulcer dyspepsia when compared to symptomatic treatment. He also found in his study that among 77 non ulcer dyspeptic patients, 50 had H pylori infection(64.9%).
Gwee KA et al (2009), showed that when compared studies conducted in western population .Indian studies states that Asian group of people have greater relief from non-ulcer dyspepsia symptoms after complete eradication of H.pylori.
71
PEPTIC ULCER DISEASE
H.pylori is the major cause of peptic ulcer and it usually occurs in middle age to older person. It has been detected in the stomach of more than 90% of gastric ulcer patients. Normally peptic ulcer may heal without any active intervention therapy but in presence of H.pylori organism the recurrence of peptic ulcer is very common. So active Eradication of H.pylori in these patients facilitates the cure of peptic ulcer and essentially prevents its recurrence. The epidemiology of gastric ulcer is immediately related to that of H.pylori infection, gastritis and gastric cancer. Studies from various literature in the world shows that prevalence of gastritis is equal to that of H.pylori prevalence.
Kolts BE et al (1993) states that by complete eradication of H.pylori from gastric mucosa helps in curing of peptic ulcer disease and thereby decrease overall prevalence of H.pylori induced peptic ulcer disease.
72
neutrophils,eosinophils,plasma cells which results in severe mucosal inflammation and all this occurs due to H.pylori bacterial products such as urease, ammonia, Vac Aproteins and other inflammatory mediators IL-8.
H.pylori produces various enzymes to safeguard itself and also produces injury to the gastric mucosa. The enzymes are urease which converts the gastric acidic medium into alkaline medium and thereby make suitable environment for its survival. Other enzymes includes protease which breaks down glycoprotein in the gastric mucus and it also secretes phospholipase which produce surface epithelial cell injury. In addition H.pylori also release enzyme lipopolysaccharide which recruit inflammatory cells into the gastric mucosa and this inflamed mucosa is more prone for acidic injury. H.pylori attracted neutrophils secretes myeloperoxides which produces hypochlorous acid and it is converted into monochloramine which cause severe damage to gastric epithelial cells and lamina propria endothelial cells.
73
layer, middle inflammatory layer predominantly composed of neutrophils and deeper layer shows active granulation tissue which rest on collagenous scar.
74
The preneoplastic condition in the development of adenocarcinoma of stomach is Gastric epithelial dysplasia (GED).
The progression of non-neoplastic lesion to carcinoma of stomach involves various steps that includes glandular atrophy, intestinal metaplasia, Dysplasia to adenocarcinoma. H.pylori plays a vital role in early stage of development of carcinoma. H.pylori associated chronic gastritis leads to severe injury to gastric mucosa which result in increased turnover of epithelial cell to compensate the epithelial degeneration. This compensatory mechanism leads to incomplete intestinal metaplasia due to DNA instability which results in subsequent development of intestinal type of adenocarcinoma.
Hence eradication of H.pylori plays an important role in various aspects to prevent the development of carcinoma .It prevents the development of intestinal metaplasia and atrophy and thereby stops the progression of disease into carcinoma and it also helps in suppression of DNA damage and subsequent proliferation of gastric epithelium.
Patients with younger age group having H.pylori infection were at increased risk for developing carcinoma of stomach compared to older ones.
75
H.pylori is designated as class I carcinogen by World health organisation. This is because it produces lifelong proinflammatory response and thereby results in continuous production of free radicals which causes damage to the DNA and produces multiple mutations required for the gastric cancer development.
Gastric lymphoma
H.pylori infection is strongly associated with gastric lymphoma and thereby involved in the pathogenesis of low grade lymphoma of Mucosa associated lymphoid tissue (MALT).
Genta et al (1993) reported in young patient about the closest link between the H.pylori infection and gastric lymphoid follicles or ‘nodular gastritis’. Histologically these nodules were composed of lymphoid follicles in the lamina propria.
76
DIAGNOSIS
The presence of H.pylori can be detected by several different methods, which can be classified as,
1. Non-invasive tests:
a. Serology
b. Urea breath tests
2. Invasive tests:
a. Histology.
b. culture.
c. Rapid urease test. (RUT)
d. Cytology.
e. polymerase chain reaction (PCR).
77
NON-INVASIVE TESTS a. Serology:
H.pylori infection elicits a local mucosal and a systemic antibody response. Circulating IgG antibodies to H.pylori can be detected by complement fixation test, enzyme linked immunosorbent assay (ELISA), immunoblot, and antibody or latex agglutination tests. These tests are simple, reproducible, and inexpensive, can be done on stored samples, and have been used widely in epidemiological studies. The accuracy of serological tests depends on the antigens used in the test making it essential that H.pylori- ELISA is locally validated. False negative results may be seen in elderly persons, with lifelong infection and underlying atrophic gastritis and in those on NSAID drugs. Antibody titres fall slowly after successful eradication of H.pylori, so serology cannot be used to determine H.pylori eradication and to measure reinfection rates.
78
b. 13C or 14C Urea breath tests:
Bacterial urease test is also the basis for these tests. The subject drinks a solution containing 13C or 14C urea along with a liquid fat meal to delay gastric emptying. If H.pylori are present in the stomach, urea is hydrolysed and labelled C02 appears in the breath, which is detected by beta-counter. These tests are non-invasive and highly sensitive for the diagnosis H.pylori as the whole stomach is sampled.
INVASIVE TESTS a) Histology
Histopathology examination is now deemed gold standard and more sensitive test for detection of H.pylori. The factors which alert the pathologist to look for this tiny pathogen include the acute inflammatory response with lymphoid proliferation, villiform transformation, regenerative changes and metaplasia.
79
inflammatory debris and polymorphonuclear cells. To further confirm the presence of these bacilli, various special stains are used such as Giemsa, Modified Giemsa, Toluidine blue, Warthin Starry stain, Gimenenz, Cresol violet, Diff - Quick, Genta and Silver based “triple stain”.
Silver based triple stain: It includes alcian blue, H & E, and Steiner silver stain. Its advantage is intestinal metaplasia can be detected due to alcian blue application at acidic ph.
In Modified Giemsa, these bacteria are seen as dark blue comma shaped structure in a pale blue background. This special stain could be counter stained with other dyes to enhance the visualization.
Toluidine blue stain is simple and cheap and the organism appear as dark purple to blue colour against a variable pale purple background.
80
Those conditions are
• Coccoid indolent forms,
• Deeper location with in cytoplasm and in gland,
• Severe inflammation and dirty necrotic debris which obscure the bacilli.
Other more specific techniques includes fluorescence and in situ hybridization using a biotinylated base pair PCR product of H.pylori as the probe can be used for the detection of very scanty organisms persisting even after eradication therapy
Immunohistochemistry - for bacterial detection have been available since 1988, and is reported as more reliable for the semi- quantitative diagnoses of H.pylori infection and for the detection of indolent forms of these bacilli9. The staining is such that, the organism stands out well, with well elaborated tissue morphology.
Helicobacter pylori Antibody
81
epitope. After labelling the antigen with a primary antibody at a dilution of 200 mcg / ml for 20 minutes at room temperature; a universal affinity purified secondary antibody is added to bind with the primary antibody forming a primary antibody - secondary antibody complex. An enzyme label is then added to bind to the secondary antibody. This detection of bound antibody is seen by a calorimetric reaction. ]
H.pylori is spiral - curved, gram - negative bacteria that are present on the surface epithelium of mucous layer of stomach. There is evidence that these bacteria play an important role in development of peptic ulcer disease. Immunohistochemistry can distinguish H.pylori from other types of curved bacteria. These small spiral organisms can be seen clearly using high power as clusters and in oil immersion (lOOx) clear morphology is noted and thus confirms the diagnosis. Both Monoclonal and Polyclonal antibodies are in existence now, with a slight variation in sensitivity / specificity. Monoclonal antibodies seem to be better than the polyclonal antibody as described by a study.
b) Culture
82
contamination makes it least sensitive method of detection. It takes several days and results are dependent on the expertise of the operator and collection methods. It is relatively expensive and difficult. However, viable bacteria are detected and antibiotic sensitivities can be obtained especially in this era of multiresistant strains.
c) Rapid urease test
Rapid urease test is otherwise known as CLO test (Campylobacter-like organism test). This is a simple biochemical test, which involves placing an endoscopic biopsy into a small amount of solution containing urea, a pH indicator (phenol red) and a bacteriostatic agent. If H.pylori are present, the bacterial urease hydrolyses the urea and produces ammonia. Alkalinisation of the medium produces a colour change from yellow to red. Results are read between lmin and 30 minutes. Thus it indicates only the presence or absence of infection. It is considered as rapid test for the diagnosis of H.pylori organism.
H.pylori and NSAIDS are independent risk factor for ulcer formation but is not clear that the patients with NSAIDS should undergo eradication therapy.
83
NSAIDS are associated with peptic ulcer disease due to impaired mucosal defence mechanism.
H.pylori can cause PANGASTRITIS leading to gastric atrophy and hypochlorhydria which leads to bacterial proliferation in stomach. Predominant gastritis is associated with duodenal ulcer formation
A) TESTS FOR DETECTION OF H.pylori INFECTION:
S.No INVASIVE
TESTS(BIOPSY)
SENSITIVITY SPECIFICITY
1 Rapid urease 80-95% 95-100%
2 Histology 80-90% >95%
3 Culture - -
. B).
S.No NON INVASIVE
TESTS(BIOPSY)
SENSITIVITY SPECIFICITY
1 Serology >80% >90%
2 Urease breath test >90% >90%
84
TREATMENT
Triple therapy
Triple therapy with proton pump inhibitors with amoxicillin plus clarithromycin or metronidazole is the first line therapy in H.pylori infection. The principle of triple therapy is to attack H.pylori with both luminal active and systematically active agents in an effort to overcome the limitations of each. Resistance to medications such as metronidazole may be prevented by the addition of a second anti-microbial drug.
Regimens containing tetracycline appear to be more effective than triple therapy using amoxicillin. Clarithromycin may be substituted for metronidazole in patients suspected of harbouring a resistant organism.
Proton pump inhibitor is frequently added to this regimen for the first 4-6 weeks in patients with acute gastric or duodenal ulceration to speed symptomatic recovery.
Dual therapy
85
The mechanism whereby acid inhibition enhances the bactericidal Activity of antibiotics directed against H.pylori is not completely understood.
Acid labile antibiotics may function more effectively in an environment
Rendered pH neutral by omeprazole. In addition, the use of a proton pump Inhibitor may prevent the denaturation of H.pylori specific immunoglobin by Gastric acid thus augmenting host defence mechanisms.
86
MATERIALS AND METHODS
1. Gastric mucosal biopsies among patients with perforated duodenal ulcer were obtained during laparotomy by passing a biopsy forceps through the perforation site.
2. Biopsy specimens were put in a urea broth for rapid urease test
3. 10% formalin for histopathology
STUDY PLACE
This study was done in the Department of General surgery in conjunction With Pathology Department, at Coimbatore medical college hospital. Coimbatore.
STUDY DESIGN
Prospective study,non randomised.
STUDY PERIOD
From Sep 2013 to Sep 2014. ,
87
INCLUSION CRITERIA
Demographic data
History dyspepsia for more than 3 months
Smoking
Use of NSAID
EXCLUSION CRITERIA
Age younger than 16
Age older than 75
Recent intake of antibiotics
H2 antagonists or proton pump inhibitor within 4 weeks before admission Sealed off perforation
Previous gastrectomy or vagotomy or patients with perforated gastric ulcer
Patients who underwent immediate definitive surgery for duodenal perforation
DATA COLLECTION
88
METHODOLOGY
Samples were sent to pathology department with records and registered. The specimens were processed routinely. Four microns sections were taken from both the tissues and stained with haematoxylin and eosin. The histopathological changes were analysed such as inflammation, glandular atrophy, neutrophil activity, intestinal metaplasia and H.pylori status. These histologic changes were graded according to the Sydney system.
Special stains like Giemsa and Toluidine blue were done subsequently to detect the organism and intensity was graded as per the Sydney system.
Immunohistochemistry was done using rabbit polyclonal anti-H.pylori antibody from Biogenex. The organism was easily identified by brown colour against blue background and the intensity was graded. 1HC positive cases were considered as H.pylori positive. All the findings were recorded.
89
Haematoxylin and Eosin Stain Procedure 1. Deparaffinize the section
2. Hydrate through graded alcohols to water.
3. If necessary remove formalin pigments.
4. Stain in Haematoxylin for 15 minutes.
5. Wash in tap water for five minutes.
6. Differentiate in 1% Acid Alcohol (70 percent alcohol in 1% Hcl) for 5-10 sec.
7. Tap water wash until the section turns blue 5 – l0 minutes.
8. Counter stain with aqueous eosin for 2 minutes.
9. Dehydrate with absolute alcohol (2-3changes).
10. Clear and mount with DPX (Dibutyl phthalate polystyrene xylene).
RESULTS
• Nuclei — Blue • Cytoplasm — Pink
• Red Cells — Red / Orange. • Fibrin - Deep pink
90
GIEMSA STAINING
Purpose: To demonstrate the H.pylori organisms -
PREPARATION OF SOLUTION
• Giemsa stock solution (commercially available) • Giemsa stain powder - 4gm
• Glycerol solution - 250 ml • Pure methanol — 250 ml
• The Giemsa powder is dissolved in glycerol at 60° c and shaked regularly. Methanol is added and mixed well. This solution is allowed to stand for 7 days and filtered just before use for better staining.
Working Solution
Working Giemsa solution is prepared while doing the procedure.
Take 4ml of Giemsa stock solution and add 96ml of acetate buffered distilled water (pH 6.8). Mix well.
PROCEDURE
1. Deparaffinize the section
2. Hydrate through graded alcohols and brings section to water.
91
4. Stain with working Giemsa solution - overnight.
5. Rinse in distilled water.
6. Dip in 0.5% aqueous acetic acid till the section is pink
7. Wash in lap water.
8. Blot until almost dry.
9. Dehydrate the section in graded alcohols
10. Clear and mount.
RESULTS:
• Organisms - Blue.
• Background - Pink to blue • Nuclei - Blue.
TOLUIDINE BLUE STAIN
SOLUTIONS
Toluidine blue in pH 6.8 phosphate buffer Sorensen’s phosphate buffer pH 6.8 20 min
92
METHOD 1. Deparaffinize.
2. Hydrate through graded alcohols to distilled water
3. Stain in buffered toluidine solution – 20min
4. Wash well with distilled water
5. Dehydrate in alcohol
6. Clear and mount
RESULTS
• Helicobacter pylori – dark blue
RESULTS AND OBSERVATIONS
1. AGE DISTRIBUTION IN SELECTED CASES
Frequency
Valid
Upto 25 Yrs 26 to 35 Yrs 36 to 45 Yrs 46 to 55 yrs Above 55 yrs
Total
Total of 52 cases were studied. The age of the patientns ranges from 25 – 55 years the mean age of 45 years. The maximum third and fourth decade.In this study
years. 0 2 4 6 8 10 12 14 16
Upto 25 yrs 9
93
RESULTS AND OBSERVATIONS
AGE DISTRIBUTION IN SELECTED CASES
Frequency Percent Valid
Percent
Upto 25 Yrs 9 17.3
26 to 35 Yrs 10 19.2 36 to 45 Yrs 12 23.1 46 to 55 yrs 15 28.8 Above 55 yrs 6 11.5
Total 52 100.00
Total of 52 cases were studied. The age of the patientns ranges 55 years the mean age of 45 years. The maximum third and fourth decade.In this study commonest age group affected are 45 to 55
26 to 35 yrs 36 to 45 yrs 46 to 55 yrs Above 55 yrs 10 12 15
AGE DISRTIBUTION
Cumulative Percent 17.3 36.5 59.6 88.5 100.0Total of 52 cases were studied. The age of the patientns ranges 55 years the mean age of 45 years. The maximum third and ted are 45 to 55
2. GENDER DISTRIBUTION IN SELECTED CASES
Valid Female Male Total
Total of 52 cas
peak number of cases were in the third and fourth with male predominance of 98.1%.
94
DISTRIBUTION IN SELECTED CASES
Frequency Percent Valid Percent
Female 1 1.9
Male 51 98.1
Total 52 100.00
ses were studied age distribution among H.pylori the peak number of cases were in the third and fourth with male predominance of 98.1%.
Female, 1
Male, 51
GENDER DISTRIBUTION
Cumulative Percent
1.9 100.00
3. H.PYLORI POSITIVE CASES IN ALCOHOLICS
Frequency Valid No
Yes Total
Total of 52 cases were studied age distribution among H.pylori the peak number of cases were in the third and fourth
predominance of 75%
39cases. Non- alcoholic patients are 25%. 0 5 10 15 20 25 30 35 40 45 95
3. H.PYLORI POSITIVE CASES IN ALCOHOLICS
Frequency Percent Valid Percent
13 25.0 25.0
39 75.0 75.0
52 100.0 100.0
Total of 52 cases were studied age distribution among H.pylori the peak number of cases were in the third and fourth
predominance of 75% in this study positive alcohol intake is about alcoholic patients are 25%.
13 39 NO YES