Arginine Methylation Increases the Stability of Human Immunodeficiency Virus Type 1 Tat
Full text
Figure
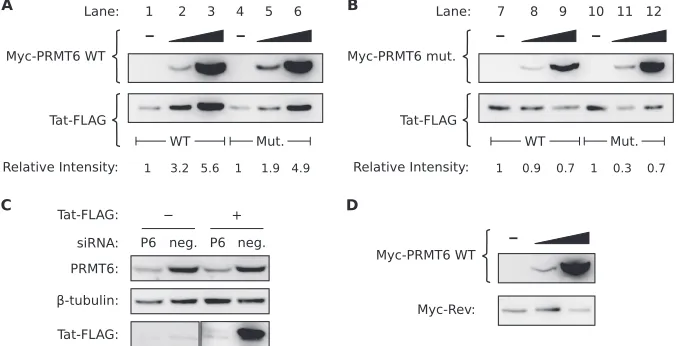
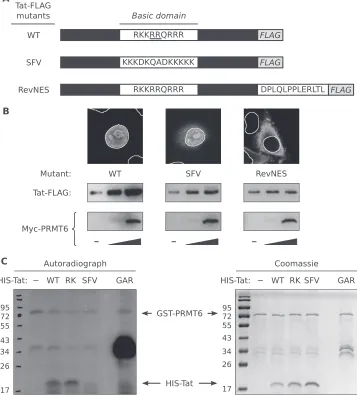

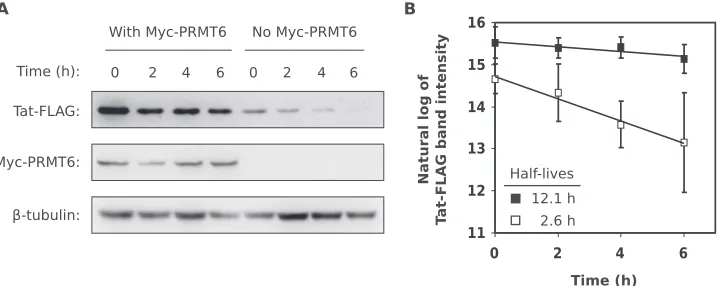
Related documents
When R2 was deleted from the chimeric constructs, none of the resultant fusion protein concentrated in the TGN; instead, the expressed protein was located entirely in endosomes,
Accumulation of mutant virus in organs of infected turnip deter- mined by dot blot analysis of total viral DNA isolated from tissues at 26 days postinfection of plants infected
An early, critical step in the human immunodeficiency virus type (HIV-1) life cycle is reverse transcription of viral RNA into proviral DNA, which can then be integrated into
(B) 35S-labeled VZV-infected cell lysate was immunoprecipitated (Immppt) with antisera to ORF 62 and ORF 47 proteins under three conditions: lane 1, in the absence of a competing
We have probed the structures of monomeric and oligomeric gpI20 glycoproteins from the LAI isolate of human immunodeficiency virus type 1 (HIV-1) with a panel of monoclonal
infected with vRVH with anti-H; lane 9, cells infected with vRVF with anti-F; lane 10, cells infected with wild-type vaccinia virus. (Wyeth) with anti-H and anti-F sera; lane
To determine which MHC antigen presented poliovirus antigens to the T lymphocytes, monoclonal antibodies to MHC class I or class II antigens were added to triplicate cultures
These data further suggest that the affinities of the EIAV and HIV-1 Tat activation domains for this cofactor vary in different cell types and that the inability of

