0022-538X/10/$12.00 doi:10.1128/JVI.01588-09
Copyright © 2010, American Society for Microbiology. All Rights Reserved.
Activation of the Inositol (1,4,5)-Triphosphate Calcium Gate Receptor
Is Required for HIV-1 Gag Release
䌤
Lorna S. Ehrlich,
1Gisselle N. Medina,
1Mahfuz B. Khan,
2Michael D. Powell,
2Katsuhiko Mikoshiba,
3and Carol A. Carter
1*
Department of Molecular Genetics and Microbiology, Stony Brook University, Stony Brook, New York 117941;
Department of Microbiology, Biochemistry and Immunology, Morehouse School of Medicine, Atlanta, Georgia 303102; and RIKEN Institute, Saitama 351-0198, Japan3
Received 30 July 2009/Accepted 18 April 2010
The structural precursor polyprotein, Gag, encoded by all retroviruses, including the human immunodefi-ciency virus type 1 (HIV-1), is necessary and sufficient for the assembly and release of particles that morpho-logically resemble immature virus particles. Previous studies have shown that the addition of Ca2ⴙto cells expressing Gag enhances virus particle production. However, no specific cellular factor has been implicated as mediator of Ca2ⴙprovision. The inositol (1,4,5)-triphosphate receptor (IP3R) gates intracellular Ca2ⴙstores. Following activation by binding of its ligand, IP3, it releases Ca2ⴙfrom the stores. We demonstrate here that IP3R function is required for efficient release of HIV-1 virus particles. Depletion of IP3R by small interfering RNA, sequestration of its activating ligand by expression of a mutated fragment of IP3R that binds IP3 with very high affinity, or blocking formation of the ligand by inhibiting phospholipase C-mediated hydrolysis of the precursor, phosphatidylinositol-4,5-biphosphate, inhibited Gag particle release. These disruptions, as well as interference with ligand-receptor interaction using antibody targeted to the ligand-binding site on IP3R, blocked plasma membrane accumulation of Gag. These findings identify IP3R as a new determinant in HIV-1 trafficking during Gag assembly and introduce IP3R-regulated Ca2ⴙsignaling as a potential novel cofactor in viral particle release.
Assembly of the human immunodeficiency virus (HIV) is determined by a single gene that encodes a structural polypro-tein precursor, Gag (71), and may occur at the plasma mem-brane or within late endosomes/multivesicular bodies (LE/ MVB) (7, 48, 58; reviewed in reference 9). Irrespective of where assembly occurs, the assembled particle is released from the plasma membrane of the host cell. Release of Gag as
virus-likeparticles (VLPs) requires the C-terminal p6 region of the protein (18, 19), which contains binding sites for Alix (60, 68) and Tsg101 (17, 37, 38, 41, 67, 68). Efficient release of virus particles requires Gag interaction with Alix and Tsg101. Alix and Tsg101 normally function to sort cargo proteins to LE/ MVB for lysosomal degradation (5, 15, 29, 52). Previous stud-ies have shown that addition of ionomycin, a calcium iono-phore, and CaCl2 to the culture medium of cells expressing Gag or virus enhances particle production (20, 48). This is an intriguing observation, given the well-documented positive role for Ca2⫹ in exocytotic events (33, 56). It is unclear which
cellular factors might regulate calcium availability for the virus release process.
Local and global elevations in the cytosolic Ca2⫹level are achieved by ion release from intracellular stores and by influx from the extracellular milieu (reviewed in reference 3). The major intracellular Ca2⫹ store is the endoplasmic reticulum
(ER); stores also exist in MVB and the nucleus. Ca2⫹release is regulated by transmembrane channels on the Ca2⫹ store
membrane that are formed by tetramers of inositol (1,4,5)-triphosphate receptor (IP3R) proteins (reviewed in references 39, 47, and 66). The bulk of IP3R channels mediate release of Ca2⫹from the ER, the emptying of which signals Ca2⫹influx (39, 51, 57, 66). The few IP3R channels on the plasma mem-brane have been shown to be functional as well (13). Through proteomic analysis, we identified IP3R as a cellular protein that was enriched in a previously described membrane fraction (18) which, in subsequent membrane floatation analyses, re-producibly cofractionated with Gag and was enriched in the membrane fraction only when Gag was expressed. That IP3R is a major regulator of cytosolic calcium concentration (Ca2⫹) is well documented (39, 47, 66). An IP3R-mediated rise in cytosolic Ca2⫹requires activation of the receptor by a ligand,
inositol (1,4,5)-triphosphate (IP3), which is produced when phospholipase C (PLC) hydrolyzes phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2] at the plasma membrane (16, 25, 54). Paradoxically, PI(4,5)P2binds to the matrix (MA) domain in Gag (8, 55, 59), and the interaction targets Gag to PI(4,5)P2 -enriched regions on the plasma membrane; these events are required for virus release (45). We hypothesized that PI(4,5)P2 binding might serve to target Gag to plasma membrane sites of localized Ca2⫹ elevation resulting from PLC-mediated PI(4,5)P2hydrolysis and IP3R activation. This idea prompted us to investigate the role of IP3R in Gag function.
Here, we show that HIV-1 Gag requires steady-state lev-els of IP3R for its efficient release. Three isoforms of IP3R, types 1, 2, and 3, are encoded in three independent genes (39, 47). Types 1 and 3 are expressed in a variety of cells and have been studied most extensively (22, 39, 47, 73). Deple-tion of the major isoforms in HeLa or COS-1 cells by small
* Corresponding author. Mailing address: Department of Molecular Genetics and Microbiology, Life Sciences Bldg., Rm 248, Stony Brook University, Stony Brook, NY 11794-5222. Phone: (631) 632-8801. Fax: (631) 632-9797. E-mail: ccarter@ms.cc.sunysb.edu.
䌤Published ahead of print on 28 April 2010.
6438
on November 8, 2019 by guest
http://jvi.asm.org/
interfering RNA (siRNA) inhibited viral particle release. Moreover, we show that sequestration of the IP3R activat-ing ligand or blockactivat-ing ligand formation also inhibited Gag particle release. The above perturbations, as well as inter-fering with receptor expression or activation, led to reduced Gag accumulation at the cell periphery. The results support the conclusion that IP3R activation is required for efficient HIV-1 viral particle release.
MATERIALS AND METHODS
Plasmids and reagents. Plasmids encoding Gag-green fluorescent protein
(GFP) (23), Gag-hemagglutinin (HA) (28), glutathioneS-transferase (GST)-IP3
“sponge” (a high-affinity binding fragment of IPR-1) (63), viral clone NL4-3 (30), and siRNA duplexes targeting IP3R types 1 and 3 (22) and Tsg101 (53) were described previously. The following antibodies and reagents were used: anti-HIV-1 CA rabbit polyclonal antibody (18), anti-anti-HIV-1 p24 mouse monoclonal antibody (DuPont NEN), IP3R type 1 (IP3R-1) rabbit polyclonal body (Affinity BioReagents), IP3R type 3 (IP3R-3) monoclonal
anti-body (BD Biosciences), anti-PI(4,5)P2monoclonal antibody (Abcam), rabbit
anti-p85␣-subunit of phosphatidylinositol 3-kinase (PI3K), CD63 and
anti-Tsg101 mouse monoclonal antibodies (Santa Cruz Biotechnology, Inc.), U73122, U73343, thapsigargin (TG), anti-IP3R type 2 and anti-GST rabbit polyclonal antibodies, actin mouse monoclonal antibody (Sigma), IRDye 800 anti-rabbit IgG (Rockland), Alexa Fluor 680 anti-mouse IgG, tetramethyl rhodamine isothiocyanate (TRITC)-tagged goat anti-mouse IgG secondary antibody (Mo-lecular Probes), IgG secondary antibody conjugated to gold particles (Jackson ImmunoResearch), and glutaraldehyde (Electron Microscopy Sciences).
Cell culture, transfection, and harvest.Cell culture, transfection with expres-sion plasmid DNA using the FuGene 6 reagent (Roche), and harvest were as previously described (18). When indicated in the text, transfection with either nontargeting control or targeted siRNA was done using Lipofectamine 2000 (Invitrogen) or DharmaFECT (Dharmacon), following the manufacturer’s guidelines, 24 h prior to DNA transfection. Forty-eight hours after DNA trans-fection, tissue culture medium was removed and saved for VLP isolation; cells
were scraped into Ca2⫹-free, Mg2⫹-free phosphate-buffered saline (PBS) with a
rubber policeman and pelleted. The cell lysate was prepared by addition of PBS with 1% Triton X-100 or by disrupting cells in hypotonic buffer using a Dounce homogenizer, as previously described (18, 22). For isolation of VLPs, the culture
medium was passed through a 0.45-m-pore-size filter and then centrifuged
through a 20% sucrose cushion. In the case of cells expressing NL4-3 provirus, infectious virus that was released into the tissue culture medium was quantified by multinuclear activation of a galactosidase indicator (MAGI) assay as de-scribed previously (30). Stock solutions of the inhibitors U73122 and U73343 were prepared in dimethyl sulfoxide (DMSO). For drug treatments, at 24 h posttransfection, medium was removed from the plates and replaced with
Dul-becco’s modified Eagle’s medium (DMEM) lacking CaCl2and glutamate and
containing the concentrations of the drug indicated in the text. DMSO control solutions consisted of medium and an amount of DMSO corresponding to the highest volume of stock solution used in the treatment set. Plates were returned to 37°C for the incubation times indicated in the text.
Western analysis.Proteins were separated by electrophoresis through 4.5% (for detection of IP3R), 10% (for detection of Gag, GST-IP3 sponge, and actin), or 15% (for detection of CA/p24) SDS-polyacrylamide gels and elec-troblotted onto nitrocellulose membrane. Following incubation with appro-priate primary and secondary antibodies, proteins were visualized using a chemiluminescence-based detection system (Lumi-light; Roche) or an infra-red-based imaging system (Odyssey; Li-Cor Biosciences). For analysis of release efficiency, densitometric measurements of bands corresponding to Gag in VLPs and Gag in cell lysates on the Western blots were made using NIH Image software. Release efficiency was defined as the ratio of the signal intensity value for the VLP-associated Gag to the sum of the values for VLP-associated Gag plus cell lysate-associated Gag.
Microinjection.DNA and antibody solutions were diluted into microinjection
buffer (20 nM Tris acetate, pH 7.4, with 20 mM NaCl, 1 mM MgCl2, 0.1 mM
EDTA, 5 mM-mercaptoethanol [-ME], and 150 mM KCl). Mixtures
contain-ing the nuclear stain DAPI (4⬘,6⬘-diamidino-2-phenylindole), Gag-GFP, and
either anti-IP3R type 2 rabbit polyclonal antibody or rabbit polyclonal antibody
against the anti-p85␣-subunit of PI3K were microinjected into the region above
the nucleus of live cells on gridded coverslips as previously described (61). After
microinjection, cells were incubated for 18 h at 37°C prior to analysis by decon-volution confocal microscopy.
Confocal microscopy.Cells that had been either treated, transfected, or mi-croinjected and plated on coverslips were fixed in 4% formaldehyde (Fisher), washed, and permeabilized with 0.1% Triton X-100 or, where indicated in the text, concurrently permeabilized and fixed with 0.075% saponin, 3.7% formal-dehyde, and 0.1% glutaraldehyde. Nuclei were stained with Hoechst for 10 min at 37°C. For indirect immunofluorescence, samples were incubated with primary antibodies for 1 h and with secondary antibodies for 30 min at 37°C. Images were captured on an inverted fluorescence/differential interference contrast (DIC) Zeiss Axiovert 200 M deconvolving fluorescence microscope operated by Axio-Vision, version 4.5 (Zeiss), software. Figures show the central image or, where indicated in the figures, one of several optical sections taken in increments of 0.4
m along thezaxis. The fluorescent data sets were deconvolved by using the
constrained iterative method (AxioVision).
Electron microscopy.Cells grown on Aclar film were fixed in 4% paraformal-dehyde–0.1% electron microscopy (EM)-grade glutaraldehyde in PBS, soaked in 2% osmium tetroxide, dehydrated in a graded series of ethyl alcohol solutions, and embedded in Durpan resin. Thin sections of 80 nm were counterstained with uranyl acetate and lead citrate and viewed with an FEI Tecanal BioTwinG2 electron microscope. For immune-EM, thin sections were incubated with anti-HIV-1 CA antibodies and subsequently with secondary antibodies conjugated to gold particles.
RESULTS
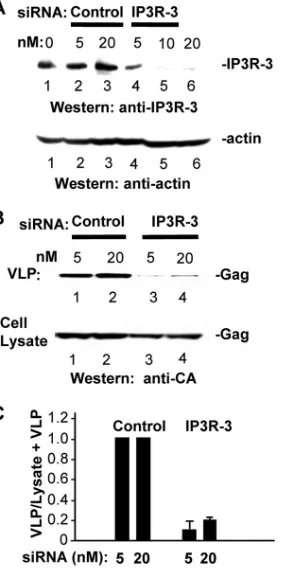
siRNA-targeted depletion of endogenous IP3R inhibits VLP release.To determine whether Gag release requires IP3R, we employed previously described targeted siRNA sequences to deplete endogenous pools of IP3R-1 and IP3R-3 from HeLa cells; these two isoforms comprise 99% of the IP3R in these cells (22). The cells were mock transfected or transfected with DNA encoding Gag-GFP 24 h after transfection with control or targeted siRNAs and incubated for an additional 48 h. Targeted siRNA reduced the steady-state level of IP3R-1 (Fig. 1) and IP3R-3 (Fig. 2) in a dose-dependent manner compared to levels in mock-treated cells or cells transfected with nontarget-ing control siRNA. The specificity of the siRNAs was con-firmed by demonstrating that expression of nontargeted pro-tein was not affected, e.g., that the endogenous level of IP3R-3 was not affected by siRNA targeting IP3R-1 (Fig. 1A) or that the actin level was not affected by siRNA targeting IP3R-3 (Fig. 2A). Both of the IP3R-targeted siRNAs reduced the number of VLPs detected in the medium relative to treatment with the nontargeting siRNA (Fig. 1B, top, compare lanes 1 and 3 to lanes 4 and 6, and 2B, top, lanes 1 and 2 to lanes 3 and 4). The reduction in the number of VLPs detected in the medium of IP3R-depleted cells reflected a defect in release as Gag accumulation in these cells was not diminished (Fig. 1B and 2B, bottom panels). Analysis of Gag release efficiency indicated that under the conditions where IP3R-1 or -3 was significantly depleted, the efficiency of VLP release was re-duced to 10 to 20% of the levels of control samples. The results indicate that steady-state levels of the major isoforms ex-pressed in HeLa cells and COS-1 cells (data not shown) are required for efficient VLP release. Simultaneous transfection with siRNAs targeted to the two isoforms did not result in the same level of depletion (22) or VLP release inhibition (data not shown) for unknown reasons.
Efficient production of infectious virus requires steady-state levels of IP3R.To test whether IP3R was required for produc-tion of infectious virus particles, HeLa cells transfected with the same siRNAs targeting type 1 or type 3 IP3R as above were transfected with a construct encoding HIV-1 NL4-3 provirus
on November 8, 2019 by guest
http://jvi.asm.org/
(30) at 24 h after siRNA transfection (Fig. 3). Cells transfected with siRNA targeting Tsg101 served as positive controls. Cel-lular levels of targeted proteins (Fig. 3A), Gag, and Gag-derived mature capsid (CA) protein (Fig. 3B) and of virus released into the tissue culture medium (Fig. 3C) were deter-mined by Western analysis of samples prepared as above. As with Tsg101, depletion of IP3R impaired virus production sig-nificantly. Calculation of release efficiency (Fig. 3D) indicated that IP3R depletion reduced the amount of virus that was released to 8% of the level produced by mock-treated cells (Fig. 3D, bar M) or 25% of the level produced by cells trans-fected with the nontargeting control siRNA (bar C), similar to the effect of Tsg101 depletion (bar T). The amount of infec-tious virus that was released into the tissue culture medium was determined in single-cycle MAGI assays (30, 31). As shown in Fig. 3E, IP3R depletion reduced the amount of infectious virus to ⱕ10% of the level produced by either mock-treated or nontargeting control siRNA-treated cells. The results indicate that a steady-state level of IP3R is required for production of infectious HIV-1 particles.
Sequestering the IP3R ligand inhibits VLP release.IP3R is activated following binding of its ligand, IP3 (39, 47). We therefore determined the effect on VLP release of sequester-ing the cytosolic pool of IP3. To achieve this, we used a pre-viously described GST-tagged fragment (amino acids [aa] 226 to 604; the sponge) (63) that contains the ligand binding region
of the IP3R-1 protein. This fragment binds IP3 with⬃ 1,000-fold greater affinity than the IP3R-1 protein due to a mutation of R to Q at amino acid 441 (R441Q) (Fig. 4A). The sponge exhibits the same specificity for IP3 as endogenous IP3R pro-teins. It distributes homogenously in the cytoplasm, competes for IP3 binding with all IP3R isoforms, and does not affect IP3 production (63). As a control, we utilized a fragment encom-passing the same residues but which is defective for IP3 bind-ing due to a different mutation (K508A). As shown in Fig. 4B, coexpression of Gag with the sponge (lanes 2 and 3) reduced VLP release in a dose-dependent manner (compare lanes 2 and 3 to lane 1). In contrast, coexpression of Gag with the nonbinding fragment did not inhibit VLP release (Fig. 4B, compare lanes 4 and 5 to lane 1). The level of intracellular accumulation of Gag was not altered, and cell viability as reflected by actin also was not affected. Analysis indicated that the efficiency of VLP release was reduced as much as 4-fold in the presence of the sponge but was not affected by the non-binding fragment (Fig. 4C). We conclude that efficient release of Gag requires endogenous levels of IP3, the activation ligand of IP3R.
[image:3.585.348.494.69.353.2]We employed confocal microscopy to examine cells coex-pressing HA-tagged Gag and the IP3-binding domain (BD) fragments. Gag was detected by indirect immunofluores-cence, and cells that received DNA encoding the sponge or
[image:3.585.91.234.69.321.2]FIG. 1. siRNA-targeted depletion of endogenous IP3R-1 inhibits Gag release. (A) IP3R-1 and IP3R-3 levels in HeLa cells mock trans-fected (lane 1) or transtrans-fected with increasing amounts of nontargeting control (lanes 2 to 4) or IP3R-1-targeting (lanes 5 to 7) siRNA. Twenty-four hours later cells were transfected with DNA encoding Gag and incubated for an additional 48 h. Cells and tissue culture medium were processed for total lysate and VLPs, respectively, as described in Ma-terials and Methods. (B) Gag VLPs released in tissue culture medium (top) and Gag accumulation in cells (bottom). Proteins were identified by Western analysis. (C) VLP release efficiency.
FIG. 2. siRNA-targeted depletion of endogenous IP3R-3 inhibits Gag release. (A) IP3R-3 and actin levels in cells mock transfected (lane 1) or transfected with increasing amounts of nontargeting control (lanes 2 and 3) or IP3R-3-targeting (lanes 4 to 6) siRNA. (B) Gag VLPs released in tissue culture medium (top) and Gag accumulation in cells (bottom). Proteins were identified by Western analysis. (C) VLP release efficiency.
on November 8, 2019 by guest
http://jvi.asm.org/
the nonbinding fragment were revealed by expression of GFP under the control of the internal ribosome entry site (IRES) element in the same vectors (63) (Fig. 4D1). As shown in Fig. 4D2 to D4, HA-tagged Gag accumulated in both the cell interior and at the cell periphery whether Gag was expressed alone (Fig. 4D2) or coexpressed with the nonbinding fragment (K508A) (Fig. 4D3) or the sponge (R441Q) (Fig. 4D4). This indicated that overexpression of
sponge did not detectably alter steady-state Gag distribu-tion.
Blocking formation of IP3 by inhibiting PLC activity inhib-its VLP release.PLC-catalyzed hydrolysis of PI(4,5)P2is the cellular mechanism by which IP3 is produced (16, 25). U73122 (6; reviewed in reference 25) is a widely used inhibitor of this event (Fig. 5A) and has also been used to inhibit PLC activity in HIV-infected cells (21). U73343 is an inactive form of the drug that must be used as a control to ensure that observed effects are specific to inactivation of the PLC enzyme activity (25). Cells were transfected with DNA encoding Gag-GFP; at 24 h posttransfection the tissue culture medium was replaced with treatment medium containing either DMSO alone or 0.5 or 1.0M U73122 or U73343 in DMSO. These concentrations are significantly lower than those previously shown to result in off-target effects (⬎3M) (reviewed in reference 25). In fact, given the short half-life of U73122 in the medium (72), this initial concentration is expected to be significantly reduced within the first few hours of the 24-h treatment. The effect of drug treatment on VLP was determined. As shown in Fig. 5B, cells exposed to U73122 (0.5M) produced significantly fewer VLPs (lane 2) than the cells incubated with the same concen-tration of U73343 (lane 3) or with the DMSO carrier (lane 1). Although the possibility of off-target effects cannot be com-pletely eliminated, the fact that the level of Gag in cell lysates was the same in U73122-, U73343-, or DMSO carrier-treated cells makes this explanation unlikely. Moreover, the level of actin was not altered, indicating that the samples contained comparable amounts of total cell protein. Analysis of VLP release efficiency (Fig. 5C) indicated that release efficiency was reduced 10-fold in the presence of U73122 compared to the levels with U73343 or the DMSO control. Examination of the treated cells by confocal microscopy (Fig. 5D) confirmed that COS-1 cells treated with U73122 (Fig. 5D3 and D4), but not U73343 (Fig. 5D2) or the DMSO carrier (Fig. 5D1), accumu-lated increased levels of PI(4,5)P2that were detected by spe-cific anti-PI(4,5)P2 monoclonal antibody under the stringent conditions used to fix and stain the cells (0.075% saponin– 0.1% glutaraldehyde–3.7% formaldehyde at 37°C for 20 min). The effect of the active inhibitor on PI(4,5)P2 accumulation
was dose dependent, as indicated by the increased detection of PI(4,5)P2 in cells treated with 1.0 M U73122 (Fig. 5D4)
compared to 0.5M U73122 (Fig. 5D3). At the same concen-trations, the inactive U73343 analog at 0.5M (Fig. 5D2) and 1.0M (data not shown) behaved like the DMSO carrier (Fig. 5D1). Under these conditions, the subcellular distribution of Gag was significantly altered. Whereas Gag puncta were dis-persed throughout the cell in 75% of 30 cells treated with the DMSO carrier (Fig. 5D1) or the inactive U73343 analog (Fig. 5D2), such was not the Gag distribution in 90% (0.5M) to 100% (1.0 M) of 40 cells treated with the active inhibitor U73122 (Fig. 5D3 and D4) (in two independent experiments). The results indicate that active PLC is required for efficient VLP release. We infer that PLC-mediated IP3 production is necessary to maintain normal Gag localization at the cell pe-riphery and elsewhere.
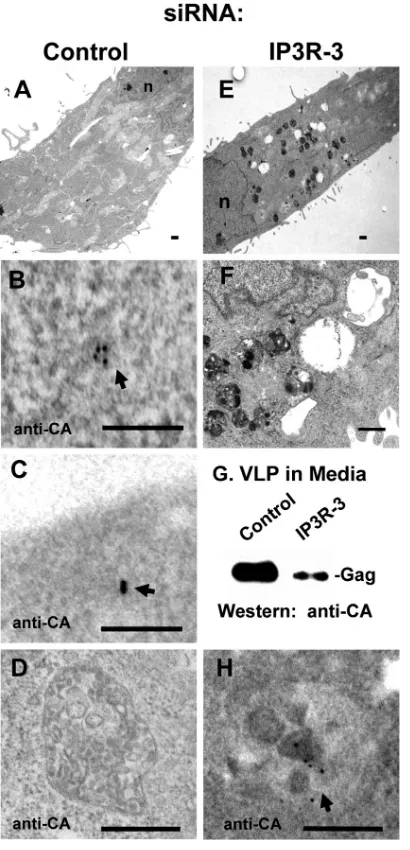
Gag accumulates in vesicles following IP3R depletion. To determine whether depletion of IP3R also altered the subcel-lular location of Gag, cultures prepared as described in the legends of Fig. 1 and 2 were examined by immunoelectron
FIG. 3. Efficient production of infectious virus requires steady-state levels of IP3R. HeLa cells were mock transfected (M) or trans-fected with control siRNA (C), siRNA targeting Tsg101 (T), siRNA targeting IP3R-1 (1) or siRNA targeting IP3R-3 (3) and 24 h later transfected with DNA encoding HIV-1 NL4-3 provirus. After an ad-ditional 48-h incubation, cells and tissue culture medium were har-vested for analysis. (A to C) Western analysis for endogenous cellular Tsg101, IP3R-1, IP3R-3, and actin (A) and for Gag and Gag-derived mature capsid (CA) proteins in the cell (B) and in the tissue culture medium (C). (D) Virus release efficiency. (E) Amount of infectious virus in the tissue culture medium determined by single-cycle MAGI assays.
on November 8, 2019 by guest
http://jvi.asm.org/
[image:4.585.77.244.68.504.2]microscopy. Figure 6 shows images of cells that had been transfected with nontargeting control (panels A to D) or tar-geted (panels E, F, and H) siRNAs. Figure 6G shows Gag, detected by Western analysis, in VLPs isolated from the me-dium of the control and targeted samples and confirms that the targeted siRNA inhibited VLP production. Cells treated with control siRNA were generally indistinguishable from un-treated cells (Fig. 6A and data not shown). Large numbers of vesicular structures characterized by a relatively consistent diameter of⬃500 nm were detected in the cytoplasm of cells treated with siRNA targeted against expression of either IP3R-1 (data not shown) or IP3R-3 (Fig. 6E; shown at higher magnification in F). Anti-CA antibodies and gold-tagged sec-ondary antibodies were employed to identify the viral protein in postembedded thin sections. No signal was detected in “un-touched” or mock-transfected cells (data not shown). In cells treated with the control siRNA, gold particles were detected throughout the cytoplasm in a manner previously described (42), i.e., in small clusters of 1 to 5 gold particles scattered throughout the cytoplasm but not associated with any apparent structure (Fig. 6B) and near the plasma membrane (panel C). None of the 500-nm vesicles in examined sections had gold particles (Fig. 6D). In contrast, in cells transfected with siRNA targeted to IP3R-1 (data not shown) or IP3R-3 (Fig. 6H), a high percentage of the gold particles was located in the vesic-ular structures, as shown in two independent experiments that examined 40 cells (Table 1). The results indicate that depletion of IP3R altered the subcellular location of Gag and suggest that the inhibitory effect of IP3R depletion on VLP release (Fig. 1 and 2) relates to this mislocalization.
[image:5.585.49.277.62.721.2]To obtain biochemical support for the notion that Gag was localized in a different membrane compartment following de-pletion of IP3R, we prepared membrane-enriched fractions (P27 and P100, the pellet fractions obtained by centrifugation at 27,000 ⫻ g and 100,000 ⫻ g, respectively) as previously described (18) from siRNA-transfected HeLa cell cultures pre-pared as described in the legends of Fig. 1 and 2 and examined them for Gag (Fig. 7). Markers of the early endosome and lysosome, early endosome antigen 1(EEA1) and cathepsin D,
FIG. 4. Depletion of the IP3R ligand inhibits VLP release. (A) Schematic representation showing preferential sequestration of IP3 by the GST-IP3 BD in sponge (shaded box) over the ligand BD in the IP3R channel (unshaded box), resulting in inhibition of IP3R activation. (B) Analysis of VLPs and cell lysates derived from COS cells transfected with DNA encoding Gag alone (lane 1, 2.5g) or cotransfected with increasing amounts of DNA (lanes 2 and 4, 5g; lanes 3 and 5, 10g) encoding the sponge BD (lane 2, 5g; lane 3, 10
g) or nonbinding control BD (lane 4, 5g; lane 5, 10g). GST-IP3 BD denotes the R441Q and K508A binding domain fragments, de-tected by probing for GST. (C) VLP release efficiency. (D) Examina-tion of cells by deconvoluExamina-tion confocal microscopy. Panel D1 is a schematic showing elements encoded in the GST-BD expression con-struct. The construct included an IRES that allowed cotranslation of GFP, which served as a transfection marker for the plasmid. Other panels show the following: cells expressing Gag-HA alone (D2); cells coexpressing control construct or Gag-HA and sponge (D3 and D4, respectively). Gag-HA was detected by indirect immunofluorescence with mouse monoclonal anti-HA antibody and TRITC-labeled second-ary antibody (red). Cell nuclei were stained with Hoechst stain (blue). Bar, 10m.
on November 8, 2019 by guest
http://jvi.asm.org/
respectively, sedimented in the P27 fraction. The plasma mem-brane marker sodium-potassium ATPase and mannose-6-phosphate receptor (M6PR), a marker of Golgi apparatus-derived vesicles, and MVB were detected mainly in the P100 fraction (18). As described above, lysates from cells transfected with control or targeted siRNAs typically contained compara-ble amounts of Gag (Fig. 7A; compare with Fig. 1B and 2B). As Gag in P27 and P100 fractions represented a very small percentage of the total Gag in the lysate, electrophoresis of the entire P27 and P100 pellet was necessary for Gag detection. Consistent with earlier observations (18), in all cases more Gag was detectable in the P27 fraction than in the P100 fraction. However, in the P27 fractions, the samples derived from the siRNA control (Fig. 7B, lanes 1 to 6), contained less Gag than the P27 fractions isolated from the cells transfected with the siRNA targeted to IP3R-1 (lanes 7 to 12) or IP3R-3 (lanes 13 to 18). The amount of Gag detected in the P27 fraction was
[image:6.585.80.501.66.435.2]directly proportional to the amount of siRNA transfected into the cell. There were no detectable changes in the Gag content of corresponding soluble fractions (S100, from centrifugation at 100,000⫻ g) (data not shown). A longer exposure of the Western blot (Fig. 7C, top) revealed a redistribution of Gag in the two membrane-enriched fractions. Comparison of the Gag levels in the fractions derived from cells treated with 20 nM control indicated a relative distribution of 60% and 40% Gag in the P27 and P100 fractions, respectively (lanes 1 and 2). This is in agreement with previous observations (18). This shifted to a relative distribution of 90 to 95% (P27) and 5 to 10% (P100) Gag, respectively, in cells transfected with targeted siRNA (Fig. 7C, lanes 3 to 6). Since, as noted above, no obvious changes were apparent in S100 fractions, we surmise that the shift reflected changes in distribution of the membrane-asso-ciated Gag population. The results support the conclusion that Gag localization was altered following IP3R depletion.
FIG. 5. Inhibition of PLC-mediated PI(4,5)P2hydrolysis inhibits VLP release. (A) Schematic representation of U73122 inhibition of PLC
catalysis of PI(4,5)P2hydrolysis into IP3 and diacyl glycerol (DAG). (B) COS cells transfected with DNA encoding Gag-GFP were treated with
a 0.5M concentration of the PLC inhibitor U73122, the nonactive analogue U73343, or the DMSO carrier (C denotes control) for 24 h at 24 h posttransfection. Medium samples were analyzed by Western blotting for VLP production, and cell lysates were examined for Gag accumulation. (C) VLP release efficiency. (D) Gag-GFP-expressing cells subjected to treatment as described in panel B and examined by deconvolution confocal microscopy. Treated cells were simultaneously fixed and permeabilized with formaldehyde, glutaraldehyde, and saponin. PI(4,5)P2was detected
by indirect immunofluorescence with mouse monoclonal antibody against PI(4,5)P2and TRITC-labeled anti-mouse secondary antibody (red). Cell
nuclei were stained with Hoechst stain (blue). Bar, 10m.
on November 8, 2019 by guest
http://jvi.asm.org/
To obtain additional support for this conclusion, cells in cultures treated with control or targeted siRNA were exam-ined by confocal microscopy (Fig. 8). In preliminary experi-ments, we established that siGLO PPIB (Dharmacon), a fluo-rescent siRNA that localizes to processing (P) bodies in the cytoplasm (27), was delivered into⬃75% of cells (n ⫽305)
under the transfection conditions used for the experiment, making it highly likely that the majority of the cells examined contained the desired siRNA molecules. Specific knockdown of IP3R expression by the targeted siRNA was confirmed by examination of VLPs in the medium collected from the control- and the targeted-siRNA-treated cultures (data not shown). In samples transfected with the siRNA control used in the experiments shown in Fig. 1 and 2 above, Gag-GFP was detected at the cell periphery and in the cell interior delineated by CD63 (red fluorescence), a commonly used marker of late endosomes and MVB which accumulates in the perinuclear region (32, 49). This is consistent with the results of previous studies (14, 34, 44, 46, 48, 58). Representative cells are shown in Fig. 8A and C. In contrast, Gag-GFP was detected in the cell interior in most of the cells in cultures transfected with siRNA targeted to IP3R-1 (Fig. 8B and D), which is indicated by the location of CD63 (Fig. 8C and D). These results were highly reproducible in three independent trials where⬎40 cells were examined, with 90 to 100% of cells in control cultures contain-ing Gag at the plasma membrane compared to⬍20% in cul-tures transfected with the specifically targeted siRNA. The results support the conclusion that steady-state levels of IP3R are necessary to maintain localization of Gag at the plasma membrane and prevent its sequestration in interior vesicles.
Interfering with ligand-receptor interaction prevents Gag accumulation at the cell periphery.Previous studies demon-strated that expression of the IP3R-binding protein, IRBIT, which binds the IP3 binding domain (BD), suppresses IP3R activity (2). Microinjection of antibody against IP3R has also been shown to interfere with release of Ca2⫹ from
[image:7.585.61.266.68.493.2]intracellular stores (12). To determine whether disrupting the IP3-IP3R interaction would interfere with Gag localiza-tion, we microinjected cells with a rabbit anti-IP3R antibody targeted to the highly conserved IP3 BD on the protein (Fig. 9A). The antibody was injected into the region above the nucleus of live COS-1 cells together with DNA encoding Gag-GFP, and the cells were examined by confocal micros-copy 18 to 24 h later. Intracellular delivery of the solution containing both DNA and antibody into the region above the nucleus maximizes expression of the DNA while still allowing the antibody to reach the cytoplasm (43, 61). In preliminary studies, TRITC-labeled anti-rabbit secondary antibody detected the primary anti-IP3R antibody in the
TABLE 1. Distribution of gold-tagged Gag in cells treated with siRNA
siRNA Fraction
No. of gold particles (% vesicle associated)
Expt 1a Expt 2b
Control Nonvesicular 21 10
Vesicular 0 (0) 0 (0)
IP3R-1 Nonvesicular 3 58
Vesicular 4 (43) 32 (35)
IP3R-3 Nonvesicular 0 5
Vesicular 12 (100) 102 (98)
a
The primary antibody was anti-CA monoclonal antibody.
b
[image:7.585.301.542.590.708.2]The primary antibody was anti-CA polyclonal antibody.
FIG. 6. siRNA-targeted depletion of IP3R sequesters Gag in interior vesicles: immunoelectron microscopy. Sections through HeLa cells in cultures treated with control siRNA (A to D) or siRNA targeted to IP3R-3 (E, F, and H) as described in the legend of Fig. 1 above were prepared for immunoelectron microscopy as described in Materials and Methods. Images in panels A and E are at low magnification, and those in panels B to D, F, and H are at high magnification. Panels D and H show vesicular structures in the cytoplasm of cells in cultures transfected with control or targeted siRNA, respectively. Arrows indicate Gag identified by probing with anti-CA monoclonal antibody and 12-nm gold particle-tagged goat anti-mouse secondary antibody. Panel G shows Gag in VLPs iso-lated from the medium of the control and targeted samples and detected by Western analysis with the anti-CA antibody. Bars, 500 nm (A, D to F, and H) and 100 nm (B and C).
on November 8, 2019 by guest
http://jvi.asm.org/
cytoplasm of injected cells 6 to 24 h later (data not shown). To control for off-target effects, additional samples were injected with a mixture containing DNA encoding Gag-GFP and a similar quantity of affinity-purified polyclonal anti-body directed at the p85 ␣-subunit of PI3K (Fig. 9B). As revealed by serial sectioning in thez-plane, Gag was mostly at the cell periphery (z of 0 m) (Fig. 9B1) with very few Gag-containing structures detected in the cell interior (zof 2.4 m) (Fig. 9B2). In contrast, when coinjected with the anti-IP3R antibody (panel C), Gag was not detectable at the cell periphery (zof 0m) (Fig. 9C1) but was mostly in the interior of the cells (z of 2.4m) (Fig. 9C2). Similar results were obtained in five independent experiments rep-resenting over 500 microinjected cells. Gag was detected at the cell periphery in⬎90% of cells microinjected with the mixture containing the control antibody and in ⬍20% of cells injected with the mixture containing the anti-IP3R antibody that recognizes the IP3 binding site. The results indicate that interference with IP3-IP3R interaction inhib-ited localization of Gag at the cell periphery. Taken to-gether with the results described above, where similar changes in Gag localization were observed following IP3R depletion or inhibition of IP3 formation, these studies sup-port the conclusion that the steady-state level of IP3R and activation of the receptor are required for productive accu-mulation of Gag near or at the plasma membrane.
Thapsigargin stimulates VLP release.As our results indi-cated that IP3R activation, i.e., release of Ca2⫹from IP3R-gated stores, promotes particle production whether directed by thegaggene alone or the entire viral genome, we determined whether thapsigargin (TG) treatment stimulated VLP release (Fig. 10). TG is a cell-permeable agent that inactivates the Ca2⫹ATPase pump in the ER membrane, effectively causing a rise in cytosolic Ca2⫹(26, 36). COS-1 cells were transfected with DNA encoding Gag-GFP, treated with thapsigargin or the DMSO carrier in calcium-free, serum-free DMEM 24 h later, and harvested following a 4-h incubation period. Treatment under the calcium-free medium condition eliminates Ca2⫹ in-flux effects (10, 51), allowing evaluation of the effect of release of stored Ca2⫹ alone. However, this condition restricts the refilling of the ER, which limits the rise in cytosolic Ca2⫹above
[image:8.585.110.473.70.337.2]its basal level. At the end of the 4-h incubation period, the medium and cells were harvested for analysis of VLPs and cell-associated Gag. As shown in Fig. 10A, the cells treated with thapsigargin exhibited a dose-dependent increase in the amount of VLP detected in the medium (lanes 2 to 4) com-pared to cells incubated with the DMSO carrier (lane 1). In contrast, the accumulation levels of cell-associated Gag and actin remained constant. Analysis of the results (Fig. 10B) indicated that thapsigargin stimulated a small but reproducible enhancement in VLP release (i.e., a 4-fold increase in VLP release efficiency at the highest dose). The results indicate that
FIG. 7. siRNA-targeted depletion of IP3R alters Gag distribution in membrane-enriched biochemical fractions. Cell lysates from the experiment described in the legend of Fig. 1 above were fractionated into membrane-enriched P27 and P100 fractions (18). (A) Unfrac-tionated cell lysate probed for Gag. (B) P27 and P100 fractions probed for Gag. A darker exposure of the blot was obtained. (C) Gag and actin bands in the 20 nM samples in lanes 1 and 2, lanes 3 and 4, and lanes 5 and 6 show the darker exposure of bands in, respectively, lanes 5 and 6, lanes 11 and 12, and lanes 17 and 18 in panel B. (D) Analysis of relative Gag accumulation in P27 and P100 fractions derived from
20 nM samples.
on November 8, 2019 by guest
http://jvi.asm.org/
release of the intracellular Ca2⫹pool stored in the ER
pro-motes VLP release. As the thapsigargin-sensitive Ca2⫹pool is the IP3-responsive Ca2⫹pool (65), we can interpret this result
as indicating that release of the IP3R-gated Ca2⫹pool in the ER promotes VLP release.
To examine the effect of thapsigargin on Gag localization, the cells were examined by confocal microscopy. As shown in Fig. 10C, Gag was detected at the cell periphery (zof 0 m) (C1, top) and in the cell interior (zof 1.2m) (C1, bottom) in
[image:9.585.97.484.69.558.2]cells treated with the DMSO carrier, as revealed by serial sectioning in thezplane. In contrast, Gag accumulation in the cell interior was significantly reduced in cells treated with thap-sigargin (compare Fig. 10C2 top and bottom panels). As shown in Fig. 10D, and consistent with the results shown above (Fig. 9), Gag localized with CD63 in the perinuclear region in the cells treated with DMSO (Fig. 10D1). However, this localiza-tion was significantly reduced in thapsigargin-treated cells, as evident by inspection of the interior z section (Fig. 10D2,
FIG. 8. siRNA-targeted depletion of IP3R sequesters Gag in interior vesicles: deconvolution confocal microscopy. HeLa cells expressing Gag-GFP in cultures treated with nontargeting control siRNA (A and C) or siRNA targeted to IP3R-1 (B and D) were examined by confocal microscopy. CD63 was detected by indirect immunofluorescence with mouse monoclonal anti-CD63 antibody and TRITC-tagged secondary antibody (red). Cell nuclei were stained with Hoechst stain (blue). Bar, 10m.
on November 8, 2019 by guest
http://jvi.asm.org/
bottom). Instead, Gag accumulated almost exclusively at the cell periphery (Fig. 10D2, top). We conclude that release of the IP3R-gated Ca2⫹pool from the ER minimizes Gag localiza-tion in the cell interior and promotes Gag release.
DISCUSSION
Previous studies showed that elevation of cytosolic Ca2⫹by
addition of CaCl2and ionomycin to the tissue culture medium enhances Gag release efficiency (20, 48). Our studies demon-strate that IP3R, a cellular factor that gates intracellular Ca2⫹ stores, is required for efficient Gag trafficking and virus particle release. We showed that experimental conditions that block Ca2⫹release through IP3R channels on the ER, i.e, depletion
of IP3R or its activating ligand, inhibited VLP release. In contrast, VLP release was stimulated following thapsigargin-induced mobilization of the IP3R-gated Ca2⫹pool from ER stores. Examination of cells by confocal or electron microscopy
indicated that interference with the IP3-IP3R interaction by all of the experimental strategies we employed except the sponge inhibited Gag accumulation on the plasma membrane. In con-trast, more Gag localized at the cell periphery after thapsigar-gin treatment. Perhaps the stoichiometric nature of sponge-IP3 interaction (i.e., 1:1), combined with the fact that sponge-IP3 is continuously generated in the cell, will always result in IP3 levels exceeding that of the sponge, and this may make sponge impact difficult to detect visually. Nevertheless, the results of our study indicate that IP3R is an important cofactor of Gag trafficking and infectious virus production.
The plasma membrane is the predominant accumulation site of the acidic phospholipid PI(4,5)P2 (70). Gag is targeted to
[image:10.585.131.452.67.445.2]the plasma membrane by PI(4,5)P2, and this event is required for viral particle release (45; reviewed in reference 69). The “hydrolysis stimulating synthesis” model postulates that PI(4,5)P2turnover is required to maintain the supply of plasma membrane PI(4,5)P2. This model states that hydrolysis and
FIG. 9. Interfering with IP3-IP3R interaction inhibits Gag targeting to the plasma membrane. (A) Schematic representation of antibody (Ab) targeting the IP3-binding site in IP3R. COS-1 cells were microinjected with a mixture consisting of DNA encoding Gag-GFP, DAPI stain (blue) to label the nucleus of the microinjected cells, and control antibody (B) or anti-IP3R rabbit antibody directed at the IP3 binding domain (C). Eighteen hours after microinjection, the samples were permeabilized and examined by deconvolution confocal microscopy. Cells coinjected with control antibody show Gag distribution at the cell periphery (B1) and the cell interior (B2). Cells coinjected with anti-IP3R antibody show low detection of Gag at the cell periphery (C1) compared to the cell interior (C2).z, section throughzplane of cell. Bar, 10m.
on November 8, 2019 by guest
http://jvi.asm.org/
synthesis of PI(4,5)P2 are tightly coupled events, such that synthesis rapidly compensates for its hydrolysis while PI(4,5)P2
hydrolysis sends signals that stimulate its production (35). As all four isoforms of PLC require Ca2⫹ for catalytic function
(25, 54) and as Ca2⫹has been found to increase both the level and activity of PIP5K1␣, the lipid kinase that is critical for synthesis of PI(4,5)P2 (74), Ca2⫹ is a key regulator of this dynamic process.
Our results suggest that PI(4,5)P2turnover also is critical for maintaining Gag at the plasma membrane. We observed that treatment of cells with U73122, an inhibitor of PLC-mediated PI(4,5)P2 hydrolysis, resulted in accumulation of Gag in the
cell interior and inhibition of VLP release even though the inhibitor was added 24 h posttransfection when a significant amount of Gag was already plasma membrane bound, as re-vealed by the control samples. This finding suggests that plasma membrane association of Gag, driven by membrane binding and targeting determinants (55, 59, 71), requires the dynamic relationship between turnover and synthesis of plasma membrane PI(4,5)P2. The fact that interfering with IP3R activation by several different approaches resulted, in almost all cases, in accumulation of Gag in the cell interior rather than at the plasma membrane suggests that Ca2⫹ sig-naling, mediated through IP3R activation, functions to oppose the Gag trafficking associated with this interior localization.
In general, cellular cargo destined for endocytic trafficking is tagged with ubiquitin to facilitate recognition by Tsg101 and other ESCRT factors (reviewed in reference 52). Tsg101, which binds HIV-1 Gag directly (17, 37, 38, 67), has been reported to increase the level of ubiquitinated Gag (41). As noted above, MVB-associated Gag has been reported by sev-eral laboratories (14, 34, 44, 46, 48, 58). Under conditions of impaired IP3R function, we found Gag associated with MVB-like vesicles. Studies by Mullock et al. (40) provide evidence for formation of an MVB-immature lysosome hybrid which eventually generates a mature hydrolytic lysosome and a re-formed late endosome. The former was suggested to contain proteins that were sorted into intralumenal vesicles for degra-dation; the reformed late endosome contained proteins on a nondegradative pathway. In their model, calcium is the deter-minant of the sorting (50). If ubiquitination signaling can be considered an “agonist” of Gag endosome association, we hy-pothesize that Ca2⫹serves as an antagonist of this event.
There at two possible sources of intracellular Ca2⫹ that
might mediate the effects on VLP production that we ob-served. It is known that IP3R-mediated release of Ca2⫹results
in an initial, transient stimulus that is followed by a second, slower Ca2⫹wave that is mediated by store-operated channels
(SOCs) in the plasma membrane (reviewed in reference 51). Channel activity is coupled to IP3R activity (4, 57, 62). One of these channels, G protein-coupled receptor extracellular Ca2⫹ (Ca2⫹
o)-sensing receptor (CaR) (62) links IP3R-mediated
Ca2⫹ signaling and ubiquitin-mediated signaling. CaR binds directly to a deubiquitinating enzyme (24) that is known to interact with ESCRT proteins and whose activity is required for retroviral budding (1). Through its ability to influence pro-teins like CaR, IP3R might regulate the ubiquitination state of plasma membrane-localized Gag, permitting the viral protein to counter nonproductive sorting signaled by polyubiquitina-tion. If Gag ubiquitination is controlled by L domain binding
partners as reported for Tsg101, in the case of HIV (41), or Nedd4, in the case of avian sarcoma virus (ASV) (64), the model predicts that these proteins would play a critical role in linking Gag to the machinery controlled by IP3R.
[image:11.585.327.510.68.449.2]Mobilization of Ca2⫹from intracellular storage pools is now a recognized factor in HIV replication (11). Nef-mediated T-cell activation, Tat-dependent HIV-1 gene expression, and gp120-mediated events are steps in virus replication for which Ca2⫹mobilization is known to be important. Previous studies demonstrated that extracellular Ca2⫹enhanced VLP release
FIG. 10. Thapsigargin stimulates VLP release. (A) COS-1 cells expressing Gag were incubated for 4 h in calcium-free, serum-free DMEM containing either DMSO carrier alone (lane 1) or thapsi-gargin (lanes 2 to 4). Tissue culture medium and cells were har-vested for analysis of VLPs and cell-associated Gag and actin. (B) Analysis of VLP release efficiency. (C and D) Examination of cells by deconvolution confocal microscopy. DMSO-treated control cells showing Gag at both the cell periphery (C1 and D1, top) and in the cell interior (C1 and D1, bottom). Thapsigargin-treated cells show Gag at the cell periphery (C2 and D2, top) but not, or at reduced levels, in the cell interior (C2 and D2, bottom). CD63 was detected by indirect immunofluorescence with mouse monoclonal anti-CD63 antibody and TRITC-tagged secondary antibody (red). Cell nuclei were stained with Hoechst stain (blue). z, section throughzplane of the cell. Bar, 10m.
on November 8, 2019 by guest
http://jvi.asm.org/
(20, 48). Our study links, for the first time, HIV-1 Gag traf-ficking and assembly with mobilization of Ca2⫹from
intracel-lular stores gated by a specific Ca2⫹channel receptor. Gag itself or any of the proteins that Gag recruits may serve as a
[image:12.585.78.504.65.664.2]physiologic trigger of IP3R activation for delivery of Ca2⫹at spatially and temporally appointed events in the Gag release pathway. Whatever the trigger, our findings identify IP3R as a new determinant in HIV-1 Gag trafficking and assembly and
FIG. 10—Continued.
on November 8, 2019 by guest
http://jvi.asm.org/
introduce IP3R-regulated Ca2⫹signaling as a potential novel cofactor in viral particle release.
ACKNOWLEDGMENTS
We thank M. Chen, T. Tian, and I. Jayatilaka for technical assistance and F. Fernandes and J. Leis for critical reading of the manuscript.
This study was supported by NIH R56 and R01 awards AI068463 (to C.C.). G.M. was supported by W. Burghardt Turner Predoctoral Fel-lowships (NSF-HRD-funded SUNY AGEP, grant 35583) and NIH Pre-Doctoral Training grant 5T32 CA-09176. M.B.K. was supported by NIH grants S06-GM08428 and SG12-RR003034 (to M.P.).
REFERENCES
1.Agromayor, M., and J. Martin-Serrano.2006. Interaction of AMSH with
ESCRT-III and deubiquitination of endosomal cargo. J. Biol. Chem.281:
23083–23091.
2.Ando, H., A. Mizutani, H. Kiefer, D. Tsuzurugi, T. Michikawa, and K. Mikoshiba.2006. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on IP3 receptor in a
phosphorylation-dependent manner. Mol. Cell22:795–806.
3.Berridge, M. J., P. Lipp, and M. D. Bootman.2000. The versatility and
universality of calcium signaling. Nat. Rev. Mol. Cell Biol.1:11–21.
4.Birnbaumer, L., G. Boulay, D. Brown, M. Jiang, A. Dietrich, K. Mikoshiba, X. Zhu, and N. Qin.2000. Mechanism of capacitative Ca2⫹entry (CCE): Interaction between IP3 receptor and TRP links internal calcium storage compartment to plasma membrane CCE channels. Recent Prog. Horm. Res.
55:127–161.
5.Bishop, N., A. Horman, and P. Woodman.2002. Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal
protein-ubiquitin conjugates. J. Cell Biol.157:91–101.
6.Bleasdale, J. E., G. L. Bundy, S. Bunting, F. A. Fitzpatrick, R. M. Huff, F. F. Sun, and J. E. Pike.1989. Inhibition of phospholipase C dependent process
by U73122. Adv. Prostaglandin Thromboxane Leukot. Res.19:590–593.
7.Booth, A. M., J. K. Fallon, J.-M. Yang, J. E. K. Hildreth, and S. J. Gould.
2006. Exosomes and HIV Gag bud from endosome-like domains of the T cell
plasma membrane. J. Cell Biol.172:923–935.
8.Campbell, S., R. J. Fisher, E. M. Towler, H. J. Isaaq, T. Wolfe, L. Phillips, and A. Rein.2001. Modulation of HIV-like particle assembly in vitro by
inositol phosphates. Proc. Natl. Acad. Sci. U. S. A.98:10875–10879.
9.Carter, C. A., and L. S. Ehrlich.2008. Cell biology of HIV-1 infection of
macrophages. Annu. Rev. Microbiol.62:425–443.
10.Chakrabarti, R., and R. Chakrabarti.2006. Calcium signaling in
non-excit-able cells: Ca2⫹release and influx are independent events linked to two
plasma membrane Ca2⫹entry channels. J. Cell Biochem.99:1503–1516.
11.Chami, M., B. Oules, and P. Peterlini-Brechot.2006. Cytobiological conse-quences of calcium-signaling alterations induced by human viral proteins.
Biochim. Biophys. Acta1763:1344–1362.
12.Davies-Cox, E. V., I. Laffafian, and M. B. Hallett.2001. Control of Ca2⫹ influx in human neutrophils by inositol 1,4,5-triphosphate (IP3) binding: differential effects of microinjected IP3 receptor antagonists. Biochem. J.
355:139–143.
13.Dellis, O., S. Dedos, S. C. Tovey, Taufiq-Ur-Rahman, S. J. Dubel, and C. W. Taylor.2006. Ca2⫹entry through plasma membrane IP3 receptors. Science
313:229–233.
14.Dong, X., H. Li, A. Derdowski, L. Ding, A. Burnett, X. Chen, T. R. Peters, T. S. Dermody, E. Woodruff, J.-J. Wang, and P. Spearman.2005. AP-3 directs the intracellular trafficking of HIV-1 Gag and plays a key role in
particle assembly. Cell120:663–674.
15.Doyotte, A., M. R. G. Russell, C. R. Hopkins, and P. G. Woodman.2005. Depletion of TSG101 forms a mammalian “Class E” compartment: a
mul-ticisternal early endosome with multiple sorting defects. J. Cell Sci.118:
3003–3017.
16.Ellis, M. V., S. R. James, O. Perisic, C. P. Downes, R. L. Williams, and M. Katan.1998. Catalytic domain of phosphoinositide-specific phospholipase C (PLC). Mutational analysis of residues within the active site and hydrophobic
ridge of PLC␦1. J. Biol. Chem.273:11650–11659.
17.Garrus, J. E., U. K. von Schwedler, O. W. Pornillos, S. G. Morham, K. H. Zavitz, H. E. Wang, D. A. Wettstein, K. M. Stray, M. Cote, R. L. Rich, D. G. Myszka, and W. I. Sundquist.2001. Tsg101 and the vacuolar protein sorting
pathway are essential for HIV-1 budding. Cell107:55–65.
18.Goff, A., L. S. Ehrlich, S. N. Cohen, and C. A. Carter.2003. Tsg101 control of human immunodeficiency virus type 1 Gag trafficking and release. J. Virol.
77:9173–9182.
19.Gottlinger, H. G., T. Dorfman, J. G. Sodroski, and W. A. Haseltine.1991. Effect of mutations affecting the p6 Gag protein on human
immunodefi-ciency virus particle release. Proc. Natl. Acad. Sci. U. S. A.88:3195–3199.
20.Grigorov, B., F. Arcanger, P. Roingeard, J.-L. Darlix, and D. Muriaux.2006. Assembly of infectious HIV-1 in human epithelial and T-lymphoblastic cell
lines. J. Mol. Biol.359:848–862.
21.Harmon, B., and L. Ratner.2008. Induction of the G␣qsignaling cascade by
the human immunodeficiency virus envelope is required for virus entry.
J. Virol.82:9191–9205.
22.Hattori, M., A. Z. Suzuki, T. Higo, H. Miyauchi, T. Michikawa, T. Naka-mura, T. Inoue, and K. Mikoshiba.2004. Distinct roles of inositol
1,4,5-triphosphate receptor types 1 and 3 in Ca2⫹signaling. J. Biol. Chem.279:
11967–11975.
23.Hermida-Matsumoto, L., and M. D. Resh.2000. Localization of human immunodeficiency virus type 1 Gag and Env at the plasma membrane by
confocal imaging. J. Virol.74:8670–8679.
24.Herrera-Vigenor, F., R. Hernandez-Garcia, M. Valdez-Sanches, J. Vazquez-Prado, and G. Reyes-Cruz.2006. AMSH regulates calcium-sensing receptor
signaling through direct interactions. Biochem. Biophys. Res. Commun.347:
924–930.
25.Horowitz, L. F., W. Hirdes, B. C. Suh, D. W. Hilgemann, K. Mackie, and B. Hille.2005. Phospholipase C in living cells: activation, Ca2⫹requirement,
and regulation of M current. J. Gen. Physiol.126:243–262.
26.Jackson, T. R., S. I. Paterson, O. Thastrup, and M. R. Hanley.1988. A novel
tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2⫹
without generation of inositol phosphates in NG115-401L neuronal cells.
Biochem. J.253:81–86.
27.Jagannath, A., and M. J. A. Wood.2009. Localization of double-stranded small interfering RNA to cytoplasmic processing bodies is Ago2 dependent and results in up-regulation of GW182 and Argonaute-2. Mol. Biol. Cell
20:521–529.
28.Jin, J., T. Sturgeon, C. Chen, S. C. Watkins, O. A. Weisz, and R. C. Mon-telaro.2007. Distinct intracellular trafficking of equine infectious anemia virus and human immunodeficiency virus type 1 Gag during viral assembly and budding revealed by bimolecular fluorescence complementation assays.
J. Virol.81:11226–11235.
29.Katzmann, D. J., M. Babst, and S. D. Emr.2001. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a
conserved endosomal protein sorting complex, ESCRT-1. Cell106:145–155.
30.Khan, M., M. Garcia-Barrio, and M. D. Powell.2001. Restoration of wild-type infectivity to human immunodeficiency virus wild-type 1 strains lacking Nef
by intravirion reverse transcription. J. Virol.75:12081–12087.
31.Kimpton, J., and M. Emerman.1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on
the basis of activation of an integrated-galactosidase gene. J. Virol.66:
2232–2239.
32.Kobayashi, T., M-H. Beuchat, J. Chevallier, A. Makino, N. Mayran, J-M. Escola, C. Lebrand, P. Cosson, T. Kobayashi, and J. Gruenberg.2002. Separation and characterization of late endosomal membrane domains.
J. Biol. Chem.277:32157–32164.
33.Li, L., and L. S. Chin.2003. The molecular machinery of synaptic vesicle
exocytosis. Cell Mol. Life Sci.60:942–960.
34.Lindwasser, O. W., and M. D. Resh.2004. Human immunodeficiency virus type 1 Gag contains a dileucine-like motif that regulates association with
multivesicular bodies J. Virol.78:6013–6023.
35.Loew, L.2007. Where does all the PIP2 come from? J. Physiol.582:945–951. 36.Lytton, J., M. Westlin, and M. R. Hanley.1991. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps.
J. Biol. Chem.266:17067–17071.
37.Martin-Serrano, J., A. Yaravoy, D. Perez-Caballero, and P. D. Bieniasz.
2003. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc. Natl. Acad. Sci.
U. S. A.100:12414–12419.
38.Martin-Serrano, J., T. Zang, and P. D. Bieniasz.2001. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle
assembly to facilitate egress. Nat. Med.7:1313–1319.
39.Mikoshiba, K.2006. Inositol 1,4,5-triphosphate (IP3) receptors and their
role in neuronal cell function. J. Neurochem.97:1627–1633.
40.Mullock, B. M., N. A. Bright, C. W. Fearson, S. R. Gray, and J. P. Luzio.
1998. Fusion of lysosomes with late endosomes produces a hybrid organelle
of intermediate density and is NSF dependent. J. Cell Biol.140:591–601.
41.Myers, E. L., and J. F. Allen.2002. Tsg101, an inactive homologue of ubiquitin ligase E2, interacts specifically with human immunodeficiency virus type 2 Gag polyprotein and results in increased levels of ubiquitinated Gag.
J. Virol.76:11226–11235.
42.Nermut, M. V., W-H. Zhang, G. Francis, F. Ciampor, Y. Morikawa, and I. M. Jones.2003. Time course of Gag protein assembly in HIV-1 infected cells: a
study by immunoelectron microscopy. Virology305:219–227.
43.Nimnual, A. S., L. J. Taylor, and D. Bar-Sagi.2003. Redox-dependent
downregulation of Rho by Rac. Nat. Cell Biol.5:236–241.
44.Nydegger, S., M. Foti, A. Derdowski, P. Spearman, and M. Thali.2003.
HIV-1 egress is gated through late endosome membranes. Traffic4:902–910.
45.Ono, A., S. D. Ablan, S. J. Lockett, K. Nagashima, and E. O. Freed.2004. Phosphatidylinositol (4,5)-biphosphate regulates HIV-1 Gag targeting to the
plasma membrane. Proc. Natl. Acad. Sci. U. S. A.101:14889–14894.
46.Ono, A., and E. O. Freed.2004. Cell-type-dependent targeting of human immunodeficiency virus type 1 assembly to the plasma membrane and the
multivesicular body. J. Virol.78:1552–1563.
on November 8, 2019 by guest
http://jvi.asm.org/
47.Patterson, R. L., D. Boehning, and S. H. Snyder. 2004. Inositol 1, 4,
5-triphosphate receptors as signal integrators. Annu. Rev. Biochem.73:437–
465.
48.Perlman, M., and M. D. Resh.2006. Identification of an intracellular
traf-ficking and assembly pathway for HIV-1 Gag. Traffic7:731–745.
49.Pols, M. S., and J. Klumperman. 2009. Trafficking and function of the
tetraspanin CD63. Exp. Cell Res.315:1584–1592.
50.Pryor, P. R., B. M. Mullock, N. A. Bright, S. R. Gray, and J. P. Luzio.2000.
The role of intraorganellar Ca2⫹in late endosome-lysosome heterotypic
fusion and in the reformation of lysosomes from hybrid organelles. J. Cell
Biol.149:1053–1062.
51.Putney, J. W., and G. S. Bird.2008. Cytoplasmic calcium oscillations and
store-operated calcium influx. J. Physiol.586:3055–3059.
52.Raiborg, C., and H. Stenmark.2009. The ESCRT machinery in endosomal
sorting of ubiquitylated membrane proteins. Nature458:445–452.
53.Razi, M., and C. E. Futter.2006. Distinct roles for Tsg101 and Hrs in
multivesicular body formation and inward vesiculation. Mol. Biol. Cell17:
3469–3483.
54.Rhee, S. G.2001. Regulation of phosphoinositide-specific phospholipase C.
Annu. Rev. Biochem.70:281–312.
55.Saad, J. S., J. Miller, J. Tai, A. Kim, R. H. Ghanam, and M. F. Summers.
2006. Structural basis for targeting HIV-1 Gag proteins to the plasma
mem-brane for virus assembly. Proc. Natl. Acad. Sci. U. S. A.103:11364–11369.
56.Savina, A., M. Furlan, M. Vidal, and M. I. Colombo.2003. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem.
278:20083–20090.
57.Semenova, S. B., K. I. Kiselev, and G. N. Mozhaeva.1999. Low-conductivity calcium channels in the macrophage plasma membrane: activation by
inosi-tol-1,4,5-triphosphate. Neurosci. Behav. Physiol.29:339–345.
58.Sherer, N. M., M. J. Lehmann, L. F. Jimenez-Soto, A. Ingmundson, S. M. Horner, G. Cicchetti, P. G. Allen, M. Pypaert, J. M. Cunningham, and W. Mothes.2003. Visualization of retroviral replication in living cells reveals
budding into multivesicular bodies. Traffic4:785–801.
59.Shkriabai, N., S. A. Datta, Z. Zhao, S. Hess, A. Rein, and M. K.
Kvaratskhe-lia.2006. Interactions of HIV-1 Gag with assembly cofactors. Biochemistry
45:4077–4083.
60.Strack, B. A., S. Calistri, E. Craig, E. Popova, and H.-G. Goettlinger.2003. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in
virus budding. Cell114:689–699.
61.Taylor, L. J., A. B. Walsh, P. Hearing, and D. Bar-Sagi.2000. Single cell
assays for Rac activity. Methods Enzymol.325:327–334.
62.Tu, C. L., W. Chang, and D. D. Bikle.2007. The role of the calcium sensing receptor in regulating intracellular calcium handling I n human epidermal
keratinocytes. J. Invest. Dermatol.127:1074–1083.
63.Uchiyama, T., F. Yoshikawa, A. Hishida, T. Furuichi, and K. Mikoshiba.
2002. A novel recombinant hyperaffinity inositol 1,4,5-triphosphate (IP3) absorbent traps IP3, resulting in specific inhibition of IP3-mediated calcium
signaling. J. Biol. Chem.277:8106–8113.
64.Vana, M. L., Y. Tang, A. Chen, G. Medina, C. Carter, and J. Leis.2004. Role of Nedd4 and ubiquitination of Rous sarcoma virus Gag in budding of
virus-like particles from cells. J. Virol.78:13943–13953.
65.Vanoevelen, J., L. Raeymaekers, J. B. Parys, H. De Smedt, K. Van Baelen, G. Callewaert, F. Wuytack, and L. Missiaen.2004. Inositol triphosphate pro-ducing agonists do not mobilize the thapsigargin-insensitive part of the
endoplasmic-reticulum and Golgi Ca2⫹store. Cell Calcium35:115–121.
66.Vermassen, E., J. B. Parys, and J.-P. Mauger.2004. Subcellular distribution of the inositol 1,4,5-triphosphate receptors: functional relevance and
molec-ular determinants. Biol. Cell96:3–17.
67.VerPlank, L., F. Bouamr, T. J. LaGrassa, B. Agresta, A. Kikonyogo, J. Leis, and C. A. Carter.2001. Tsg101, a homologue of ubiquitin-conjugating (E2)
enzymes, binds the L domain in HIV type 1 Pr55Gag. Proc. Natl. Acad. Sci.
U. S. A.98:7724–7729.
68.von Schwedler, U. K., M. Stuchell, B. Muller, D. M. Ward, H. Y. Chung, E. Morita, H. E. Wang, T. Davis, G. P. He, D. M. Cimbora, A. Scott, H.-G. Krausslich, J. Kaplan, S. G. Morham, and W. I. Sundquist.2003. The
protein network of HIV budding. Cell114:701–713.
69.Waheed, A. A., and E. O. Freed.2009. Lipids and membrane microdomains
in HIV-1 replication. Virus Res.143:162–176.
70.Watt, S. A., G. Kular, I. N. Fleming, C. P. Downes, and J. M. Lucocq.2002. Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the
pleckstrin homology domain of phospholipase C␦1. Biochem. J.363:657–
666.
71.Wills, J. W., and R. C. Craven.1991. Form, function, and use of retroviral
Gag proteins. AIDS5:639–654.
72.Wilsher, N. E., W. J. Court, R. Ruddle, Y. M. Newbatt, W. Aherne, P. W. Sheldrake, N. P. Jones, M. Katan, S. A. Eccles, and F. I. Raynaud.2007. The
phosphoinositide-specific phospholipase C inhibitor U73122 (1-(6-(17
-3-me-thoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole-2,5,-dione) spontane-ously forms conjugates with common components of cell culture medium. Drug
Metab. Dispos.35:1017–1022.
73.Wojcikiewicz, R. J. H.1995. Type I, II, and III inositol 1,4,5-triphosphate receptors are unequally susceptible to down-regulation and are expressed in
markedly different proportions in different cell types. J. Biol. Chem.270:
11678–11683.
74.Xie, Z., S. M. Chang, S. D. Pennypacker, E. Y. Liao, and D. D. Bikle.2009.
Phosphatidylinositol-4-phosphate 5-kinase 1␣mediates extracellular
calcium-induced keratinocyte differentiation. Mol. Biol. Cell20:1695–1704.