Epithelia and Adult Oral Epithelia
Sharof M. Tugizov,a,bRossana Herrera,aPiri Veluppillai,bDeborah Greenspan,bVanessa Soros,cWarner C. Greene,cJay A. Levy,a and Joel M. Palefskya,b
Department of Medicineaand Department of Orofacial Sciences,bUniversity of California San Francisco, San Francisco, California, USA, and Gladstone Institute of Virology and Immunology, University of California, San Francisco, California, USAc
While human immunodeficiency virus (HIV) transmission through the adult oral route is rare, mother-to-child transmission
(MTCT) through the neonatal/infant oral and/or gastrointestinal route is common. To study the mechanisms of cell-free and
cell-associated HIV transmission across adult oral and neonatal/infant oral/intestinal epithelia, we established
ex vivo
organ
tissue model systems of adult and fetal origin. Given the similarity of neonatal and fetal oral epithelia with respect to epithelial
stratification and density of HIV-susceptible immune cells, we used fetal oral the epithelium as a model for neonatal/infant oral
epithelium. We found that cell-free HIV traversed fetal oral and intestinal epithelia and infected HIV-susceptible CD4
ⴙT
lym-phocytes, Langerhans/dendritic cells, and macrophages. To study the penetration of cell-associated virus into fetal oral and
in-testinal epithelia, HIV-infected macrophages and lymphocytes were added to the surfaces of fetal oral and inin-testinal epithelia.
HIV-infected macrophages, but not lymphocytes, transmigrated across fetal oral epithelia. HIV-infected macrophages and, to a
lesser extent, lymphocytes transmigrated across fetal intestinal epithelia. In contrast to the fetal oral/intestinal epithelia, cell-free
HIV transmigration through adult oral epithelia was inefficient and virions did not infect intraepithelial and subepithelial
HIV-susceptible cells. In addition, HIV-infected macrophages and lymphocytes did not transmigrate through intact adult oral
epithe-lia. Transmigration of cell-free and cell-associated HIV across the fetal oral/intestinal mucosal epithelium may serve as an initial
mechanism for HIV MTCT.
E
pidemiologic data indicate that the risk of genital HIV
trans-mission in adults is substantially higher than the risk of oral
transmission (38, 40, 46, 52). However, HIV mother-to-child
transmission (MTCT) via the neonatal oral and/or
gastrointesti-nal route is not uncommon and was even less so in the
pre-antiretroviral-treatment era (13, 29, 33).
HIV MTCT in the fetus/neonate may occur
in utero
or during
labor from exposure to HIV-containing amniotic and
cervico-vaginal fluids (24, 27, 32, 35, 42). Furthermore, HIV MTCT may
result from breastfeeding milk containing HIV (4, 5, 37, 47, 49,
59). While the rate of HIV MTCT has been reduced to less than 2%
with antiretroviral therapy (ART) in developed countries, HIV
MTCT in developing African and Asian countries may be as high
as 25% to 30% (13, 29). Analysis of HIV transmission in mother/
child pairs has shown that the majority of HIV-1 strains
transmit-ted from mother to child are R5 tropic (7–9).
Using a single-layer, polarized epithelial cell model, we recently
showed that HIV can traverse both adult and fetal oral epithelia;
however, virions that transmigrated through adult epithelial cells
were rendered noninfectious, whereas those that passed through
fetal epithelial cells remained highly infectious (54). We further
found that HIV inactivation by adult oral epithelial cells was
me-diated by high-level expression of the anti-HIV innate proteins
beta-defensin 2 (HBD2) and HBD3. Thus, high-level antiviral
in-nate protein expression may contribute to epithelial resistance to
HIV transmission across the adult oral epithelium, in contrast to
the fetal oral epithelium, which lacks expression of these innate
proteins and allows transcellular passage of infectious virions.
In the current study, we further investigated the mechanisms
of HIV transmigration through mucosal epithelia by using
ex vivo
oral tissue explants. We show that the more highly stratified adult
oral epithelium limits viral penetration more efficiently than does
the less-stratified fetal oral epithelium. The greater efficiency of
HIV transmission across fetal versus adult oral epithelia may
re-flect a reduced barrier function of fetal epithelia associated with
paucistratification. We also show that R5-tropic-HIV-infected
macrophages can penetrate into fetal mucosal epithelia from the
apical surface, suggesting that this may be one of the predominant
mechanisms of transmission of R5-tropic HIV from mother to
child (7–9).
MATERIALS AND METHODS
Collection of tissues and establishment of polarized oriented tissue ex-plants.One or two fresh biopsy specimens of nonkeratinized buccal mu-cosae were obtained using 6-mm-diameter biopsy punches from healthy, HIV-seronegative volunteers (age range, 30 to 41 years) who had no in-flammation in the oral cavity. Each biopsy specimen was cut into two or more pieces and used for propagation of tissue explants. Fetal buccal, oropharyngeal, and small intestinal (jejunal region) tissue explants con-taining the mucosal epithelium and lamina propria were obtained from fetuses 18 to 24 weeks old that had been subjected to elective termination for nonmedical reasons from HIV-uninfected women. The tissues were placed in a tube with 2 ml of RPMI medium containing 10% heat-inactivated fetal bovine serum, 20 mM HEPES, 100 mM glutamine, 20 g/ml gentamicin, 200 U/ml penicillin, and 200g/ml streptomycin.
To establish polarized organ cultures, adult oral biopsy specimens were used approximately 30 min after biopsy procedures. Fetal oral and
Received15 October 2011Accepted19 December 2011
Published ahead of print28 December 2011
Address correspondence to Sharof Tugizov, sharof.tugizov@ucsf.edu.
Copyright © 2012, American Society for Microbiology. All Rights Reserved.
doi:10.1128/JVI.06578-11
on November 7, 2019 by guest
http://jvi.asm.org/
intestinal biopsy specimens were used approximately 2 to 3 h after abor-tion procedures. Explants were placed with the mucosal side facing up in the top chamber of Millicell filter inserts (Millipore) (diameter, 12 mm; pore size, 0.4m). The lateral edges of the explants were sealed with 3% agarose, as described previously (11, 30, 31). The orientation of the ex-plants was monitored using a stereomicroscope (Stereomaster; Fisher Sci-entific).
Infant buccal and tonsil tissues from 4 infant cadavers (newborn, 2 days old, 53 days old, and 3 months old) were used for immunostaining of HIV-susceptible cells. Approval for collection of adult, infant, and fetal biopsy tissues was obtained from the Institutional Review Board at the University of California, San Francisco.
Detection of insoluble tight junction proteins in adult and fetal oral epithelia.To detect membrane-associated, insoluble tight junction pro-teins in oral epithelium, the epithelial portion of the mucosa was isolated from the submucosa by the use of a surgical scalpel under a stereomicro-scope (Stereomaster; Fisher Scientific). The stratified epithelium was ho-mogenized and extracted with Triton X-100 buffer (1% Triton X-100, 100 mM NaCl, 10 mM HEPES [pH 7.2], 2 mM EDTA) containing a cocktail of protease inhibitors consisting of the following ingredients: phenylmeth-ylsulfonyl fluoride (PMSF) (1 mM), aprotinin (10g/ml), leupeptin (10 g/ml), and pepstatin A (10g/ml) (36). The extracts were centrifuged at 15,000⫻gfor 30 min. The supernatant and the pellet were considered to be the Triton X-100-soluble and -insoluble fractions, respectively. Protein concentrations were measured using the Bradford method, and both sol-uble and insolsol-uble fractions were mixed with 2⫻sample buffer (4% so-dium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS], 0.75 M Tris [pH 8.8], 20% glycerol, 20 mM dithiothreitol). Proteins were sepa-rated using denaturing SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and 16% gels, and claudin-1 and occludin were detected using a Western blot assay and rabbit antisera (Zymed).
Electron microscopy.Tissues were fixed with 2% glutaraldehyde– 4% formaldehyde in 0.1 M sodium cacodylate buffer, pH 7.3. Tissues were postfixed with 1% osmium tetroxide alone or with 1.5% potassium ferri-cyanide followed by 2% uranyl acetate and then dehydrated with ethanol. Tissues were embedded in Eponate 812 or EmBed 812. Ultrathin sections were stained with 2% uranyl acetate and examined at 120 kV using a JEOL 1400 transmission electron microscope.
Viruses.Green fluorescent protein (GFP)-labeled HIV-1 virions were produced by cotransfection of 293T cells with proviral DNA of either X4-tropic HIV-1NL4-3or R5-tropic HIV-181Aand an expression vector encoding a GFP-Vpr fusion protein, as described previously (45). After 48 h, the released virions were concentrated by ultracentrifugation at 20,000 rpm in an SW41 rotor for 2 h at 4°C. p24 antigen in the viral stocks was quantified using a p24 enzyme-linked immunosorbent assay (ELISA) and stored in aliquots at⫺80°C. The R5-tropic HIV-1SF170and X4-tropic HIV-192UG029primary clinical isolates were grown in peripheral blood mononuclear cells (PBMCs) from HIV-uninfected individuals. PBMCs were activated with 2.5g/ml phytohemagglutinin (Sigma) and 1g/ml interleukin-2 (BD Bioscience) for 3 days.
Cell-free HIV penetration into adult and fetal oral mucosal epithe-lia.To analyze penetration of cell-free HIV, GFP-labeled X4-tropic HIV-1NL4-3and R5-tropic HIV-181Avirions (100 ng of p24 per explant [ap-proximately 108virions]) were added to the upper chambers, and filter inserts with explants were incubated at 37°C or 4°C. In parallel experi-ments, matching tissues were pretreated with 10M colchicine for 2 h at 37°C, washed, and then used for virion penetration experiments. To dis-rupt tight junctions, explants were incubated in culture medium contain-ing 10 mM EDTA for 2 h, extensively washed, and then used for virus penetration experiments. The duration of transcytosis in most experi-ments was 4 h and for some experiexperi-ments was 8 or 20 h. Cross-sections of tissues were cut to 7m thickness with a horizontal orientation and ex-amined for GFP virus or immunostained for cellular proteins. To amplify the GFP signal of GFP-labeled virions, sections were stained with rabbit anti-GFP antibodies conjugated with fluorescein isothiocyanate (FITC)
(Invitrogen) (5g/ml). Penetration of GFP virus into the epithelium was confirmed by immunostaining of sections with mouse or goat anti-HIV-1 p24 antibodies (Virostat) (5g/ml).
To examine the infectivity of HIV that penetrated into intact and disrupted epithelium, adult buccal biopsy specimens were cut into 2 pieces, which were then used for polarized oriented explants. One of the matching explants was treated with 10 mM EDTA for 2 h, and the second one was not treated and served as a control. In parallel experiments, R5-tropic HIV-1SF170and X4-tropic HIV-192UG029primary clinical isolates were then applied to the surfaces of intact and EDTA-disrupted tissue explants at 100 ng of p24/insert. The infectious activity of input R5-tropic HIV-1SF170and X4-tropic HIV-192UG029stock viruses was measured us-ing a reverse transcriptase (RT) assay as described previously (21). The RT values of HIV-1SF170and HIV-192UG029stock viruses were 982,000 and 588,000 cpm, respectively. After 4 h of incubation, explants were washed with trypsin to remove virions from mucosal surfaces as described previ-ously (50), and tissues were homogenized in 0.5 ml of RPMI 1640 media. Tissue homogenates were centrifuged for 10 min at 2,000 rpm. Superna-tant (200 ml) was used for infection of 106PBMCs, and after 1, 2, and 3 weeks, 200l of media from each of the PBMC cultures was tested using an ELISA for HIV-1 p24. The infectivity of HIV-1SF170and HIV-192UG029 viruses that penetrated into intact fetal buccal and intestinal tissues was evaluated using a similar approach.
Assay for inhibition of cell-free HIV penetration into fetal oral epi-thelium.For antibody-mediated inhibition of HIV-1 penetration into fetal oral epithelium, the following antibodies were used: rabbit poly-clonal anti-galactosyl ceramide (anti-GalCer) (Chemicon) (25g/ml), mouse monoclonal anti-heparan sulfate proteoglycan (anti-HSPG) (Seikagaku) (25g/ml), mouse monoclonal anti-CXCR4 (pool of clones 44708, 44716, 44717, and 12G5) (10g/ml of each), and anti-CCR5 (pool of clones 45531, 45549, and 2D7) (50g/ml of each). Antibodies were added to the apical surfaces of polarized oriented fetal oral tissue explants, which were then incubated for 1 h prior to the addition of virus. A parallel set of experiments was performed in which tissues were treated with ap-propriate isotype controls for the anti-GalCer (rabbit IgG), -HSPG (mouse IgM), and -CXCR4 and -CCR5 (mouse IgG2a and IgG2b) anti-bodies. The concentrations of isotype antibodies were similar to the con-centrations of specific antibodies. After 4 h of incubation, tissues were fixed and immunostained for HIV detection using a pool of human monoclonal antibodies composed of antibodies against HIV gp41 (2F5, D50) and gp120 (2G12), as described by Ganor et al. (15). Quantification of viral penetration was performed by counting epithelial cells containing HIV-specific signals. Inhibition of viral penetration was defined as the percentage of HIV-infected epithelial cells exposed to receptor-specific antibodies relative to HIV-infected epithelial cells exposed to isotype con-trol antibodies. All antibodies to CXCR4, CCR5, and HIV proteins were provided by the NIH AIDS Reagent Program.
Cell-associated HIV penetration into adult and fetal oral mucosal epithelia.To examine cell-associated HIV penetration into oral epithe-lium, PBMCs from heparinized blood were isolated using a Ficoll-Paque Plus density gradient (Sigma). CD4⫹T lymphocytes and CD14⫹ mono-cytes were then isolated by positive selection using CD4 and anti-CD14 Microbeads (Miltenyi Biotec), respectively (Miltenyi Biotec). To propagate R5-tropic HIV-1SF170cell-associated virus, monocyte-derived macrophages were established. To induce differentiation of monocytes into macrophages, purified monocytes were cultured in the presence of 20 ng/ml macrophage colony-stimulating factor for 7 days. To confirm mac-rophage differentiation, cells were immunostained for CD68, and CD68-positive macrophages were infected with R5-tropic HIV-1SF170at 20 ng of p24 per 106cells. To propagate cell-associated X4-tropic HIV-1
92UG029, CD4⫹T lymphocytes were infected with HIV-192UG029at 20 ng of p24 per 106cells. Infection was confirmed after 7 to 10 days by detection of p24 released from macrophages or lymphocytes by ELISA. HIV-infected CD68⫹macrophages were dissociated from the plastic using enzyme-free cell dissociation buffer containing 5 mM EDTA and a cell scraper, as
HIV Transepithelial Migration via Oral Epithelium
on November 7, 2019 by guest
http://jvi.asm.org/
described previously (25, 26). To detect penetration of HIV-infected mac-rophages and lymphocytes into oral epithelium, infected cells were incu-bated with 10M carboxyfluorescein diacetate succinimidyl ester (CFSE) for 10 min as described in the manufacturer’s protocol (Invitrogen), en-abling transmigrated HIV-infected cells to be distinguished from the intra- and subepithelial macrophages and lymphocytes present in the tis-sues at the time of collection. When CFSE penetrates the cells, its acetate groups are cleaved by intracellular esterases to yield a highly fluorescent green signal. HIV-infected, CFSE-labeled macrophages and lymphocytes were collected, washed, and added to the mucosal surfaces of polarized oriented explants at 106cells per explant. In some experiments, incuba-tion of HIV-infected cells with tissues was done in the presence of breast milk from HIV-uninfected, healthy, breastfeeding mothers. A pool of whole breast milk from 5 donors was used for experiments. Cells were resuspended in breast milk at a 1:2 dilution and added to the mucosal surfaces of explants. After 4 h, tissue explants were fixed and sectioned, and cross-sections were examined for CFSE-labeled cells coimmunos-tained with goat anti-HIV-1 antiserum.
Confocal immunofluorescence.Immunostaining of tissue sections was performed as described previously (53, 54). The following antibodies were used for detection of HIV-1 and cellular proteins: mouse anti-HIV-1 p24 (NIH AIDS Research reagent program) (5g/ml); goat anti-HIV-1 p24 (Virostat) (5g/ml); goat anti-HIV-1 (US Biological) (5g/ml); mouse anti-CD3 (BD Bioscience), goat anti-CD68 (R&D), rat anti-CD1a (Biosource), and mouse anti-dendritic cell specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN) (BD Bioscience) (1g/ml of each); and rabbit anti-ZO-1 (1g/ml), -occludin (1 g/ml), and -claudin-1 (5 g/ml) (all from Zymed). Secondary antibodies labeled with fluorescein isothiocyanate (FITC), tetramethyl rhodamine isothio-cyanate (TRITC), or cyanine 5 (Cy5) were purchased from Jackson Im-munoResearch. The specificity of each primary antibody was confirmed by negative staining with the corresponding isotype control antibody. Cell nuclei were counterstained with TO-PRO-3 iodide (Molecular Probes) (blue). Immunostaining for each experiment was performed from 3 to 10 times. Cells were analyzed using a krypton-argon laser coupled with a Bio-Rad MRC2400 confocal head. The data were analyzed using Laser Sharp software.
For quantitative analysis of cell-free HIV penetration into adult oral epithelia, the sections of adult buccal explants exposed to GFP-labeled HIV were immunostained with anti-HIV p24 and anti-occludin antibod-ies, and the GFP- and p24-positive (colocalized) cells were counted. To examine penetration of HIV into virus-susceptible cells, sections were coimmunostained for HIV p24 and for CD4, CD68, or CD1a, which are markers for lymphocytes, macrophages, and Langerhans cells (LCs), re-spectively.
For quantitative evaluation of HIV penetration into fetal oral epithe-lia, tissue sections were immunostained for CD45 (clone T29/33; Dako), which is a marker of white blood cells, including dendritic cells/LCs, mac-rophages, and CD3⫹lymphocytes. The same sections were costained for HIV p24, which confirms the presence of GFP-labeled HIV in CD45⫹ immune cells. HIV-GFP⫹p24⫹CD45⫹intraepithelial and subepithelial immune cells in tissue explants were counted under various experimental conditions.
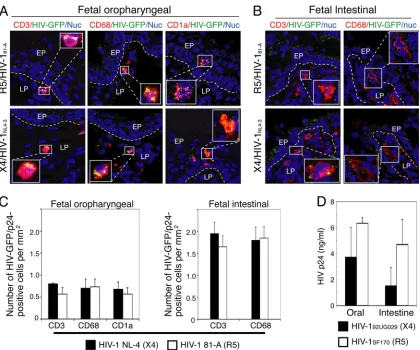
To quantitate HIV-infected immune cells, fetal oropharyngeal ex-plants exposed to GFP-labeled X4-tropic HIV-1NL4-3or R5-tropic HIV-181Avirus were immunostained with antibodies to HIV-1 p24 and the following immune cell markers: CD3 for T lymphocytes, CD68 for mac-rophages, and CD1a for LCs. HIV-GFP/p24-positive cells expressing im-mune cell markers within the epithelium and lamina propria were counted.
For quantitative analysis of cell-associated HIV penetration into oral epithelia, sections were immunostained with goat anti-HIV-1 antiserum, and CFSE-labeled HIV-infected and CFSE-unlabeled HIV-infected cells were counted.
The number of HIV-positive epithelial and immune cells in adult and
fetal mucosal epithelia was counted in at least 3 sections of each explant and 10 randomly chosen separate fields in each section. Results are pre-sented as the percentage of positive cells or the average number of positive cells per square millimeter. Standard errors of the means of the results determined for positive cells were calculated using Microsoft Excel soft-ware.
Statistical analysis.Comparisons of differences in X4- and R5-tropic virus infection between appropriate target cells and infectivity of tissue-penetrated X4- and R5-tropic viruses in PBMC cells were performed us-ing a cutoff ofP⬍0.05 with Student’sttest.
RESULTS
Adult, infant, and fetal mucosal epithelial cells have tight
junc-tions.
To determine whether there were differences in the
integ-rity of junctions among adult, infant, and fetal oral epithelia, we
analyzed tight junction protein expression in these tissues by the
use of a confocal immunofluorescence assay. We also examined
fetal intestinal epithelia, since fetal and neonatal intestinal
epithe-lia may also be portals for HIV MTCT. Tissue sections were
im-munostained for the tight junction proteins zonula occludens 1
(ZO-1) (Fig. 1A), occludin, and claudin-1 (data not shown). Tight
junction proteins were detected in multistratified (10- to 30-layer)
adult, 9-day- and 3-month-old infant, and 18- to 24-week-old
fetal buccal epithelia (Fig. 1A). Tight junctions were also detected
in infant tonsil and fetal oropharyngeal epithelia (data not
shown). The thicknesses of infant and fetal oral epithelia were
comparable. Infant oral epithelia had 3 to 7 layers of stratification,
and fetal epithelia had 2 to 5 layers of stratification. However,
some areas of infant and fetal oral epithelia had up to 10 cell layers.
The monostratified columnar fetal intestinal epithelium was also
positive for the tight junction proteins ZO-1 (Fig. 1A) and
occlu-din (data not shown).
By electron microscopy, tight junction structures appeared as
highly electron-dense regions between membranes of juxtaposing
epithelial cells of the granulosum and spinosum layers of the adult
oral epithelium (Fig. 1B, insets). Electron microscopy of fetal oral
(Fig. 1C, red arrowheads in the inset) and fetal intestinal (Fig. 1D,
red arrowheads in the inset) epithelia also revealed tight junctions.
Western blot analysis of claudin-1 and occludin from adult and
fetal oral tissue extracted with Triton X-100 showed that these
proteins were predominantly associated with the insoluble
frac-tions of junctional complexes (Fig. 1E). These findings indicate
the presence of well-formed tight junctions in both the adult and
fetal oral epithelia. Together, these findings demonstrate that
adult, infant, and fetal oral and fetal intestinal epithelia all contain
well-developed tight junctions that support a barrier function.
Penetration of cell-free HIV into adult oral mucosal
epithe-lium is limited to the surface granulosum layers.
To study the
penetration of HIV from the surface of the adult epithelium, we
mounted adult buccal explants from 11 different donors in
aga-rose media, allowing interaction of HIV with only the mucosal
epithelial surface (Fig. 2A). Green fluorescent protein
(GFP)-labeled X4-tropic HIV-1
NL4-3and R5-tropic HIV-1
81Avirions
were added to the epithelial surfaces of the explants, and explants
were incubated at 37°C for 4 h. Tissues were then fixed and
sec-tioned, and viral penetration was examined by detection of GFP.
Since the GFP signal was weak, signals were amplified by
immu-nostaining of sections with anti-GFP antibodies.
Migration of GFP-labeled virions into the surface layers of the
adult oral epithelium was detected in explants from 7 of 11 donors
(63%). Confocal microscopy analysis revealed the presence of
on November 7, 2019 by guest
http://jvi.asm.org/
GFP-labeled virions within the most superficial 2 to 5 layers of the
oral epithelium (Fig. 2B). The HIV-GFP signal could be seen in
both small and large particles, which may represent unclustered
and clustered virions, respectively. Viral penetration was
incon-sistently detected using lower concentrations of GFP-labeled
viri-ons (i.e., 10 ng or 50 ng of p24 per explant), but HIV-GFP signal
was consistently detectable when incubating the epithelium with
the viral equivalent of 100 ng of HIV p24 per explant.
Immuno-staining of tissue sections for HIV-1 Gag showed p24
colocaliza-tion with the GFP signals, confirming penetracolocaliza-tion of GFP-labeled
virions into the epithelium (Fig. 2B). Incubation of explants with
virus for 8 or 20 h at 37°C did not lead to further penetration of
virions into the deeper layers of the epithelium (data not shown).
Quantitative analysis of HIV-GFP/p24-positive epithelial cells
re-vealed that about 10% to 25% of cells of the granulosum layers
contained virions (Fig. 2C). The penetration of X4-tropic
HIV-1
NL4-3and that of R5-tropic HIV-1
81Aviruses into epithelia
oc-curred at similar levels. To confirm the confocal microscopy data,
HIV-exposed tissues were examined by electron microscopy,
which revealed the presence of virions in the epithelial cells of
surface granulosum layers (Fig. 2D).
To determine whether HIV virions that transmigrate across
mucosal epithelia can reach intraepithelial and subepithelial
HIV-susceptible immune cells, tissues were examined for the presence
of GFP/p24-positive virions in intraepithelial Langerhans cells
(LCs), macrophages, and T lymphocytes. Confocal microscopy
revealed that LCs, macrophages, and CD3
⫹lymphocytes were
mostly localized within the basal and parabasal layers and did not
contain GFP/p24-positive virions (data not shown). These
find-ings indicate that virions from the granulosum layers did not
reach the HIV-susceptible immune cells of the basal/parabasal
layers.
Paracellular penetration of HIV into adult oral epithelia with
disrupted tight junctions.
In the above-described experiments
(Fig. 1), we showed that adult and fetal oral epithelia have
well-developed tight junctions, which may prevent HIV penetration
via intercellular spaces. Disruption of tight junctions may
there-fore allow viral penetration via these spaces. To examine this
pos-sibility, we experimentally disrupted the tight junctions of adult
buccal epithelia by incubating explants in media containing 10
mM EDTA (Fig. 3). GFP-labeled R5-tropic HIV-1
81Avirions were
then added to the mucosal surfaces for 4 h at 37°C, and their
penetration was examined. Confocal microscopy of untreated
ep-ithelia with intact tight junctions revealed virion penetration
lim-ited to the topmost 2 to 5 layers of the epithelial surface, i.e.,
stratum granulosum (Fig. 3A, left panel). Analysis of ZO-1 (Fig.
FIG 1Detection of tight junction proteins in adult, infant, and fetal oral and fetal intestinal epithelia. (A) Representative immunofluorescence images of ZO-1 tight junction protein by confocal microscopy. Buccal tissues from a 41-year-old adult, 3-month-old infant, and 22-week-old fetus, as well as intestinal tissue from a 22-week-old fetus, were immunostained for ZO-1 (in red). Nuclei are stained in blue. Dashed white lines separate epithelium from the lamina propria. EP, epithelium; GR, granulosum; SP, spinosum; LP, lamina propria. Original magnification,⫻600. (B, C, and D) Detection of tight junctions by electron microscopy. Adult buccal (B), fetal buccal (C), and fetal intestinal (D) epithelia were examined by electron microscopy. (B) Images are taken from the granulosum and spinosum layers. Insets in panel B and insets and red arrows in panels C and D show the electron-dense tight junction regions of lateral membranes. (E) Detection of insoluble tight junction proteins in adult and fetal buccal epithelia. The presence of tight junction proteins claudin-1 and occludin was examined by Western blotting of Triton X-100-soluble (S) and -insoluble (P) fractions of buccal epithelial extracts.
HIV Transepithelial Migration via Oral Epithelium
on November 7, 2019 by guest
http://jvi.asm.org/
[image:4.585.84.504.70.374.2]3A, right panel) expression in EDTA-treated tissues showed that
the tight junctions were disrupted, and virions in these disrupted
tissues were detected within the deeper part of the epithelium.
Virions were detected in about 65%, 43%, and 10% of cells of the
spinosum, granulosum, and parabasal layers, respectively, of
dis-rupted epithelium (Fig. 3B). These data demonstrate that intact
tight junctions prevent paracellular penetration across both adult
and fetal oral epithelia and that their disruption facilitates virion
access between epithelial cells, allowing for entry of HIV into
deeper layers of epithelium.
To determine whether HIV that had penetrated into oral
epi-thelium was infectious, we applied R5-tropic HIV-1
SF170or
X4-tropic HIV-1
92UG029primary clinical isolates to intact and
EDTA-disrupted buccal explants propagated from 3 independent adult
donors. After 4 h, explants were homogenized, and HIV infectivity
was examined in PBMCs. ELISA analysis of PBMCs after 1 week
did not detect HIV-1 p24; however, after 2 and 3 weeks, p24 was
detected in PBMCs exposed to the supernatants of tissue
homog-enates from two of the three EDTA-treated explants (Fig. 3C). p24
was not detected in PBMCs exposed to the supernatants of two of
the three control tissues not treated with EDTA. A low level of p24
was detected after 3 weeks in PBMCs infected with supernatant of
homogenate from one of the untreated tissue explants.
Analysis of HIV-susceptible cells in fetal and infant mucosal
epithelia.
It has been shown that the adult oral mucosa has
HIV-susceptible T lymphocytes, macrophages, and Langerhans cells
(10, 12, 17, 18, 56, 57). However, the presence of these cells in
infant and fetal oral mucosae has not previously been investigated.
To detect HIV-susceptible lymphocytes, macrophages, and
den-dritic cells in infant and fetal oral mucosae, fetal buccal and
oro-pharyngeal tissues and infant buccal and tonsil tissues were
im-munostained for CD3, CD68, and CD1a. Confocal microscopy
FIG 2Penetration of HIV-1 into adult buccal mucosal epithelia. (A) A schematic model of the polarized oriented tissue explant system for studying cell-free HIV transmission. To establish polarized oriented mucosal epithelia, tissue explants were placed with the mucosal side facing up in the top chamber of a Millicell filter insert. The lateral edges of the explants were sealed with 3% agarose, and GFP-labeled HIV virions were added to the apical surfaces. After 4 h, tissues were fixed, sectioned, and examined for virus penetration by confocal immunofluorescence microscopy. (B) Representative fluorescence microscopy images of adult oral tissues exposed to GFP-labeled HIV-1. Adult buccal explants exposed to HIV-1NL4-3(X4) virions for 4 h at 37°C were immunostained for rabbit anti-GFP (green)
and goat anti-HIV-1 p24 (red, upper panels) or normal goat IgG (red, lower panels). Yellow in merged panel indicates colocalization of HIV-Gag with GFP-labeled (green) virions. GR, granulosum; SP, spinosum; LP, lamina propria; BL, basal. White lines indicate the border between the lamina propria and the mucosal epithelium. Original magnification of images,⫻400. Insets show GFP signals from virions at higher magnification. (C) To quantify HIV penetration into the adult buccal epithelium, GFP-labeled HIV-1NL4-3(X4) and HIV-181-A(R5) virions were added to the apical surfaces of polarized oriented matching adult
tissue explants obtained from 3 independent donors. Tissues were sectioned and immunostained for HIV p24 and occludin. Quantitative evaluation of HIV penetration into epithelia was performed by counting HIV-GFP- and p24-positive cells within the granulosum layers (1 to 5 layers), where virus penetration was detected. Cells were counted in a minimum of 3 sections and 10 fields of each section. Error bars show⫾standard errors of the means (⫾SEM). (D) For detection of HIV penetration into adult oral epithelium by electron microscopy, adult buccal tissues exposed to HIV-181-A(R5) virions were examined by electron
microscopy. Mature HIV virions are visible in the cytoplasm of granulosum layers of oral epithelium (red arrowheads and inset).
on November 7, 2019 by guest
http://jvi.asm.org/
[image:5.585.82.503.66.396.2]analysis showed that both the fetal and infant oral mucosae
con-tained all three cell types (Fig. 4A). T lymphocytes and
macro-phages were detected predominantly within the lamina propria,
and Langerhans cells were present within the epithelium. We also
immunostained fetal intestinal epithelium for CD3, CD68, and
dendritic cell-specific intercellular adhesion molecule 3
(ICAM-3)-grabbing nonintegrin (DC-SIGN) to identify T lymphocytes,
macrophages, and dendritic cells, respectively (Fig. 4A). This
staining revealed the presence of all three cell types within the
lamina propria of fetal intestine. Quantitative analysis of HIV
tar-get cells in fetal and infant oral epithelia revealed that their
num-bers were comparable between the two epithelia (Fig. 4B to E).
However, the number of HIV-susceptible cells in fetal intestinal
epithelium was about 2-fold higher than that in fetal oral
epithe-lium.
Cell-free HIV transmigration across fetal oral and intestinal
mucosal epithelia.
To determine whether HIV crosses the
strati-fied (2- to 5-layer) fetal oral and monostratistrati-fied columnar
intes-tinal epithelium, polarized oriented explants from 10 buccal, 18
oropharyngeal, and 6 intestinal tissues obtained from
indepen-dent fetuses at 18 to 24 weeks of gestation were exposed to
GFP-labeled X4-tropic HIV-1
NL4-3and R5-tropic HIV-1
81Avirions at
their apical surfaces. Examination of fetal buccal (data not shown)
and fetal oropharyngeal and intestinal epithelia incubated with
these virions at 37°C for 4 h revealed virus penetration of both the
epithelium and the lamina propria (Fig. 5A, left panels). To
deter-mine whether the viral penetration was due to endocytosis and
transcytosis of virions from the apical surface of epithelia,
exper-iments were performed at 4°C or in tissues pretreated with
colchi-cine, which inhibits HIV endocytosis and transcytosis (3, 34).
Penetration of virions into the lamina propria was not observed in
fetal tissues incubated at 4°C (Fig. 5A, middle left panels) or in
tissues pretreated with colchicine (Fig. 5A, middle right panels).
Transmigration of GFP-labeled virions was detected in 8 of 10
buccal tissues (80%), 16 of 18 oropharyngeal tissues (88%), and 6
of 6 intestinal tissues (100%). To quantitate HIV transmigration
through fetal epithelia, we counted cells costained for CD45—a
marker of white blood cells, including LCs, macrophages, and
CD3
⫹lymphocytes (23, 61, 62)—and HIV p24. HIV infection of
CD45
⫹immune cells has been shown previously (55). The
pres-FIG 3Paracellular penetration of HIV into adult oral epithelia. (A) Adult buccal explants from 3 independent donors were incubated in media containing 10 mM EDTA for 2 h (disrupted junctions). One matching explant was not treated and served as a control (intact junctions). GFP-labeled HIV-181-A(R5) was added
to the surfaces of explants for 4 h. Tissues were fixed, sectioned, and immunostained for GFP and ZO-1 (red). Cells were analyzed by confocal microscopy, and representative images from three independent tissues are shown. Green indicates GFP-labeled virions. Nuclei are stained in blue. GR, granulosum; SP, spinosum; LP, lamina propria; EP, epithelium; BL, basal. Original magnification,⫻600. Insets show GFP-labeled virions at higher magnification. (B) For quantitative evaluation of HIV paracellular penetration, HIV-containing epithelial cells were counted within the granulosum, spinosum, and parabasal layers of untreated and EDTA-treated explants. Average numbers of HIV-containing epithelial cells from explants of 3 independent donors are presented. Cells were counted in a minimum of 10 fields. Error bars show⫾SEM. (C) To examine the infectivity of HIV that had penetrated into adult oral epithelium, polarized oriented explants were propagated from buccal biopsy specimens of three independent adult donors. One of the matching explants was treated with EDTA, and one was left untreated. R5-tropic HIV-1SF170or X4-tropic HIV-192UG029viruses were applied to the surfaces of intact and EDTA-disrupted tissue explants for 4 h. Explants
were homogenized, and 200 ml of supernatant from tissue homogenate was used for infection of 106PBMCs. After 2 and 3 weeks, HIV-1 p24 was detected in
PBMCs by the use of an ELISA p24 assay. *, not detected.
HIV Transepithelial Migration via Oral Epithelium
on November 7, 2019 by guest
http://jvi.asm.org/
[image:6.585.84.506.65.367.2]FIG 4Distribution of HIV-susceptible lymphocytes, macrophages, and dendritic/Langerhans cells in infant and fetal oral and fetal intestinal epithelium. (A) Tonsil tissue from a 3-month-old infant and oropharyngeal tissue from a 22-week-old fetus were immunostained for CD3, CD68, and CD1a markers for T
on November 7, 2019 by guest
http://jvi.asm.org/
[image:7.585.93.495.64.697.2]ence of HIV-GFP
⫹p24
⫹CD45
⫹immune cells in the tissue
ex-plants is an indicator of initial viral transmigration across mucosal
epithelia. In oropharyngeal tissues, about 1.5 intraepithelial and
subepithelial CD45
⫹cells per mm
2were positive for HIV-GFP/
p24 (Fig. 5B, upper panel). The frequency of CD45
⫹cells positive
for HIV-GFP/p24 in the intestinal tissues was about 2 cells per
mm
2(Fig. 5B, lower panel). The numbers of HIV-GFP-positive
CD45
⫹cells in tissues exposed to X4- and R5-tropic viruses were
similar, indicating that the levels of initial transmigration of
X4-and R5-tropic viruses across epithelia were also similar.
HIV-GFP/p24-positive CD45
⫹cells were almost exclusively detected
only in the tissues incubated at 37°C, and only minimal levels
lymphocytes, macrophages, and Langerhans cells, respectively. Infant tissues were also stained with isotype control antibodies for each marker. Intestinal tissues from a 22-week-old fetus were immunostained for CD3 and CD68, as well as for DC-SIGN, which is a marker for dendritic cells. All cell markers are in red, and nuclei are in blue. Representative images are shown. EP, epithelium; LP, lamina propria. A dashed yellow line indicates the boundary between epithelium and lamina propria. Original magnification,⫻400. (B to E) Quantitative evaluation of HIV target cells within infant and fetal oral and fetal intestinal epithelia. (B, C, and D) CD3-, CD68-, and CD1a-positive cells in infant and fetal buccal mucosa were counted. (E) In the fetal intestinal mucosae, CD3-, CD68-, and DS-SIGN-positive cells were counted. (B to E) WKS, weeks. Cells were counted in a minimum of 10 separate fields, and results are presented as the average number of cells per square millimeter. Error bars show⫾SEM.
FIG 5Penetration of HIV-1 into fetal oral and intestinal mucosal epithelia. (A) Representative confocal immunofluorescence images of fetal oropharyngeal and intestinal tissues exposed to cell-free HIV-1. GFP-labeled HIV-1NL4-3(X4) virions were added to the apical surfaces of polarized oriented fetal oropharyngeal and
intestinal tissue explants. Tissues were incubated at 37°C or 4°C for 4 h. In parallel experiments, fetal tissues were pretreated with 10M colchicine for 2 h at 37°C and then used for virion penetration at 37°C. Tissue sections were coimmunostained for GFP and occludin. Original magnification,⫻600. (B) For quantitation of HIV penetration into fetal oropharyngeal and intestinal epithelia exposed to HIV-1NL4-3(X4) or HIV-181A(R5) virions, tissue sections were immunostained
for HIV-p24 and CD45⫹, which is a marker for white blood cells, including HIV-susceptible immune cells. Virus penetration into epithelia was evaluated by counting p24-positive CD45⫹cells. Cells were counted in a minimum of 10 fields. Error bars show⫾SEM. Average numbers of HIV-infected CD45⫹cells per square millimeter from 3 independent tissues are presented. (C and D) For electron microscopy detection of HIV penetration into fetal oral and intestinal epithelium, oropharyngeal and intestinal tissues were exposed to HIV-181-A(R5) virions for 4 h and then fixed and examined by electron microscopy. Mature
virions are present in the cytoplasm of fetal oral and intestinal epithelium (red arrowheads and inset). (E) To examine the role of HIV-1 coreceptors in HIV transmission through fetal oral mucosal epithelia, polarized oriented oropharyngeal tissue explants from two independent donors were exposed to antibodies against GalCer, HSPG, CXCR4, or CCR5. After the explants were washed, R5-tropic HIV-1SF170or X4-tropic HIV-192UG029viruses were applied to the surfaces
of tissues for 4 h. Explants were fixed and immunostained for HIV by the use of pooled antibodies against gp41, gp120, and p24. Quantitative analysis was performed by counting HIV-positive epithelial cells in the explants treated with specific and control antibodies. HIV-positive epithelial cells were counted in at least 10 fields. Inhibition of viral penetration by antibodies is expressed as the percentage of HIV-positive epithelial cells in the tissues exposed to specific antibodies relative to HIV-positive cells in the tissues exposed to the isotype control antibodies. The average percentage of inhibition determined for two tissue explants obtained from two independent donors is presented. Error bars show⫾SEM.
HIV Transepithelial Migration via Oral Epithelium
on November 7, 2019 by guest
http://jvi.asm.org/
[image:8.585.81.502.64.384.2]were detected in tissues incubated at 4°C or in those pretreated
with colchicine, which had frequencies of 0.1 to 0.2 cells per
mm
2(Fig. 5B).
Electron microscopy analysis of oropharyngeal and intestinal
epithelia incubated with R5-tropic HIV-1
81Avirions at 37°C
showed that viral particles had penetrated into oral (Fig. 5C) and
intestinal (Fig. 5D) epithelial cells from their surfaces.
We have shown that the fetal oral epithelium expresses the HIV
coreceptors CXCR4, CCR5, galactosyl ceramide (GalCer), and
heparan sulfate proteoglycan (HSPG) (54). To determine whether
these coreceptors play a role in HIV penetration into fetal oral
epithelia, we incubated polarized oriented explants from two
in-dependent fetuses with antibodies against GalCer, HSPG, CXCR4,
and CCR5. Penetration of R5-tropic HIV-1
SF170and X4-tropic
HIV-1
92UG029viruses into these epithelia was then examined.
Immunostaining of these tissues for GalCer, HSPG, CXCR4,
and CCR5 confirmed their expression in these tissues (data not
shown). Quantitative analysis showed that antibodies to
Gal-Cer and HSPG inhibited both X4- and R5-tropic HIV
penetra-tion into these epithelial cells by about 45% and 35%,
respec-tively (Fig. 5E). However, antibodies to CXCR4 and CCR5 did
not significantly inhibit X4- and R5-tropic viral penetration,
respectively.
HIV transmigration through the fetal mucosal epithelium
leads to infection of virus-susceptible cells.
Immunostaining of
LCs, macrophages, and CD3
⫹lymphocytes for GFP-labeled
viri-ons in fetal oral tissues exposed to X4-tropic HIV-1
NL4-3or
R5-tropic HIV-1
81Aviruses revealed that both viruses were present in
these cells (Fig. 6A). These HIV-GFP-positive immune cells were
mainly localized within the epithelium and the adjacent lamina
propria, near its border with basal cells. Analysis of CD3
⫹lym-phocytes and macrophages for GFP-labeled virions in the
intesti-FIG 6Detection of HIV-infected immune cells in fetal oral and intestinal epithelium. (A) For detection of HIV-infected immune cells, fetal oropharyngeal epithelia were exposed to GFP-labeled HIV-181-A(R5) virions (upper panels) and HIV-1NL4-3(X4) virions (lower panels) for 4 h at 37°C. Tissue sections were
coimmunostained for GFP and CD3⫹, CD68⫹, or CD1a⫹cells (all in red). (B) Fetal intestinal epithelium exposed to GFP-labeled HIV-1
81-A(R5) virions (upper
panels) and HIV-1NL4-3(X4) virions (lower panels) for 4 h at 37°C was coimmunostained for GFP and CD3⫹or CD68⫹cells (all in red). (A and B) Insets show
GFP signals from virions at higher magnification. Representative images from 3 fetal buccal and intestinal tissues are shown. Original magnification of images, ⫻600. EP, epithelium; LP, lamina propria. (C) For quantitative analysis of HIV-infected immune cells, tissue sections were immunostained for HIV-p24, as well as for CD3, CD68, and CD1a, which are markers for T lymphocytes, LCs, and macrophages, respectively. HIV-1 p24-positive immune cells expressing CD3, CD68, or CD1a were counted in at least 10 fields. Average data are presented from three tissue explants obtained from three independent donors. Error bars show⫾SEM. (D) To determine the infectivity of HIV within the fetal oral and intestinal epithelium, polarized oriented oral and intestinal explants from three independent donors were exposed to R5-tropic HIV-1SF170or X4-tropic HIV-192UG029viruses. After 4 h, explants were extensively washed and homogenized.
PBMCs were infected with 200 ml of supernatant of tissue homogenate, and after 1 week, HIV-1 p24 was detected in PBMCs by the use of an ELISA p24 assay. Average data from three independent tissue explants are presented.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:9.585.83.503.63.413.2]nal epithelium also showed HIV-infected lymphocytes and
mac-rophages (Fig. 6B).
To quantitate HIV-infected CD3
⫹T lymphocytes, CD68
⫹macrophages, and CD1a
⫹LCs, HIV-GFP-positive cells were
counted in three independent oropharyngeal tissues exposed to
X4-tropic HIV-1
NL4-3or R5-tropic HIV-1
81Avirus (Fig. 6C, left
panel). We found that CD3
⫹T lymphocytes, macrophages, and
LCs were positive for both X4- and R5-tropic HIV. The number of
HIV-infected cells was about 0.6 to 0.8 cells per mm
2. The
differ-ences between X4-tropic and R5-tropic virus infection rates in
FIG 7Cell-associated HIV-1 does not penetrate into the adult oral mucosal epithelium. (A) A schematic model of the polarized oriented tissue explant system for studying cell-associated HIV transmission. R5-tropic HIV-infected macrophages and X4-tropic HIV-1-infected lymphocytes were labeled with 10M CFSE for 10 min. HIV-infected and CFSE-labeled macrophages and lymphocytes were added to the mucosal surfaces of polarized oriented explants. After 4 h, tissue explants were fixed and sectioned, and cross-sections were examined for CFSE-labeled cells (green) coimmunostained with goat anti-HIV-1 antiserum (red). Colocalization of the red HIV signal with the green CFSE signal generates a yellow signal. Cells with yellow signals represent transmigration of HIV-infected cells through the mucosal epithelium. Cells containing only red signal represent HIV-infected fetal cells, which might have been infected by cell-free virions released from apically added adult lymphocytes or macrophages. (B) X4-tropic HIV-192UG029- and R5-tropic HIV-1SF170-infected CD4⫹lymphocytes and CD68⫹
macrophages, respectively, were labeled with CFSE (green) and added to the mucosal surfaces of adult buccal tissues. After 4 h, tissues were fixed, sectioned, and immunostained with goat anti-HIV-1 antiserum (red). Merged panels are shown. (C) To determine the integrity of cell junctions in adult oral epithelia exposed to HIV-infected lymphocytes and macrophages, sections of buccal explants incubated with HIV-infected lymphocytes and macrophages were immunostained for the tight junction protein ZO-1 (red). Cell nuclei are stained in blue. (B and C) Original magnification,⫻400. Images are representative of explants from 3 independent donors.
HIV Transepithelial Migration via Oral Epithelium
on November 7, 2019 by guest
http://jvi.asm.org/
[image:10.585.136.456.67.535.2]both CD3
⫹T lymphocytes and macrophages were not statistically
significant. Quantitative analysis of LCs also showed no significant
difference between infection rates by the two viruses.
Analysis of intestinal tissues also showed that HIV-infected
CD3
⫹T lymphocytes and CD68
⫹macrophages were positive for
both X4- and R5-tropic HIV (Fig. 6C, right panel). However, the
number of HIV-infected cells was about 3-fold higher than it was
in oropharyngeal tissues.
To measure the infectivity of HIV entering the fetal oral and
intestinal epithelium, we applied R5-tropic HIV-1
SF170or
X4-tropic HIV-1
92UG029primary viruses to fetal oropharyngeal and
intestinal explants propagated from three independent fetal
do-nors. The infectivity of HIV that penetrated into these mucosal
epithelia was tested in PBMCs by infecting the PBMCs with
su-pernatants of tissue homogenates. Detection of viral p24 using an
ELISA after 1 week showed that the HIV that penetrated into the
oral and intestinal epithelia was infectious both at both locations.
However, significant differences between R5-tropic and X4-tropic
viruses were not observed.
HIV-infected lymphocytes and macrophages do not
pene-trate into the adult oral epithelium.
To investigate transmission
of cell-associated HIV, CD4
⫹lymphocytes and CD68
⫹macro-phages were infected with X4-tropic HIV-1
92UG029and R5-tropic
HIV-1
SF170viruses, respectively, and the cells were labeled with
5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE)
(Fig. 7A). Cells were added to the apical surfaces of adult and fetal
buccal epithelia for 4 h. Confocal microscopy analysis of adult oral
tissues with HIV-infected CD4
⫹lymphocytes and CD68
⫹macro-phages showed that no lymphocytes or macromacro-phages were bound
to the mucosal surface or penetrated into the epithelium (Fig. 7B).
To determine the integrity of the mucosal epithelia examined
here, adult buccal explants exposed to HIV-infected lymphocytes
and macrophages were immunostained for the tight junction
pro-teins ZO-1, occludin, and claudin-1. Confocal microscopy
re-vealed that ZO-1 (Fig. 7C), occludin, and claudin-1 (data not
shown) were present as rings encircling epithelial cells, a typical
pattern for intact tight junctions. These data indicate that
incuba-tion of adult oral mucosa with HIV-infected lymphocytes and
macrophages for 4 h did not lead to disruption of epithelial
junc-tions.
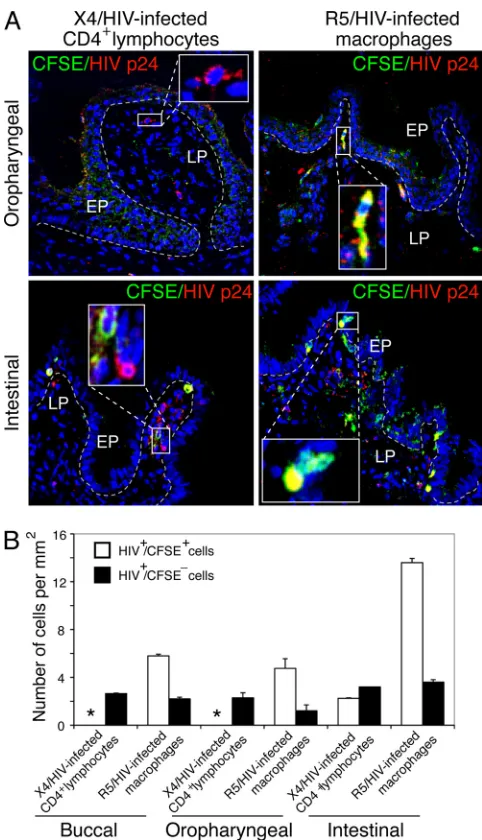
Transmigration of cell-associated HIV into the fetal oral
ep-ithelium.
Analysis of the fetal epithelium incubated with
HIV-infected/CFSE-labeled lymphocytes showed that these cells were
bound to the surface of the fetal oral epithelium but did not
pen-etrate into the epithelium (Fig. 8A, upper left panel). However,
HIV-infected/CFSE-unlabeled cells were detected within the
lam-ina propria (Fig. 8A, upper left panel, inset) in these tissues,
sug-gesting penetration of cell-free virions, which were released from
HIV-infected lymphocytes. Notably, HIV-infected macrophages
were bound to the mucosal surface of fetal oral mucosa (Fig. 8A,
upper right panel) and transmigrated through the epithelium into
the lamina propria (Fig. 8A, upper right panel, inset). Quantitative
evaluation of penetration of HIV-infected macrophages into the
fetal epithelium showed that about 6 HIV-infected macrophages
FIG 8Penetration of cell-associated HIV-1 into fetal oral mucosal epithelium. (A) CFSE-labeled X4-tropic HIV-192UG029- and R5-tropic HIV-1SF170-infected
CD4⫹lymphocytes and CD68⫹macrophages, respectively, were added to the mucosal surfaces of fetal buccal tissues. After 4 h, tissues were fixed, sectioned, and immunostained with goat anti-HIV-1 antiserum (red). Merged panels are shown. CFSE-labeled lymphocytes and macrophages are in green. HIV-infected cells are in red. Yellow indicates colocalization of CFSE-labeled and HIV-infected cells. (B) For quantitative analysis of transmigration of HIV-infected lymphocytes and macrophages into fetal oral epithelia, HIV-infected and CFSE-labeled cells (HIV⫹/CFSE⫹cells) were counted. HIV-infected and CFSE-unlabeled cells (HIV⫹/CFSE⫺cells) were also counted. Cell numbers per square millimeter are presented. In each section, cells were counted in a minimum of 10 fields. Average numbers of HIV⫹/CFSE⫹and HIV⫹/CFSE⫺cells per square millimeter are presented from 3 independent tissues. *, not detected. Error bars show⫾SEM. (C) To examine the tight junctions of fetal oral epithelium exposed to HIV-infected lymphocytes and macrophages, tissue sections were immunostained for the tight junction protein ZO-1 (red). Cell nuclei are stained in blue. (A and C) Original magnification,⫻400. Images are representative of three independent experiments.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:11.585.81.507.69.315.2]per mm
2penetrated into the epithelium (Fig. 8B). The number of
HIV-infected/CFSE-unlabeled cells was about 2 cells/mm
2.
Analysis of tight junction protein expression in fetal explants
revealed that ZO-1 (Fig. 8C), occludin, and claudin-1 (data not
shown) were intact, indicating that incubation of fetal oral
muco-sea with HIV-infected lymphocytes and macrophages for 4 h did
not cause disruption of epithelial junctions.
Since postnatal HIV MTCT occurs during breastfeeding, we
examined cell-associated HIV penetration into fetal oral and
in-testinal epithelia in the presence of breast milk. HIV-infected
CD4
⫹lymphocytes and CD68
⫹macrophages were resuspended
in breast milk and applied to the mucosal surfaces of fetal buccal,
oropharyngeal, and intestinal epithelia for 4 h. Analysis of
pene-tration of lymphocytes into buccal (data not shown) and
oropha-ryngeal (Fig. 9A, upper left panel) epithelia revealed that
HIV-infected/CFSE-labeled lymphocytes were not present within the
lamina propria. However, HIV-infected/CFSE-unlabeled cells
were detected within the lamina propria (Fig. 9A, upper left panel,
inset) of these tissues, potentially due to the penetration of
cell-free virions produced by HIV-infected lymphocytes from the
mu-cosal surfaces of oral explants. HIV-infected/CFSE-labeled
mac-rophages were found to readily transmigrate across the oral
epithelium and penetrated into lamina propria (Fig. 9, upper
right panel, inset). The number of macrophages that
transmi-grated through the fetal oral epithelium was about 4 to 5 per
mm
2(Fig. 9B).
Notably, penetration of both HIV-infected/CFSE-labeled
lym-phocytes and HIV-infected/CFSE-labeled macrophages was
de-tected in the intestinal tissues (Fig. 9A, lower panels); however, the
rate of macrophage transmigration was about 5- to 6-fold higher
than the rate for lymphocytes (Fig. 9B).
HIV-infected/CFSE-unlabeled cells were also detected within the lamina propria of
intestinal tissues (Fig. 9A, lower panels, insets) exposed to both
HIV-infected lymphocytes and macrophages, which could have
been due to spread of virus from transmigrated cells and/or from
cell-free virus released by apically applied lymphocytes and
mac-rophages.
DISCUSSION
In a previous study, we showed that experiments detecting HIV
transmission across monostratified, polarized adult and fetal oral
epithelial cells gave different results. Only virions that passed
through the fetal cells—and not those that passed through the
adult cells—remained infectious (54). To understand further the
potential mechanisms in adult mucosae that prevent HIV
trans-mission, we established a clinically relevant model system, i.e.,
ex
vivo
organ tissue explants of fully developed adult and
underde-veloped fetal oral mucosal epithelia (Fig. 1). Both the thickness of
the fetal oral epithelium and the distribution of HIV target cells
within the fetal mucosa were comparable to those seen with infant
oral mucosa (Fig. 1 and 4). Thus, these fetal oral tissues serve, in
particular, as suitable models for the study of HIV MTCT in
in-fants, which plays a critical role in viral transmission during
breastfeeding.
In this report, we have shown that the efficiency of HIV
trans-mission depends on the thickness of the oral mucosal epithelium.
Penetration of virions into intact oral mucosae was found to occur
only through a few layers (1 to 5 layers) of the adult oral
epithe-lium (Fig. 2). Notably, HIV-infected immune cells were not
de-tected in the adult epithelium. This finding indicates that HIV
transiting through a few superficial layers of adult multistratified
oral epithelium has little chance of reaching HIV-susceptible
im-mune cells, which reside within the basal/parabasal/suprabasal
layers of the oral epithelium. However, disruption of the epithelial
tight junctions facilitated penetration of virus into deeper parts of
FIG 9Penetration of cell-associated HIV-1 into fetal oral and intestinal mu-cosal epithelium in the presence of breast milk. (A) X4-tropic HIV-192UG029
-and R5-tropic HIV-1SF170-infected CD4⫹lymphocytes and CD68⫹
macro-phages, respectively, were labeled with CFSE (green) and resuspended in breast milk. Cells with breast milk were then added to the mucosal surfaces of fetal buccal and intestinal epithelia for 4 h. Tissues were fixed, sectioned, and im-munostained with goat anti-HIV-1 antiserum (red). Merged panels are shown. Green shows CFSE-labeled lymphocytes or macrophages. Red indi-cates HIV-infected cells. Yellow indiindi-cates colocalization of CFSE-labeled and HIV-infected cells. Original magnification,⫻400. Representative images from 3 independent tissues are shown. (B) For quantitative evaluation, HIV-infected and CFSE-labeled cells (HIV⫹/CFSE⫹cells) and HIV-infected and CFSE-unlabeled cells (HIV⫹/CFSE⫺cells) were counted. Cell numbers per square millimeter are presented. In each section, cells were counted in a min-imum of 10 fields. Average numbers of HIV⫹/CFSE⫹and HIV⫹/CFSE⫺cells per square millimeter are presented from 3 independent tissues. *, not de-tected. Error bars show⫾SEM.
HIV Transepithelial Migration via Oral Epithelium
on November 7, 2019 by guest
http://jvi.asm.org/
[image:12.585.299.540.63.483.2]the adult epithelium (Fig. 3). Thus, a critical role for tight
junc-tions is apparent in preventing paracellular transmission of HIV.
Penetration of cell-free HIV through intact fetal oral mucosal
epithelia with tight junctions suggests that the migration of HIV
across epithelia occurs by transcytosis (2, 3, 19, 20, 34, 43, 54). The
inhibition of viral transmission at 4°C and by colchicine-induced
depolymerization of microtubules (3, 34) also supports
transcel-lular transmission of virus (Fig. 5). However, we cannot
com-pletely rule out the possibility of paracellular passage of some
vi-rions through microscopically undetectable disruptions in the
tight junctions.
Expression of the HIV coreceptors GalCer, HSPG, CXCR4,
and CCR5 and their functional role in viral transmission have
been demonstrated in endometrial, vaginal, and intestinal
epithe-lial cells (2, 3, 14, 19, 20, 28, 34, 43, 51, 54, 63, 64). Reductions in
cell-free HIV transmission through the fetal oral epithelium by
antibodies against GalCer and HSPG (Fig. 5) suggest that these
molecules also play critical roles in cell-free HIV MTCT via the
fetal/infant oral epithelium. However, antibodies against CCR5
and CXCR4 did not significantly affect cell-free viral transmission
through this epithelium, suggesting that chemokine receptors do
not play important roles in HIV MTCT through fetal/infant oral
mucosal epithelia.
HIV that penetrated into the limited layers of intact adult oral
epithelium were not infectious, in contrast to virions that
pene-trated into intact fetal oral epithelium (Fig. 3 and 6). These data
are consistent with our other recent findings that a high level of
expression of the anti-HIV innate proteins HBD2 and HBD3 in
adult oral epithelia may inactivate virus during its transcellular
transmission (54). Furthermore, lack of these innate proteins in
fetal oral epithelia facilitates transmigration of infectious virions
(54).
HIV-infected dendritic/Langerhans cells, macrophages, and T
lymphocytes are present in fetal oral and intestinal epithelia (Fig.
6). This finding indicates that transcellular passage of infectious
virions through the stratified fetal oral and nonstratified intestinal
mucosal epithelium can lead to infection of intraepithelial and
subepithelial HIV-susceptible immune cells and thereby initiate
systemic infection.
Another mechanism by which HIV may traverse epithelia is by
migration of HIV-infected cells through the intact epithelium. To
test this possibility, we examined transmigration of HIV-infected
lymphocytes and macrophages through adult and fetal oral
mu-cosal epithelia. The absence of HIV-infected lymphocytes and
macrophages that transmigrated through the adult oral
epithe-lium after 4 h indicates that cell-associated HIV transmission in
adults has little chance to initiate infection through the oral route
(Fig. 7). In terms of the fetal oral epithelium, R5-tropic
HIV-1-infected macrophages but not X4-tropic HIV-1-HIV-1-infected
lympho-cytes transmigrated across this epithelium (Fig. 8). During these
studies, epithelial tight junctions were intact in both adult and
fetal mucosa, indicating no disruption of the epithelium.
HIV-infected macrophages may migrate through paracellular
spaces of intact mucosal epithelia (1). Similarly, HIV-infected
macrophages may transmigrate via the paracellular spaces of
po-larized endometrial epithelial cells (6). In contrast, HIV-infected
T-lymphoid cells (3) do not penetrate the epithelium below tight
junctions. These data are consistent with our findings showing
that HIV-infected macrophages can transmigrate across fetal oral
and intestinal mucosal epithelia.
The breast milk of infected mothers contains
HIV-infected lymphocytes and macrophages (22, 39, 41, 44, 47–49),
which may initiate HIV MTCT during breastfeeding. R5
HIV-1-infected macrophages and not X4-tropic HIV-1-HIV-1-infected
lympho-cytes were the primary vehicle of infection in the presence of
breast milk (Fig. 9), suggesting that the HIV-infected
macro-phages in breast milk lead to MTCT. Thus, cell-associated
R5-tropic HIV-1 macrophages may be the most likely cells to
trans-migrate across fetal oral and intestinal mucosal epithelia.
Predominant dissemination of R5-tropic HIV-1 in MTCT has
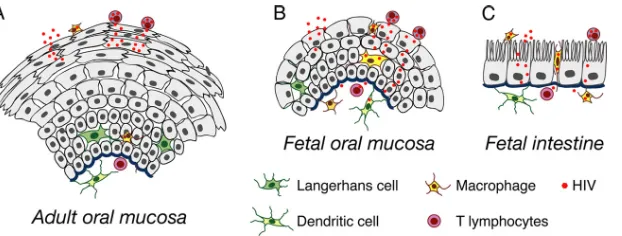
FIG 10Model of HIV transmigration in adult (A) and infant/fetal oral (B) and infant/fetal intestinal (C) epithelial cells. The adult oral mucosal epithelium is multistratified, with 10 to 30 epithelial layers, whereas the infant/fetal oral mucosal epithelium is incompletely stratified, with only about 2 to 7 epithelial layers. Transmigration of cell-free HIV across the intact adult oral epithelium may occur only to a limited extent (2 to 5 layers) within the stratum granulosum, preventing spread of virus into the stratum spinosum, stratum basale, and lamina propria. However, disruption of tight junctions of the adult oral epithelium diminishes its barrier function and therefore facilitates HIV penetration to the deeper parts of the epithelium. Cell-free HIV transmigration across the paucis-tratified fetal and infant mucosal epithelium leads to passage of virions into the intraepithelial and subepithelial virus-susceptible cells. Cell-free HIV also transmigrates across monostratified fetal intestinal epithelia. infected lymphocytes and macrophages do not penetrate into adult oral mucosae. HIV-infected lymphocytes also do not transmigrate across fetal oral epithelia; however, HIV-HIV-infected macrophages penetrate into fetal epithelia and reach the lamina propria. Both HIV-infected lymphocytes and macrophages transmigrate across fetal intestinal epithelia; however, macrophage transmigration is substantially higher than lymphocyte transmigration. Transcellular spread of cell-free and HIV-infected macrophages across fetal and infant oral and intestinal mucosal epithelia may lead to infection of intraepithelial and submucosal HIV-susceptible cells, including T lymphocytes, Langerhans/dendritic cells, and macrophages. Thus, fetal/infant oropharyngeal and intestinal mucosal epithelia may serve as key portals of entry for HIV MTCT. The increased rate of HIV transmission across fetal/infant versus adult oral epithelia may reflect a combination of reduced barrier function (associated with paucistratification) and lower levels of innate immune response proteins (54).
on November 7, 2019 by guest
http://jvi.asm.org/
[image:13.585.138.449.64.182.2]been well documented (7–9, 58, 60). This finding could reflect the
greater migratory activity of macrophages over lymphocytes.
In summary, we found that while HIV can readily transmigrate
across fetal oral mucosal epithelia, efficient viral transmission
through adult mucosal epithelia is unlikely (Fig. 10). This
obser-vation could be due to the differences in levels of stratification
between fetal/infant and adult oral epithelia, which are
paucis-tratified and multispaucis-tratified, respectively. Moreover, fetal and
in-fant oral epithelia lack expression of the anti-HIV innate proteins
HBD2, HBD3, and SLPI, and the adult oral epithelium expresses
high levels of these proteins (54). Thus, the adult oropharyngeal
stratified epithelium may have two lines of defense against HIV:
(i) a mechanical barrier of stratified epithelia with tight junctions
that prevent penetration of virions into the deeper layers of the
epithelium, and (ii) antiviral innate proteins that inactivate those
virions that penetrate into the first 1 to 5 layers of epithelium.
These defense mechanisms can play a key role in reducing HIV
oral transmission in adult populations. Lack of these lines of
de-fense in the fetal/infant oral epithelium may facilitate HIV MTCT.
Approaches designed to increase the levels of innate defense
pro-teins in infant mucosal epithelia and to inhibit transmigration of
HIV-infected cells may be of value in reducing the risk of HIV
MTCT in neonates and infants.
ACKNOWLEDGMENTS
We thank Richard Jordan (University of California San Francisco, De-partments of Orofacial Sciences and Pathology) for histological evalua-tion of tissues, Philip Ursell (University of California San Francisco, De-partment of Pathology) for providing the infant tissues, Larry Ackerman (University of California San Francisco, Diabetic Center) for electron mi-croscopy, and Matthew Petitt for editorial assistance.
This project was supported by National Institutes of Health grants R21 DE016009 and R21 DE021011, the UCSF ARI Carl L. Gaylord Estate fund, and a UCSF CFAR grant (to S.M.T.).
REFERENCES
1.Anderson DJ, et al.2010. Targeting Trojan Horse leukocytes for HIV prevention. AIDS24:163–187.
2.Bobardt MD, et al.2007. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J. Virol.81:395– 405.
3.Bomsel M.1997. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med.3:42– 47. 4.Böttcher MF, Jenmalm MC, Bjorksten B.2003. Cytokine, chemokine and secretory IgA levels in human milk in relation to atopic disease and IgA production in infants. Pediatr. Allergy Immunol.14:35– 41. 5.Böttcher MF, Jenmalm MC, Bjorksten B, Garofalo RP.2000.
Chemoat-tractant factors in breast milk from allergic and nonallergic mothers. Pe-diatr. Res.47:592–597.
6.Carreno MP, et al. 2002. Binding of LFA-1 (CD11a) to intercellular adhesion molecule 3 (ICAM-3; CD50) and ICAM-2 (CD102) triggers transmigration of human immunodeficiency virus type 1-infected mono-cytes through mucosal epithelial cells. J. Virol.76:32– 40.
7.Casper C, et al.2002. Coreceptor change appears after immune deficiency is established in children infected with different HIV-1 subtypes. AIDS Res. Hum. Retroviruses18:343–352.
8.Casper CH, et al.2002. Link between the X4 phenotype in human im-munodeficiency virus type 1-infected mothers and their children, despite the early presence of R5 in the child. J. Infect. Dis.186:914 –921. 9.Clevestig P, et al.2005. The X4 phenotype of HIV type 1 evolves from R5
in two children of mothers, carrying X4, and is not linked to transmission. AIDS Res. Hum. Retroviruses21:371–378.
10. Colasante A, et al.1992. Distribution and phenotype of immune cells in normal human gingiva: active immune response versus unresponsiveness. J. Oral Pathol. Med.21:12–16.
11. Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P. 2000.
Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat. Med.6:475– 479.
12. Daniels TE, et al.1987. Absence of Langerhans cells in oral hairy leuko-plakia, an AIDS-associated lesion. J. Investig. Dermatol.89:178 –182. 13. De Cock KM, et al.2000. Prevention of mother-to-child HIV
transmis-sion in resource-poor countries: translating research into policy and prac-tice. JAMA283:1175–1182.
14. Dwinell MB, Eckmann L, Leopard JD, Varki NM, Kagnoff MF.1999. Chemokine receptor expression by human intestinal epithelial cells. Gas-troenterology117:359 –367.
15. Ganor Y, et al.2010. Within 1 h, HIV-1 uses viral synapses to enter efficiently the inner, but not outer, foreskin mucosa and engages Langerhans-T cell conjugates. Mucosal Immunol.3:506 –522.
16. Reference deleted.
17. Handschel J, et al.2001. Late effects of radiotherapy on oral mucosa in humans. Eur. J. Oral Sci.109:95–102.
18. Handschel J, et al.2001. Increase of RM3/1-positive macrophages in radiation-induced oral mucositis. J. Pathol.193:242–247.
19. Hocini H, et al.2001. Active and selective transcytosis of cell-free human immunodeficiency virus through a tight polarized monolayer of human endometrial cells. J. Virol.75:5370 –5374.
20. Hocini H, Bomsel M.1999. Infectious human immunodeficiency virus can rapidly penetrate a tight human epithelial barrier by transcytosis in a process impaired by mucosal immunoglobulins. J. Infect. Dis.179(Suppl. 3):S448 –S453.
21. Hoffman AD, Banapour B, Levy JA.1985. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology147:326 –335.
22. Ichikawa M, et al.2003. Breast milk macrophages spontaneously produce granulocyte-macrophage colony-stimulating factor and differentiate into dendritic cells in the presence of exogenous interleukin-4 alone. Immu-nology108:189 –195.
23. Ismail SA, et al.2007. Immunohistochemical staining for CD45R iso-forms in paraffin sections to diagnose mycosis fungoides-type cutaneous T-cell lymphoma. J. Am. Acad. Dermatol.56:635– 642.
24. Jaspan HB, Robinson JE, Amedee AM, Van Dyke RB, Garry RF.2004. Amniotic fluid has higher relative levels of lentivirus-specific antibodies than plasma and can contain neutralizing antibodies. J. Clin. Virol.31: 190 –197.
25. Karlsson KR, et al.2008. Homogeneous monocytes and macrophages from human embryonic stem cells following coculture-free differentia-tion in M-CSF and IL-3. Exp. Hematol.36:1167–1175.
26. Kitani H, Takenouchi T, Sato M, Yoshioka M, Yamanaka N.2011. A simple and efficient method to isolate macrophages from mixed primary cultures of adult liver cells. J. Vis. Exp.2011:2757. doi:10.3791/2757. 27. Kourtis AP, M Bulterys. 2010. Mother-to-child transmission of HIV:
pathogenesis, mechanisms and pathways. Clin. Perinatol37:721–737, vii. 28. Kumar RB, Maher DM, Herzberg MC, Southern PJ.2006. Expression of HIV receptors, alternate receptors and co-receptors on tonsillar epithe-lium: implications for HIV binding and primary oral infection. Virol. J. 3:25.
29. Luzuriaga K.2007. Mother-to-child Transmission of HIV: A Global Per-spective. Curr. Infect. Dis. Rep.9:511–517.
30. Maher D, Wu X, Schacker T, Horbul J, Southern P.2005. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proc. Natl. Acad. Sci. U. S. A.102:11504 –11509.
31. Maher D, Wu X, Schacker T, Larson M, Southern P.2004. A model system of oral HIV exposure, using human palatine tonsil, reveals exten-sive binding of HIV infectivity, with limited progression to primary infec-tion. J. Infect. Dis.190:1989 –1997.
32. Maiques V, Garcia-Tejedor A, Perales A, Cordoba J, Esteban RJ.2003. HIV detection in amniotic fluid samples. Amniocentesis can be performed in HIV pregnant women? Eur. J. Obstet. Gynecol. Reprod. Biol.108:137– 141.
33. Mandelbrot L, et al.1999. Frequent detection of HIV-1 in the gastric aspirates of neo