0022-538X/79/11-0583/10$02.00/0
Salmonella Bacteriophage
Glycanases: Endorhamnosidases
of
Salmonella typhimurium Bacteriophages
STEFAN B.SVENSON,1* JORGEN LONNGREN,2 NILSCARLIN,' AND ALFA. LINDBERG'
Departmentof Bacteriology,NationalBacteriological Laboratory,S-10521Stockholm,'andDepartment of
Organic
Chemiistry,
ArrheniusLaboratory,
University of Stockholm,
S-10691
Stockholm,2
SwedenReceived forpublication15 May 1979
Twelve bacteriophages lysing only smooth Salmonella typhimurium strains wereshowntohave similar morphology-anicosahedric head to which a short,
noncontractile tail carrying sixspikes was attached. Allphages degraded their
lipopolysaccharide (LPS)receptors asshownby their ability to cleave off
['4C]-galactosyl-containingoligosaccharidesfrom S. typhimurium cells labeled in their
LPS. The oligosaccharidesinhibited the a-D-galactosyl-specific Bandeiraea
sim-plicifolia
lectinagglutination of humantype B erythrocytes, indicating thatall12phageglycanaseswereof endorhamnosidase specificity, i.e.,hydrolyzedthe a-L-rhamnopyranosyl-(1 -- 3)-D-galactopyranosyllinkage in the S. typhimurium
0-polysaccharide chain.Twoof thephages,28Band 36, were studied in more detail.
Whereas the phage 28B
glycanase
hydrolyzed the S. typhimurium LPS intododeca- and octasaccharides, the phage 36 glycanase in addition cleaved off
tetrasaccharides. Both phageenzymeshydrolyzedthe0-polysaccharidechains of
LPS fromSalmonella belongingtoserogroups A, B, andDl, which arebuiltup
of tetrasaccharide-repeating units identical except for the nature of the
3,6-dideoxyhexopyranosylgroup(R). R
al
- 2)-a-D-Manp-(1-- 4)-a-L-Rhap-(1-+ 3)-a-D-Galp-(l
The phage 28Band36endorhamnosidases hydrolyzed also an LPS from which
the3,6-dideoxyhexosyl substituents had previouslybeen hydrolyzed off. However,
neither of theenzymes wasactiveonLPSpreparations in which the C2-C3 bond
of theL-rhamnopyranosyl ring had been opened by periodateoxidation.
Glucosyl-ationat0-6 of the D-galactopyranosyl residues in the S. typhimurium LPS was
foundtobeincompatible with hydrolysisby both enzymes. However, in an LPS
glucosylated at 0-4 of the D-galactopyranosyl residues, the adjacent
a-L-rham-nopyranosyllinkageswerefoundtobepreferentiallycleaved.
Receptors forseveral
bacteriophages specific
for smooth
gram-negative
bacteria have been shown to be located in the lipopolysaccharide (LPS) moiety of the bacterialoutermembrane (12). Morerecently,
it has been revealed thatsuchphage
adsorption
oftenis concomitantwithan
enzymatic
hydrolysis
ofthepolysaccharide
receptor.
Thus, adsorption
ofphages
P22 andE'5totheirLPS receptors (seeFig. 1forgeneral
structureofSalmonellaLPS)ofsusceptible
Sal-monella species is accompanied by hydrolytic cleavage of a-L-rhamnosyl linkages within the LPS (7, 11). Likewise, one Escherichia coli
phage
Qi8
cleavesa-D-mannopyranosyl linkagesof the E. coli0-8LPS, and theShigella-phage
Sf6 cleaves the Shigella flexneriLPSatthe
a-L-rhamnopyranosyl linkages(13, 17).
The phage
glycanase
activities have so far been showntobeexclusively
associatedwith thephage tail. In some instances
(P22
and coli-phagesf18 and29),
thephage
enzymes have beenpurified
andidentifiedastail structuralproteins
(2, 7, 17).
Theelucidation of
properties
andspecificities
of
phage
glycanases
is of interest for studiesofphage-receptor interactions and host
specifici-ties.Furthermore,phage
glycanases
may alsobeimportant
as tools forspecific
degradation
ofpolysaccharides.
In a search for
phages
possessing
glycanase
activity, 120-antigen-specific
S.typhimurium
phageswerefound tohaveLPSreceptorcleav-ingproperties.In thepresent paperwereporton
the substrate
specificities
oftwoof thesephage
583
on November 10, 2019 by guest
http://jvi.asm.org/
enzymes (no. 28B and 36) and the isolation of phage 36subviralstructurescarrying glycanase activity.
MATERIALS AND METHODS
Bacterial strains. S. typhimurium strainsSH4305
(0-antigens 4, 5, and 122) and SH4809 (0-antigens4, 5, and 12), S. enteritidis SH1262 (his- thr-thy-, 0-antigens9and 12)wereobtained from P. H. Miikela,
Central Public Health Laboratory, Helsinki, Finland. S.paratyphiAvar.durazzo(0-antigens2and12),S.
typhimurium LT2,and S.flexnerivar.Ywerefrom the collectionattheDepartmentofBacteriology, Na-tional Bacteriological Laboratory, Stockholn, Swe-den. StrainS. typhimurium SL3622 and the uridine
diphosphate-galactose epimerase-defective strain LT2-M1werefrom thecollection of B. A. D.Stocker,
Departmentof Medical Microbiology, Stanford Uni-versity,Stanford, Calif. S.typhimurium strains 1, 2, 3, 4, 5a, 6a,6b, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 18, 19, 20, 21, 22,and23a werethephagetypingreference strains used at the Department of Bacteriology, National
Bacteriological Laboratory, Stockholm,Sweden.
Bacteriophages. The clearplaquemutant P22c2 was obtained from B. A. D. Stocker. Salmonella smoothspecificphages 2, 4, 8, 28B, 30, 31, 32, 33, 34, 36, 37, and 39 were those used for S. typhimurium phagetypingattheDepartment of Bacteriology, Na-tionalBacteriological Laboratory, Stockholm,Sweden
(15).
Preparation of bacteriophage stocks. All phages were grown in submerged culture on their corresponding S. typhimurium host strain (Table 1). Log-phase cells, approximately5x 108 cells per ml, grownat37°C in Marvin medium (16),wereinfected at amultiplicity of infection of1to5.The individual cultureswereheavilyaeratedfor5 to 6horuntillysis wasevident. Completelysisofcellswasimposed by theaddition of chloroform.Cell debriswassedimented bycentrifugationat2,500xgfor20min.Some of the phage stocks were further purified by polyethylene glycol (PEG) precipitation. PEG (average molecular weight, 6,000) and sodium chloridewereadded to the clear supernatantto givefinal concentrations of 8%
(wt/vol) and0.5M,respectively (23). After sedimen-tationfor 24 h at 4°C, aggregated phages were col-lectedbycentrifugationat4,000xgfor20minat4°C.
The phage pelletswere suspendedin M-9 base me-dium (1) toapproximately 1/100of the original vol-ume,and thePEGwasprecipitated by repeated chlo-roform additions and removedbylow-speed centrifu-gations. Theresulting phage stocks had titers of 5 x
1010to 5 x 1013plaque-forming units (PFU) per ml. Thephages28Band 36wereinadditionpurified by ultracentrifugation in CsCl gradients (Spinco SW 40 rotor at160,000xg for16h). Gradientswere fraction-ated from the bottom in about
250-Al
portions, and the densities of the phage bands were determined (for 28B,8=1.48; for 36, 8=1.49).The phage-containing fractions werefinally dialyzed against M-9base me-dium and titrated on their respective host strains (phage 28B,4.7x 10'3PFUml';phage 36,2.2 x1013PFU*ml-').
Isolation ofphage36endoglycanase.Aheavily
aerated,5-literculture oflogarithmicallygrowingcells
of S. typhimurium strain 22 was infected at a multi-plicityofinfection of1.5.After5hof propagation at 37°C, the culture waslysed by the addition of a few
milliliters of chloroform and 0.5% (wt/vol) Sarkosyl. Cell debriswassedimentedat2,500xgfor 20 minat 4°C. Ammonium sulfatewasaddedto50%saturation of the clearsupernatant, andprecipitationwasallowed for24hat4°C.After sedimentation at 2,000xg,the precipitate was suspended in 500 ml of phosphate-buffered saline(PBS)anddialyzedagainst PBS over-night. The precipitation with 50% saturated ammo-niumsulfatewasrepeatedtwice. Afterfinaldissolution anddialysis againstPBS,thematerialwassubjected
to high-speed centrifugation to sediment remaining phageparticles.The enzymeactivityof the
superna-tantpreparationwasdeterminedby itsability to re-lease [14C]galactose-containing material from forma-linized S. typhimurium LT2-M1 cellslabeled in the LPS. Glycanase-active preparationswerealso exam-ined by electronmicroscopy.
PreparationofLPS. Bacteriaweregrownin sub-merged culture, and LPSwasextractedbythe
phenol-watermethod fromformaldehyde-killedbacteria(22).
Some of the LPSpreparationsweremademorewater soluble bytreatmentwith0.15Msodiumhydroxide
for2hat1000C.Thistreatmenthydrolyzes phosphate
bonds and fatty acid ester linkages in the lipid A moiety.Preparationofabequose-deficient
polysaccha-ridewasperformed by heatingLPS fromS.
typhimu-riumSH4809 in50%acetic acidat90°Cfor8h.This treatment hydrolyzed the
2-keto-3-deoxy-D-manno-octulusonic acid linkages in thecoreregion of the LPS as well as all a-1,3-abequosyl linkages. Sugar and methylation analyses of the dialyzed preparation
showed the presence of theexpected methylethersof D-mannose, L-rhamnose, and D-galactose. Sodium
metaperiodate-sodium borohydridetreatmentof the S.typhimurium LPSwasperformedasdescribed(8).
LPS fromKlebsiella 012 and thecapsular
polysac-charidefrom KlebsiellaK47were thesame asused earlier(3,6).
Screening for phage glycanase activities. Screening for phage glycanase activities was per-formedbythemethod of Erikssonetal.(7).Inshort,
mid-log-phase cellsof the uridine diphosphate-galac-tose-4-epimeraseless mutant, S. typhimurium
LT2-Ml,werelabeled with
D-galactose-1-14C
(0.5,uCi/ml,2mg/ml) in their LPS for3h. To measureglycanase
activities,the14C-labeledcells,twice washed inPBS, weremixed with dilutions of the phagetobe tested. After60min of incubationat 37°C, cellswere sedi-mented at 2,000 x g for 20 min in the cold, and portions of thesupernatantwerewithdrawn and
as-sayed for14Cactivity.
Screeningforendorhamnosidaseactivities. To testwhetheraspecific phage glycanase possessed the abilitytocleavethea-L-rhamnosyllinkages of the S.
typhimurium SH4809 LPS,the dialyzablecrude
oli-gosaccharide preparation(seebelow)wastestedforits
inhibitoryactivityonBandeiraeasimplicifolialectin
agglutination of human typeB erythrocytes. Inhibi-tion 50% or more of that obtained with melibiose
(calculatedonamolarbasisassuminga mean molec-ular weightof the crude oligosaccharide of -1,200) wasconsideredaspositive.
on November 10, 2019 by guest
http://jvi.asm.org/
585
Electron microscopy. Thepreparation tobe ex-aminedwasstainedwithuranylacetatebythe follow-ingprocedure:adropletof thepreparationwasplaced
on a grid (200-mesh copper with carbon-shadowed Parlodion film), andexcessfluidwassucked offwith filterpaper.Adrop offreshlyprepared 1% (wt/vol) uranylacetate wasthenappliedto the grid. Aftera
few minutes,excessfluidwasagainremoved by touch-ing the gridtothecornerofanadsorbent filterpaper.
Thepreparationswereexamined inaPhilips EM-200 electronmicroscope. The estimation ofphage dimen-sionswasmadeby comparisonwithacalibrationgrid. Methylation analyses.Methylation analyseswere performedasdescribed earlier(14). The oligosaccha-rideswerereduced with sodium borohydride,andafter methylation and subsequent work-up, the materials
werehydrolyzed. The resulting monosaccharideswere
transformedintoalditolacetatesandanalyzed by gas-liquidchromatography (GLC) andmassspectrometry. Forgas-liquid chromatography, a Perkin-Elmer 990 instrumentfitted witha3%OV-225colunmwasused. Gas-liquid chromatography-mass spectrometry was performedwithaVarianMAT311instrument.
Test for phage glycanase hydrolysis of
capsu-larorLPS. Thephagetobeassayedwasmixedata
ratio of 109to1010PFU/mg ofpolysaccharide in5mM ammoniumcarbonatebuffer, pH7.0,and incubatedat
37°Cwithinadialysis bag immersedin 10times the volume ofthesame buffer.After48hofincubation,
the surrounding dialysis fluid was concentrated to
drynessandheatedto50°C undervacuumtoremove
theremaining ammonium carbonate. Theamountof hexose inthe reaction mixture and the surrounding
dialysis fluid wasdetermined by the phenol-sulfuric acidmethod (5).
Miscellaneous methods. Gel chromatographyof oligosaccharides wasperformedon acolumn (170 by
2.5cm) ofBio-GelP-2 (200to400mesh)eluted with water,usingtrichlorobutanol (0.05%)asthe
bacterio-cidalagent.SeparationsweremonitoredbyaWaters
403refractometer,and the fractionswereassayed for
carbohydrate content by the phenol-sulfuric acid
method. For nuclearmagnetic resonancerecordings,
aJeol FX-100 instrument operatedin the PFT mode
wasused. Thespectrawererecorded for solutionsin
D20at850C, using extemaltetramethylsilaneas stan-dard.
RESULTS
Screening for phage glycanase activity. Twelve phageswereselected fromasetused for phage typing ofclinical isolates of S. typhimu-rium (15). The lytic spectraof these phageson the reference set of S. typhimurium strains is given in Table 1. The various phages were in-cubated withformaldehyde-treated S. typhimu-rium LT2-M1 cellswhich had been labeled with
['4C]galactose in theirLPS (7). After centrifu-gation, '4C-labeled material was found in all supernatants,indicating that all 12phages pos-sessed glycanase activities (Table 2). Thesame batch of ['4C]galactose-labeled LT2-M1 cells was also subjected to hydrolysis by the P22 endorhamnosidase byincubating the cellswith TABLE 1. Lyticspectra of S.typhimuriumphagesa
Bacteria 2 4 8 28B 30 31 32 33 34 36 37 39
1 + + + + + + + + +b +
2 + + + + + + + + +
3 + + i + + + + +
4 + + +b + + + + +
5a + +b + + + + + +
6a + + + + + + + +
6b +b + + + + + +
7 + + + + + +
8 + + + + + + +
9 + + + + + +
10 + + + + + + +
11 + +b+
12 + + +b + +
13 +b
14 +b + +
15 + + +
16 + +b
18 +b
19 + +
20 + + + +
21 + +
22 + +b +
23a + + + + + +
aPhagesinvestigated on the standard set of S. typhimurium strains used for phage typing of clinical isolates.
+,Sensitivity to phage indicated. bStrain was used forpropagation. VOL. 32,1979
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.507.52.449.410.635.2]TABLE 2. Glycanase activitiesof S.typhimurium phages assayedby
["4C]galactose
release'Phage Titer %Releaseb
2 5 x107 10 (4)
4 1.5 x
1010
49 (33)8 3.2x1012 50 (48)
28B 3.1 x1012 55 (48)
30 6.7 x 1010 43 (48)
31 1 x109 46 (12)
32 1.6 x1010 45 (33)
33 1.4x 1012 64 (48)
34 1.3 x1010 65 (32)
36 1x 109 68 (12)
37 1x 1010 39 (30)
39 1x 109 54 (12)
a14C-labeled material was released from S. typhi-murium LT2-M1 cells.To 100-,ulportions of washed S. typhimurium LT2-M1 cells previously labeled in their LPS with [14C]galactose were added
900-pl
amounts ofthevarious phagepreparations. After in-cubation for 1 h at 37°C, cellswere sedimented by centrifugation, 500-,ul portions of the supernatantswere withdrawn, and the released 14C activity was
determined.The same batch of
[14C]galactose-labeled
S. typhimurium LT-2 M-1wasalso assayed against aPEG-purified P22c2 phage stock. Maximal release (48% oftotal) was obtained at 5x 1010PFU per assay.
bValues in parentheses were obtained by using equal numbers of PFU of a purified phage P22c2 preparation.
differentamounts ofa
PEG-purified phage
P22stock and the percentage ofrelease
compared
with those obtained
by
the 12phages
underinvestigation
(Table 2).In allinstances(except
phage 30), the crude
phage
lysates
showedhigher release activities per PFU than did the
purified
phage
P22stock. To confirm that therelease of 14C-labeled material from the S.
ty-phimurium LT2-M1 cellswasdue to
phage-as-sociatedglycanase
activities,
crudelysates
of therespective propagation strains were tested for
hydrolytic properties. About 5 x 1011 cells of eachstrainwere
lysed by
sonication(three
times 10 s at25W ina Labsonic 1510sonicator),
andthe crude bacterial
lysates
wereincubatedwith[14C]galactose-labeled
S.typhimurium
LT2-M1cells. Noneofthe bacterial
lysates
releasedany14C-labeled
material. In a controlexperiment,
the variousphage lysates were, after sonication asdescribedabove,
foundtobeequally
activeasbefore sonication.
The
PEG-purified phage
28B and 36 stockswerefurtherpurified
by
density gradient
ultra-centrifugation.Inbothcases,the
phage-contain-ingfractions
displayed
thehighest
release activ-ities of[14C]galactose-labeled
material from S.typhimurium LT2-M1 cells. The 50% maximal release (45% of total label was
maximally
re-leased) values were, for
phage
28B and36, 8.8J. VIROL.
x
109
and 1.1 x 108PFU, respectively.
Thesedata indicated that the glycanase activities of
phages
28B and 36 were associated with thephage
particles.
Screening
for endorhamnosidasespeci-ficities. In another series ofexperiments, the various
phages
were incubated withpartially
delipidated
LPS from S.typhimurium SH 4809within
dialysis
sacksimmersedinbuffer.All 12phages
degradedtheLPStodialyzableoligosac-charides asevidenced byrecovery of 30to50%
of the total carbohydrate in the surrounding
dialysis
fluid(data
notshown).
Glycanases
cleaving the a-L-rhamnosyllink-agesin the S.
typhimurium
SH4809 LPS(Fig. 1, Table4)
shouldgive
rise tooligosaccharides
possessingaterminal, nonreducing
ca-D-galacto-syl
group. The agglutination ofhumantype Berythrocyteswith the a-D-galactosyl-specificB.
simplicifolia
lectin was inhibited by the crudeoligosaccharide
preparations(obtained fromthedialysis
experiments described above),thussug-gesting
that all 12phages exhibitedendorham-nosidase activity.
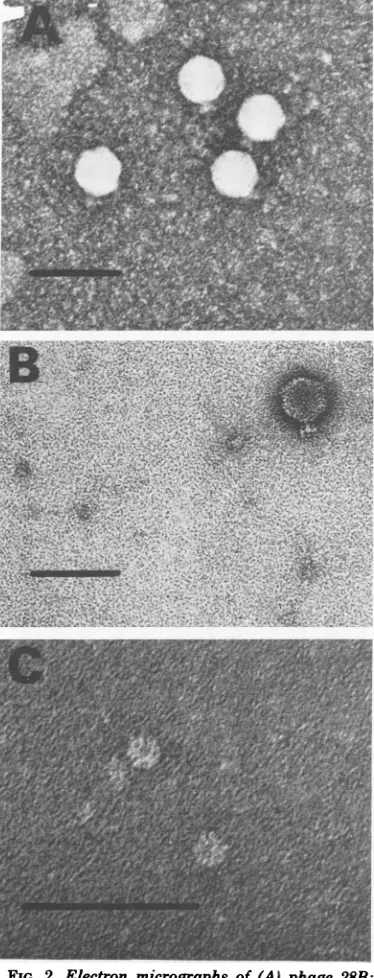
Electron microscopic examination of phages.InFig. 2,themorphology typical forall
12
phages
isshown. Theyall havea headwithhexagonal symmetry about50 nmindiameterto
which a short, noncontractile tail carrying six
spikesisattached. Thisappearanceistypicalfor type C phages, based on the classification of
Bradley (4).
Two ofthe phages, 28B and 36, were chosen
foramoredetailed study. Phage28B waschosen
for itsnarrowlyticspectrum (Table1) and ease ofpropagation. Phage36,however,had abroad lyticspectrum(Table 1) alsolysingtheS. typhi-murium SL3622 (Fig. 1 and Table 4).
Further-more,
phage
36showedahigh
'4C
releaseactiv-R D-Glcp
a
a3i
4/6[a-D-Ma1nP-(l1- 4)-a-L-RhaP-(l1- 3)-D-Gaip]n
-+2)-R
1J1
al 3
a-D-Manp-(1
4)-a-L-Rhap-(l
3)-a-D-Galp-(1 -*CORE LipA
FIG. 1. Generalstructureof LPS fromSalmonella serogroups A, B, andDl. Rindicates abequose (3,6-dideoxy-D-xylo-hexose) in Salmonella B, ortyvelose (3,6-dideoxy-D-arabino-hexose)andparatose (3,6-di-deoxy-D-ribo-hexose) in Salmonella Dl andA, re-spectively. Broken arrow indicates substitution oc-curring onlyin some strains. n,Numberof repeating units in0-chain,varyingfrom4to30.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.507.66.257.92.224.2] [image:4.507.267.462.491.586.2].--; i-'! - * < =, *S t In%
*
4;'r
''1'v'" '.
.''
'" ' -,;,.,
--.''
'"
FIG. 2. Electron micrographs of(A)phage 28B; (B)phage 36; and (C) isolated baseplate-like
struc-tures of phage 36. Each line shown represents 100
nm.
ity as compared with the phage P22 standard (Table 2); i.e., it could be overproducing the glycanase.
Isolation ofphage 36 subviral elements possessing glycanase activity. Subviral
structuresexhibitingglycanase activitywere
iso-lated by ammonium sulfate precipitation of a
crude lysateofphage36. More than 60%ofthe total glycanase activity was recovered in the precipitate fraction. To remove contaminating intact phages and ghosts, the dialyzed
precipi-tate fraction was subjected to high-speed
cen-trifugations. The final preparation contained <105PFU ofproteinper mg.
Electron microscopic examination of the su-pernatantfraction revealed thepresence oflarge numbers ofdoughnut-shaped structures (15 to 20 nm in diameter), each with a central hole (Fig. 2c). Thesestructuresresemblewhole phage tails or baseplates. No ghosts or intact phage particles were seen. The glycanase activity of the supernatant fractionwas about 1,000 times higher (per milligram ofprotein) than that of
PEG-purified phage36particles.
Phage 36- and 28B-mediated hydrolysis of S. typhimurium SH4809 LPS. Purified phage36and28B wereincubated withpartially
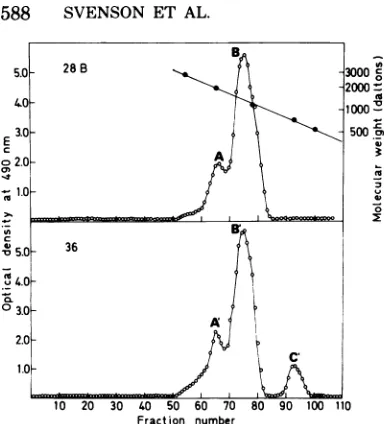
delipidated LPS from S. typhimurium SH4809. The crude dialyzable oligosaccharide
prepara-tions obtainedweresubjectedtogel chromatog-raphy (Fig. 3a andb). The phage 28B-cleaved material showed peaks corresponding to a do-deca- (A) and an octasaccharide (B),
respec-tively. Phage 36-cleavedmaterialshowed
essen-tially the same elutionpattern, but in addition to the dodeca-
(A')
and octasaccharide(B1),
atetrasaccharide
(C')
wasalso isolated.Toconfirm the estimated sizes ofthedifferent oligosaccharides, the individual peaks were
pooled,
andafteranadditionalchromatography
on the same column the saccharides were
re-duced and subjected to methylation analysis (Table 3). The only difference compared with
the
analyses
oforiginal LPSwasthepresence of2,3,4,6-tetra-O-methyl-D-galactose,
demonstrat-ing that the
a-L-rhamnosyl
linkages
had beenselectively
cleaved in the S.typhimurium
SH4809 LPS.Fromtheratio of tetra-O-methyl-D-galactosetotri-O-methyl-D-galactose,the size
of theoligosaccharidescanbeestimated.Thus, fractionsAandBfrom thephage28B
hydroly-satecorrespondtododeca-and
octasaccharides,
respectively.
Similarly,
A', B',
andC'
fromthephage-36
hydrolysate
correspond
tododeca-,
octa-, and tetrasaccharides, respectively. All analyses showed the presence of significant amountsof3,4,6-tri-O-methyl-D-mannose.
This was most likelydue to acidichydrolysis of theabequosyllinkage duringthework-up.
Oligosaccharides B and Bl and the
octasac-charide obtained by phage P22 endorhamnosi-dase-mediated hydrolysis of S. typhimurium
SH4809 LPSwere shown tobeidenticalby
'H
nuclear magnetic resonance (8). Signals wereon November 10, 2019 by guest
http://jvi.asm.org/
[image:5.507.56.243.78.567.2]E z
-4
-I
o*0C
0'
Fraction number
FIG. 3. Gelchromatography ofcrude oligosaccha-ride preparationsobtained after phage 28B (upper panel)andphage36(lower panel) hydrolysis of par-tially delipidatedLPSfromS.typhimuriumSH4809. The crudeoligosaccharideswereappliedtoacolumn (170 by2.5cm) ofBio-GelP-2(200to400mesh)and eluted withwater(8.5 ml/h).Thefractionvolumewas
5ml,and the total hexosecontentwasestimatedby the phenol-sulfuric acid method. For molecular
weightcalibration ofthecolumn,thefollowing
sac-charideswereused:melibiose, stachyose, cellohexose,
S. typhimurium dodecasaccharide, and fluorescein isothiocyanate-labeleddextran ofmolecular weight 2,900.
obtained, inter alia, at 8 4.9 to 5.4 (anomeric protons), 8 2.0 to2.2 (H-3 of abequosylgroup),
and 8 1.2 to1.5 (H-6 of abequosylgroupand L-rhamnosyl residue).
Under the conditionsused, both phageswere foundtoproducemoreoctasaccharide than do-decasaccharide. However, with the phage 36 en-dorhamnosidase, tetrasaccharides were also formed. The approximative molar proportions ofdodeca-, octa-, and tetrasaccharide were for phage 28B, 2:8:0,and forphage 36, 2:6:2.
Substrate specificities of phage 36 and
28B endorhamnosidases.In additiontothe S.
typhimurium (SH4809,serogroup B)LPS,both phages cleaved the S. enteritidis (SH1262,
se-rogroupD1) and S.paratyphi A(serogroup A)
LPS (Table 4). Since Salmonella bacteria be-longingtoserogroupsA, B,and Dlallshare the same trisaccharide backbone in their tetrasac-charide-repeating unit (Fig. 1) and differ only in the type of3,6-dideoxyhexosyl group linked to
[image:6.507.64.256.56.268.2]0-3 of the D-mannosyl residue, these results show that different 3,6-dideoxyhexosyl groups linkedtothispositionareaccepted by the phage 36and28B endorhamnosidases.
TABLE 3. Methylation analyses ofoligosaccharide fractions obtained by phage-mediatedhydrolysis of
S. typhimurium SH4809 LPS
Methylated Mol%C
Hydrolysis sugar (major Tb
components)a A B A' B' C'
Phage 28 2,4-Abed 0.28 7 5 medi- 2,3-Rha 0.92 24 18 ated 2,3,4,6-Gal 1.19 10 16 3,4,6-Man 1.82 15 19 2,4,6-Gal 2.03 24 19 4,6-Man 2.92 19 22
Phage 36 2,4-Abed 0.28 5 5 7
medi- 2,3-Rha 0.92 23 23 5
ated 2,3,4,6-Gal 1.19 10 15 48 3,4,6-Man 1.82 12 4 18 2,4,6-Gal 2.03 24 19 2
4,6-Man 2.92 26 34 19
a2,4-Abe indicates
2,4-di-O-methyl-abequose;
2,3-Rha indicates2,3-di-0-methyl-L-rhamnose, etc.bRetention time of the corresponding alditol ace-tates relative to
1,5-di-O-acetyl-2,3,4,6-tetra-0-methyl-D-glucitolon anOV-225 column. cFractionsaccordingtoFig.2.See text.
d
Most
ofthis volatile compound and derivativeswerelost during evaporations.
LPS from S. typhimurium SH4809in which
the
3,6-dideoxyhexosyl
groups had beenre-movedby mild acidic
hydrolysis
wasalso hydro-lyzed by theenzymes,although
less efficiently. However, apreparation
of S.typhimurium
SH4809 LPSinwhich the
L-rhamnosyl
residues had beenmodifiedby periodate
oxidation-boro-hydride reduction was found resistant to bothenzymes.
Different glucosylations of the D-galactosyl residues in the S. typhimurium 0-side chain occur. Partial
glucosylation (about 40%)
at 0-6 of theD-galactosyl
residuesinthe LPSof theS.typhimurium
P22lysogen
strain SL3622 loweredbothenzymeactivities asmeasuredby the deg-radation of LPS intodialyzable oligosaccharides.
Upon
methylation analysis
of theseoligosaccha-rides,
only
the2,3,4,6-tetra-0-methyl-galactose
etherwasfound.Hence,the absence of2,3,4-tri-0-methyl-galactose
indicated that none of thephageenzymes could cleavea-L-rhamnosyl link-agesadjacentto0-6-glucosylated galactosyl
res-idues.
Also, LPS from the S. typhimurium strain SH4305 which is
glucosylated
(about70%)
at0-4 of its D-galactosyl residues was subjected to
degradationbythe
phage
enzymes. Portions of theLPSwere mixed withphage 28B and36indialysis bags.. After 48 h ofhydrolysis with si-multaneousdialysis,boththedialyzable saccha-rides aswellasthose retained within thedialysis
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.507.266.455.87.259.2]+.
+ + + + +
+ + + + +
+
16
1
t
T
TI
T T T_
T
T
Tt
XI
t
I
I
-T
T
T T TQ > t t
O~~~~
J = J J
J~~~~~~~~~~~~~a
,a
':
="t
1
~~~~~c1
t
t
_
I
_I_I
0 N N 2 a) 'a) ct * CO < C. -A)C L. C
CiCli
.0 6-a'g
la N .0 C. C. .0.~ I-C.t's ~ a
ci5c' a) 4) co *4j a) a) a) 10
0.
CI) C. 'a) c#i C. *D CI)'04) S)to0C)0A4 a)
04 04 0o 04 a) 40. 0 a) Ci) C4 CA) co t3 co a) 1.s -9 C. CO 'a) a) *0-Co0 a4) C.)1 c) P.) C. Co '0
F-t
II
0 0a
a 4Ib1-4 Ql. IVT
T
a)I
I
I
T-, ,I
I
T
-1-I
rt
1-Ca 1I CI)t
1-C.. 'a) QliI
"4'" I¢ 4
W: ta) t z
t
II r--C',t
ri ._ _) a) a) 0 a) C.) oo00
.0, a) a) a)C.) 4.) 4.3a)
a)"a)C6q'X
o oa)
C. C.) .
o~~~~~~~~~ a
4)4 )
cI CI Qo L.a -Cl c4' C'1
.E .@ a)
Co Co "I
C
li
on November 10, 2019 by guest
http://jvi.asm.org/
590 SVENSON ET AL.
bag weresubjected to methylation analysis.
Ta-ble 5shows the molar percentage of the various
methyl ethers ofD-galactosein these fractions. The presence of 2,3,6-tri-O-methyl-D-galactose
in thedialyzable fractions showed that the phage enzymescleaved linkages adjacent to galactosyl residues glucosylated at 0-4. Furthermore, in both cases thesmallincrease in 2,3,4,6-tetra-0-methyl-D-galactosecompared with that of
2,3,6-tri-0-methyl-D-galactoseshowed that
a-L-rham-nosyllinkages adjacent to 0-4-substituted D-ga-lactosyl residues were preferentially cleaved. The same pattern was also seen in similar
ex-periments in which the enzymes were given
equalamountsof unglucosylatedLPS (S.
typhi-murium SH4809) andLPS glucosylated at 0-4
(S. typhimurium SH4305) as substrates (data notshown).
The high specificities of the present endo-rhamnosidases were also evidenced by their
in-ability to degrade three other polysaccharides
(S. flexneri var.Y,Klebsiella 012, and
Klebsi-ella K47) containing a-L-rhamnosyl residues
(Table4).
DISCUSSION
It has been shown that phages infecting
en-capsulatedorsmoothbacteria likeSalmonella, Klebsiella,E.coli, andShigellahaveglycanase
activities associated with their tail structures
(forareview, see reference 12). These glycanases seem toassist in the penetration of the outer-most layer of carbohydrates surrounding the
bacterial cell,thus allowing the phage to bind to
postulatedsecondary receptors close to the cy-toplasmic membrane from where injection of the phage nucleic acid then occurs. Because
cleav-TABLE 5. Methylation analyses ofsaccharides after phage-mediatedhydrolysisof S.typhimurium
SH4305LPS
Mol % of total
galac-Methyl tose content
Hydrolysis ethersof Tb
Undi-D-galac- alyzable Dialyza-tose' saccha- ble sac-rides charides Phage 28 me- 2,3,4,6-Gal 1.19 13 19
diated 2,4,6-Gal 2.03 53 0
2,3,6-Gal 2.22 0 28
2,6-Gal 3.14 34 53
Phage 36 me- 2,3,4,6-Gal 1.19 20 22
diated 2,4,6-Gal 2.03 43 0
2,3,6-Gal 2.22 0 38
2,6-Gal 3.14 37 40
See footnote a to Table 3.
bRetention time of thecorrespondingalditol acetates rel-ative to1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl-D-glucitolon an OV-225 column.
age of the impeding
carbohydrate
layer
is anecessity forsuccessful
infection,
the substratespecificities of these
phage
glycanases
set thelimits to their host range.
Phage
P22 causesglycosylation at 0-6 of
D-galactosyl
residues. Astrainwhich is
lysogenic
with thisphage
isre-sistant to the
endorhamnosidase
andconse-quentlyto
multiple
infectionby
thisphage.
Inthisstudy, 12S.typhimurium
smoothspe-cific phages,
originally
isolatedby
Lilleengen
(15) and used in the routine
phage
typing
ofclinical S.
typhimurium
isolatesinSweden,
wereinvestigated.
Thephageswerescreenedfor
glycanase
activ-ity by their
ability
to release1"C-labeled
LPS
fragments fromwhole S.typhimurium
LT2-M1 cells (7). All were shown todegrade
theLPS
(Table2). When
partially
delipidated
LPS
fromS.
typhimurium
SH4809 wasmixed with crudelysates ofthe various
phages,
dialyzable
oligo-saccharides
couldbeisolated,
giving
furthercon-firmation to the
hydrolytic
capacity
of the phages.Theinabilityof crudelysatesof the
propaga-tion strains to cleave off
oligosaccharides
from14C-labeled
S.typhimurium
LT2-M1 cellssug-gested that the
glycanase
activities were ofphage and not of bacterial
origin.
In the in-stanceswhere the phageswerepurified by
den-sity gradient
ultracentrifugation,
theglycanase
activities werefoundassociatedwith the
phage
particles, indicatingthat the enzymes are
inte-gralpartsof thephage
particles
rather than viralexitenzymes.
All phages (except phage 30) showed
higher
14C release values (per PFU) whenphage
P22 glycanase activity wasusedas standard(Table
2). The higher activity could be accounted forbythe presence of
enzymatically
active butnotviableghostsor
overproduction
ofenzymatically
active phageprotein
or both. The isolation of subviralbaseplate-like
structures ofphage
36(Fig. 2c) exhibitinga
1,000-fold higher
glycanase
activity(permilligram of
protein)
than the PEG-purified wholephage 36particles,
showed thatthehigh release value obtained with the crude lysate of thisphage mostlikely was causedby overproduction of thephageenzyme.
Inhibition of the
a-D-galactosyl-specific
B.simplicifolialectinagglutinationof humantype
B erythrocytes with the crude
oligosaccharide
preparations obtained after phagedegradation
of S. typhimuriumLPSsuggested
thatthe gly-canasesofall
12phageshadendorhamnosidase specificity.Phages 28B and 36, which wereexamined in moredetail, had
essentially
equal substrate pro-files(Table4). Hydrolysisoccurredirrespectiveof thetypeof3,6-dideoxyhexosylsubstituentin
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.507.66.259.499.640.2]the polysaccharide. Bothenzymes were shown to preferentially cleave at galactosyl residues
glucosylated
at 0-4(S. typhimurium SH4305)
(Table5). Thiswas evidentby the preferential
appearance of2,3,6-tri-0-methyl-D-galactosein
the methylation analysis of the oligosaccharide
preparations obtained after degradation of the
S.
typhimurium
SH4305 LPS(Table
5). Also,when theS. typhimurium 4305LPS wasmixed
atequal ratio with the unglucosylated S.
typhi-muriumSH4809, thesame methyl ether
(2,3,6-tri-0-methyl-D-galactose)
wasdominating (datanotshown). In contrast, glucosylation at 0-6 of
theD-galactosyl residueswas anobstacle to the enzymes, as demonstrated by the rather low
degradation of the LPSfromtheP22lysogen S.
typhimurium
strainSL3622(Table 4).
No 2,3,4-tri-0-methyl-D-galactose could be
detected in the
methylation
analysis of the oli-gosaccharides prepared by degradation of this LPS, indicating thatglucosylation
at0-6 ofga-lactosyl residues makes such
a-L-rhamnosyl
linkages resistantto
hydrolysis.
Abequose-deficient LPS from S.
typhimurium
SH4809 is cleaved
by
bothphage
28Band36. Inthisrespect,these
phages
differ fromphage P22, which does nothydrolyze
LPS preparations lacking 3,6-dideoxyhexosyl groups (8). Modifi-cationof theL-rhamnosyl
residuesby
periodate-borohydride treatment
made,
asexpected,
the S.typhimurium
substrate inerttobothenzymes.In parallel with
phage
P22,phage
28B and36endorhamnosidases were unable to cleave the
a-L-rhamnosyl
linkages occurring
in theS.flex-neri var. Y and the Klebsiella 012 LPS or in
the
capsular
polysaccharide
ofKlebsiella
K47(Table 4). Although the
phage
28B endorham-nosidase, likephage P22,
mainly yields
octasac-charides, phage 36inaddition alsoproduced20
mol %tetrasaccharides.
Although the enzymes of the 28B and 36
phageshavesubstrate
profiles
similartothatofphage P22 (8), differences are discernible. This
setof
phage
enzymescould be of value forprep-aration of
0-antigen-specific oligosaccharides
from
Salmonella.
Sucholigosaccharides
havebeen used for the elucidation of the size of 0-antigen determinants and also for the
prepara-tion ofartificial Salmonella vaccines
(9, 18-21;
H. J.
Jorbeck,
S.B.Svenson,
andA. A.Lindberg,
J.Immunol., inpress).ACKNOWLEDGMENTS
Thisworkwasinpartsupported by grantsfrom the Swed-ishMedicalResearch Council (no.B77-16X-00656) andthe Swedish Board of TechnicalDevelopment(no. 75-5809).
Weareindebted to BertLarsson, Birgitta Sundberg,and Viveka Eriksson for technical assistance. Ulla Eriksson is gratefullyacknowledgedfor valuablediscussions.
LITERATURE CITED
1. Adams, M. H. 1959. Bacteriophages. Interscience Pub-lishers, Inc., New York.
2. Bessler, W., F. Fehmel, E. Freund-Molbert, H. Kniifermann, and S. Stirm. 1975. Escherichia coli capsule bacteriophages. IV. Free capsule depolymerase 29. J. Virol.15:976-984.
3. Bjorndahl, H.,B.Lindberg, J. Ldnngren, K.-G. Ro-sell,and W.Nimmich. 1973. Structural studies of the Klebsiella type K47 capsularpolysaccharide. Carbo-hydr. Res. 27:373-378.
4. Bradley, D. E. 1967. Ultrastructure of bacteriophages and bacteriocins. Bacteriol. Rev. 31:230-314. 5. Dubois, M.,K.Gilles, J. K. Hamilton, P. A. Rebers,
and F. Smith. 1951.A colorimetric method for the determination of sugars. Nature (London) 168:167. 6. Erbing, C.,B.Lindberg, J. Lonngren, and W.
Nim-mich. 1977. Structural studies on the Klebsiella 0 group 12 lipopolysaccharide. Carbohydr. Res. 56:337-381.
7. Eriksson, U., and A. A. Lindberg. 1977. Adsorption of phage P22 toSalmonella typhimurium. J.Gen. Virol. 34:207-221.
8. Eriksson, U., S. B.Svenson,J.Ldnngren, and A. A. Lindberg. 1979. Salmonella phage glycanases: sub-stratespecificity of the phage P22 endo-rhamnosidase. J. Gen.Virol. 43:503-511.
9. Jorbeck, H., H.E. Carlsson, S. B. Svenson, A. A. Lindberg, G. Alfredsson, P. J. Garegg, S. Svens-son, and N. H. Wallin. 1979. Immunochemistry of Salmonella0-antigens:specificity and cross-reactivity of factor0-9serumand of antibodies against tyvelose
1 3
1 mannosecoupled to bovine serum albumin.
a
Int. Arch.Allergy Appl. Immunol. 58:11-19.
10.Hayes,C.E., and I. J. Goldstein.1974.An a-D-galac-tosyl-binding lectin from Bandeiraea simplifolia seeds. J. Biol. Chem. 240:1904-1914.
11.Kanegasaki, S.,and A.Wright. 1973. Studiesonthe mechanism ofphageabsorption: interaction between phage E15 and itscelularreceptor.Virology 52:160-173. 12.Lindberg, A. A. 1977. Bacterial surface carbohydrates andbacteriophage adsorption. InI. W.Sutherland(ed.), Surface carbohydrates of the prokaryotic cell. Academic PressInc., New York.
13.Lindberg, A. A., R. Wollin, P. Gemski, and J. A. Wohlhieter.1978.Interactionbetweenbacteriophage Sf6 andShigella flexneri.J. Virol.27:38-44. 14.Lindberg, B. 1972.Methylation analysisof
polysaccha-rides. MethodsEnzymol.28B:178-195.
15. Lilleengen,K.1948.TypingofSalmonellatyphimurium by meansofbacteriophage. Acta Pathol. Microbiol. Scand.Suppl.77:1-125.
16. Marvin,D.A.,and H. Schaller. 1966.Thetopologyof DNAfrom thesmallfilamentousbacteriophagefd. J. Mol. Biol.15:1-7.
17. Prehm, P., and K. Jann. 1976. Enzymatic action of coliphageQ8 and itspossiblerole in infection. J. Virol. 19:940-949.
18. Svenson,S.B.,and A. A.Lindberg.1977.
Oligosaccha-ride-protein conjugate: a novel approach for making Salmonella0-antigen immunogens.FEMS Microbiol. Lett.1:145-148.
19. Svenson,S. B., andA. A.Lindberg. 1978. Immuno-chemistryofSalmonella0-antigens: preparationofan
octasaccharide-bovineserumalbuminimmunogen rep-resentativeofSalmonellaserogroupB0-antigenand characterizationof theantibodyresponse.J.Immunol. 120:1750-1757.
20. Svenson,S.B.,and A. A.Lindberg.1979.Couplingof acidlabileSalmonellaspecificoligosaccharidesto
mac-romolecularcarriers. J. Immunol.Methods25:323-335. 21. Svenson, S.B., M.Nurminen,andA. A.Lindberg.
on November 10, 2019 by guest
http://jvi.asm.org/
1979.ArtificialSalmonella vaccines:0-antigenic oligo-saccharide-proteinconjugates induce protection against infection withSalmonellatyphimurium.Infect.Immun. 25:863-873.
22. Westphal, O.,0.Luderitz,and F.Bister. 1952.Uber die Extraktion von Bakterien mit Phenol/Wasser. Z.
Naturforsch.7:148-155.
23. Yamamoto, K. R., and B. M. Alberts. 1970. Rapid
bacteriophage sedimentation in the presence of
poly-ethylene- glycoland itsapplicationtolargescale virus
purification.Virology40:734-744.
![TABLE 2. Glycanase activities ofS. typhimuriumphages assayed by ["4C]galactose release'](https://thumb-us.123doks.com/thumbv2/123dok_us/1505291.103205/4.507.66.257.92.224/table-glycanase-activities-ofs-typhimuriumphages-assayed-galactose-release.webp)