0022-538X/89/093769-08$02.00/0
Copyright
© 1989, American
Society
for Microbiology
Herpes
Simplex
Virus Ribonucleotide
Reductase: Expression
in
Escherichia
coli and Purification
to
Homogeneity of
a
Tyrosyl
Free
Radical-Containing,
Enzymatically Active
Form
of
the
38-Kilodalton
Subunit
ROLF
INGEMARSON,1*
ASTRIDGRASLUND,1
ALLAN DARLING,2 AND LARS THELANDER1Department
of Medical Biochemistry
andBiophysics,
Universityof
Umead,S-901
87 Umea', Sweden,1 andMedicalResearch
Council
Virology
Unit,
Institute
of
Virology,
Church
Street,
Glasgow
GlSJR,
United
Kingdom2
Received30January1989/Accepted 16May 1989
Infection of
mammalian cells with herpes simplex virus (HSV) induces a virus-encoded ribonucleotidereductase which
isdifferent from
the cellularenzyme.This essential viralenzymeconsists oftwononidentical subunitsof 140
and38 kilodaltons
(kDa) which have notpreviously been purifiedtohomogeneity. The small subunit ofribonucleotide
reductasesfrom
other species containsatyrosylfree radical essential for activity. Wehave cloned the gene
for
the small subunitof
HSV-1
ribonucleotide reductase intoa tac expression plasmidvector.
After
transfection of Escherichia
coli, expression of the 38-kDa proteinwas detected in immunoblotswitha
specific
monoclonal antibody. About 30,ug
of proteinwasproducedperliter of bacterial culture. The38-kDa
protein
waspurified
tohomogeneity
in analmostquantitative
yield by immunoaffinitychromatogra-phy. It
contained
a tyrosylfree
radical which gave aspecific
electron paramagnetic resonance spectrumidentical
tothatwehave observed inHSV-infected mammalian
cells and clearlydifferent
from that producedby
the E. coliand mammalianribonucleotide
reductases. Therecombinant
38-kDa subunit
hadfull activity
when
assayed
in
thepresenceof HSV-infected
cellextractsdeficient
in the native38-kDa
subunit.Ribonucleotide reductase (EC 1.17.4.1) reduces all four
ribonucleotides
tothe
corresponding
deoxyribonucleotides
(34).
The enzymes fromEscherichia coli
and mammaliancells,
aswellasthoseencodedby
certainbacteriophages
andviruses, have been shown
tobe composed of
twononiden-tical subunits
which
show similarities between thedifferent
species.
The E. coli and mammalian enzymes have beenpurified
tohomogeneity
andarethebest-characterized
ones(38, 40, 41).
Inthese,
thelarge
subunits contain sites for allosteric effectors and the small subunits haveatyrosyl
freeradical
interacting
with apair
of ferric ions.Both
the ironcenterand the
radical,
which is stableonly
inthepresenceof theiron
center, areessential forenzymeactivity
(34).
Mammalian cells
infected with differentherpes viruses,
including herpes simplex
virus type 1(HSV-1), herpes
simplex
virustype2, Epstein-Barr virus,
andpseudorabies
virus, contain
aribonucleotide
reductaseactivity different
from the
activity
inuninfected cells(10, 11, 22, 26).
None of these enzymes have beenpurified
tohomogeneity.
The HSV-1 ribonucleotidereductase
consists ofalarge
subunit of 140 kilodaltons(kDa)
andasmall subunit of 38 kDa(4, 17,
24). Experiments
withthepseudorabies
virus- and the HSV-1-induced enzymesindicate
that both lack the allostericregulation
characteristic of the E. coli and mammalianen-zymes
(3, 26).
Thepseudorabies
virus enzyme also has atyrosyl
free radical whichgives
an electronparamagnetic
resonance
(EPR)
signal
different from those observed for themammalian and E. colienzymes.
The small subunit of HSV-1 ribonucleotide reductase is
encoded
by
a 1.2-kilobase(kb) transcript,
and thelarge
subunit is encoded
by
a5-kbtranscript.
Thetranscripts
arecolinear, sharing
thesame3'end,
butthetranslatedpartsdo notoverlap (28).
Thecorresponding
DNA has beense-* Correspondingauthor.
quenced (13, 28, 29).
Wepreviously produced monoclonal
antibodies
against
each HSV-1ribonucleotide
reductase subunit andused themtoshow that theenzymeis builtas atight complex
of thea2P2
type. Inthis
complex
the twosubunits,
eachconsisting
of two identicalpolypeptide
chains,
bindstrongly
to one another(24).
Anonapeptide
corresponding
to thecarboxyl
end of the 38-kDa subunit inhibitsenzymeactivity by interfering
with thisbinding (9,
14, 31).
The HSV ribonucleotide reductase seems to be essential for virusgrowth,
atleast innondividing
cells(7, 19,
32, 33).
Attempts
toseparately
expressenzymatically
active HSV-2ribonucleotide
reductase subunits in cultured human cells have beenreported, although they
haveresulted inverylowyields (23).
We have cloned the geneencoding
the small subunit of HSV-1 ribonucleotide reductase into a bacterialexpression
vector. After transfection of E.coli, expression
of the HSV-1 38-kDa
protein
wasdetected in immunoblotswith a
specific monoclonal antibody.
Theprotein
has beenpurified
tohomogeneity
and isenzymatically
active. Itcontains a
tyrosyl
freeradical, giving
aspecific
EPRsignal
which is identical to the EPR spectrum observed from
HSV-infected mammalian cells. This spectrum is
clearly
different from those
arising
fromthe E. coliand mammalianreductases.
MATERIALS AND METHODS
Plasmids. The
plasmid pSG 124, containing
the 23-kb EcoRI Afragment
ofthe HSV-1 strain KOS DNA clonedinto
pBR 325,
waskindly supplied by
M. Levine of theUniversity
ofMichigan,
Ann Arbor(18).
Theplasmid pDR
540 is an
expression
vector constructedby
Russel andBennet
(35)
and iscommercially
available fromPharmacia,
Inc., Piscataway,
N.J. Theplasmid
contains the strongtacpromoter, which is
composed
of the -35region
of the trp3769
on November 10, 2019 by guest
http://jvi.asm.org/
3770 INGEMARSON ET AL.
promoter and the -10 region, operator, and
ribosome-binding
site of thelau
UV-5 promoter. The promoteris
controlled
by thelactose repressor,and
transcription
canbe
induced by the addition of
isopropyl-4-D-thiogalactoside.
Plasmid
DNA was preparedfrom
overnight cultures of
infected
E.coli
cellsgently
lysed by
treatmentwith
lyso-zymeandthen withTriton X-100.The DNA was
purified by
two
consecutive
CsCl gradientultracentrifugations.
Bacterial strains and media. The
plasmid pDR
540
waspropagated
in E.(oli
K-12JM109
(43).Transfection
of
E.coli
was performed asdescribed by Hanahan (21). Bacteriawere grown in LB mediumat
37°C,
andbacteria containingplasmids
were grown in the presence of 50 pLgof carbacillin (Astra) per ml.Extraction of bacteriaforenzymepurification andassay. E.
c0li JM109
cells containing the38-kDa
protein expression
plasmids weregrowntoanoptical density at590nm
(OD,,)
of
3.0. The cellswerethenpelletedat4°C,
washedonce in25mM
4-(2-hydroxyethyl)-1-piperazine
sulfonic acidbuffer,
pH 7.6, suspended in the same buffer to anOD,9(
of
325,and
frozen
in liquid nitrogen. After the cells were thawed,KCI
and phenylmethylsulfonyl fluoride were added tofinal
con-centrations
of80 and 1 mM, respectively. Egg whitelyso-zyme(Sigma ChemicalCo., St. Louis, Mo.) wasadded toa
concentration of 300
[tg/ml,
and the mixture was incubated onice
for 20min.
After another cycle of freezing andthawing,
cell debriswasremoved bycentrifugationat44,000x gfor60
min
at4°C.
Enzymes. Restriction endonucleases werepurchased from
IBI
(BamnHI
and HindIII) and Boehringer GmbH,Mann-heim,
Federal Republic of Germany) (EcoRl and NcoI). T4ligase, the Klenow fragment of DNA polymerase, alkaline
phosphatase, and mung bean nuclease all came from
Boeh-ringer
GmbH.DNA sequencing. A309-base-pair
HindIII
restrictionfrag-mentcontainingthepromoterandthefirst nucleotides ofthe
herpesvirus
DNA insert was isolated from the plasmid pRI10 (see Fig. 1B) and subcloned in M13 mpl9 (30). The
sequence was determined by the dideoxy method (37). The
plasmid
pRI 9 (see Fig.1B)
was sequenced directly at theplasmid
DNAlevel by themethod of Chen and Seeburg(8).A 15-mer oligonucleotide corresponding to the sequence
between
the -35 and -10region of the promoter was used as a primer. The primer was synthesized by Symbicom,Ume'a,
Sweden.Antibodies. The mouse monoclonal antibody 535 directed
against
the 38-kDa subunit ofHSV-1 ribonucleotide reduc-tase was purified from ascitic fluid by ammonium sulfatefractionation
(24) followedby chromatography on aproteinA-Sepharose
column (Pharmacia). A 5-mg portion ofanti-body
waslinkedto1 mlof CNBr-activated Sepharose4B bythe
methods recommended by the manufacturer(Pharma-cia).
This column could bind at least 0.5 mg of 38-kDaproteinpermlof sedimented Sepharose. Polyclonal
antibod-ies
directed against a nonapeptide corresponding to thesequence ofthe nine carboxyl-terminal amino acid residues
of
the HSV-1 38-kDa subunit wereinduced byinjecting 0.5mgof peptidelinkedto1.6 mgof hemocyanin (Sigma) intoa
rabbit. The couplingwasmade in 0.1 M NaPO4, pH 8.0, in
thepresenceof 6.7mM glutaraldehyde for1 hat
37°C.
Afterequilibration
with 0.1 M sodium phosphate, pH 7.6, on aSephadexG-25 column, the peptide solution was combined
with an equal volume of complete Freund adjuvant. The
boosting was made with the same amount of peptide in
incomplete Freund adjuvant, and then antibodies were
pu-rified
fromthe rabbit serum by ammonium sulfatefraction-ation (see above)
followed
by
dialysis
against
0.2
Msodium
citrate
buffer, pH
6.5.
Theantibodies
werethen
linked
toCNBr-activated Sepharose 4B as
described above
by
using
9 mg ofantibodies permlof Sepharose. The
binding
capacity
was around 80
[tg
of 38-kDaprotein
perml
of
sedimented
Sepharose. Thenonapeptide
wassynthesized by
M.Carl-qvist at the Department of
Biochemistry, Karolinska
Insti-tute, Stockholm, Sweden.
Polyacrylamide gel electrophoresis and
immunoblotting.
Sodium
dodecylsulfate
(SDS)-polyacrylamide
gel
electro-phoresis was carried out as described
previously
(16).Pel-leted bacteria were
lysed
at100°C
for
5min
in gel sample
buffer consisting
of
0.125 M Trischloride, pH 6.8, 0.18
M 2-mercaptoethanol,1.1%
SDS, and25% glycerol.
Toreduce
the viscosity of the samples, DNase I was added to a
final
concentration of 0.03
mg/ml,
and
themixture
wasincubated
for 5
min
at room temperature. In theimmunoblots,
proteins
were transferred from the gel to a nitrocellulose membrane for 2 h at 130 mA by using a
semidry electroblotter
from
Ancos, Denmark (25). After blocking, the membranes were incubated in a solution containing the mousemonoclonal 535 antibody and then in asolution
containing rabbit anti-mouse
antibodies conjugated to alkaline phosphatase (Sigma) as described previously (24).
Partially purified 140-kDa subunit of HSV-1 ribonucleotide reductase. Partially purified 140-kDa subunit of HSV-1 ribo-nucleotide reductase was obtained from BHK-21 cells in-fected with HSV-1 strain 17
ts1222.
This strain has a ts mutation in the 38-kDa subunit of ribonucleotide reductase and cannot make a functional protein when grown at the nonpermissive temperature (12, 32). The cells were infected at a multiplicity of infection of 10 PFU per cell, incubated at39.5°C,
and harvested at 6 h postinfection. After sonication, nucleic acids were removed by precipitation withstreptomy-cin
sulfate
and proteins were precipitated by the addition of ammonium sulfate to85%
saturation. Finally, the precipitate was dissolved and dialyzed extensively against 50 mM Tris chloride, pH 8.Protein determinations. The protein concentration in the cell extracts was determined by the Coomassie brilliant blue method of Bradford (6), using bovine serum albumin as a standard. The concentration of the 38-kDa subunit in bacte-rial extracts was determined after immunoprecipitation with an excess of Sepharose-linked 535 antibody followed by SDS-polyacrylamide gel electrophoresis of the dissolved precipitate. After electrophoresis, the gels were stained with Coomassie brilliant blue and the 38-kDa protein bands were measured with a laser densitometer (LKB Instruments, Inc., Rockville,
Md.),
using known amounts of bovine serum albumin as standards.Assay of HSV-1 ribonucleotide reductase. Ribonucleotide reductase activity was determined by measuring the reduc-tion of
[3H]CDP
as described previously (16) by using the following incubation mixture. Protein, 15 nmol of[3H]CDP
(Dupont, NEN Research Products, Boston, Mass.; specific activity, 128,000 cpm/nmol), 1.5 pmol ofMgCl,,
1.5Vxmol
of dithiothreitol, 3 nmol ofFeCI3,
and 6 pLmol of4-(2-hydroxy-ethyl)-1-piperazine
sulfonic acid buffer, pH 7.6, were incu-bated in a final volume of 150jtl
for 30min
at37°C.
One unit of ribonucleotide reductase activity is defined as the amount of enzyme or subunit which, in the presence of the othersubunit,
catalyzes the formation of1 nmol of dCDP permin
at 370C.EPR measurements. A general background for the use of this technique is given in reference 26. A bacterial extract containing
120
p.g of the 38-kDa HSV-1 ribonucleotideJ. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
A HERPES DNA
Ncol
site BamHlsite
PLASMID VECTOR
tac BamHl
CATO. T .
_ 1.7kB
_0
Digestwfith
I--- Bam Hl
ligation of BamHl ends fiN in wfth Klenow enzyme ! ligationofbluntends
B S/D S/D met
Plasmid pRI 10 .CACAGGAAACAGGATCCATG.
of
\\" " 9 .CACAGGAAACAGGATCGATCCATG.
AmSL
pRI105.7 kB
BamHl
FIG.
1. (A)Constructionof the expression vector pRI 10. The entire 38-kDa subunit was encoded within a 1.7-kb Ncol-to-BamnHI HSV-1 DNAfragment (HERPESDNA). Thefirst deoxynucleotide C and the start codon ATG are shown. The translation stop codon TGA is shown 1kbdownstream. This insert was ligated to the expression plasmid vector pDR 540 by using theBacmHlrestrictionendonuclease site located 5basepairsdownstream from the tacpromoter. Both the Ncol site and theBainiiHI
site happenedto be recreated in theblunt-end ligationatthe 5' end of the insert. The resulting plasmid was named pRI 10. (B) Deoxyribonucleotide sequences of the initiation sites for protein
synthesis intwo differentplasmids. The Shine-Dalgarno boxes AGGA and the start codons ATG are underlined. pRI 10 is the originally
designed plasmid,andpRI9wasextended 4 nucleotides byfillingin thesticky ends of the originalBanmHIsite situated 5 basepairs upstream from the start codon ATG by using the Klenow fragment of DNA polymerase I and religating. TheBainHIsite at the 3' end of theinsert had
previouslybeen destroyed by mung beannuclease treatment after partial
BanmHl
digestion of the plasmid.reductase subunit was
incubated
with200
,ul of sedimented
535
antibody-Sepharose
for3
h at4°C. The Sepharose
waspelleted by centrifugation,
washed once with 50 mM Trischloride, pH 7.6,
transferredtoanEPRtube,resedimented,
and frozen in
liquid nitrogen.
The EPRexperiments
wereperformed
inaBruker ER-200spectrometerequipped
witha10-in.
(25.4-cm)
magnet and anOxford
cryostatfor
low-temperaturemeasurements.Quantitation
wasmade
by
com-parison
of the doubleintegral
of the EPRspectrum at32 K withthat of
aCu2+
solution of known
concentration.Protein
concentration was determined
by extracting
a known vol-ume ofantibody-Sepharose
withSDS-sample
buffer fol-lowedby
SDS-polyacrylamide gel electrophoresis.
RESULTS
Isolation ofaDNAfragmentencoding the 38-kDasubunit of
HSV-1 ribonucleotide reductase and its subcloninginto atac
expression
vector.The38-kDasubunitgeneislocated within the 23-kb EcoRI Afragment
of HSV-1 DNA(2.
5,17).
To isolate the gene, theplasmid
pSG
124containing
the 23-kbfragment
wasdigested
withBam2HI
and with NcoI whichcuts
just upstream from the
startcodon ATG. The
resulting
1.7-kb
DNAfragment
wasligated into
theBalnHI site
of
theexpression
vector asindicated in
Fig.
1.After transfection of
E.coli JM109, the
nucleotide
se-quence
between the
tacpromoter
and the ATG
startcodon
was
determined in
plasmid DNA from a number of
colonies,
and
aplasmid
called
pRI
10had the
expected
sequence
(Fig.
1B).
Tofurther
testtheinfluence of the nucleotide sequence
between
theShine-Dalgarno
sequence and
theATG
startcodon
ontranslation,
this sequence
wasmodified
asindi-cated in
Fig. 1B.
Theresulting
plasmid, pRI
9, contained
anextra 4
nucleotides
compared
with
pRI
10 and
had
anAGGA
sequence
located
7base
pairs
upstream from the
startcodon
(Fig. 1B).
Expression
in E. coli andimmunological
detection of theHSV-1
ribonucleotide reductase subunit.Bacteria
containing
plasmid pRI
9 orpRI
10 weregrown for
4 heither
in theabsence
orin
thepresence of
1 mMisopropyl-4-D-thiogalac-toside
(IPTG)
added
at anOD9()
of
0.7. The cells werepelleted
andlysed,
and the extracts wereanalyzed
by
immunoblotting
(Fig.
2). Plasmid
pRI
9expressed
apolypep-tide
showing
the samemobility
as the38-kDa subunit
on November 10, 2019 by guest
http://jvi.asm.org/
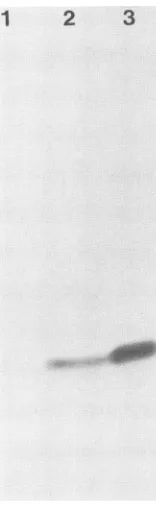
[image:3.612.126.478.63.396.2]3772 INGEMARSON ET AL.
2
1 2 3
:0
N
[image:4.612.323.559.67.281.2]0
FIG. 2. Analyses ofextractsfrombacteria containing the 38-kDa subunit expression vector constructs and of extract from HSV-1-infectedmousecells byimmunoblotting with themouse
monoclo-nal 535 antibody (see Materials and Methods). The proteins were
separated ina7.5%polyacrylamide gel. Lanes: 1,extract(0.5mgof
protein) from bacteria containing plasmid with the insert in the
wrong orientation; 2, extract (0.5 mg of protein) from bacteria
containingplasmid pRI 9 withnoIPTG induction; 3,extract(40FLg ofprotein) fromHSV-1-infected mouse3T3 BALB cells (24).
present
in
HSV-1-infected
mammalian cells (Fig. 2, lanes 2
and
3). No band
wasobserved in cells
containing
aplasmid
with the
wrongorientation of the insert (Fig. 2, lane 1). Cells
containing
thepRI
9plasmid showed
a strongerexpression
than the
pRI 10
plasmid.
Furthermore,the pRI 9
plasmid
showed
strongexpression both in the absence and in the
presence
of IPTG
(data
notshown). Therefore, the
pRI 9
cells
without IPTG induction
wereused inall further
exper-iments.
To quantify the
amountsof the 38-kDa protein in the
E. coli extracts,
cell
lysates wereimmunoprecipitated by
using
the535
Sepharose-linked antibody. After
SDS-poly-acrylamide gel electrophoresis of the dissolved precipitate
followed
by Coomassie
brilliant bluestaining,
protein
con-centration was
determined
asindicated in
Materials and
Methods.
About 30
,ug of 38-kDa subunit
wereobtained
perliter of stationary-phase bacterial culture (about 3 OD590 units per
ml).
The 38-kDa
HSV-1
ribonucleotide reductase subunitpro-duced in E.coli is enzymatically active. As
shown earlier,
the 535monoclonal antibody does
notneutralize the activity of
the
HSV-1-induced ribonucleotide reductase
but only binds the 38 kDa subunitwith high affinity
(24).Therefore,
thisantibody
linked
toSepharose
was usedtobind
andconcen-tratethe
38-kDa
subunit
presentin
abacterial
extract made from astationary-phase bacterial
culture. After a wash toremove
unbound protein,
the ribonucleotide reductaseac-tivity of the
immobilized
protein was measured in thepresence
of
an extractfrom
BHK cellsinfected
with the HSV-1 ts1222mutant. Extractsfrom such cellsgrownatthenonpermissive
temperature lackedafunctional 38-kDa viral0 1 2 3 4 5 6
[image:4.612.144.222.69.323.2]Bacterial extract
(ml)
FIG. 3. Proportionality between increasingamounts ofE.
coli-produced 38-kDa subunit and HSV-1 ribonucleotide reductase
ac-tivity inareconstitution experiment. Increasingamountsofextract
from plasmid pRI 9-containing bacteria were mixed with 20 i,l of
sedimented 535 antibody-Sepharose inaseries of tubes and
incu-batedfor 3hat4°Cunderconstantmixing.TheSepharosewasthen
pelleted by centrifugationandwashedoncein50 mM Trischloride, pH 7.6.Immediately beforetheassay wasperformed,300Fgof the
ts1222-infected BHK cell extract wasadded to each tube. A1-ml quantity of bacterial extract corresponds to 100 ml of bacterial
culture(2
OD590
unitsperml).ribonucleotide
reductase subunit(12).
The results fromexperiments
inwhichincreasing
amountsofaplasmid pRI
9bacterial extract were incubated with an excess of 535
antibody-Sepharose
followedby
the addition of aconstant amount of ts1222-infected-cell extract are shown inFig.
3. The enzymeactivity increased
rapidly
withincreasing
amountsof
bacterial extract, but then the increase becameslower,
which indicated that thelarge
subunit islimiting,
since the 535
antibody-Sepharose
was still far fromsatura-tion. No
activity
wasobserved whencomparable
amountsof each subunitwereassayed separately
(Table 1).
Thespecific
activity
of the immobilized 38-kDa subunitwas 1.4U/mg.
Purification of HSV-1 38-kDa
ribonucleotide
reductase sub-unitproduced
in E. coli.Unfortunately,
the 535antibody
bindstoo
strongly
tothe38-kDa subunitto allowelution of theboundprotein
inanactiveform(24).
For thepurification
we instead used anaffinity column
containing
Sepharose-TABLE 1. Ribonucleotide reductaseactivityobtainedbymixing the38-kDa subunit produced in E. coli with the 140-kDa subunit
presentints1222-infected BHK cells
Antibody-
Free
38-kDa s1222-infected Enzyme Sp.act.Sepharosebound ,, BHKcell
38-kDasubunit(k,g) subunit (p.g) extract(p.g) activity (mU) (U/mg)
3.5 350 4.8 1.4
3.5 0
350 0
0.1 350 1.5 15.0
0.3 350 2.8 9.3
0.5 350 3.7 7.4
"Containing0.44 mgof bovineserumalbumin per ml as a carrier.
J. VIROL.
llim"
.::.,:,.
am
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.612.321.559.619.708.2]1
2
Mr
116
_io
66
46
36
29
FIG. 4. SDS-polyacrylamide gel electrophoresis of the 38-kDa HSV-1 ribonucleotide reductase subunit produced inE. coli after elution from polyclonal antibody-Sepharose. The gel contained 7.5% polyacrylamideandwasstained withCoomassie brilliantblue. Lanes: 1, 1.3 jig of the eluate analyzed after precipitation with trichloroacetic acid(16): 2. molecularweight markers
(3-galactosi-dase [116 kDa], bovine serum albumin [68 kDa], ovalbumin [46 kDa], glyceraldehyde-3-phosphate dehydrogenase 136 kDa]. and carbamic anhydrase [29 kDa] [Sigma]).
linked rabbit polyclonal antibodies directedagainst the
car-boxyl-terminal nonapeptide of the 38-kDa subunit. This nonapeptide (YAGAVVNDL) is quite different from the
carboxyl-terminal sequence of the small subunit of the E.
coli
ribonucleotide reductase and wasalso used toelute the38-kDa subunit from the column.
Acrudeextractfrom 7.3 liters ofstationary-phasebacteria
containing the pRI9 plasmid was passed through a 2.7-ml
antibody column at4°C.Thecolumnwaswashed with30ml of 50 mM Tris chloride,
pH 7.6,
5.4 ml of the same buffercontaining 0.5 MKCI, and 7 ml of 50 mM Tris
chloride,
pH 7.6, all at 4°C. The column was then moved from the coldroom to room temperature and was immediately washed with 6ml of
carefully degassed
50 mM Trischloride, pH
7.6.at
25°C. Finally,
boundprotein
was eluted in 3.5 ml of thesame buffer containing 1 mM nonapeptide at 25°C. An
aliquot of the eluate was analyzed by SDS-polyacrylamide
gel electrophoresis (Fig. 4). Only one protein band was
observed, and this
migrated
at 38 kDa (lane1),
but laserdensitometry scanning
revealedanadditionalveryfaint bandat around 80 kDa which possibly was the dimer. After the
immunoaffinity chromatography
step, 113 p.g of 38-kDaprotein was obtained, resulting in a recovery of 70%. This
was estimated from immunoprecipitation of the bacterial
extract and SDS-polyacrylamide gel electrophoresis of the eluate. Direct measurement ofprotein concentration in the eluatewas prevented by the presence of thenonapeptide.
The
nonapeptide
is known toinhibit
enzyme activityandtherefore
had to be removed. Direct fractionation with ammonium sulfate could notbe used
because the
nonapep-tide precipitated together with the protein. Instead,bovine
serum albumin was added as a carrier protein to the eluate (0.7
mg/ml),
and then the solution was passed through aSephadex G-50
column (sample volume to columnvolume
ratio,
1:10) equilibrated with 50 mM Trischloride,
pH 7.6. This gave a quantitative recovery of the 38-kDa proteinand
a clear separation from the
peptide.
The concentration of 38-kDa protein in the Sephadex G-50 eluate was determined inaportion by SDS-polyacrylamide
gel electrophoresis,and
the rest of the solution was
kept
frozen at -70°C.The
specific activity
of the purified 38-kDa subunit wasmeasured
in the presenceof large subunit
fromts1222-infected
cells, giving
avalue of 15 U/mg (Table
1). No activity wasobserved when either
the 38-kDa subunit or the t.s1222 extracts were assayed separately. For comparison, apartially purified
HSV-1-infected Vero cell extract assayed inparallel
had aspecific activity of 0.04 U/mg. Furthermore, a homogeneous preparation of the small subunit ofmouseribonucleotide reductase
had aspecific activity of
55 U/mgwhen assayed
inthe presence of a large excess of pure largesubunit (41).
In our assay,the
activity did
notincrease in
a linearway when the amount ofpurified 38-kDa subunit wasincreased
in the presenceof
a constant amount of the large subunit(Table 1).
This indicated that the amount oflarge subunit inthe
ts1222-infected
cell extract waslimiting
in the assay.EPRspectroscopyof the 38-kDa subunit of HSV ribonucle-otide reductase
produced
in E.coli. An EPR spectrum at32
K of 535antibody-Sepharose-linked
38-kDa subunit is shownin
Fig.
Sa.For
comparison,the
corresponding
spectraof the
tyrosyl
free radicals of the small subunits of ribonucleotidereductase
inHSV-1-infected Vero cells (Fig. Sb), in
mouse fibroblast cells(Fig. Sc),
and in E. coli cells(Fig. Sd)
areshown.
Foreach
EPR spectrum, theoverall
spectral shape
reflects the
hyperfine interactions of the radical.
In ourinterpretation
(20), differences in hyperfine
structure among theseradical
spectra are to alarge
extentdependent
ontheangle between the
3-methylene hydrogens of the
tyrosineand the
plane
of its
aromatic
ring.
Inthis
respect,the
EPRsignal from
therecombinant
38-kDa
proteinis identical
tothe
EPRsignal
inHSV-infected Vero cells but
isclearly
different from the
signals originating from
the noninfectedmammalian and
E. colicells.
When the
microwavesatura-tion behaviors
of thesignals
arecompared,
the HSV-1ribonucleotide reductase produced in
E.coliis
verydifferent
from the
ordinary
E.
coli enzymeand is in fact
morelike the
mammalian
one(36).
By
quantitation
of
the EPRsignal,
theconcentration of
thetyrosyl free radical in
theSepharose-bound
38-kDa
protein
was
estimated
to be3
p.M.
Thisvalue
did notincrease after
incubation with iron-dithiothreitol,
a treatmentknown
toregenerate
the radical
in themammalian
smallsubunit of
ribonucleotide reductase
(41).
By
using
amolecular
weight
of
76,000,
thedimer
concentration of the 38-kDa
protein
in the EPR tube was estimated to be 7.9F.M,
whichcorre-sponds
to0.4 radicals per dimer.DISCUSSION
Because
of
thetight
intersubunitbinding
in the HSVribonucleotide
reductase(24)
and the low abundance of enzyme, it hasnotbeenpossible
topurify
theindividual
140-and38-kDa subunits
tohomogeneity
from
HSV-infected
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.612.145.212.62.338.2]3774 INGEMARSON ET AL.
FIG. 5. EPR spectra at 32 K and nonsaturating microwave
powerconditions of the following substances. (a)A0.12mgsample of HSV-1 38-kDa ribonucleotide reductase subunitproduced in E.
coli and immobilized in 535 antibody-Sepharose. The microwave
power was 3.9 mWand the modulation amplitude was 2.0G. (b)
HSV-1-infected Vero cells. Confluent monolayers of Vero cells
wereinfected with HSV-1 at amultiplicity of infection of 10, and
after 9.5 hat37°C the cellswereharvested,packed inanEPRtube,
frozen, and stored in liquid nitrogenas described in reference 26.
The EPRspectrometer conditions wereessentially the sameas in panela. (c)Hydroxyurea-resistantmousefibroblast 3T6 cells which
overproduced the small subunit of mammalian ribonucleotide
reduc-tase (1). (d) E. coli KK546 cells which overproduced the E. coli ribonucleotide reductase (39).
cells.
The
apparentlack of allosteric regulation (3), the
presence
of
alarge subunit of 140 kDa compared with
oneof
around
90 kDa
inother species (34) and
theveryinteresting,
highly specific protein-protein interaction observed between
the
140-kDa
subunitand
anonapeptide corresponding
tothe
C-terminal sequenceof the small subunit
(31)
made itdesir-able to obtain
sufficient
amountsof
pure subunits toallow
further studies.
We have therefore used a tac
expression
vector topro-duce the 38-kDa
subunit
in E.coli cells. Our best DNA
constructexpressed around
30 p.gof protein
perliter of
culture,
and thisexpression
wasindependent of IPTG
induc-tion.
E.
coli lacks the
posttranslational machinery of
mamma-lian
cells. The 38-kDa protein
produced in E.coli
had thesame
mobility in SDS-polyacrylamide
gel electrophoresisasthe
corresponding protein from HSV-infected
Vero cells. Thisfact together with
theenzymatic activity
of therecom-binant
protein strongly
arguesagainst
anymajorposttrans-lational
modification of
the protein in mammalian cells. There aredata in the literature
suggesting that the HSVribonucleotide reductase
subunits are phosphorylated ininfected cells (5, 27,
42),but
thesignificance
of these findingsought
tobefurther
studied.
Wecannotexclude thepresenceofa
protein kinase in the partially purified
140-kDa subunitpreparation from HSV-1
ts1222-infected cells. However,
since no ATP
waspresent
during the assay to serve as a
phosphate donor, such an enzyme would not be able to
phosphorylate the 38-kDa
protein.
The
38-kDa
protein produced in
E.coli
wasHSV
specific
and
wasdistinguished
frompotentially contaminating E.
coli
ribonucleotide reductase
B2subunit in the
following
ways.
Enzyme
activity
wasobtained in reconstitution assays with
the
38-kDa subunit
immobilized
tothe
535 monoclonal
antibody-Sepharose;
this
antibody
has
noaffinity
for the E.
coli
B2protein.
Furthermore,
activity
wascompletely
de-pendent upon
the
addition of the HSV-specific 140-kDa
subunit.
Finally,
the EPRspectrum
of the recombinant
38-kDa
protein
wasidentical to the spectrum of the
ribonu-cleotide reductase small subunit present in HSV-infected
Vero cells
(shown for the first time in this paper). This
spectrum
is
completely
different from the
corresponding
spectra
of the small subunits of ribonucleotide reductase in
E.
coli and mammalian cells (36, 39, 41) in both
shape and
saturation
behavior.
Despite the activity of the
antibody-immobilized
38-kDa
subunit,
weassumed that
amolecule free
insolution without
any
steric hindrance would be
moreactive.
Therefore,
the
38-kDa
protein
waspurified
tohomogeneity by
immunoaf-finity chromatography. After removal of the
eluting
non-apeptide, the 38-kDa
protein
wasalmost
asenzymatically
active
asthe pure M2 subunit
of mammalian
ribonucleotide
reductase. The
free 38-kDa
protein
wasabout 10 times
asactive
asthe
antibody-immobilized protein (Table
1). The
specific activity
wasdependent
onthe
amountof
partially
purified
140-kDa subunit present in the assay,
in that
higher
specific activity
wasobserved with
decreasing
amountsof
the
38-kDa
protein.
A
very
interesting
observation is that the 38-kDa HSV
subunit
wasable
togenerate
its
tyrosyl free radical in
E.coli.
Our data indicate the presence of about
0.4tyrosyl
free
radicals per 38-kDa dimer.
Considering
the accuracy
of the
protein
determinations and the
possible loss of free radical
during
the
extraction of the
bacteria, binding
toantibody-Sepharose, washing,
and
freezing, this figure is close
tothe
maximal value of
1free
radical per dimer determined for the
pure
E.coli B2
protein
(38). The extraction was made in the
absence of iron-dithiothreitol to prevent radical
generation
outside the bacteria. There
aredata
indicating
aspecific
tyrosyl
free radical
regeneration
system
in
E.coli (34).
However,
since the amino acid sequence
similarity between
the
E.ccli B2
protein
and
the HSV-1 38-kDa
protein is
notvery
pronounced (15) (optimal score,
61[determined by
using
the
amino acid
align
program
of
DNASTAR
Inc.]
compared
with
scoresof
121for
B2 versusthe
M2subunit of
the
mammalian ribonucleotide reductase and 316 for the
38-kDa
protein
versusM2),
wefind it
morelikely
that the
radical formation in
the38-kDa
protein is
anintrinsic
prop-erty
of the
protein
itself
onceit is
supplied
with
ferrous iron
and oxygen. The
environment inside
E.coli fulfills this
requirement.
This convenient and reproducible way of preparing
the38-kDa subunit of HSV-1
ribonucleotide reductase resulted
in
20 to 30p.g
of pure
protein
per liter
of bacterial culture and
should enable
moredetailed
studies of this very
interesting
and
medically important
form of
ribonucleotide
reductase.
ACKNOWLEDGMENTS
This work was supported by grants from the Swedish Natural Science Research Council, Magn. Bergvalls Stiftelse, the Kempe J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.612.79.279.68.328.2]Foundation, the Medical Faculty at the University ofUme'a.and the Medical Research Council of Great Britain.
LITERATURE CITED
1.
Akerblom,
L., A. Ehrenberg, A. Graslund, H. Lankinen, P.Reichard, and L. Thelander. 1981. Overproduction of the free
radical of ribonucleotide reductase in hydroxyurea-resistant
mouse fibroblast 3T6 cells. Proc. Natl. Acad. Sci. USA 78: 2159-2163.
2. Andersson, K. P., R. J. Frink, G. B. Devi, B. H. Gaylord, R. H.
Costa, and E. K. Wagner. 1981. Detailed characterization of the mRNA mapping in the
Hindlll
fragment K region of the herpes simplex type 1 genome. J. Virol. 37:1011-1027.3. Averett, D. R., C. Lubbers, G. B. Elion, and T. Spector. 1983. Ribonucleotide reductase induced by herpes simplex type 1 virus: characterization of a distinct enzyme. J. Biol. Chem. 258:9631-9638.
4. Bacchetti, S., M. J. Evelegh, and B. Muirhead. 1986.
Identifica-tion and separaIdentifica-tion of the two subunits of the herpes simplex ribonucleotide reductase. J. Virol. 57:1177-1181.
5. Bacchetti, S., M. J. Evelegh, B. Muirhead, C. S. Sartori, and D.
Huszar. 1984. Immunologicalcharacterization of herpes simplex virus type 1 and 2 polypeptides involved in viral ribonucleotide reductase activity. J. Virol. 49:591-593.
6. Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254.
7. Cameron, J. M., I. McDougall, H. S. Marsden, V. G. Preston, M. D. Ryan, and J. H. Subak-Sharpe. 1988. Ribonucleotide
reductase encoded by herpes simplex virus is a determinant of the pathogenicity of the virus in mice and a valid antiviral target. J. Gen. Virol. 69:2607-2612.
8. Chen, E., and P. H. Seeburg. 1985. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA 4: 165-170.
9. Cohen, E. A., P. Gaudreau, P. Brazeau, andY.Langelier. 1986.
Specific inhibition of herpesvirus ribonucleotide reductase by a nonapeptide derived from the carboxyl terminus of subunit 2. Nature (London) 321:441-443.
10. Cohen, G. H. 1972. Ribonucleotide reductase activity of syn-chronized KB cells infected with herpes simplex virus.J. Virol. 9:408-418.
11. Cohen, G. H., M. N. Factor, and M. Ponce de Leon. 1974.
Inhibition of herpes simplex virus type 2 replication by thymi-dine.J. Virol. 14:20-25.
12. Darling, A. J., E. M. McKay, R. Ingemarson, and V. G. Preston.
1988. Reconstitution of herpes simplex virus type 1 ribonucle-otide reductase activity from the large and smallsubunits. Virus Genes 2:163-170.
13. Draper, K. G., R. J. Frink, and E. K. Wagner. 1982. Detailed
characterization of an apparently unspliced ,B herpes simplex type 1 gene mapping in the interior of another. J. Virol. 43:1123-1128.
14. Dutia, B. M., M. C. Frame, J. H. Subak-Sharpe, W. N.Clark, and H. S. Marsden. 1986. Specific inhibition of herpesvirus
ribonucleotide reductase by synthetic peptides. Nature (London) 321:439-441.
15. Elledge, S. J., and R. W. Davis. 1987.Identification and isolation of the gene encoding the small subunit ofribonucleotide reduc-tase from
Sacchalromvces
cerevisiae: DNA damage-inducible gene required for mitotic viability. Mol. Cell.Biol. 7:2783-2793.16. Engstrom, Y., S. Eriksson, L. Thelander, and M.
Akerman.
1979. Ribonucleotide reductase from calfthymus. Purification and properties. Biochemistry 18:2941-2948.17. Frame, M. C., H. S. Marsden, and B. M. Dutia. 1985. The
ribonucleotide reductaseinducedby herpessimplex virus type 1 involves minimally a complex oftwo polypeptides (136K and 38K). J. Gen. Virol. 66:1581-1587.
18. Goldin, L. R., M. Sandri-Goldin, M. Levine,andJ. C.Glorioso. 1981. Cloning of herpes simplex virus type 1 sequences
repre-senting the whole genome. J. Virol. 38:50-58.
19. Goldstein, D. J., and S. K. Weller. 1988. Factor(s) present in herpes simplex type 1-infected cells cancompensate for the loss
of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology 166: 41-51.
20. Graslund, A., A. Ehrenberg, and L. Thelander. 1982. Charac-terization of the free radical of mammalian ribonucleotide reductase. J. Biol. Chem. 257:5711-5715.
21. Hanahan, D. 1983. Studies on transformation of E. coli with plasmids. J. Mol. Biol. 166:557-580.
22. Henry, B. E., R. Glaser, J. Hewetson, and D.J. O'Callaghan. 1978.Expression of an alteredribonucleotide reductaseactivity
associatedwiththereplication of the Epstein-Barr virus. Virol-ogy89:262-271.
23. Huang, A., G. Jacobi, Y. Haj-Ahmad, and S. Bacchetti. 1988. Expression of the HSV 2 ribonucleotidereductase subunits in adenovirus vectors or stably transformed cells: restoration of enzymatic activity by reassociation of enzyme subunits inthe absence of other HSVproteins. Virology 163:462-470. 24. Ingemarson, R., and H. Lankinen. 1987. The herpes simplex
virus type 1 ribonucleotide reductase is a tight complex of the typeca2I32 composed of 40 K and 140 Kproteins,of which the latter shows multiple forms due to proteolysis. Virology 156: 417-422.
25. Kyhse-Andersen, J. 1984. Electroblotting of multiple gels: a
simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J. Biophys. Biochem. Methods 10:203-209.
26. Lankinen, H., A. Graslund, and L. Thelander. 1982. Induction
ofa new ribonucleotide reductase after infection ofmouse L cells with pseudorabies virus.J. Virol. 41:893-900.
27. Marsden, H.S., N. D. Stow,V.G.Preston,M.C.Timbury,and N. M. Wilkie. 1978. Physical mappingofherpes simplex
virus-induced polypeptides. J. Virol. 28:624-642.
28. McLauchlan, J., and J. B. Clements. 1983. Organization ofthe
herpes simplex virus type 1 transcription unit
encoding
twoearly proteins with molecular weights of 140000 and 40000. J. Gen. Virol. 64:997-1006.
29. Nikas, I., J.McLauchlan, A. J. Davison,W.R.Taylor,andJ.B.
Clements. 1988. Structural features of ribonucleotide reductase. Proteins Struct. Funct. Genet. 1:376-384.
30. Norrander, J., T. Kempe, andJ. Messing. 1983. Construction of improved M13 vectors using oligonucleotide-directed mutagen-esis. Gene 26:101-106.
31. Paradis, H., P. Gaudreau, P. Brazeau, and Y. Langlier. 1988.
Mechanism of inhibitionofherpes simplex virus ribonucleotide
reductase by a nonapeptide corresponding to the
carboxyl
terminus of its subunit 2. J. Biol. Chem. 263:16045-16050. 32. Preston, V. G., A. J. Darling, andI. M. McDougall. 1988. Theherpes simplex virus type 1 temperature-sensitive mutant ts
1222 has a single base pair deletion in the small subunit of ribonucleotide reductase. Virology 167:458-467.
33. Preston, V. G., J. W. Palfreyman, and B. M. Dutia. 1984. Identification of a herpes simplex virus type 1
polypeptide
which is a component of the virus-induced ribonucleotidere-ductase. J. Gen. Virol. 65:1457-1466.
34. Reichard, P. Interactions between
deoxyribonucleotide
and DNA synthesis. 1988. Annu. Rev. Biochem. 57:349-374. 35. Russel, D. R., and G. R. Bennett. 1982. Construction andanalysis of in vivo activity of E. coli promoter
hybrids
and promoter mutants that alter the -35 to -10spacing.
Gene 20:231-243.36. Sahlin, M., L. Petersson, A. Graslund, A.
Ehrenberg,
B. M.Sjoberg,andL. Thelander. 1987.
Magnetic
interaction betweenthe tyrosyl free radical and the
antiferromagnetically
coupled
iron center in ribonucleotide reductase.Biochemistry
26:5542-5548.
37. Sanger, F., S.Nicklen, and A. R. Coulson. 1977. DNA sequenc-ing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467.
38. Sjoberg, B. M., and A. Graslund. 1983. Iron
binding
proteins
without cofactors or sulphur clusters. p. 87-110. In G. L.Eichhorn. L. Marzilli. and E. C. Theil
(ed.).
Advances ininorganic biochemistry. vol. 5. Elsevier Science
Publishing,
Inc.. New York.on November 10, 2019 by guest
http://jvi.asm.org/
3776 INGEMARSON ET AL.
39. Sjoberg, B. M., P. Reichard, A. Graslund, and A. Ehrenberg.
1978.The tyrosine free radical in ribonucleotide reductase from Escherichia coli. J. Biol. Chem. 253:6863-6865.
40. Thelander, L., S. Eriksson, and M.Akerman. 1980. Ribonucle-otidereductase from calf thymus. Separation of theenzymeinto
twononidentical subunits, proteins Ml and M2.J.Biol. Chem. 255:7426-7432.
41. Thelander, M., A. Graslund, and L. Thelander. 1985. Subunit M2of mammalianribonucleotide reductase. Characterizationof
a homogeneous protein isolated from overproducing mouse
cells.J. Biol. Chem. 260:2737-2741.
42. Wilcox,K.W.,A.Kohn, E.Sklyanskaya,and B.Roizman.1980. Herpes simplex phosphoproteins. 1. Phosphate cycles on and offsomeviralpolypeptidesandcanalter theiraffinityforDNA.
J. Virol. 33:167-182.
43. Yannish-Peron, C., J. Viera, and J. Messing. 1985. Improved
M13 phage cloning vectors and host strains: nucleotide se-quencesof the M13 mp18 and pUC 19vectors. Gene33:103.
J. VIROL.

![FIG.4.elutioncarbamic7.5%trichloroaceticdasekDa],HSV-1Lanes: SDS-polyacrylamide gel electrophoresis of the 38-kDa ribonucleotide reductase subunit produced in E](https://thumb-us.123doks.com/thumbv2/123dok_us/1324569.86239/5.612.145.212.62.338/elutioncarbamic-trichloroaceticdasekda-polyacrylamide-electrophoresis-ribonucleotide-reductase-subunit-produced.webp)
