Copyright © 2004, American Society for Microbiology. All Rights Reserved.
Characterization of Nucleocapsid Binding by the Measles Virus and
Mumps Virus Phosphoproteins
Richard L. Kingston,* Walter A. Baase, and Leslie S. Gay
Howard Hughes Medical Institute, Institute of Molecular Biology, University of Oregon, Eugene, Oregon 97403
Received 20 January 2004/Accepted 27 May 2004
We report an analysis of the interaction between the P protein and the RNA-associated N protein (N-RNA) for both measles and mumps viruses with proteins produced in a bacterial expression system. During this study, we verified that the C-terminal tail of the N protein is not required for nucleocapsid formation. For both measles and mumps virus N, truncated proteins encompassing amino acids 1 to 375 assemble into nucleo-capsid-like particles within the bacterial cell. For measles virus N, the binding site for the P protein maps to residues 477 to 505 within the tail of the molecule, a sequence relatively conserved among the morbilliviruses. For mumps virus N, a binding site for the P protein maps to the assembly domain of N (residues 1 to 398), while no strong binding of the P protein to the tail of N was detected. These results suggest that the site of attachment for the polymerase varies among the paramyxoviruses. Pulldown experiments demonstrate that the last 50 amino acids of both measles virus and mumps virus P (measles virus P, 457 to 507; mumps virus P, 343 to 391) by themselves constitute the nucleocapsid-binding domain (NBD). Spectroscopic studies show that the NBD is predominantly␣-helical in both viruses. However, only in measles virus P is the NBD stable and folded, having a lesser degree of tertiary organization in mumps virus P. With isothermal titration calorimetry, we demon-strate that the measles virus P NBD binds to residues 477 to 505 of measles virus N with 1:1 stoichiometry. The dissociation constant (Kd) was determined to be 13M at 20°C and 35M at 37°C. Our data are consistent
with a model in which an␣-helical nucleocapsid binding domain, located at the C terminus of P, is responsible for tethering the viral polymerase to its template yet also suggest that, in detail, polymerase binding in morbilliviruses and rubulaviruses differs significantly.
Measles and mumps are ubiquitous and serious diseases of early childhood in many regions of the world. The large, en-veloped viruses from which these diseases originate are among the most familiar members of the paramyxovirus family. The genome of these viruses is a nonsegmented single-stranded RNA molecule approximately 15,000 nucleotides in length, in which the viral genes are arranged in a linear sequence. The viral nucleocapsid (N) protein packages the genomic RNA into a helical protein-RNA complex, termed the nucleocapsid. Within the infected cell, the viral polymerase uses the nucleo-capsid (N-RNA) as a template for transcription of mRNAs encoding the viral proteins as well as replication of the viral genome. The viral polymerase has two components, the large (L) protein and the phosphoprotein (P). All of the catalytic activities associated with the polymerase reside within L (2,183 amino acids in measles virus, 2261 amino acids in mumps virus), which is always found in complex with P. The P protein binds the polymerase to the nucleocapsid, through its interac-tion with N-RNA (21, 38). The replicainterac-tion of paramyxoviruses has been comprehensively reviewed (6, 10, 45).
Measles and mumps virus nucleocapsids, as visualized by transmission electron microscopy, are flexible rods, approxi-mately 20 nm in diameter, with a hollow central cavity approx-imately 5 nm in width (3, 14, 33, 54). RNA is tightly bound within the nucleocapsid, which is not believed to dissociate during RNA synthesis. Expression of the N protein from
mea-sles virus and mumps virus in a variety of heterologous systems results in the formation of nucleocapsid-like particles (3, 26, 44, 46, 47, 52, 53). For the measles virus N protein, it has been shown that these structures result from the nonspecific encap-sidation of cellular RNA (47), a result which is likely to hold for the mumps virus N protein as well, based on the reported densities of mumps virus nucleocapsid-like particles in CsCl (44, 46). These nucleocapsid-like particles are a useful model for the authentic viral nucleocapsid.
The N protein (525 amino acids in measles virus, 549 amino acids in mumps virus) can be divided into two regions. An N-terminal assembly domain, encompassing the first 400 amino acids of the protein, is responsible for RNA binding and formation of the helical nucleocapsid. A C-terminal tail, vary-ing widely in length and sequence among the paramyxoviruses, is located on the nucleocapsid exterior and is thought to be dispensable for nucleocapsid assembly. In Sendai virus, the tail of N encompasses a binding site for the P protein and hence for the viral polymerase (5, 9, 43), but for other paramyxovi-ruses, less is known. For measles virus, it has been established that the tail of N binds to the P protein (30, 32), and the binding site has been tentatively mapped to residues 457 to 525 (2). For mumps virus, nothing is known from experiments regarding this interaction.
The P protein does not have a compact structure but con-tains a number of functional elements separated by intrinsi-cally disordered sequences (10, 45). Thus, the size of P varies considerably among paramyxoviruses, ranging from approxi-mately 250 to 600 amino acids in length. All paramyxovirus P proteins are likely to be oligomeric, containing heptad se-quence repeats in the central region of the molecule,
charac-* Corresponding author. Mailing address: Howard Hughes Medical Institute, Institute of Molecular Biology, University of Oregon, Eu-gene, OR 97403. Phone: (541) 346 5867. Fax: (541) 346 5870. E-mail: richard@uoxray.uoregon.edu.
8630
on November 8, 2019 by guest
http://jvi.asm.org/
Downloaded from
on November 8, 2019 by guest
http://jvi.asm.org/
Downloaded from
on November 8, 2019 by guest
http://jvi.asm.org/
teristic of helical coiled coils (8). The structure of this region of the Sendai virus P protein has been determined and is a tet-rameric coiled coil (50), but it is not known if all paramyxoviral P proteins are similarly tetrameric. The extreme C terminus of P is critical for nucleocapsid binding, as short deletions at the C terminus of P from Sendai virus (40), human respiratory syncytial virus (15), and human parainfluenza virus 3 (12) elim-inate or dramatically decrease binding to N-RNA. The final 50 amino acids of P are predicted to form a trihelical structural motif in many paramyxoviral P proteins, including those of measles virus and mumps virus (8). A very recent crystal struc-ture of this domain from measles virus P (24) shows it to be a compact bundle of three␣-helices. For measles virus, the C-terminal region of P, including the putative coiled coil and downstream sequences, can bind to the N protein (18, 22, 31, 32). For mumps virus, there is no direct evidence, but for the closely related La Piedad Michoacan Mexico virus (LPMV), the equivalent region of P may be involved in nucleocapsid binding (49).
The N, P, and L proteins of measles virus are functionally equivalent to those of mumps virus, yet sequence homology between proteins from these viruses is limited, and the viruses have been placed in different genera (Morbillivirusand
Rubu-lavirus, respectively). We began to study components of the
replication complex from both viruses in order to clarify the similarities and the differences in the organization of these familiar pathogens. In this paper, we define the regions of the P and N proteins that mediate association of the polymerase with the nucleocapsid for both measles and mumps viruses. The binding site for the P protein maps to a different region of N in the two viruses. We show, however, that in each case the last 50 amino acids of the P protein constitute the nucleocap-sid-binding domain, and we report a comparative analysis of the secondary and tertiary structure of this domain. For mea-sles virus, we characterized the binding process by isothermal titration calorimetry and report the binding parameters.
MATERIALS AND METHODS
Isolation of viral genomic RNA and cloning of the N and P genes.Measles virus (Moraten vaccine strain) and mumps virus (Jeryl Lynn vaccine strain, major component) were supplied by Merck Research Laboratories (West Point, Pa.). For each virus, genomic RNA was isolated (High Pure Viral RNA kit; Roche), and the N and P genes were amplified with reverse transcription-PCR. Amplified DNA segments were ligated into pET expression vectors (Novagen) with stan-dard procedures, and these plasmids were used as the template for all subsequent DNA amplification and manipulation (described below). Complete nucleotide sequencing of the N and P genes revealed no differences from published se-quences for measles virus, Moraten vaccine strain, GenBank accession number AF266287 (36) and mumps virus, Jeryl Lynn vaccine strain major component, GenBank accession number AF338106 (1). In the case of mumps virus, the nucleotide sequence that encodes the P protein differs from the genomic RNA sequence as a result of a cotranscriptional editing mechanism (37). Accordingly, two guanine nucleotides were inserted at the appropriate site within the cloned mumps virus P gene. This mutation was made with the QuikChange site-directed mutagenesis protocol (Stratagene), incorporating the modifications suggested in reference 51. Similar procedures were used for all of the mutagenesis procedures described in this paper.
Construction of P protein expression plasmids.Constructs encoding the full-length measles virus and mumps virus P proteins (MEP and MUP, respectively) were made by ligating the P genes into plasmid pET-22b(⫹) (Novagen) between the unique NdeI and XhoI sites. Plasmids for expression of truncated P pro-teins lacking the final 49 amino acids of the wild-type sequence (MEP1-458and MUP1-342) were derived by introducing a stop codon (TAA) at the appropriate point in the coding sequence. Variants of the all these constructs carrying a
20-amino-acid N-terminal His tag (MGSSHHHHHHSSGLVPRGSH) were cre-ated by subcloning the coding sequences into pET28b(⫹) (Novagen)
Construction of GST-P fusion protein-expression plasmids.To prepare pro-teins for both biophysical analysis and glutathioneS-transferase (GST) pulldown experiments, the C-terminal region of the P protein (MEP457-507and MUP 343-391) was fused to the C terminus of GST, with an interleaving tobacco etch virus protease cleavage site allowing release of the P fragments if required. Exploiting a recent finding that a number of side chains can be tolerated in the P1⬘position of the tobacco etch virus protease recognition sequence (25), the constructs were designed so that the proteins released after protease digestion did not retain any nonnative residues at the N terminus. First plasmid pET41a(⫹) was modified, replacing the sequence GGTTCA, between the end of the GST gene and the SpeI site, with the sequence GGCAGTGACTACGATATCCCA, encoding GSDYDIP. DNA encoding the desired regions of the P proteins was amplified by PCR and ligated into the modified plasmid between the unique SpeI and XhoI sites. In each case, PCR primers were designed to append the sequence GAGAACCTCTATTTCCAG, encoding ENLYFQ, between the SpeI site and the 5⬘end of the P coding sequence. The resulting vectors encode the GST fusion proteins GST-MEP457-507and GST-MUP343-391. For GST-MEP457-507, cleavage by tobacco etch virus protease was found to be greatly improved by mutating the P2⬘position from Pro to Gly. For this construct, the protein released after protease cleavage was MEP457-507, with a Pro-to-Gly mutation at residue 458 (MEP457-507/P458G)
Construction of N protein expression plasmids.Constructs encoding the full-length measles virus and mumps virus N proteins (MEN and MUN, respectively), with and without an 8-amino-acid polyhistidine tag at the C terminus (LEHHHHHHHH), were made by ligating the N genes into pET-26b(⫹) be-tween the unique NdeI and XhoI sites. To create truncated variants for analysis of nucleocapsid assembly, a SalI site was introduced at the desired location within N. A SalI-XhoI restriction digest excised the unwanted 3⬘end of the N gene, and the compatible ends of the plasmid were religated. Plasmids created in this fashion encode the truncated N proteins with an 8-amino-acid tag appended at the C terminus (VEHHHHHHH). Untagged, truncated measles virus and mumps virus N proteins for GST pulldown analysis were created by introducing a stop codon at the appropriate point within the N coding sequence.
Construction of GST-N fusion protein expression plasmids.To create fusion proteins for GST pulldown experiments, DNA encoding the tail of the N protein (MEN374-525and MUN374-549) was amplified by PCR and ligated into plasmid pGEX-4T-1 (Amersham Biosciences) between the EcoRI and XhoI sites, cre-ating vectors encoding the GST fusion proteins GST-MEN374-525 and GST-MUN374-549. Variants truncated at the C terminus of N were created by intro-duction of stop codons within the N coding sequence. Plasmids encoding GST fused to short fragments of the tail of measles virus N (MEN477-505and MEN 495-505) were created in a different fashion which allowed release and purification of the tail fragment. Starting with the plasmid encoding GST-MUP343-391, de-scribed above, a KpnI site was introduced directly after the tobacco etch virus protease recognition sequence. The N coding sequences were subcloned into the modified plasmid with the KpnI and XhoI sites. If the fusion proteins were treated with tobacco etch virus protease, tail peptides were released, carrying a nonnative Thr-Ser dipeptide at their N terminus. To allow quantitation of the peptide for calorimetric studies, a variant was produced in which a tyrosine was appended to the C terminus.
Protein expression. High-level expression of N, which contains multiple codons rarely used by Escherichia coli, required the presence of a separate compatible plasmid carrying additional copies of the rare tRNA genes (plasmid pRARE; Novagen). Accordingly, N or GST-N protein expression plasmids were transformed into Rosetta (DE3) (for pET-based vectors) or Rosetta (for pGEX-based vectors). P and GST-P protein expression plasmids were transformed into BL21-Star(DE3) (Invitrogen). Transformed bacteria were grown in Luria broth with selective antibiotics at 37°C until induction. Protein expression was induced by addition of isopropylthiogalactopyranoside (IPTG) (0.1 to 1 mM final con-centration). After induction, bacteria were maintained at 16 to 28°C for 4 to 16 h before harvest. Cell pellets were stored at⫺80°C before use. Uniformly15 N-labeled proteins (GST-MEP457-507, GST-MUP343-391, and GST-MUP343-391/ C356S) were prepared by growing transformed bacteria in minimal medium (see reference 35 for medium composition) with15NH
4Cl (Spectra Stable Isotopes) as the sole nitrogen source. Following induction, bacteria were maintained at 28°C for 14 to 18 h before harvest.
GST pulldown assay.For GST pulldown assays, cell pellets were resuspended in phosphate-buffered saline buffer (10 mM Na2HPO4/KH2PO4[pH 7.4], 137 mM NaCl, 2.7 mM KCl; 1 ml/100-mg pellet) and lysed by sonication. Lysates were clarified by centrifugation in a benchtop centrifuge. The assays were carried out with MicroSpin columns containing 50l of glutathione-Sepharose 4B
on November 8, 2019 by guest
http://jvi.asm.org/
ersham Biosciences); 400l of clarified lysate containing GST or the GST fusion protein was applied to the column and incubated 5 min at room temperature with occasional mixing. The column was washed twice with 400l of PBS buffer, and 400l of lysate containing the potential binding partner was applied to the column, incubated, and washed in the same fashion. Bound proteins were eluted in 100l of 50 mM Tris-HCl (pH 8.0) containing 20 mM reduced glutathione, and the results were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry. GST, for use as a negative control in the pulldown assay, was produced with plasmid pGEX-4T-1 (Amer-sham Biosciences) or plasmid pET41a(⫹) (Novagen) into which a stop codon had been introduced at the end of the GST gene.
Electron microscopy.Samples were prepared for electron microscopy by ap-plying them to carbon- and Formvar-coated electron microscopy grids and neg-atively staining with 2% (wt/vol) sodium phospotungstate (pH 7.5). Images of the specimens were recorded with a Phillips CM-120 electron microscope.
Western blotting.Proteins resolved on SDS-PAGE gels were transferred onto a polyvinylidene difluoride membrane (Hybond-P; Amersham Biosciences) by electroblotting. Primary and secondary antibody hybridization was done accord-ing to standard protocols. The primary antibody was a mouse immunoglobulin G recognizing a polyhistidine tag (Penta-His antibody; Qiagen). The secondary antibody was an anti-mouse immunoglobulin G whole antibody conjugated to horseradish peroxidase (Amersham Biosciences). Antibody binding was detected with the enhanced ChemiLuminesence system (ECL; Amersham Biosciences), and the results were recorded on Kodak XAR photographic film.
Mass spectrometry.MALDI-TOF mass spectra were obtained with a Voyag-er-DE instrument (Applied Biosystems). The matrix (ferulic acid dissolved a 1:1 mixture of 2-propanol and water, with addition of 0.25%[vol/vol] trifluoroacetic acid) was mixed directly with the sample and air dried before data collection.
Purification of truncated N proteins for analysis of nucleocapsid assembly.All constructs used for analysis of nucleocapsid assembly carried a C-terminal poly-histidine tag, which was used to isolate the expressed proteins. Frozen cell pellets were resuspended in primary-amine-free BugBuster (Novagen) a proprietary lysis buffer, to which was added a protease inhibitor cocktail (complete, EDTA-free; Roche Diagnostics), and small quantities of lysozyme and DNase I. The mixture was stirred gently at 4°C for 45 min. The lysate was clarified by centrif-ugation, and the supernatant was added to Talon resin (Clontech), preequili-brated with buffer A (25 mM morpholinepropanesulfonic acid [MOPS]-NaOH [pH 7], 200 mM NaCl, 0.5 mM TCEP-HCl, 5 mM imidazole). The resin was thoroughly washed with buffer A, and then the bound protein was eluted by increasing the imidazole concentration to 500 mM. Samples were analyzed by SDS-PAGE and Western blot to assess N protein expression and by electron microscopy to assess nucleocapsid assembly.
Purification of the nucleocapsid-binding domain from measles virus and mumps virus P. The nucleocapsid-binding domains from measles virus and mumps virus P (constructs MEP457-507, MUP343-391,MUP343-391/C356S, and MEP457-507/P458G) were initially isolated as GST fusion proteins. Frozen cell pellets were resuspended in buffer B (50 mM Tris-HCl [pH 7.9], 200 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA; 5 ml/g of pellet), with addition of a protease inhibitor cocktail (Roche Diagnostics), and cells were disrupted by sonication. The lysate was clarified by centrifugation, and the supernatant was passed over glutathione-Sepharose 4B resin (Amersham Biosciences), preequilibrated with buffer B, which was then washed extensively with the same buffer. Tobacco etch virus protease (Invitrogen) was added and gently mixed with the resin-bound fusion protein. Following overnight incubation at 16 to 24°C, the released P proteins were eluted by washing the resin with buffer B. P proteins were further purified by cation-exchange chromatography, by first binding them to SP Sepha-rose HP resin (Amersham Biosciences) buffered with 25 mM MOPS-KOH (pH 7.0)–25 mM NaCl and then displacing them with a linear salt gradient.
Purification of the measles virus N tail peptide.The tail peptide MEN477-505 was first isolated as a GST fusion protein. The initial purification of the fusion protein and proteolytic cleavage to release the peptide were performed as de-scribed above. The peptide was further purified by cation-exchange chromatog-raphy, by first binding it to SP Sepharose HP resin (Amersham Biosciences) buffered with 25 mM malic acid-KOH (pH 3.7)–50 mM NaCl and then displacing it with a linear salt gradient.
Circular dichroism spectroscopy and thermal unfolding measurements.For measurement of circular dichroism, the proteins (1 to 2M) were dialyzed into 5 mM Na2HPO4-NaH2PO4buffer (pH 7.0), with or without 10 mM NaCl (for MUP343-391and MEP457-507/P458G, respectively). Peptide concentrations were estimated by measuring sample absorbance at 190 nm with a Varian 2290 spec-trophotometer flushed with dry nitrogen gas, and assuming a per residue molar extinction coefficientε190⫽10,000 M⫺1cm⫺1, which is characteristic of folded
proteins (20). Circular dichroism spectra were collected on samples in a 0.1-cm optical cell at a temperature of 2°C with a J-600 JASCO spectrophotometer configured for circular dichroism measurement. Thermal unfolding of the pep-tides (0.3 to 0.7M) in the same buffers was monitored by following the circular dichroism signal at 220 nm as a function of temperature, as previously described (11).
Nuclear magnetic resonance spectroscopy.For nuclear magnetic resonance spectroscopy, concentrated protein samples were dialyzed into 10 mM Na2HPO4-NaH2PO4buffer (pH 5.7) with 50 to 100 mM NaCl; 10% (vol/vol) 2H
2O was added to each sample before data acquisition. HSQC spectra (27, 57) were acquired on a Varian Inova 600 MHz spectrometer equipped with a triple-resonance pulsed-field gradient probe.1H chemical shifts were referenced with an external DSS standard.15N chemical shifts were referenced indirectly with standard frequency ratios (56). Nuclear magnetic resonance data were analyzed with the programs NMRPipe and NMRDraw (13).
Dynamic light scattering and size exclusion chromatography.Dynamic light scattering measurements were made at 25°C with a DynaPro99 instrument (Pro-tein Solutions Inc.). Translational diffusion coefficients were calculated from the measured autocorrelation function, and the hydrodynamic radius was calculated via the Stokes-Einstein equation. Size exclusion chromatography was carried out on a Hi Load 16/60 Superdex 75 column (Amersham BioSciences). For both measurements, the starting protein concentration was equivalent to that used for nuclear magnetic resonance spectroscopy, and the sample buffer was 12.5 mM MOPS-KOH (pH 7.0)–100 mM NaCl.
Isothermal titration calorimetry. For isothermal titration calorimetry, the purified proteins (MEN477-505and MEP457-407/P458G) were dialyzed extensively against 10 mM Na2HPO4-NaH2PO4buffer (pH 7.0)–100 mM NaCl–0.01% (wt/ vol) sodium azide. Protein concentrations were calculated from UV absorption measurements at 280 nm (16). Before use, protein solutions were outgassed at 1/3 atm for 4 min. Isothermal titration calorimetry measurements were made with a VP-ITC microcalorimeter (MicroCal, Inc.) operated at 20 and 37°C. MEP457-407/P458G was used as the injectant in the titrations, with the injection syringes carefully dried to avoid dilution effects. A 3% correction was applied to account for dilution of MEN477-505 upon loading into the VP-ITC reaction chamber. For each titration, an initial injection of 2l was followed by 23 injections of 12l at intervals of 240 s. Baseline mixing heats were determined by duplicate injections of MEP457-407/P458G into buffer at both temperatures, and these were subtracted from the binding heat profiles before model fitting. The standard multiple independent binding-site model was fitted to the data with the Origin software package, version 5.0 (MicroCal).
RESULTS
C-terminal tail of the N protein is not required for assembly of nucleocapsid-like particles in E. coli. We first sought to verify that heterologous expression of the measles virus and mumps virus N proteins inE. coliresulted in the formation of nucleocapsid-like particles and to directly define the region of N that is responsible for nucleocapsid assembly. We con-structed expression plasmids encoding both the full-length N proteins and a series of C-terminally truncated variants (1 to 500, 1 to 450, 1 to 400, 1 to 375, 1 to 350, 1 to 325, and 1 to 300). Proteins were expressed inE. coliand purified by metal affinity chromatography with a hexahistidine tag appended to the C terminus of each protein. To assay for assembly into nucleo-capsid-like particles, samples were negatively stained and then examined by transmission electron microscopy. The visualiza-tion of flexible rods with a characteristic herringbone appear-ance, similar to nucleocapsids isolated from virus-infected mammalian cells (14, 33, 54), was considered diagnostic of assembly.
The full-length N proteins and many of the truncated vari-ants assembled into nucleocapsid-like particles when expressed
in E. coli. For both measles virus and mumps virus, all
con-structs encompassing residues 1 to 375 of the N protein re-mained assembly competent. The other constructs that were tested (1 to 300, 1 to 325, and 1 to 350) showed high-level
on November 8, 2019 by guest
http://jvi.asm.org/
expression in E. coli but were not soluble and could not be isolated from cell lysates. In addition to helical nucleocapsids, ring structures were also observed which correspond to single turns of the helical nucleocapsid (3). While only the first 375 amino acids of N are strictly required for nucleocapsid forma-tion, modifications to the tail of the molecule did affect assem-bly in more limited fashion. The shortest of the assemassem-bly- assembly-competent variants (1 to 375, 1 to 400, and 1 to 425) gave rise to long and regular nucleocapsid-like particles. The longer variants (1 to 475, 1 to 500, and the wild-type proteins) gave rise to shorter, more fragmented particles and increased num-bers of subnucleocapsid rings.
P protein binds to the nucleocapsid-like particles resulting from heterologous expression of the N protein. Before pro-ceeding with a detailed investigation of the interaction be-tween the P protein and the nucleocapsid, we needed to verify that the N and P proteins produced in our heterologous ex-pression system bound one another. To establish this, the N and P proteins from measles virus and mumps virus were individually expressed inE. coli. The measles virus and mumps virus N proteins were wild-type, while the corresponding P proteins carried a polyhistidine tag at their N termini. We used these proteins in a polyhistidine tag pulldown assay, confirming the association between N and P from each virus (data not shown).
The measles virus P protein binds to the nucleocapsid via a short sequence within the tail of the measles virus N protein (MEN374-525).Since the measles virus P protein was already
known to associate with the nucleocapsid via the tail of N protein, we fused this region of N to the C terminus of
Schis-tosoma japonicumGST and assayed for binding to P. For the
purpose of our experiments, we defined the tail of the N protein as the entire sequence that was earlier shown to be dispensable for nucleocapsid assembly (i.e., MEN374-525). With
a GST pulldown assay, we showed that MEN374-525retained
the ability to bind measles virus P (Fig. 1A, lanes 5 and 6), consistent with prior observations (2, 24, 30, 32).
We suspected that the binding site for the measles virus P protein might reside near the end of the tail. To test this idea, a series of fusion proteins were constructed in which the tail of N, fused to GST, was truncated. Deletions of 10 (MEN374-515)
and 20 (MEN374-505) amino acids from the tail had no effect on
the ability of the molecules to bind to P in a pulldown assay (Fig. 1B). However, a third construct in which 30 amino acids were deleted (MEN374-495) lost the ability to bind the P protein
(Fig. 1B). This suggested that the region from 495 to 505 within the tail of N was critical for binding P.
To further map the binding region, two short tail fragments were tested for their ability to bind to P. The fragments en-compassed 11 and 29 residues of the tail of N, ending in each case at residue 505. These fragments were fused to the C terminus of GST, and the results of a GST pulldown experi-ment are shown in Fig. 1C. While the shorter of the two fragments (MEN495-505) did not bind to P, the longer tail
fragment (MEN477-505) retained binding ability.
Last 51 amino acids of the measles virus P protein (MEP457-507) mediate binding to the tail of the measles virus N
protein (MEN374-525).Having mapped the binding site for the
measles virus P protein to a short and contiguous sequence within the tail of the N protein, we sought to establish if the
trihelical motif located at the C terminus of the P protein was directly mediating the interaction. We had previously demon-strated that deletion of the 49 amino acids at the C terminus of P, which encompass this motif, eliminated binding to the tail of N (Fig. 1A, lanes 7 and 8). We then sought to ascertain if this region of P was not only necessary but also sufficient for bind-ing to the tail of N.
We fused MEP457-507to the C terminus of GST and assayed
for binding to nucleocapsid-like particles resulting from the expression of full-length and truncated measles virus N pro-teins (Fig. 2). MEP457-507 fused to GST bound to the
full-length N protein and bound weakly to a truncated variant (1 to 500) lacking a small part of the previously identified P protein binding site (477 to 505). Binding to truncated variants from which the P protein binding site had been completely elimi-nated (1 to 450 and 1 to 398) did not exceed background levels. This experiment confirms our previous observations and showed that MEP457-507alone had an intrinsic ability to bind to
the tail of N. The same point was independently demonstrated by a recent study of the changes in circular dichroism that occur on titration of the tail of the N protein with MEP459-507
(24). Our experiment additionally indicates that binding of the P protein to the nucleocapsid occurs exclusively through its association with the tail of N.
Last 49 amino acids of the mumps virus P protein (MUP343-391) bind to the assembly domain of the mumps virus
N protein (MUN1-398). Having studied the interactions
be-tween the P protein and the nucleocapsid of measles virus, we attempted to characterize the same interaction for mumps virus. The tail of the mumps virus N protein (MUN374-549) was
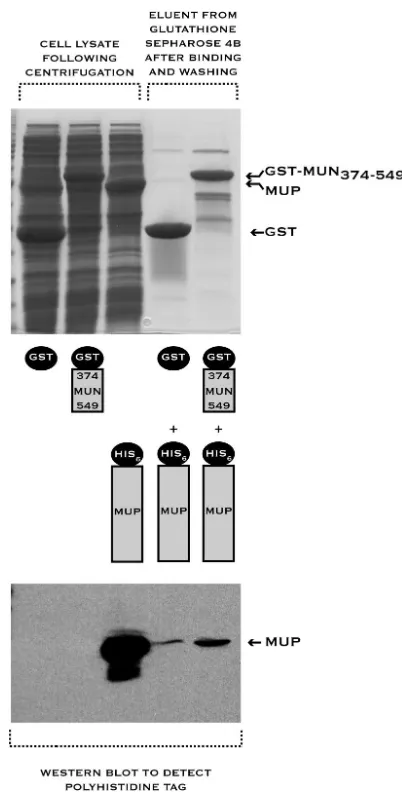
fused to the C terminus of GST and assayed for the ability to bind to the mumps virus P protein. Binding of MUN374-549to
the P protein in a pulldown assay was extremely weak, unde-tectable on Coomassie blue-stained SDS-PAGE gels and barely detectable by Western blotting with an antibody specific for the affinity tag carried by P (Fig. 3). This is in direct contrast to the situation for measles virus, where binding of P to the tail of N was readily detected in an equivalent assay (Fig. 1A, lanes 5 and 6). Hence it appeared that the principal binding site for the mumps virus P protein might not reside within the tail of the N protein.
With a polyhistidine tag pulldown assay, we showed that deletion of the last 49 amino acids of P abolished binding to N (data not shown). This established that the putative trihelical motif at the C terminus of P was critical for nucleocapsid binding. We then fused the last 49 amino acids of the mumps virus P protein (MUP343-391) to the C terminus of GST and
assessed binding of MUP343-391 to a series of truncated N
proteins (Fig. 4). MUP343-391bound specifically to all the N
constructs tested, including one (MUN1-398) in which the tail
had been completely deleted. This is strikingly different from the results for measles virus (Fig. 2). These experiments (Fig. 3 and Fig. 4) demonstrate that the last 49 amino acids of the mumps virus P protein directly mediate binding to the nucleo-capsid, just as was the case for measles virus. However, unlike the measles virus P protein, the mumps virus P protein does not bind strongly to the tail of N but instead binds strongly to the assembly domain of N (amino acids 1 to 398).
Secondary and tertiary organization of the nucleocapsid-binding domain from measles virus and mumps virus P.Our
on November 8, 2019 by guest
http://jvi.asm.org/
FIG. 1. Short sequence (amino acids 477 to 505) within the tail of the measles virus N protein (MEN374-525) is responsible for measles virus P protein (MEP) binding, demonstrated by a GST pulldown assay. The results of this pulldown assay (and all others described in this paper) were analyzed by SDS-PAGE, and protein masses were verified by MALDI-TOF mass spectrometry. Constructs used in the pulldown assay are illustrated schematically below or above each lane. The leftmost lanes (labeled “Cell lysate following centrifugation”) show the crude cell lysates for each component used in the pulldown assay. (A) Measles virus P protein binds to the tail of the measles virus N protein. The last 49 amino acids of measles virus P are necessary for binding, as a construct in which this region is deleted (MEP1-458) does not bind. (B) Binding of measles virus P protein to the tail of measles virus N protein is abolished when 30 amino acids are deleted from the tail. (C) A measles virus N protein tail fragment encompassing amino acids 477 to 505 retains the ability to bind the measles virus P protein.
on November 8, 2019 by guest
http://jvi.asm.org/
experiments showed that the last ⬇50 amino acids of both measles virus and mumps virus P constituted the nucleocapsid-binding domain. Hence we carried out a spectroscopic charac-terization of these domains to assess their secondary and tertiary structure. For this structural analysis, the C-terminal regions of measles virus and mumps virus P (MEP457-507and
MUP343-391) were initially isolated as GST fusion proteins. The
P fragments were released from GST through proteolytic cleavage and purified to homogeneity by cation-exchange chro-matography (see Materials and Methods). Because the GST-MEP457-507 fusion protein was not cleaved efficiently, a
Pro458Gly mutation was introduced into this construct to fa-cilitate isolation of the P fragment. Correspondingly, some structural work was carried out with the mutated protein (MEP457-507/P458G).
Circular dichroism spectra of the two P fragments (Fig. 5) showed negative bands near 222 and 208 nm and a positive band near 192 nm. Such spectra are characteristic of proteins that are predominantly ␣-helical (17). By means of a simple and approximate method (17), we estimate the␣-helical con-tent to be⬇80% for MEP457-507and⬇70% for MUP343-391on
the basis of the magnitude of the circular dichroism signal at 208 nm. The thermal stability of the two proteins was examined by monitoring the change in the circular dichroism signal at 220 nm as a function of temperature (2 to 95°C). For both proteins, the circular dichroism signal increased sigmoidally as the temperature was raised, indicative of loss of the helical structure (data not shown). In both cases, the␣-helical struc-ture persisted at physiological temperastruc-tures. For MUP343-391,
the transition to the unfolded state appeared to be complete within the studied temperature range, with little evidence of
cooperative unfolding. For MEP457-507/P458G, the transition
was incomplete at 95°C, the limit imposed by the instrumen-tation, indicating a much higher thermal stability of this pro-tein relative to MUP343-391.
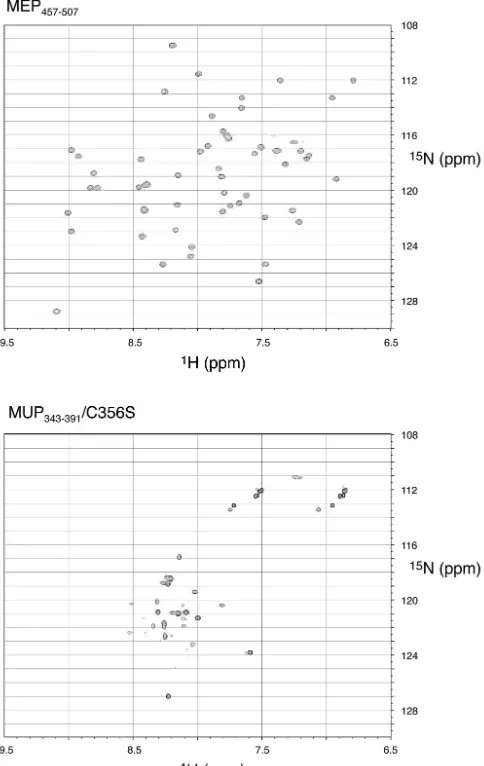
We also studied the proteins uniformly isotopically labeled with15N by solution nuclear magnetic resonance spectroscopy.
2D1H-15N HSQC spectra for the two proteins are shown in
Fig. 6. For a small, folded domain that does not aggregate,
1H-15N HSQC spectra should show well-defined and dispersed
peaks for most backbone and side chain amide protons. For MUP343-391we studied the wild-type protein at first but found
that introducing a conservative Cys-to-Ser mutation (C356S) gave rise to improved nuclear magnetic resonance spectra, presumably through elimination of intermolecular disulfide bond formation.
For MEP457-507the presence of exactly the expected number
of resonance peaks, all roughly equivalent in intensity, and the high degree of chemical shift dispersion showed that this pro-tein had a well-defined tertiary structure. The spectroscopic data are consistent with the very recently determined X-ray crystal structure of this domain (24). Sequence-specific assign-ment of the chemical shifts is in progress. For MUP343-391/
[image:6.603.98.490.68.326.2]C356S, interpretation of the spectra was more complicated. Chemical shift dispersion in the proton dimension was limited and does not deviate markedly from that expected from a random coil structure (55). There were also large variations in resonance intensity, with some resonances broadened and oth-ers apparently absent. This is the result of exchange processes, which could correspond to intermolecular aggregation or con-formational fluctuations within the molecule itself. To discrim-inate between these possibilities, we examined the aggregation
FIG. 2. Last 51 amino acids of the measles virus P protein (MEP457-507) are sufficient for binding to the tail of the measles virus N protein (MEN374-525), demonstrated by a GST pulldown assay. MEP457-507specifically binds to nucleocapsid-like particles resulting from expression of measles virus N protein, but binding is lost when the tail of N is truncated and the binding site (amino acids 477 to 505) is eliminated.
on November 8, 2019 by guest
http://jvi.asm.org/
state of the molecule by both dynamic light scattering and size exclusion chromatography. Dynamic light scattering measure-ments were consistent with a single light-scattering species, with a hydrodynamic radius of 1.9 nm. Applied to size exclu-sion columns, the protein eluted in a single symmetric peak following RNase A (hydrodynamic radius, 1.2 nm). These ob-servations suggested that aggregation is not the source of the exchange broadening seen in Fig. 6 (bottom). It is therefore most probable that MUP343-391 is partially structured in its
native state, containing persistent helical secondary structure but lacking a high level of tertiary organization.
Interaction between the measles virus P nucleocapsid-bind-ing domain and MEN477-505 studied by isothermal titration
calorimetry.To complete this study, we obtained some quan-titative data on the interaction between the measles virus P NBD and MEN477-505 with isothermal titration calorimetry,
monitoring the heat generated as the two purified proteins were titrated. Analysis of such data yields the binding
param-eters, including the stoichiometry of binding (n), the strength of the association (which we express in terms of the dissocia-tion constantKd), as well as the enthalpic (⌬H) and entropic
(⌬S) contributions to the Gibbs free energy of association. The data for one such titration, performed at 20°C, is shown in Fig. 7. When the data were fit with the standard multiple independent binding-site model, the binding parameters were determined to ben⫽ 0.93,Kd⫽13M,⌬H⫽ ⫺1.1⫻ 104
cal/mol, and⌬S⫽ ⫺14 cal/K.mol. A second titration, carried out at 37°C, resulted in fitted parametersn⫽ 0.87,Kd⫽ 35
M,⌬H⫽ ⫺1.9⫻104cal/mol, and⌬S⫽ ⫺42 cal/K.mol. In
both cases, the fit of the model to the experimental data was very good. The deviation of the experimentally determined stoichiometry from unitary values could arise from a large number of factors (e.g., small errors in the estimated protein concentration) and is not significant.
DISCUSSION
The principal aim of this study was to define the regions of the P and N proteins that mediate association of the polymer-ase with the nucleocapsid in both measles virus and mumps virus. Before investigating this interaction, we wanted to es-tablish, through direct experiment, the minimal region of N capable of supporting nucleocapsid assembly, particularly for the relatively uncharacterized mumps virus N protein. It has previously been shown that heterologous expression of measles virus N in bacterial (26, 52) and insect (3, 23) cells and of mumps virus N in yeast cells (44, 46) results in the formation of nucleocapsid-like particles.
Consistent with a large body of prior data, our study shows directly that for both measles virus and mumps virus, the tail of N is dispensable for nucleocapsid assembly. The minimal re-gion found to be capable of supporting assembly (amino acids 1 to 375 in each case) is slightly shorter than had been previ-ously supposed. Modifications to the tail did, however, influ-ence the length of the nucleocapsid-like particles that were produced. Constructs in which most or all of the tail was removed (1 to 375, 1 to 400, and 1 to 425) produced the longest particles. Very similar observations were recently reported for the Newcastle disease virus N protein (28).
The major objective of this study was an analysis of the interaction between the P protein and the nucleocapsid for both measles virus and mumps virus (see summary in Fig. 8). We mapped the P protein-binding site within the measles virus nucleocapsid to a short and contiguous sequence (amino acids 477 to 505) near the end of the N protein (Fig. 3). Among the morbilliviruses, this corresponds to a relatively conserved se-quence within the largely hypervariable tail of N (4, 29, 39). It has previously been shown that the tail of measles virus N, which is intrinsically disordered, undergoes a structural tran-sition upon binding the P protein (24, 32). This trantran-sition has been hypothesized to involve helix formation by residues 489 to 504 of N (24). The minimal binding sequence that we have defined encompasses this region. Additionally, we have shown that the binding of MEN477-505to MEP457-507occurs with low
affinity and is entropically disfavored at both temperatures studied. This is consistent with binding of MEN477-505 being
coupled with folding.
Our delimitation of the measles virus P binding site is
sup-FIG. 3. Tail of the mumps virus N protein (MUN374-549) binds weakly to the mumps virus P protein (MUP), demonstrated by a GST pulldown assay. The Western blot shown at the bottom of the figure was specific for the tag carried on the P protein.
on November 8, 2019 by guest
http://jvi.asm.org/
[image:7.603.62.263.68.464.2]ported by a recent study which examined the ability of trun-cated measles virus N to act as a template for transcription and replication, with a reverse genetics system (58). While deletion of the last 23 amino acids of N (ending at residue 502) did not attenuate basal activity in this system, further truncation of N caused activity to decline precipitously. The authors suggested that this result could be explained by loss of the polymerase-binding site, an interpretation that is fully consistent with the data that we have presented.
As the tail of the N protein is arrayed on the exterior of the nucleocapsid, its involvement in binding the P protein makes sense on the basis of accessibility. In Sendai virus, the P bind-ing site is also located within the tail of the N protein (5, 9, 43).
However, in contrast to these results, we found that the mumps virus P protein binds specifically to the assembly domain of mumps virus N (residues 1 to 398). Some support for this finding comes from a recent study of interactions between the N and P proteins from LPMV, a porcine rubulavirus closely related to mumps virus (proteins from the two viruses exhibit 45 to 55% amino acid identity) (49). On the basis of a series of pulldown experiments, it was concluded that the last 236 amino acids of the LPMV P protein bound to residues 1 to 333 of the N protein.
We suggest that the assembly domain of mumps virus N is the primary site of attachment for the P protein within the nucleocapsid. Several factors suggest that this is plausible. The tail of N is likely to be unstructured and may allow access to assembly domain (Fig. 8) in a way that might not be possible if the tail were fully folded. Additionally, the nucleocapsid is an inherently flexible structure, and it may be unwound during RNA synthesis (19), which could facilitate binding of the poly-merase to the assembly domain. It would therefore be of in-terest to know whether a tailless mumps virus N protein can serve as a template for RNA synthesis.
[image:8.603.99.493.68.325.2]We have also shown, for both the measles virus and mumps virus P proteins, that the last⬇50 amino acids constitute the nucleocapsid-binding domain (NBD). This domain was found to be both necessary and sufficient for binding to nucleocapsid-like particles. For measles virus, this domain was shown by others to be capable of inducing a structural transition with tail of the N protein (24), consistent with our data. The Sendai virus P protein has previously been subject to very detailed analysis. A deletion of 30 amino acids at the C terminus of Sendai virus P abolishes nucleocapsid binding (40), in accord with the results for measles virus and mumps virus. However,
FIG. 4. Last 49 amino acids of the mumps virus P protein (MUP343-391) bind to the assembly domain of mumps virus N (MUN), demonstrated by a GST pulldown assay. Truncation of the tail of mumps virus N protein does not eliminate binding.
FIG. 5. Circular dichroism spectra of MUP343-391and MEP457-507/ P458G, collected at 2°C.
on November 8, 2019 by guest
http://jvi.asm.org/
[image:8.603.49.280.536.702.2]in a cosedimentation assay, the last 95 amino acids of Sendai virus P, encompassing the NBD, did not associate with nucleo-capsids under conditions where the full-length P protein was able to bind (42). On the basis of deletion analysis, it was concluded that two noncontiguous regions of Sendai virus P were required for nucleocapsid binding (41, 42), the C-termi-nal 90 amino acids (479 to 568), and an upstream sequence (345 to 412) which is now known to be part of the coiled-coil domain (50).
It is possible to reconcile the experimental results for mea-sles virus, mumps virus, and Sendai virus through the following model (Fig. 8), initially suggested by Curran (7). First, it is supposed that the binding of the P protein to the nucleocapsid occurs in each case through the NBD at the very C terminus of P, but this association is intrinsically weak, as required for movement of the viral polymerase along the nucleocapsid dur-ing RNA synthesis. Within the P protein, the coiled-coil oli-gomerization domain effectively holds the downstream NBDs in close proximity and in the correct orientation for binding to the nucleocapsid. The sequence that interleaves the coiled coil
[image:9.603.47.289.74.457.2]and the NBD is likely to be unstructured (34). By tethering the NBDs together in this fashion, the coiled coil modulates the association of the NBD with the nucleocapsid. In this model, the role of sequences upstream of the NBD is a general one, restricted to the localization and positioning of the binding domain, thereby enabling the polymerase to walk along the
[image:9.603.308.528.76.522.2]FIG. 6. 2D 1H-15N HSQC NMR spectra of MEP457-507 and MUP343-391/C356S collected at 15°C.
FIG. 7. Binding of the measles virus P nucleocapsid-binding do-main (amino acids 457 to 505) to the measles virus N tail peptide (amino acids 477 to 505) studied by isothermal titration calorimetry at 20°C. (A) Raw binding data obtained for 23 automatic injections of MEP457-507/P458G (each injection, 12l; protein concentration, 1.44 M) into a cell containing MEN477-505(initial volume, 1,800l; initial protein concentration, 0.24M). Proteins were suspended in 10 mM phosphate buffer (pH 7.0)–100 mM NaCl. (B) The integrated titration curve obtained from the raw data in panel A after baseline subtraction. The solid squares represent the experimental data, while the solid line corresponds to the standard multiple independent binding-site model that was fitted to the data.
on November 8, 2019 by guest
http://jvi.asm.org/
nucleocapsid during RNA synthesis. This model (Fig. 8) can account for weak nucleocapsid binding by the isolated NBD and the much stronger binding by the full-length P protein.
Our calorimetric results for measles virus contribute to this model in several ways. First, they show that each NBD from the measles virus P protein will bind the tail of a single N protein. Second, the results demonstrate that binding of the tail by the measles virus P NBD is indeed weak, with a disso-ciation constant of 35M at 37°C. This is consistent with the processive movement of the P protein along the nucleocapsid during RNA synthesis. Finally, they suggest that burial of hy-drophobic amino acids is likely to be driving the binding pro-cess, since the enthalpy change ⌬H associated with binding becomes increasingly negative at higher temperature, which is characteristic of such interactions (48). Indeed, residues 491 through 502 of measles virus N protein are predominantly hydrophobic.
In this regard, the very recent X-ray crystal structure of the measles virus P nucleocapsid-binding domain (amino acids 459 to 507) is revealing (24). This structure was reported after the experiments described in this paper were completed. The bind-ing domain consists of three␣-helices, arranged in an antipa-rallel bundle, and it is proposed that a hydrophobic cleft on the surface of the molecule might accommodate a short helix con-tributed by the tail of the measles virus N protein (24). This model of binding is consistent with the data that we have presented here, especially in its suggestion that the interaction with N involves the burial of a hydrophobic surface.
The NBD from measles virus P is a stable and folded do-main. The analogous region of the Sendai virus P protein is also highly structured (34). However, this finding does not
extend to the mumps virus P NBD, which retains helical sec-ondary structure (Fig. 5) but lacks a defined tertiary structure (Fig. 6). This implies that the binding domain from mumps virus P, unlike its counterpart from measles virus, cannot act as a template into which a sequence from the N protein simply docks. It seems likely that the mumps virus P NBD will be stabilized upon binding to the N protein, representing an in-version of the situation found for measles virus. We speculate that this difference may be related to the migration of the binding site for the P protein, which is in the relatively unstruc-tured tail of N in measles virus and within the strucunstruc-tured assembly domain of N in mumps virus.
In conclusion, we have shown for both measles virus and mumps virus that a 50-amino-acid helical domain at the C terminus of the P protein engages the nucleocapsid. However, the binding domains have different levels of tertiary organiza-tion in measles virus and mumps virus P and attach to distinct sites within the nucleocapsid. Isothermal titration calorimetry binding studies of the proteins from measles virus has provided the first quantitative information on the interaction between P and the nucleocapsid. While our results support previously proposed models for polymerase attachment in many aspects, they also suggest that there may be some significant differences among the paramyxoviruses.
ACKNOWLEDGMENTS
This work was carried out in the laboratory of Brian Matthews, and we are most grateful for his continued support, encouragement, and constructive criticism. We thank Damon Hamel for assistance with nuclear magnetic resonance spectroscopy. We also thank Wendy Breyer and Zac Wood for comments on the manuscript. Merck Re-search Laboratories generously provided vaccine strains of the measles and mumps viruses.
REFERENCES
1. Amexis, G., S. Rubin, V. Chizhikov, F. Pelloquin, K. Carbone, and K. Chu-makov.2002. Sequence diversity of Jeryl Lynn strain of mumps virus: quan-titative mutant analysis for vaccine quality control. Virology300:171–179. 2. Bankamp, B., S. M. Horikami, P. D. Thompson, M. Huber, M. Billeter, and
S. A. Moyer.1996. Domains of the measles virus N protein required for binding to P protein and self-assembly. Virology216:272–277.
3. Bhella, D., A. Ralph, L. B. Murphy, and R. P. Yeo.2002. Significant differ-ences in nucleocapsid morphology within the Paramyxoviridae. J. Gen. Virol. 83:1831–1839.
4. Blixenkrone-Moller, M., G. Bolt, E. Gottschalck, and M. Kenter.1994. Comparative analysis of the gene encoding the nucleocapsid protein of dolphin morbillivirus reveals its distant evolutionary relationship to measles virus and ruminant morbilliviruses. J. Gen. Virol.75:2829–2834.
5. Buchholz, C. J., C. Retzler, H. E. Homann, and W. J. Neubert.1994. The carboxy-terminal domain of Sendai virus nucleocapsid protein is involved in complex formation between phosphoprotein and nucleocapsid-like particles. Virology204:770–776.
6. Conzelmann, K. K.1998. Nonsegmented negative-strand RNA viruses: ge-netics and manipulation of viral genomes. Annu. Rev. Genet.32:123–162. 7. Curran, J.1998. A role for the Sendai virus P protein trimer in RNA
synthesis. J. Virol.72:4274–4280.
8. Curran, J., R. Boeck, N. Lin-Marq, A. Lupas, and D. Kolakofsky.1995. Paramyxovirus phosphoproteins form homotrimers as determined by an epitope dilution assay, via predicted coiled coils. Virology214:139–149. 9. Curran, J., H. Homann, C. Buchholz, S. Rochat, W. Neubert, and D.
Kola-kofsky.1993. The hypervariable C-terminal tail of the Sendai paramyxovirus nucleocapsid protein is required for template function but not for RNA encapsidation. J. Virol.67:4358–4364.
10. Curran, J., and D. Kolakofsky.1999. Replication of paramyxoviruses. Adv. Virus Res.54:403–422.
11. Dao-Pin, S., W. A. Baase, and B. W. Matthews.1990. A mutant T4 lysozyme (Val 131—-Ala) designed to increase thermostability by the reduction of strain within an alpha-helix. Proteins7:198–204.
[image:10.603.60.264.68.226.2]12. De, B. P., M. A. Hoffman, S. Choudhary, C. C. Huntley, and A. K. Banerjee. 2000. Role of NH2- and COOH-terminal domains of the P protein of human
FIG. 8. Schematic model of polymerase attachment to the nucleo-capsid in measles virus and mumps virus, incorporating the results presented in this paper. A helical nucleocapsid-binding domain (NBD) at the C terminus of the P protein (amino acids 457 to 507 in measles virus P and 343 to 391 in mumps virus P) directly mediates binding of the polymerase to the nucleocapsid. In measles virus, the NBD at-taches to a short sequence (amino acids 477 to 505) within the tail of the RNA-associated N protein. In mumps virus, the NBD binds to the assembly domain (amino acids 1 to 398) of the N protein. Based on our results for measles virus, each NBD is capable of weakly associating with a single N molecule. A coiled coil within P holds the downstream NBDs in close proximity. The sequence interleaving the coiled coil and the downstream NBD is likely to be unstructured. The P protein is depicted as a tetramer, but the oligomerization state of measles virus and mumps virus P has not been determined.
on November 8, 2019 by guest
http://jvi.asm.org/
parainfluenza virus type 3 in transcription and replication. J. Virol.74:5886– 5895.
13. Delaglio, F., S. Grzesiek, G. W. Vuister, G. Zhu, J. Pfeifer, and A. Bax.1995. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR6:277–293.
14. Finch, J. T., and A. J. Gibbs.1970. Observations on the structure of the nucleocapsids of some paramyxoviruses. J. Gen. Virol.6:141–150. 15. Garcia-Barreno, B., T. Delgado, and J. A. Melero.1996. Identification of
protein regions involved in the interaction of human respiratory syncytial virus phosphoprotein and nucleoprotein: significance for nucleocapsid as-sembly and formation of cytoplasmic inclusions. J. Virol.70:801–808. 16. Gill, S. C., and P. H. von Hippel.1989. Calculation of protein extinction
coefficients from amino acid sequence data. Anal. Biochem.182:319–326. 17. Greenfield, N., and G. D. Fasman.1969. Computed circular dichroism
spec-tra for the evaluation of protein conformation. Biochemistry8:4108–4116. 18. Harty, R. N., and P. Palese.1995. Measles virus phosphoprotein (P) requires
the NH2- and COOH-terminal domains for interactions with the nucleopro-tein (N) but only the COOH terminus for interactions with itself. J. Gen. Virol.76:2863–2867.
19. Heggeness, M. H., A. Scheid, and P. W. Choppin.1980. Conformation of the helical nucleocapsids of paramyxoviruses and vesicular stomatitis virus: re-versible coiling and uncoiling induced by changes in salt concentration. Proc. Natl. Acad. Sci. USA77:2631–2635.
20. Hennessey, J. P., Jr., and W. C. Johnson, Jr.1981. Information content in the circular dichroism of proteins. Biochemistry20:1085–1094.
21. Horikami, S. M., and S. A. Moyer.1995. Alternative amino acids at a single site in the Sendai virus L protein produce multiple defects in RNA synthesis in vitro. Virology211:577–582.
22. Huber, M., R. Cattaneo, P. Spielhofer, C. Orvell, E. Norrby, M. Messerli, J. C. Perriard, and M. A. Billeter.1991. Measles virus phosphoprotein retains the nucleocapsid protein in the cytoplasm. Virology185:299–308. 23. Hummel, K. B., D. D. Erdman, J. Heath, and W. J. Bellini.1992. Baculovirus
expression of the nucleoprotein gene of measles virus and utility of the recombinant protein in diagnostic enzyme immunoassays. J. Clin. Microbiol. 30:2874–2880.
24. Johansson, K., J. M. Bourhis, V. Campanacci, C. Cambillau, B. Canard, and S. Longhi.2003. Crystal structure of the measles virus phosphoprotein do-main responsible for the induced folding of the C-terminal dodo-main of the nucleoprotein. J. Biol. Chem.278:44567–44573.
25. Kapust, R. B., J. Tozser, T. D. Copeland, and D. S. Waugh.2002. The P1⬘
specificity of tobacco etch virus protease. Biochem. Biophys. Res. Commun. 294:949–955.
26. Karlin, D., S. Longhi, and B. Canard.2002. Substitution of two residues in the measles virus nucleoprotein results in an impaired self-association. Vi-rology302:420–432.
27. Kay, L. E., P. Keifer, and T. Saarinen.1992. Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with im-proved sensitivity. J. Am. Chem. Soc.114:10663.
28. Kho, C. L., W. S. Tan, B. T. Tey, and K. Yusoff.2003. Newcastle disease virus nucleocapsid protein: self-assembly and length-determination domains. J. Gen. Virol.84:2163–2168.
29. Kreis, S., E. Vardas, and T. Whistler.1997. Sequence analysis of the nu-cleocapsid gene of measles virus isolates from South Africa identifies a new genotype. J. Gen. Virol.78:1581–1587.
30. Liston, P., R. Batal, C. DiFlumeri, and D. J. Briedis.1997. Protein interac-tion domains of the measles virus nucleocapsid protein (NP). Arch. Virol. 142:305–321.
31. Liston, P., C. DiFlumeri, and D. J. Briedis.1995. Protein interactions en-tered into by the measles virus P, V, and C proteins. Virus Res.38:241–259. 32. Longhi, S., V. Receveur-Brechot, D. Karlin, K. Johansson, H. Darbon, D. Bhella, R. Yeo, S. Finet, and B. Canard.2003. The C-terminal domain of the measles virus nucleoprotein is intrinsically disordered and folds upon bind-ing to the C-terminal moiety of the phosphoprotein. J. Biol. Chem.278: 18638–18648.
33. Lund, G. A., D. L. Tyrrell, R. D. Bradley, and D. G. Scraba.1984. The molecular length of measles virus RNA and the structural organization of measles nucleocapsids. J. Gen. Virol.65:1535–1542.
34. Marion, D., N. Tarbouriech, R. W. Ruigrok, W. P. Burmeister, and L. Blanchard.2001. Assignment of the 1H, 15N and 13C resonances of the nucleocapsid-binding domain of the Sendai virus phosphoprotein. J. Biomol. NMR21:75–76.
35. Marley, J., M. Lu, and C. Bracken.2001. A method for efficient isotopic labeling of recombinant proteins. J. Biomol. NMR20:71–75.
36. Parks, C. L., R. A. Lerch, P. Walpita, H. P. Wang, M. S. Sidhu, and S. A. Udem.2001. Comparison of predicted amino acid sequences of measles virus strains in the Edmonston vaccine lineage. J. Virol.75:910–920.
37. Paterson, R. G., and R. A. Lamb.1990. RNA editing by G-nucleotide inser-tion in mumps virus P-gene mRNA transcripts. J. Virol.64:4137–4145. 38. Portner, A., K. G. Murti, E. M. Morgan, and D. W. Kingsbury.1988.
Anti-bodies against Sendai virus L protein: distribution of the protein in nucleo-capsids revealed by immunoelectron microscopy. Virology163:236–239. 39. Rima, B. K., R. G. Wishaupt, M. J. Welsh, and J. A. Earle.1995. The
evolution of morbilliviruses: a comparison of nucleocapsid gene sequences including a porpoise morbillivirus. Vet. Microbiol.44:127–134.
40. Ryan, K. W., and D. W. Kingsbury.1988. Carboxyl-terminal region of Sendai virus P protein is required for binding to viral nucleocapsids. Virology167: 106–112.
41. Ryan, K. W., E. M. Morgan, and A. Portner.1991. Two noncontiguous regions of Sendai virus P protein combine to form a single nucleocapsid binding domain. Virology180:126–134.
42. Ryan, K. W., K. G. Murti, and A. Portner.1990. Localization of P protein binding sites on the Sendai virus nucleocapsid. J. Gen. Virol.71:997–1000. 43. Ryan, K. W., A. Portner, and K. G. Murti.1993. Antibodies to paramyxovirus nucleoproteins define regions important for immunogenicity and nucleocap-sid assembly. Virology193:376–384.
44. Samuel, D., K. Sasnauskas, L. Jin, S. Beard, A. Zvirbliene, A. Gedvilaite, and B. Cohen.2002. High level expression of recombinant mumps nucleo-protein in Saccharomyces cerevisiae and its evaluation in mumps IgM serol-ogy. J. Med. Virol.66:123–130.
45. Sedlmeier, R., and W. J. Neubert. 1998. The replicative complex of paramyxoviruses: structure and function. Adv. Virus Res.50:101–139. 46. Slibinskas, R., A. Zvirbliene, A. Gedvilaite, D. Samuel, L. Jin, S. Beard, J.
Staniulis, and K. Sasnauskas.2003. Synthesis of mumps virus nucleocapsid protein in yeast Pichia pastoris. J. Biotechnol.103:43–49.
47. Spehner, D., R. Drillien, and P. M. Howley.1997. The assembly of the measles virus nucleoprotein into nucleocapsid-like particles is modulated by the phosphoprotein. Virology232:260–268.
48. Stites, W. E.1997. Protein-protein interactions: interface structure, binding thermodynamics, and mutational analysis. Chem. Rev.97:1233–1250. 49. Svenda, M., B. Hjertner, T. Linne, and M. Berg.2002. Both the P and V
proteins of the porcine rubulavirus LPMV interact with the NP protein via their respective C-terminal unique parts. Virus Res.83:31–41.
50. Tarbouriech, N., J. Curran, R. W. Ruigrok, and W. P. Burmeister.2000. Tetrameric coiled coil domain of Sendai virus phosphoprotein. Nat. Struct. Biol.7:777–781.
51. Wang, W., and B. A. Malcolm.1999. Two-stage PCR protocol allowing introduction of multiple mutations, deletions and insertions using QuikChange site-directed mutagenesis. BioTechniques26:680–682. 52. Warnes, A., A. R. Fooks, A. B. Dowsett, G. W. Wilkinson, and J. R.
Stephen-son.1995. Expression of the measles virus nucleoprotein gene in Escherichia coli and assembly of nucleocapsid-like structures. Gene160:173–178. 53. Warnes, A., A. R. Fooks, and J. R. Stephenson.1994. Production of measles
nucleoprotein in different expression systems and its use as a diagnostic reagent. J. Virol. Methods49:257–268.
54. Waters, D. J., and R. H. Bussell.1974. Isolation and comparative study of the nucleocapsids of measles and canine distemper viruses from infected cells. Virology61:74–79.
55. Wishart, D. S., C. G. Bigam, A. Holm, R. S. Hodges, and B. D. Sykes.1995. 1H, 13C and 15N random coil NMR chemical shifts of the common amino acids. I. Investigations of nearest-neighbor effects. J. Biomol. NMR5:67–81. 56. Wishart, D. S., C. G. Bigam, J. Yao, F. Abildgaard, H. J. Dyson, E. Oldfield, J. L. Markley, and B. D. Sykes.1995. 1H, 13C and 15N chemical shift referencing in biomolecular NMR. J. Biomol. NMR6:135–140.
57. Zhang, O., L. E. Kay, J. P. Olivier, and J. D. Forman-Kay.1994. Backbone 1H and 15N resonance assignments of the N-terminal SH3 domain of drk in folded and unfolded states using enhanced-sensitivity pulsed field gradient NMR techniques. J. Biomol. NMR4:845–858.
58. Zhang, X., C. Glendening, H. Linke, C. L. Parks, C. Brooks, S. A. Udem, and M. Oglesbee.2002. Identification and characterization of a regulatory do-main on the carboxyl terminus of the measles virus nucleocapsid protein. J. Virol.76:8737–8746.
on November 8, 2019 by guest
http://jvi.asm.org/
ERRATUM
Characterization of Nucleocapsid Binding by the Measles Virus and
Mumps Virus Phosphoproteins
Richard L. Kingston, Walter A. Baase, and Leslie S. Gay
Howard Hughes Medical Institute, Institute of Molecular Biology, University of Oregon, Eugene, Oregon 97403
Volume 78, no. 16, p. 8630–8640, 2004. Page 8638, legend to Fig. 7, line 6: “M” should read “mM.” Page 8638, legend to Fig. 7, line 7: “0.24M” should read “0.12 mM.”