JOURNAL OFVIROLOGY, Aug. 1994, p. 5036-5044 Vol.68, No. 8 0022-538X/94/$04.00+0
Copyright © 1994, American Society for Microbiology
Direct
Injection of
a
Recombinant Retroviral
Vector
Induces
Human
Immunodeficiency
Virus-Specific Immune Responses in
Mice
and
Nonhuman
Primates
MICHAEL J. IRWIN,* LISA S. LAUBE, VIRGINIA LEE, MELISSA AUSTIN, SUNIL CHADA, CAROL-GAYANDERSON, KAYTOWNSEND,DOUGLAS J. JOLLY,
ANDJOHN F. WARNER
Departmentof Immunobiology and Department of Viral Therapeutics,
Viagene,
Inc.,
SanDiego, Califomia
92121Received 27 December 1993/Accepted 25 April 1994
The cytotoxic T-lymphocyte(CTL)response plays animportant role in controlling theseverityandduration of viral infections. Immunization by directinvivoadministration of retroviral vectorparticlesrepresents an efficient means ofintroducing and expressinggenes and, subsequently, the proteins they encode in vivo in mammalian cells. In this manner foreign proteins can be provided to the endogenous, class I major
histocompatibility complex antigen presentation pathway leading to CTL activation. A nonreplicating recombinant retroviral vector, encoding the humanimmunodeficiencyvirus type1 (HIV-1) IIIBenvelopeand rev proteins, has been developed and examined for stimulation of immune responses in mouse, rhesus macaque, and baboon models. Animals were immunized by direct intramuscularinjection ofthe retroviral vector particles. Vector-immunized mice, macaques, and baboons generated long-lived CD8+, major histo-compatibility complex-restricted CTL responses that were HIV-1 protein specific.The CTL responses were foundtobedependentontheabilityofthe retroviralvector totransduce cells. The vectoralso elicited HIV-1 envelope-specificantibodyresponses inmiceand baboons.These studiesdemonstrate theability ofaretroviral vector encoding HIV-1 proteins to stimulate cellular and humoral immune responses and suggest that retrovector immunization may provide an effective means of inducing or augmenting CTL responses in HIV-1-infected individuals.
Inmanyviralinfections the cellular immune response plays animportant role in eliminating virus-infected cells. Cytotoxic T lymphocytes (CTL) specifically lyse infected cells and are important in limiting the severity and duration of many viral diseases(11-13, 18,28, 29,31). Many individuals infected with the human immunodeficiency virus type 1 (HIV-1) exhibit cellular immune responses. CTL specific for different HIV-1 proteins have been found inHIV-1seropositiveindividuals(7, 14, 23, 33,38-40). Specific CTL have also been demonstrated
earlyinthecourseofHIVinfection, priortothe appearance of
antibody,and arepresumedtocontrol the initial viremia often observed early in the disease (37). The presence ofspecific
CTL in seropositive individuals has been associated with slowerprogression toAIDS (5, 16). Because of the potential
role of CTL in controlling HIV-1 infection and disease, numerous approaches have been suggested for activating the cellular immune responseincluding inactivated virus (9), sub-unit vaccines(1, 2), and viralvectors(42).
Many conventional immunization procedures involving the administration of recombinant proteins orkilled virus prepa-rations are currently being evaluated to combat HIV infection and disease. These procedures primarily deliver foreign pro-teins to the exogenous class IImajor histocompatibility com-plex(MHC) antigen presentation pathway. The class II path-way favors activation of CD4+ T cells leading to antibody production (8). Although soluble proteins have been shown to induce CTL activity (10, 15), efficient activation of class I-restricted, CD8+ CTL generally occurs by intracellular syn-thesis offoreign proteins and subsequent antigen processing
*Corresponding author. Phone: (619) 452-1288. Fax: (619)
623-3428.
andpresentationthroughtheendogenous,class Ipresentation
pathway (8, 20). Retrovector immunization is a means of introducing foreign genes into the genome of host cells and, subsequently, foreign proteins into the intracellular antigen-processing compartment. This should provide these immuno-gens for class I MHC-restricted antigen presentation (41),
favoringstimulation ofCD8+ CTL.
We have been studying the induction of CTL responses specific for HIV-1 proteins in mice and nonhuman primates
usingimmunization witharecombinant retroviralvector.This
nonreplicatingvector(N2 IIIBenv)encodes theenvelope (env) and rev proteins from HIV-1 IIIB and has previously been
employed to provide endogenous production ofthese HIV-1 proteins for antigen presentation in murine and nonhuman primate cells. These studies have demonstrated the induction of antigen-specific, class I MHC-restricted, CD8+ CTL re-sponses in mice and rhesus monkeys following injection of ex-vivo vector-transduced autologous fibroblasts (6, 17, 41).
Furthermore, HIV-infected patientstreated with vector-trans-duced autologous fibroblasts have shown augmented HIV-1 IIIBenv-specificCD8+ CTLresponses (43).
As an extension of thesestudies,we haveexaminedvector immunization involving direct administration of theN2
IIIB-env retroviral vector. Direct, in vivo administration of the vector provides a more feasible approach for retrovector immunization compared with using ex-vivo-transduced cells. This study demonstrates that HIV-1-specific CD8+ CTL
re-sponses are induced in mice, rhesus monkeys, and baboons
following administration of this retroviralvector. Inaddition, antibodyresponses areelicitedinmice andbaboonsfollowing vector immunization. This study represents the first direct
5036
on November 9, 2019 by guest
http://jvi.asm.org/
administration of a recombinant retroviral vector leading to the induction of a cell-mediated immune response.
MATERIALS AND METHODS
Animals. Six- to eight-week old, female BALB/c mice (H-2"), obtained from Harlan Sprague-Dawley (Indianapolis, Ind.), were used in these experiments. Rhesus macaques and baboons were maintained at White Sands Research Center (Alamogordo, N. Mex.). Blood samples from the rhesus ma-caques and baboons were obtained at White Sands and shipped onice via overnight airdelivery for testing.
Retroviralvectors. Thenonreplicating, amphotropicmurine N2 IIIBenv retroviral vectorcarries the HIV-1 IIIB env and rev genes and aneomycinphosphoryltransferase gene (Neor) conferring resistance to the antibiotic geneticin (G418) (6). The HIV-1 env and rev genes wereexcised from this provector by digestion with XhoI and ClaI, and this vector backbone, whichretained the Neor marker, was usedin the construction of additional retroviral vectors described below. The N2 III Brev vector,which encodes HIV-1 rev and Neor, was gener-ated byinserting the rev cDNAcoding sequence from pcREV (19) (a generousgift from B.Cullen) into the N2 backbone at theXhoI-ClaIrestriction sites. The bacterial lacZ gene encod-ing 3-galactosidase (a generous gift from J. Kwang) (27) was cloned intothe N2 backbone as described above to generatean N2 ,B-galactosidase vector.
Aliquots ofvector(0.2 ml each) were tested for the presence of replication-competent retrovirus by an extended S+/L-assay (26).Thevector-producing cell line and vector prepara-tions were also tested by cocultivation with Mus dunni cells, and the supernatant generated was assayed for replication-competent retrovirus by a hygromycin marker rescue assay (22). All vector-producing cells andvectorpreparations were shown to befree of detectable replication-competent retrovi-rus by usingthese assay systems.
Vector production.Novel retroviralpackagingcell lines have beendeveloped for thesestudies on the basis of theexpression of murine leukemia virus gag/pol and env sequences in the D-17 caninecell line(ATCC CCL183). The packaging cell line (DA) wasdeveloped by the principle ofsplitting the retroviral genome, removing the viral long terminal repeat sequences, and replacing them with thecytomegalovirus immediate-early promoter (3)to reduce thepotentialof generating replication-competentretrovirus (37a). TheDA/N2 IIIBenvproducer cell line wasgenerated bytransductionof vesicular stomatitis virus G pseudotyped vector (4) into the DA packaging cell line.
DA/N2 IIIBenvproducer cells were grown, and the superna-tantcontaining the replication-defectiveN2IIIBenvvector was collected, filtered (pore size, 0.45 ,um), and stored at -80°C.
The vector titer was determined by serial dilution and CFU assay on HT 1080 indicator cells selected in G418-containing
medium. Titers were typically between
106
and107 CFU/ml.CTL targetcelldevelopment.Themurinefibroblastcellline BC1OME (H-2d) (25) was transduced with the previously
described amphotropic recombinant retroviral vectors encod-ing HIV-1 IIIB env/rev, HIV-1 IIIB rev, or ,B-galactosidase, resulting in the development ofthe corresponding target cell linesBC-env/rev, BC-rev, and
BC-3gal.
The murine fibroblast cell line BL/6(H-f2) (34) was transduced with a recombinant retroviralvector encoding HIV-1 IIIB env/rev togenerate the control targetcell line BL/6-env/rev.In certain experiments the murine effector cellsweretested for lysis of BC target cellsthat werecoatedwithpeptideduring
radioisotopelabeling. Either thep18IIIBpeptide(35)derived from the envelope gpl20 V3 loop region of the HIV-1 IIIB
virus isolate [amino acids 315 to 329; RIQRGPGRAFVT
IGK(C); ChironMimotopes, Clayton,Australia] orthe anal-ogous peptide derived from the HIV-1 MN strain (36), p18 MN[amino acids 315 to 329; RIHIGPGRAFYTIKN(C)]was added(10,uM)tocellsduring radioisotopelabeling. Following a1-hincubation, peptide-coatedtargetcellswerewashed four times to remove both unboundpeptide and excess radioiso-tope. Inotherexperiments,targetcellsinfected with recombi-nantvaccinia virusvectors (Vac-gpl6OandVac-LacZ [gener-ous giftsfrom B. Moss and D. Kuritzkes,
respectively])
were employedaspreviouslydescribed(6).Rhesus macaque and baboonperipheralbloodmononuclear cells (PBMC)were isolated by Ficollgradient centrifugation (Histopaque; Sigma,St.Louis, Mo.).Washed PBMC(4 x 106
per well) were added to 24-well plates
(Coming, Coming,
N.Y.) and transformed by the addition ofpurifiedherpesvirus
papio derived from the549S-1X1055cell line
(32) (a
generousgiftfrom KrishnaMurthy)at amultiplicityof infection of 3:1
plusPolybrene(2
,ug/ml)
(Sigma). TheestablishedBlympho-blastoid cell lines(BLCL)weregrownin RPMI1640 medium
supplementedwith20% fetal bovineserum (HyClone, Logan, Utah), 1 mM sodium pyruvate (Irvine
Scientific,
Santa Ana, Calif.), 10 mM HEPES(N-2-hydroxyethylpiperazine-N'-2-eth-anesulfonic acid) buffer (Irvine Scientific), and 50
jig
ofgentamicinperml(RPMI 20% growth
medium).
The nonhu-man primate BLCL were vectortransduced by cocultivation withaxenotropic producercell lineHX/N2IIIBenvas a sourceof the N2 IIIBenvvector.TheHX/N2IIIBenvproducercells
(106)
weregammairradiated(10,000 rads of60C)
and added(in 2 ml of RPMI 20% growth medium) to 6-cm-diameter tissue culture dishes
containing
106 BLC. The cultures wereincubated for 5daysat37°Cin5%
C02,
washed,andplaced
in selective medium (G418; 800pug/ml).
The transduced BLC weretestedfor HIV-1envexpression byWestern blot(immu-noblot) analysis.
Vector immunization. Mice were injected either
intramus-cularly(i.m.)with 0.2 ml(0.1mlateach oftwomuscle
sites)
ofthe N2 IIIBenv vector
(>106
CFU/ml) orintraperitoneally
with 1 ml of vector,onday0 andday7. Sevendaysafter the final injection mice were sacrificed and the spleens were
removed. Invitro cultures ofprimed splenocyteswere estab-lished as previously described
(41).
Briefly,
splenocytes(3
x106
cells per ml) were cultured for 7 days in vitro withgamma-irradiated(10,000radsof
'Co)
BC-env/revstimulator cells(6x104/ml)
in10 mlofRPMIgrowth
mediumcontaining
10%fetal bovineserumin T-25flasks(Coming).
Theresulting
effector cellswere
subsequently
tested forcytotoxic
activity.
In certain experiments, murine CTL lines weregenerated
andpropagated by multiple in vitro restimulations. CTL effector lines
(106
cells perml) were restimulated withgamma-irradi-ated BC-env/rev cells (6 x
104/ml)
andgamma-irradiated
(3,000rads) BALB/c splenocytefiller cells (2 x106/ml)
every 9to 12 days. RPMI 10%growthmedium(10
ml)
wassupple-mented with recombinant human interleukin 2
(2
U/ml)
Boehringer GmbH,Mannheim, Germany).
CTL effector lines wereassayedforcytotoxicactivity
7days
after restimulation.Rhesusmonkeysandbaboonswereinjected i.m. with upto
atotalof 3 ml ofvector(107
CFU/ml)
distributedtofourtosix different muscle sites. These animalswereinjected
threetimes at 2-week intervals. Peripheral blood was collectedprior
toeach
injection,
andPBMCwereisolated fromaFicollgradient.
The PBMC
(106/ml)
were stimulated in vitro with irradiated(10,000rads), autologous, vector-transduced BLC
(106/ml)
in T-25culture flasks inRPMI20%growth
medium(10 ml).
Theresultingeffector cellswereharvested7
days
later andtested ina
51chromium
releasecytotoxicity
assay.on November 9, 2019 by guest
http://jvi.asm.org/
5038 IRWIN ET AL.
Cytotoxicity assay. The
"tchromium
release cytotoxicityassay was performed for murine and nonhuman primate effector cells as described previously (17, 41). Briefly, the
Na25"CrO4-labeled
syngeneic (murine) and autologous(ma-caque andbaboon)targetcells
(104)
wereincubated for4hat37°C
with various numbers of effector cells (104 to106)
in afinal volume of 200
RI
ina96-well round-bottomplate.Culture supernatants(100
jl)
wereharvested and assessedforspecific
51Crrelease(counts
perminute) by using
a gammaspectrom-eter. Spontaneous release was determined as counts per minute from targetsplusmedium andwastypically 10to20%
of the maximum release. The maximum release was deter-minedascountsperminute from targetsplus 1MHCl.Target
cell
lysis
(percent)
wascalculated as (experimental release-spontaneous
release)/(maximum
release - spontaneousre-lease)
x 100. In some cases, results are presentedaspercentnettargetcell
lysis (specific
target celllysis-control target celllysis).
T-celldepletion. Mousecytotoxiceffector cellsweretreated with CD4 or CD8
T-cell-specific
monoclonalantibody
and then with complement(Low-Tox;
Cedarlane,Westbury,
N.Y.)
to
deplete
therespective
T-cellpopulations,
aspreviously
described
(41).
Macaque cytotoxic effector cellswere treatedwith
CD8-specific
monoclonalantibody (T8;
Dako,Carpente-ria, Calif.), washed, and treatedwith a fluoresceinated
anti-mouse
immunoglobulin
(Cappel, Durham, N.C.). These CD8-enriched(CD8+)
and CD8-depleted(CD8-)
effector cellpopulations
wereisolatedbyflow cytometry. Babooncytotoxiceffector cellswere incubated witha CD8-specificmonoclonal
antibody (Dynal,
Great Neck,N.Y.).
The cells were treatedwith anti-mouse
immunoglobulin-conjugated
magnetic beads(Dynal).
Cells thatbound the ironconjugate
were separatedwith a magnet,
yielding
CD8-enriched andCD8-depleted
effector cell
populations.
Effector cellswerewashed and then incubated with target cells.Antibodydetection.BALB/cmicewere
injected
i.m. with 0.1 ml of the N2 IIIBenv vector and boosted twice at weeklyintervals. Sera were collected
(retro-orbital
venous plexusduring
metaphane-inducedanesthesia) priortoeachinjection.Baboonswereinjectedwithvector asdescribedabove,andsera were collected pre- and postimmunization. By using an
en-zyme-linked
immunosorbent assay(ELISA),
serawereexam-ined for bindingtopurified HIV-1 IIIB
gpl20
protein (25 ngper
well)
(ABT, Cambridge, Mass.),
and the assaywasdevel-oped
with the ABTS system(Kirkegaard
&PerryLaboratories,
Gaithersburg, Md.)
andanalyzed
at 450nm on amicroplate
reader
(Molecular Devices,
MenloPark,
Calif.).RESULTS
The
generation
ofHIV-1-specific
CTL responses directedagainstthe geneproducts deliveredbythe N2 IIIBenv vector
(HIV-1
env, HIV-1 rev, andNeor)
was evaluated in BALB/cmice.
Splenocytes
obtained from vector-immunized mice (2 x105
CFU)
weretested forcytolyticactivityonsyngeneic target cells expressing HIV-1 env and rev proteins and the Neor marker(BC-env/rev).
HIV-1-specific cytotoxic activity from a representativeindividualmousewasdemonstrated on BC-env/rev target cells but not control target cells (Fig. 1A). To indicate the approximate frequency with which N2
IIIBenv-induced CTL responses occur,Fig. 1D showsacompilationof
recent
experiments involving
76 vector-injected mice. Thisfigure
indicates the netspecific
target cell lysisat an effector/target ratio of 100:1 for each individual mouse tested and reveals that 64 of these mice (response frequency, 84%)
generated netspecifictarget celllysisofgreater thanor
equal
to 15%.
The ontogeny of the N2 IIIBenv-induced CTL response in mice has a relatively rapid onset and is long-lived. Mice
injectedasingletime andsacrificed 7dayslater have occasion-ally exhibited specific CTL activity aftera7-day in vitro culture
(datanotshown); however,twovectoradministrations 1 week apart, followed by in vitro culture, generate CTL responses withafrequencyofabout 84%(Fig. 1D).TheCTL response is relatively long-lived,asmicesacrificed17, 20,25, and 27 weeks after thefinal vectorinjection revealed net CTL responses of 50 to 99% at an effector-to-target ratio of 100:1 (data not
shown). Two mice, sacrificed 36 weeks after the final vector
administration, demonstrated relatively low net lysis (13 and 14%at 100:1),butthe CTL responses inindividual micewere
notfollowed overtime, so no conclusionscan be made as to whether these mice possessed low CTL activity at all times postinjectionorthe CTL responsewas droppingtolow levels atthis extended timepoint.
Induction ofcytotoxic activity by direct injection of the N2 IIIBenv retroviralvector was also examined in naive, healthy
nonhuman primates. Rhesus macaques and baboons were
injected with the N2 IIIBenv vector, and cellular immune responses specific for HIV-1 env/rev-expressing target cells were assessed. Both rhesus macaques and baboons (Fig. 1B andC, respectively) generated HIV-1 env/rev-specificcytotoxic
responses after vector injection, as shown by the lysis of autologous HIV-1 env/rev-expressing target cells compared withnontransduced control targets in these selected individu-als. Table 1 shows CTL responses from all vector-injected macaques and baboons at a single effector/target ratio and
sampled at various time points during the study. PBMC obtained prior to vector injection from all treated macaques and baboons (week 0) and in vitro stimulated with vector-transduced cells did not lyse HIV env/rev-expressing autolo-gous targetcells,indicatingthat in vivoprimingwasnecessary for induction ofcytotoxic responses in these animals. These data indicate that six of six macaques and four of four baboons generated specific CTL responses (Table 1).
The ontogeny of the CTL response innonhuman primates was assessed both directly and indirectly. Eight of the 10 nonhuman primates injected with N2 IIIBenv exhibited spe-cific target celllysis with the firstpostinjectionbloodsampling, 2 to 4 weeks following vector administration (Table 1). To
directly examine the onset of specific cytotoxicity and the subsequent loss of this response, macaque R188wasinjected once atfour i.m. sites andperipheral blood lymphocyteswere subsequently sampled for specific CTL activity. This animal showed nospecifictargetcell lysispriorto vector administra-tion but exhibited CTLactivity 3 weeks after injection (Table
1).Macaque R188maintained demonstrablespecifictarget cell lysis 28 and 32 weeks postinjection, but the CTL response dropped by week 36.
Theeffector cellpopulationsinducedbyN2IIIBenv admin-istration tomice and nonhuman primateswerecharacterized for the CD4/CD8 T-cell phenotype. Results indicated that mouse,rhesusmacaque,andbaboon effector cellpopulations
(Fig. 2A, B, and C, respectively) were CD8+ CD4- CTL, as shownbyabrogationofcytotoxicactivityfollowing depletion of CD8+Tcells,whereas CD4+T-celldepletion (orenrichment ofCD8+Tcells)of the effectorcellpopulation hadanegligible effect on the lysis of target cells compared with the lysis by untreatedcontrol effector cells.
The vector-induced CD8+ CTLwere subsequently evalu-ated for MHC restriction requirements. The HIV-specific
BALB/c
(H-2d)
mouse CTL recognized and lysed syngeneicJ. VIROL.
on November 9, 2019 by guest
http://jvi.asm.org/
-0- BC-env/rev
-a.-- BC-Bgal
100-A.
80
- 60- 40-
20-3:1 10:1 30:1 100:1
EFFECTOR:TARGETRATIO
-0- B36env/rev
0-B36 ca
"4
C.
I.-_
X
100-
80-
60-
40-
20-A
1*
103:1 101 30:1 100:1 EFFECTOR:TARGET RATIO
* -- M178-env/rev
-0--- M178
I 1
3:1 10:1 30R1 100:1
EFETOR:TARGET RATIO
D.
3.. I
I.
I!I
1.
I'
I..
Mice Macaques Baboons CTL RESPONSEFROM INDIVIDUAL ANIMALS
FIG. 1. Retrovector immunizationwith N2 IIIBenvinducescytolytic activity in mice and nonhumanprimates. N2IIIBenv vector-generated
effector cells froma mouse (A),amacaque (B),or ababoon (C)weretested for lytic activityontargetcellsexpressing HIV-1 env/rev antigens
orcontroltargetcells.Syngeneictargetcellswereemployedto testmouseeffector cells(A),andautologousBlymphoblasttargetswereusedto testmacaqueM178(B)andbaboonB36(C)effector cells. Themeanof thepercent targetcelllysis from triplicateassaywells isplottedateach effector-to-target cell ratio shown.The standarderrorsof themeanforalldatumpointswereless than 5%(A), 7% (B), and 13% (C). Nettarget celllysisisshown for 76injected mice,allmacaques(six),and all baboons(four)that have beenvector-immunized(D). Each dotrepresents net targetcelllysisataneffector/targetratio of 100:1for individualmice,macaques,and baboons. Thepeak CTLresponsefornonhuman primates
isdepicted,asthese animalsweretestedatvarious timepoints postimmunization.
targets (BC-env/rev; H-2d) but not allogeneic target cells expressing HIV-1 env and rev proteins (BL6-env/rev; H-2b)
(Table 2), indicating that the mouse CTL were MHC
re-stricted. Insubsequentmouseexperiments, humantargetcells (HT1080)transfected with the murine class I H-2Ddgeneand infected with a recombinant vaccinia virus expressing HIV-1
gpl60 were specifically lysed byvector-induced mouse CTL
(datanotshown). These dataconfirm that themouse effector
cellswere restricted tothe H-2DdMHC molecule. In prelim-inaryexperiments,the CTL frommacaquesand baboonslysed autologous target cells butwere unable to lyse heterologous
macaqueand baboon targetcells, respectively, suggestingthat thesecytotoxicresponses werealsoself-MHC restricted(Table 2). Therefore, conventional class I MHC-restricted, CD8+ CTL were generated in mice and nonhuman primates as a
result of the direct administration of the N2 IIIBenv retroviral
vector.
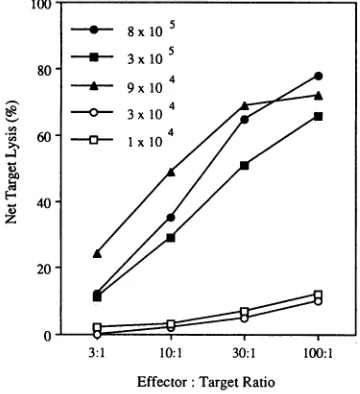
Examination of CTL induction inBALB/cmice withrespect to vector dose demonstrated a threshold relationship. By
injecting mice with serial dilutions of the N2 IIIBenvvector
rangingfrom8x 105to9 x 104 CFU, splenocyte effectors that caused equivalent target cell lysis were generated (Fig. 3).
Miceinjectedwithasmallerquantityofvector(3x 104or1 x
104 CFU) exhibited negligible targetcell lysis. These studies
wereextended by injectinggroupsofmice(fivetoseven) with
asinglebatch ofvectorthatwasdilutedto
107,
106,105,and104CFU/mland confirmed by titer assay. All mice injectedwith 107 and 106 CFU/ml (sevenof seven and five offive,
respec-tively) generatedCTLresponses(greaterthan15%netlysisat
aneffector/targetratio of100:1).Two of six miceinjectedwith
105 CFU/ml generated CTLresponsesof 31 and43% specific
netlysis,andnomiceinjectedwith104CFU/mlexhibitedCIL
responses. Several other experiments, involving injection of differentpreparationsof the N2 IIIBenv vector, demonstrated
asimilar threshold phenomenonforCILinduction. Analysis of these data indicates that, in mice, atiter ofapproximately
105 CFU/mlwas necessaryforCTL induction.
The induction of an immune response by recombinant
100
-80
60
40 20 -0_
B.
100 1-,
Q
Ez Ez
80. 60. 40
-20
-0
0---- -0
on November 9, 2019 by guest
http://jvi.asm.org/
[image:4.612.116.497.75.435.2]5040 IRWIN ET AL.
TABLE 1. CTLresponses fromrhesus macaques and baboons
injectedwith N2 IIIB env
%Specificnettarget celllysisb (wk)
Animala
Wk0 Wk 2 Wk 2-4 Wk 6 Wk 16 Extended
Macaques
R168 3 56 47 38(22),ND
R178 0 14 60 28(18),9 (20)
R180 0 7 30 33(26),6(30)
R188 1 50 34(28) 14(32),9(36)
R190 4 40 34 ND, ND
R156 4 2 30 8 (25), 7(27)
Baboons
B36 7 22 45 11(10)
B38 6 16 18 4 (10)
B40 0 17 12 38(18)
B42 0 17 34 45(14)
aTimes of injection (weeks): R168, 0, 2, 4, 16,and18;R178,0, 12, 14, and 16; R180,0, 4, 8, 12,18,and22; R188, 0; R190,0, 4, 8,and12;R156, 0,2, 4, 10, 12, 14, 23,and25;B36 andB38,0 and4;B40andB42, 0,2,and 4.
bLysisof HIV-1 env/rev-expressingautologous targets- lysis of autologous herpesvirus papio-transformed targetsataneffector-to-target cell ratioof100:1. Effector CTLwereobtainedfromperipheral bloodlymphocytesattheindicated samplingtimes before(week 0)andafter administration of N2 IIIB env.ND,not done.
retroviral vectors could be mediated by transduction and de novo protein synthesis or, potentially, byforeign vector-asso-ciated proteins. To determine whether the observed CTL responsesweredependent upon N2 IIIBenv-mediated in vivo transduction orthe potential presence of soluble HIV-1 pro-teinscontained in thevectorpreparationcouldstimulate CTL, vector particles were inactivated prior to immunization. The vector was exposed to either Nonidet P-40 (0.05%) or heat
(68°C, 30 min) treatment so as to not denature proteins presentin the vectorpreparation buttoinactivate transduction
by the vector. Treated N2 IIIBenv and untreated control vectorsweretitered andadministeredtomicetoevaluate CTL
induction. These treatments did inactivate the vector, as the
titers were substantially reduced (<104 CFU/ml) compared
with the untreated controlvector(8 x 105 CFU/ml).Neither the Nonidet P-40-treated nor the heat-treated vector gener-ated CTLactivity (0% nettarget cell lysis at 100:1), whereas the untreatedvectorinduced HIV-1-specific CTLactivity (52
and54% net target celllysis at 100:1). These results suggest that transduction of host cells by the recombinant retroviral vectoris essential forgenerating the observed CTL responses. The N2IIIBenv vector encodes differentproteins (env,rev, and Neor). Therefore, N2 IIIBenv-induced CTLwere tested on various target cells to evaluate the specificity of the
vector-induced CTL response. CTLlines,generatedby
prim-ing mice with theN2 IIIBenvvectorandrestimulating in vitro with irradiated BC-env/rev cells, were tested on targets ex-pressing individual HIV-1 proteins. The lack oflysis of
BC-,Bgaltargets compared withBC-env/rev targetsindicated that the cytotoxic activity was specific for vector-encoded HIV-1 proteins and not an irrelevant protein or the Neor marker, whichis shared by both target cells (Fig. 1A and Table 3). The HIV-1 env protein is produced in the absence of the rev
protein in target cells infected with a recombinant vaccinia virus that encodes HIV-1 IIIB gpl60
(Vac-gpl6O)
(6). N2 IIIBenv-induced CTL lines recognized and lysed BC target cellsinfected withVac-gpl60(BC+Vac-gpl60)butnottargetsinfected with acontrol recombinant vaccinia virus expressing the bacterial LacZ gene (BC+Vac-LacZ) (6), indicating that
1-1 rA
rA ;04 04 04
04 w
u
1-w
0 lx
E-4 F4 w
z 80
-60 -40
20
-
0-80
60
40
20 0
F-z 80
-60
-40 -20
--*- Untreated * CD4-Depleted
A.
A--- CD8-Depleted
I I T
3:1 10:1 30:1 100:1 EFFECTOR:TARGET RATIO
1:1 3:1 10:1 30:1 EFFECTOR:TARGET RATIO
Untreated CD8-selected CD4-selected
C.
1:1 3:1 10:1 30:1 EFFECTOR:TARGET RATIO
FIG. 2. Cytolyticeffector cells generated by N2 IIIBenv immuniza-tionareCD8+ T cells. Cytolytic effector cells from animals injected with the N2 IIIBenvvector weretreatedwithspecific antibodies and separated intoCD8-enriched (CD8+)orCD8-depleted (CD4+) cell populations. Control effector cell populations were not antibody treated(untreated). Thenetof themeanofspecifictargetcelllysis is plottedfor mouse(A),macaque(B),andbaboon(C)effector cells at the indicatedeffector-to-targetcell ratios.
HIV-1env-specificCTL werepresentin thepolyclonal effector cell population. To investigate whether these CTL lines also contained rev-specific effector cells, target cells transduced witharetroviral vectorencoding onlyHIV-1 rev(BC-rev)were employed. N2 IIIBenv-induced CTLlines showedrev-specific target celllysis,asBC-revtargetsandBC-env/rev targetswere lysedwhileBC-,galcontroltargetswere not(Table3). There-fore, N2 IIIBenvvectoradministration to BALB/cmice gen-erated CTL responses specific for both env and rev protein epitopes.
J.VIROL.
on November 9, 2019 by guest
http://jvi.asm.org/
[image:5.612.63.304.94.247.2]TABLE 2. MHCrestriction of N2 IIIBenv-inducedCTIL
%Nettargetcell lysis AnimalandE,T
Syngeneicorautologous Allogeneicor heterologous
Mouse
100:1 79 0
30:1 60 2
10:1 35 1
3:1 14 2
Macaque R168
25:1 28 0
8:1 19 0
3:1 11 0
1:1 2 0
BaboonB42
100:1 45 4
30:1 35 2
10:1 18 2
3:1 4 0
CTLweretested forlysisofMHC-compatibleand-incompatibletarget cells expressingHIV-1env/rev.Vector-inducedBALB/c(H-2?d)CTLweretested for
lysisof syngeneic(BC-env/rev;H-2d) and allogeneic (BU6-env/rev;H-2")targets at the indicated effector/target (EBT) ratios. Rhesus macaque and baboon vector-inducedCTL weretestedfor lysis of autologous andheterologousHIV-1 env/rev-expressing targets.
To further characterize the env-specific component of the mouse CTL response,a peptide (p18 IIIB) corresponding to the HIV-1 IIIB hypervariable V3 loop region of the env protein was usedtopulse-labeltargetcells. Thispeptide is an
immunodominantCTLepitope,recognizedby BALB/c mouse Tcellsspecificfor the HIV-1 IIIBenvprotein(35).Target cells
pulsedwith thispeptide (BC+pl8 IIIB)were recognizedand lysed by N2 IIIBenv-induced CTLlines, in contrast to control BC targets(Fig.4).Direct N2 IIIBenvvectoradministrationto
80 -
I-._
2
60
-40
20
-3:1 10:1 30:1 100:1
[image:6.612.52.293.84.273.2]Effector: Target Ratio
FIG. 3. Miceinjectedwith serialdilutions of N2 IIIBenv exhibita
threshold response for CTL induction. Mice were immunized with serial dilutions of the N2 IIIBenvvectorandtested forantigen-specific
CTL induction. Themeanofthenetpercent targetcelllysisisplotted
for effector cellsgeneratedwith the indicatedvectordoses(CFU)and attheindicatedeffector-to-targetcellratios. The standarderrorof the
meanfortriplicateassaywellswaslessthan3%ateach datumpoint.
TABLE 3. HIV-1 IIIB env and rev specificities of N2
IIIBenv-inducedCTLf
%Lysis of target cells expressing:
BIT"
BC-env/rev BC-,Bgal BC-rev BC+Vac-gpl6O BC+Vac-LacZ
30:1 68 0 44 62 9
10:1 45 0 20 44 4
3:1 18 0 8 12 0
aCTL lines weregeneratedfrom mice that wereinjectedwith the N2IIIBenv vector.TheBC-env/revtarget cell expresses the HIV-1 env and revproteinsfrom
HIV-1IIIB,whereas the BC-rev target cell expressesonlythe revprotein.The
BC-,3gal targetcell expresses bacterial ,-galactosidase.BC1OME targetcells werealsoinfectedwith recombinant vacciniavirusesencodingthe HIV-1 IIIB
gpl60genebutnotthe rev gene(BC+Vac-gp160)orthebacterialLacZgene (BC+Vac-LacZ).
bEf, effector/targetratio.
mice inducedacross-reactiveCTL response in two of fiveCTL
lines, as demonstrated by lysis of targets coated with an equivalent peptide derived from the V3 loop region of the HIV-1 MNviral isolate (Fig. 4). These results suggested that thevector-induced(HIV-1 IIIBenv-specific)CTLpopulation was capable ofrecognizing cross-reactive determinants of an envprotein fromadifferentHIV-1 isolate.
HIV-1 env-specificantibody responseswere also examined for mice and nonhuman primates immunized with the N2
IIIBenv retroviral vector. Serum samples obtained from ani-mals prior to and afterinjection of N2 IIIBenv were tested by ELISAforHIV-1 gpl20-specificantibody. High-titerantibody responses wereobserved in six BALB/c mice after the second administration ofvectorandpersisted for at least 12 months after the third injection (Fig. 5A). Preinjection mouse sera showednoanti-gpl20 specificantibody, nor didseracollected after the first injection. Sera from one of four vector-immu-nized baboons have shown gpl20-specific antibody titers by ELISA(Fig.SB),but rhesus macaque serahave, thus far, been negative for anti-gpl20 reactivity (data not shown). More recent attempts at generating antibody responses in mice injected with N2 IIIBenv have not been successful. Interest-ingly, vector preparations that successfully induced antibody in mice were supernatants from producer cells, which possess
quantities of soluble env protein detectable byWestern blot
analysis.Current vectorpreparationswhich have failedtoelicit antibody responses contain undetectablequantitiesof soluble envprotein, by Westernblot analysis. Wearecurrently
inves-tigating particular vector compositions capable of inducing antibodyresponses.
DISCUSSION
Retrovirus-mediated gene transfer is an efficient means of
deliveringgenesencodingforeignproteins.Directinjectionof the N2 IIIBenvretroviralvector leadsto activation of class I MHC-restrictedCD8+ T-cell responsesspecificfor the HIV-1 IIIBenvandrevproteinsencodedbythevectorin eachof the three animal models examined. CTL inductionwas shownto bedependenton the vectortransduction process andwas not
theresult of associated soluble
gpl60/120
protein.Induction of CTL memory cell activity was also apparent, because CTLresponses have beenobserved at least 6 months after vector
administration in mice and macaques and4months
postinjec-tion in ababoon.Therefore, theN2IIIBenv retroviralvector
appearstobequitecapableofinducinglong-lived CTLactivity
in the different animal models.
The
CTL
response in HIV-infected individuals likely con-* 8x 105* 3x105
-*-9x104
--O~-
3x104
01- x
104
c
on November 9, 2019 by guest
http://jvi.asm.org/
[image:6.612.313.554.93.156.2] [image:6.612.84.264.460.657.2]5042 IRWIN ET AL.
A.
I
0
9:1 27:1 81:1
Effector:Target Ratio
SERUM DILUTION(1/X)
80
-*._
q
3-VU
60
-40
-20
--0- BC-env/rev -U--- BC+p18
BC+MN18
-0.--
BC-B.
3:1 9:1 27:1
Effector:Target Ratio
FIG. 4. N2 IIIBenv-induced CTLcanrecognizetargetcells coated
withapeptide from the HIV-1 IIIB envelope protein andcross-react
withtargetcells coated with the analogous peptide fromHIV-1 MN.
Twoindependentlyderived CTL lines(AandB)weregenerated byin
vitrorestimulation of N2 IIIBenv-primed splenocyteswithBC-env/rev
cells and recombinant interleukin 2. Effector cells were tested for
recognition ofBC1OME target cells (BC)orBC-env/revtarget cells,
whichexpress theHIV-1 IIIB envelope and rev proteins. BC1OME targetcellswerealso coated withpeptidesderived from the V3loop region of either the HIV-1 envelope protein of the IIIB strain
(BC+pl8 IIIB)orthe MN strain(BC+pl8 MN). Standarderrorof themean fortriplicate assaywellswasless than 3% for each datum
point.
tributes to maintenance of the asymptomatic phase of the disease (5). Current approaches to enhance HIV-specific im-munityinHIV-infectedindividuals have included administer-ing soluble recombinant HIV-1 gpl60/120 protein (10, 15). Thesetreatmentshaveshown increasedCD8+andCD4+CTL
responses as well as antibody responses. CD4+ CTL, in
contrast to CD8+ CTL, have been shown in vitro to lyse non-HIV-infected targets that have surface-bound HIV-1 gpl20 protein (24, 33). CD4+CTLlysisofgpl20-bound CD4+ T cells could result in the elimination of non-HIV-infected
0.8
I
!, 0.6
S
0.4
- 0.2
0
0.0
B.
[image:7.612.340.537.70.480.2]SERUM DILUTION(1/X)
FIG. 5. HIV-1 envelope gpl20-specific antibody is generated in
miceand baboonsbyadministration of N2 IIIBenv.(A) BALB/cmice
were injectedi.m. (0.1mlpermusclesite) into each hind limb three times with the N2 IIIBenvvector(1.6x 106CFU/ml). Open symbols,
serumantibody responsesspecificfor gpl20 priorto immunization;
solidsymbols, postimmunization serum antibodyresponses for each individualmouse.(B)Baboonswereinjectedi.m. with 3 ml of N2 IIIB
env(1.0 x 107CFU/ml)distributedtosix muscle sitesevery2weeks foratotal of threeinjections.Serumsampleswerecollected fromall animalspriortoeachinjectionand testedbyELISA for thepresence
of HIV-1envelopegpl20-specificantibody.Serumantibodyresponses
fromonebaboonpriortoinjection (0)and after the first(A),second
(U), and third(0) injectionsare shown. Serumsamplesfrom three other immunized baboons have failedtogeneratedetectableantibody
responses.
targetcells invivo,anargumentinvokedtopossibly explainthe
loss ofCD4+TcellsduringHIVinfection.Conversely, CD8+ CTLappeartospecifically recognize virallyinfected cells and do not exhibit lysis of targets that are exogenously protein
coated. Therefore, specific induction ofCD8+ CTL may be
preferred to CD4+ CTL induction in the HIV infection and diseaseprocess.
Theabilitytoinduce broadreactivity against different HIV
80
-0- BC-env/rev - BC+IIIB p18
-*- BC+MNp18 -0-- BC-
60-
40-20
._
EV
0
J. VIROL.
on November 9, 2019 by guest
http://jvi.asm.org/
[image:7.612.97.270.71.485.2]isolates is an essential component of potential HIV vaccines and immunotherapeutics. The HIV-1 env/rev-specific CTL generated in some mice by administration of N2
IIIBenv
exhibited cross-reactivity on targets coated with an immuno-dominant env peptide derived from the V3 hypervariable region of the HIV-1 IIIB and HIV-1 MN env proteins (36). Previous studies employing immunization with ex-vivo vector-transduced autologous fibroblasts have generated similar V3 peptide cross-reactive CTL (41). A more comprehensive anal-ysis, employing target cells infected with different prototypic and clinicalHIV-1 isolates demonstrated extensive cross-strain recognition and lysis with CTL induced by injection of N2 IIIBenv-transduced syngeneic fibroblasts (6). CTL recognition of commonHIV-1 determinants by N2IIIBenv
vector-induced CTL could be important in eliminating cells infected with diverseHIVisolates as well as variant, sequential intrapatient viruses.CTL specific for the HIV-1 rev protein have been shown following N2 IIIBenv immunization of
BALB/c
mice. Also, preliminary evidence suggests that the baboon CTL response contains effectors directed at a peptide epitope derived from the revprotein (data not shown). The CTL response specific for rev determinantsmay be of particular importance. Firstly, the revprotein is expressed early in virus infection, and lysis of virus-infectedcells prior to mature virion production could be beneficial in controlling virus spread. Secondly, CTL specific for rev determinants may also be cross-reactive with cells infectedwith different HIV-1 isolates because the rev protein appears to be conserved (21). Immune responses directed at conserved determinants are likely to provide the broadest specificity and, thus, be effective over the prolonged time course typically seen in HIV infections.HIV-1 antibody responses are likely important for clearing freevirus. HIV-1 env-specific antibody responses can lead to direct virus neutralization or could contribute to antibody-dependent cellular cytotoxicity (30). The
gp120-specific
anti-bodyresponsegenerated in vector-injected mice indicates that humoral immunity can also be involved in the immune re-sponse induced by the N2 IIIBenv vector. High-titer serum antibody, specific for gpl20, persisted in N2IIIBenv
vector-treatedanimals for at least 1 year after immunization (data not shown), suggesting potential long-term gene expression in theseanimals. Studies are under way to determine the longev-ity ofintegratedvector DNA as a potential explanation for the persistence of env-specific antibody.The vectorpreparation used in the mouse antibody studies (Fig. 5A) was a filtered vector
supernatant
and contained soluble proteins including fetal bovine serum and vector-generatedHIV envelope protein. Current vector preparations are column purified and have markedly reduced free-protein levels. Interestingly, envelope-specific antibody responses in miceand macaques to these vector preparations are undetect-able. An HIV-1-specific antibody response in one of the four baboons injectedwith purified vector has been detected (Fig. 5B), but the titerwas relatively low and other baboon immune serum sampleshave been envelope-specific antibody negative. Rhesusmacaques do generate substantialHIV-1-specific
anti-body responses following injection of 5 x 107 N2 IIIBenv-transduced autologous fibroblasts (17), as do similarly treated mice (41). The induction of an antibody response probably requires moresoluble envelope protein than that provided by N2 IIIBenv in vivo-transduced cells, under the present condi-tions. Studies are under way to determine under what condi-tions the presentvector preparations can generate an antibody response. Furthermore, we are investigating which cell types havebeen transducedin vivo and which cells subsequently actas the
antigen-presenting
cells that drive envelope-specificimmune
responses.
Induction of an immune response involving both cellularimmunity
and humoral immunity is likely toprovide
thegreatest
impact
on the HIV disease process.Retrovector immunization
may
represent at least oneap-proach
forconsistently
activating
these immune responses.ACKNOWLEDGMENTS
We thank the
following
individuals for their valuable assistance tothis
project: Stephen
Chang
and Elizabeth Song for discussions and critical review ofthemanuscript;
TammiHoward for animal care andexpert
technicalassistance;
and Gloria Peters, Greg Ronlov, and AndreaLynn
forstimulating
discussions and additional technical assistance.Sincere thanks to Sharon Kerins for assistance with graph-ics and to the members ofViagene
associated with the VectorDevelopment,
VectorProduction,
Process Development,and QualityAssurance/Controlgroups.
This
study
was funded inconjunction
with The Green Cross Corp., Osaka, Japan.REFERENCES
1.
Arthur,
L.O.,
J. W.Bess,
Jr., D. J. Waters, S. W. Pyle, J. C.Kelliger,
P. L.Nara,K.Krohn,W. G.Robey,A. J. Langlois, R. C.Gallo,
et al. 1989.Challenge
of chimpanzees (Pan troglodytes)immunized with human immunodeficiency virus envelope
glyco-protein
gpl20.
J. Virol. 63:5046-5053.2.
Berman,
P.,
J.Groupman,
T.Gregory,
P.Clapman, R. Weiss, R.Ferriarri,
L.Riddle,
C. Shimasaki, C. Lucas, L. Lasky, and J.Eichberg.
1988. Human immunodeficiencyvirus type 1 challengeof
chimpanzees
immunized with recombinantgpl20.
Proc. Natl. Acad. Sci. USA 85:5200-5204.3.
Boshart, M.,
F.Weber,
G. Jahn, K. Dorsch-Hasler, B.Flecken-stein,
and W. Schaffner. 1985. Averystrong enhancer is locatedupstream
of an immediateearlygene of human cytomegalovirus.Cell 41:521-530.
4.
Burns,
J.C.,
T.Friedmann,
W.Driever,M.Burrascano,and J.-K.Yee. 1993. Vesicular stomatitis virus G glycoproteinpseudotyped
retroviral vectors: concentration to very high titer and efficient
gene
transfer into mammalian and nonmammalian cells. Proc.Natl. Acad. Sci. USA 90:8033-8037.
5.
Carmichael, A.,
X.Jin,
P. Sissons, and L. Borysiewicz. 1993.Quantitative
analysis
of thehuman immunodeficiencyvirus type1
(HIV-1)-specific
cytotoxic
Tlymphocyte
(CTL) response atdiffer-ent
stages
of HIV-1 infection: differential CTL responsesto HIV-1and
Epstein-Barr
virus in late disease. J. Exp. Med.177:249-256.
6. Chada, S., C. E.
Dejesus,
K.Townsend,W. T. L. Lee, L. Laube,D.J.
Jolly,
S. M. W.Chang,
andJ.F.Warner. 1993.Cross-reactivelysis
of humantargets
infected withprototypicand clinicalhumanimmunodeficiency
virustype
1(HIV-1)
strains by murineanti-HIV-1
IIIB
env-specific cytotoxic
Tlymphocytes.J.Virol. 67:3409-3417.7.
Culman, B.,
E.Gomard,
M. P. Kieny, B. Guy, F. Dreyfus, A. G.Saimot,
D.Sereni,
andJ. P.Levy.
1989. An antigenic peptide ofthe HIV-1 nef
protein
recognized
by
cytotoxic T lymphocytes ofseropositive
individuals in association with different HLA-Bmol-ecules. Eur. J. Immunol. 19:2383.
8.
Germain,
R.N.,
and D. H.Margulies.
1993. The biochemistry andcell
biology
ofantigen
processing
and presentation. Annu. Rev. Immunol. 11:403-450.9.
Gibbs,
C.J., Jr.,
R.Peters,
M. Gravell, B. K. Johnson, F. C.Jensen,
D.J.Carlo,
andJ.Salk.
1991. Observationsafter humanimmunodeficiency
virus immunization and challenge of humanimmunodeficiency
virusseropositive
and seronegativechimpan-zees. Proc. Natl. Acad. Sci. USA 15:3238-3242.
10.
Hammond,
S.A.,
R.C.Bollinger,
P. E. Stanhope, T.C. Quinn, D.Schwartz,
M. L.Clements,
and R. F. Siliciano.1992. Comparativeclonal
analysis
of humanimmunodeficiencyvirus type1
(HIV-1)-specific
CD4+
andCD8+
cytolytic
Tlymphocytes isolated fromseronegative
humans immunized with candidate HIV-1 vaccines.J.Exp. Med. 176:1531-1542.
on November 9, 2019 by guest
http://jvi.asm.org/
5044 IRWIN ET AL.
11. Hou, S.,P. C.Doherty,M.ZUlstra,R.Jaenisch,andJ.M. Katz. 1992. Delayed clearance ofSendai virus inmice lackingclass I MHC-restrictedCD8+Tcells.J. Immunol. 149:1319-1325. 12. Kast,W.M.,A. M.Bronkhorst,L. P. DeWaal,andC.J.M.Melief.
1986.CooperationbetweencytotoxicandhelperTlymphocytesin
protection against lethal Sendai virus infection. J. Exp. Med.
164:723-738.
13. Klavinskis, L. S., J. L. Whitton, and M. B. A. Oldstone. 1989.
Molecularly engineeredvaccine whichexpressesan
immunodom-inant T-cellepitopeinducescytotoxicTlymphocytes thatconfer
protectionfromlethalvirus infection.J.Virol. 63:4311-4316.
14. Koenig, S.,T. R.Fuerst,L.V.Wood,R. M.Woods,J.A.Suzich,
G.M.Jones,V.F. De LaCruz,R. T.Davey, Jr.,S.Venkatesan,B.
Moss,W. E. Biddison, and A. S. Fauci. 1990. Mapping the fine specificityofacytolyticTcell responsetoHIV-1 nef protein.J. Immunol. 145:127-135.
15. Kundu, S.K.,D.Katzenstein, L. E.Moses, andT. C. Merigan. 1992. Enhancement of human immunodeficiency virus
(HIV)-specific CD4+
and CD8+ cytotoxic T-lymphocyte activities inHIV-infected asymptomatic patients given recombinant gp160
vaccine. Proc. Natl.Acad. Sci.USA89:11204-11208.
16. Kundu,S. K., and T. C.Merigan. 1991. Inverse relationshipof
CD8+CD11+
suppressor T cells with humanimmunodeficiencyvirus (HIV)-specific cellular cytotoxicity and natural killer cell
activity
in HIVinfection.Immunology74:567-571.17. Laube,L.S.,M.Burrascano,C. E.DeJesus,B.D.Howard,M. A.
Johnson,W.T. L.Lee,A. E. Lynn,G.Peters,G. S.Ronlov,K. S.
Townsend,R. L.Eason,D.J.Jolly,B.Merchant,andJ.F. Warner.
1994. Cytotoxic T lymphocyte (CTL) and antibody responses
generated in rhesus monkeys immunized with retroviral
vector-transduced fibroblasts expressing HIV-1 IIIB env/rev proteins.
Hum.GeneTher.5:853-862.
18. Lin, Y.-L.,and B. A. Askonas. 1981. Biological propertiesofan
influenzaA virus-specific killerT cell clone: inhibition of virus
replication
in vivo and induction ofdelayed-type hypersensitivityreactions.J.Exp. Med.154:225-234.
19. Malim, M.H., J. Hauber, R Fenrick,and B. R. Cullen. 1988.
Immunodeficiency
virusrevtrans-activator modulates theexpres-sion ofthe viralregulatorygenes.Nature(London)335:181-183.
20. McMichael,A.J. 1992. Role of class I molecules of the major
histocompatibility
complexin cytotoxicT-cell function inhealthanddisease.
Springer
Semin. Immunopathol. 14:1-16.21. Meyers, G.,J.A.Berzofsky,B. Korber,R. F. Smith,andG. M. Pavlakis.1992. Humanretrovirusesand AIDS:acompilationand
analysis
ofnucleic acid andamino acid sequences.Los AlamosNationalLaboratory, LosAlamos,N.Mex.
22. Miller, A. D., and C. Buttimore. 1986. Redesign of retrovirus
packaging
cell linestoavoid recombinationleadingtohelpervirusproduction.Mol.Cell.Biol.6:2895-2902.
23. Nixon, D. F., A. R. M. Townsend, J.G. Elvin, C. R Rizza, J.
Gallwey,
andA.J.McMichael. 1988. HIV-1gag-specific cytotoxicT
lymphocytes
definedwithrecombinant vaccinia virusandsyn-thetic
peptides.
Nature(London)
336:484-487.24. Orentas,R. J.,J.E. K.Hildreth,E. Obah,M.
Polydefids,
G. E.Smith, M. L. Clements, and R. F. Siliciano. 1990. An HIV
envelope protein
vaccineinduces CD4+humancytolyticTcellsactive
against
HIV-infected cells. Science248:1234-1237. 25. Patek, P. Q., J. L. Collins, and M. Cohn. 1982. Activity anddexamethasonesensitivityof naturalcytotoxiccellsubpopulations.
Cell. Immunol. 72:113-121.
26. Peebles,P. T. 1975. An invitrofocus induction assayfor
xeno-tropic
murine leukemia virus, feline leukemia virus C and thefeline-primate
viruses RD-114/CCC/M-7.Virology67:288-291.27. Price, J.,D.Turner,and C.Cepko. 1987.Lineage analysisin the vertebrate nervous system byretrovirus-mediatedgene transfer. Proc. Natl. Acad. Sci. USA 84:156-160.
28. Reddehase,M.J.,W.Mutter,K.Munch,H.-J.Buhring,and U. H.
Koszinowski. 1987.CD8-positiveTlymphocytes specificfor
mu-rinecytomegalovirus immediate-earlyantigensmediateprotective
immunity.J. Virol. 61:3102-3108.
29. Reusser,P., S. R. Riddell,J. D. Meyers,and P. D. Greenberg.
1991.Cytotoxic T-lymphocyte response tocytomegalovirus after humanallogeneic bonemarrowtransplantation:patternof
recov-ery and correlation with cytomegalovirus infection and disease. Blood 78:1373-1380.
30. Rook,A.H.,H. C.Lane,T.Folks,S.McCoy,H.Alter,and A. S. Fauci. 1987. Sera fromHTLV-III/LAVantibody-positive individ-uals mediate antibody dependent cellular cytotoxicity against
HTLV-IIIILAV-infected T cells. J. Immunol. 138:1064-1067. 31. Rouse, R.T., S. Norley, and S. Martin. 1988. Anti-viralcytotoxicT
lymphocyte induction and vaccination. Rev. Infect. Dis. 10:16-33. 32. Sharp, R. M., C. L. Durocher, and J. L. Parmenter. 1990.
Production of baboon (Papio hamadryas)monoclonal antibodies by herpesvirus papio immortalized baboonlymph node cells. J. Immunol. Methods126:287-294.
33. Siliciano, R.F., T. Lawton, C.Knall, R. W.Karr,P.Berman,T. Gregory, and E. L. Reinherz. 1988.Analysis of host-virus interac-tions in AIDS with anti-gpl20 T cell clones: effect of HIV sequencevariation andamechanism for CD4+ celldepletion.Cell
54:561-575.
34. St.Louis, D., and I. M. Verma. 1988. An alternativeapproachto
somaticcell gene therapy. Proc. Natl. Acad. Sci. USA 85:3150-3154.
35. Takahashi, H., J. Cohen, A. Hosmalin, K. B.Cease, R.Houghton,
J. J. Cornette, C. DeLisi, B. Moss, R. N. Germain, and J. A.
Berzofsky. 1988. An immunodominant epitope of the human immunodeficiency virus envelope glycoproteingpl60 recognized
by class I major histocompatibility complex molecule-restricted murine cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 85:3105-3109.
36. Takahashi, H., S. Merli, S. D. Putney, R. Houghton, B. Moss, R. N. Germain, and J. A. Berzofsky. 1989. A single amino acid inter-change yields reciprocal CTL specificities for HIV-1 gpl60.
Sci-ence246:118-121.
37. Tsubota, H., C. I. Lord, D. I. Watkins, C. Morimoto, and N. L. Letvin. 1989. Acytotoxic T lymphocyte inhibits acquired
immu-nodeficiency syndromevirusreplication in peripheral blood
lym-phocytes. J. Exp. Med. 169:1421-1434.
37a.Viagene, Inc. April 1992. U.S. patent application WO92/05266.
38. Walker,B.D.,S.Chakrabarti, B.Moss, T. J. Paradis, T. Flynn, A. G.Durno,R. S.Blumberg,J. C. Kaplan, M. S. Hirsch, and R. T. Schooley. 1987. HIV-specific cytotoxic T lymphocytes in seropos-itiveindividuals.Nature(London)328:345-348.
39. Walker, B. D.,C. Flexner, K. Birch-Limberger, L. Fisher, T.J.
Paradis, A. Aldovini, R. Young, B. Moss, and R. T. Schooley. 1989. Long-term culture and fine specificity of human cytotoxic
T-lymphocyteclones reactive with human immunodeficiencyvirus type 1. Proc. Natl. Acad. Sci. USA 86:9514-9518.
40. Walker,B.D.,C.Flexner,T.J. Paradis, T. C. Fuller, M. S.Hirsch,
R. T.Schooley, and B. Moss. 1988. HIV-1 reverse transcriptase is
a target for cytotoxic T lymphocytes in infected individuals. Science 240:64-66.
41. Warner, J. F.,C.-G.Anderson,L.Laube, D. J. Jolly, K. Townsend, S.Chada,and D.St. Louis. 1991.Induction ofHIV-specificCTL andantibodyresponses in miceusing retroviral vector-transduced cells. AIDS Res. Hum. Retroviruses7:645-655.
42. Zarling, J. M., J. W. Eichberg, P. A. Moran, J. McClure, P.
Sridhar,and S. L.Hu.1987.ProliferativeandcytotoxicTcellsto
AIDS virus glycoproteins in chimpanzees immunized with a recombinant vaccinia virusexpressingAIDSvirusenvelope
glyco-proteins.J. Immunol. 139:988-990.
43. Ziegner, U. H. M., D. J. Jolly, S. J. Mento, J. Galpin, C. E.
Prussak, D. E.Hartnett, C.Bohart,J. R. Barber, G. Peters, W.
Klump, N.
Sajjadi,
B. Merchant, and J. F. Warner. Genetic immunization ofasymptomatic HIV-1-infected patientswithau-tologousretroviralvectortransduced fibroblastsexpressingHIV-1 IIIBenv/revproteins.Submitted forpublication.
J.VIROL.