JOURNAL OFVIROLOGY, May 2005, p. 6358–6367 Vol. 79, No. 10 0022-538X/05/$08.00⫹0 doi:10.1128/JVI.79.10.6358–6367.2005
Copyright © 2005, American Society for Microbiology. All Rights Reserved.
Stimulation of Poliovirus Synthesis in a HeLa Cell-Free In Vitro
Translation-RNA Replication System by Viral Protein 3CD
pro
David Franco,
1Harsh B. Pathak,
2Craig E. Cameron,
2Bart Rombaut,
3Eckard Wimmer,
1and Aniko V. Paul
1*
Department of Molecular Genetics and Microbiology, School of Medicine, Stony Brook University, Stony Brook, New York 117901; Department of Biochemistry and Molecular Biology, Pennsylvania State University,
University Park, Pennsylvania 168022; and Department of Microbiology and Hygiene,
Vrije Universiteit Brussel, B-1090 Brussels, Belgium3
Received 20 August 2004/Accepted 30 December 2004
The plus-strand RNA genome of poliovirus serves three distinct functions in the life cycle of the virus. The RNA is translated and then replicated, and finally the progeny RNAs are encapsidated. These processes can be faithfully reproduced in a HeLa cell-free in vitro translation-RNA replication system that produces viable poliovirus. We have previously observed a stimulation of virus synthesis when an mRNA, encoding protein
3CDpro
, is added to the translation-RNA replication reactions of poliovirus RNA. Our aim in these experiments
was to further define the factors that affect the stimulatory activity of 3CDpro
in virus synthesis. We observed
that purified 3CDpro
protein also enhances virus synthesis by about 100-fold but has no effect on the translation
of the polyprotein. Optimal stimulation is observed only when 3CDpro
is present early in the incubation period.
The stimulation, however, is abolished by a mutation either in the RNA binding domain of 3CDpro
, 3Cpro
R84S/ I86A, or by each of two groups of complementary mutations R455A/R456A and D339A/S341A/D349A at
interface I in the 3Dpol
domain of 3CDpro
. Surprisingly, virus synthesis is strongly inhibited by the addition of
both 3Cpro
and 3CDpro
at the beginning of incubation. We also examined the effect of other viral or cellular proteins on virus synthesis in the in vitro system. No enhancement of virus synthesis occurred with viral
proteins 3BC, 3ABC, 3BCD, 3Dpol
, and 3Cpro
or with cellular protein PCBP2. These results suggest that 3CDpro
has to be present in the reaction at the time the replication complexes are assembled and that both the 3Cpro
and 3Dpol
domains of the protein are required for its activity that stimulates virus production.
The RNA genome of poliovirus (PV), a prototype of Picor-naviridae, is used in three important processes in the viral life cycle: (i) it is an mRNA, which directs the synthesis of a polyprotein; (ii) it serves as a template for RNA synthesis; and (iii) the progeny RNA associates with the nonstructural pro-teins during virus assembly. In the past, several different ap-proaches have been used to elucidate the individual steps in poliovirus replication. The most complex and difficult method involves studying biochemical reactions in the infected cell itself. The second in complexity uses crude replication com-plexes isolated from infected cells to decipher the stages in the replication of the viral RNA (vRNA). The simplest system utilizes purified components to conduct detailed biochemical analyses of individual reactions in RNA synthesis. The disad-vantage of the latter approach is that it has not yet been possible to reconstitute active replication complexes that syn-thesize VPg-linked RNAs of plus and minus polarity or to produce infectious virions. The discovery more than a decade ago that authentic PV can be made de novo in HeLa cell-free translation RNA-replication reactions (22) has provided an important new tool to study individual steps in the life cycle of the virus, except those involving virus attachment, entry into the cell, and uncoating.
The coupled translation-RNA replication system utilizes
ex-tracts from uninfected HeLa cells, which support the transla-tion of input viral RNA, yielding all of the necessary viral proteins (22), the formation of membranous replication com-plexes (3, 4, 11, 23), the uridylylation of VPg (24, 33), the synthesis of plus- and minus-strand RNAs (4, 22), and the encapsidation of the progeny viral RNA (22). Although the in vitro cell-free translation-RNA replication reactions mimic, in large part, the processes observed in virus-infected cells, there are also significant differences between the two systems. The in vitro reactions are programmed with large amounts of viral RNA (⬃0.5 g of RNA or 1 ⫻ 1011 RNA molecules per
reaction) when compared to the RNA of a single or of a few viruses, which are initially replicated in an infected cell. In spite of this, the yield of virus in the in vitro reaction is low com-pared to PV-infected HeLa cells. The low yield of virus in the in vitro system, compared to in vivo, might be attributed to differences in the membranous structures where RNA replica-tion takes place (11) or to a lack of sufficient quantities of active viral proteins for efficient RNA synthesis or encapsida-tion to occur. Particle instability might also contribute to the low titers of infectious virus in the in vitro reactions.
Viral RNA replication in the infected host cell is carried out primarily by the RNA-dependent RNA polymerase 3Dpol in
conjunction with other viral and cellular proteins (reviewed in reference 34). Replication takes place on small vesicles, which are derived from membranes of the host cell and which are associated with the nonstructural proteins of the virus. The incoming viral RNA is first transcribed into a complementary minus strand yielding a double-stranded replicative
intermedi-* Corresponding author. Mailing address: Department of Molecular Genetics and Microbiology, School of Medicine, Stony Brook Univer-sity, Stony Brook, NY 11790. Phone: (631) 9777. Fax: (631) 632-8891. E-mail: apaul@notes.cc.sunysb.edu.
6358
on November 8, 2019 by guest
http://jvi.asm.org/
ate. In the next step, the minus strand is used as a template for the synthesis of the progeny plus strands. In addition to the RNA polymerase, the other viral proteins most likely involved in RNA replication are a small membrane-bound protein, 3A, and its precursor, 3AB, the terminal protein and primer for RNA synthesis VPg, and the multifunctional proteinase 3Cpro/
3CDpro.
Protein 3CDpro, the precursor of both proteinase 3Cproand
RNA polymerase 3Dpol, is derived from the P3 domain of the
poliovirus polyprotein (Fig. 1), primarily by a proteolytic cleav-age at a Q/G cleavcleav-age site between 3CDproand 3AB. 3CDpro
is a multifunctional polypeptide, with roles both in polyprotein processing and in RNA replication. As a proteinase, 3CDprois
required for the processing of the structural precursor domain P1 of the polyprotein (19, 46) and for the cleavage of a cellular protein (36). Its role in replication is related to its ability to bind RNA and to form ribonucleoprotein complexes withcis -acting RNA elements of the viral genome. It forms complexes with the 5⬘-terminal cloverleaf structure of the PV RNA either in the presence of 3AB (16) or in the presence of cellular protein poly(rC) binding 2 protein (PCBP2) (1, 2, 13, 28). The 3CDpro/PCBP2 complex has been proposed to have a role both
in the switch from translation to replication (12) and also in the circularization of the genome (17). The RNA binding activity of 3CDprois also important for an interaction with thecre(2C)
RNA element, during the uridylylation of VPg by 3Dpol (33,
45). Finally, 3CDpro, in a complex with 3AB, interacts with the
3⬘ nontranslated region (NTR)-poly(A) segment of the PV genome (16), but the biological significance of this interaction has not yet been determined.
In the in vitro cell-free system, translation of the polyprotein and processing of the viral proteins are optimal during the first 3 h postincubation, but RNA replication is relatively slow, reaching a maximum between 8 and 9 h (41). The formation of infectious virus reaches a peak after a 12-h incubation period. Since 3CDprohas been speculated to play a role in the switch
from translation to replication (12), we reasoned that at least
one of the reasons for the observed imbalance between trans-lation and RNA synthesis or encapsidation in the in vitro system could be the lack of sufficient quantities of 3CDpro.
Indeed, when we cotranslated 3CDpro mRNA with the viral
RNA (vRNA) template, we observed a 100-fold increase in virus yield compared to reactions in which only the template RNA was added (41). However, under the same conditions, only a 3-fold enhancement of viral RNA synthesis by 3CDpro
mRNA was observed (41), which we attributed to an imbal-ance between translation and replication in vitro. An alternate explanation of the findings is that the large increase in virus yield is due the effect of 3CDproon an additional step or steps
of the viral life cycle.
The aim of this study was to further define how 3CDpro
enhances virus yield in the in vitro cell-free translation-RNA replication reactions programmed with viral RNA. We have now shown that 3CDproprotein can stimulate virus production
just as well as the translation products of its mRNA. Our results suggest that the RNA binding activity of 3CDpro is
required for its stimulatory activity and that both the 3Cproand
3Dpol domains of the protein are required for function. In
addition, we have tested several mature and precursor proteins of the P3 domain of the viral polyprotein and cellular protein PCBP2 and found that they all lacked the ability to stimulate virus synthesis in vitro.
MATERIALS AND METHODS
Cells and viruses.HeLa R19 cell monolayers and suspension cultures of HeLa S3 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal bovine calf serum. Poliovirus was amplified on HeLa R19 cells as described before. The infectivity of virus stocks was determined by plaque assays on HeLa R19 monolayers as described by Lu et al. (21).
Preparation of poliovirus RNA.Virus stocks were grown and they were puri-fied by CsCl gradient centrifugation (21). Viral RNA was isolated from the purified virus stocks with a 1:1 mixture of phenol and chloroform. The purified RNA was precipitated by the addition of 2 volumes of ethanol.
Preparation of HeLa cytoplasmic extracts.HeLa S10 extracts were prepared as previously described (7, 22), except for the following modifications: (i) packed
FIG. 1. Genomic structure of poliovirus and processing of the P3 domain of the polyprotein. The single-stranded RNA genome of poliovirus is shown with the terminal protein VPg attached to the 5⬘end of the RNA and the poly(A) tail at the 3⬘end. The 5⬘NTR and the 3⬘NTR are shown with single lines. The polyprotein (open box) contains structural (P1) and nonstructural (P2, P3) domains that are cleaved into individual polypeptides. Processing of the P3 domain by proteinase 3Cpro/3CDprois shown enlarged.
VOL. 79, 2005 STIMULATION OF PV SYNTHESIS BY VIRAL PROTEIN 3CDpro 6359
on November 8, 2019 by guest
http://jvi.asm.org/
cells from 2 liters of HeLa S3 cells were resuspended in 0.8 to 1.0 volume (relative to packed cell volume) of hypotonic buffer; (ii) the final extracts were not dialyzed.
Translation-RNA replication reactions with HeLa cell extracts and plaque assays.Viral RNA (500 ng) was translated at 34°C in the presence of unlabeled
methionine, 200M each CTP, GTP, UTP, and 1 mM ATP in a total volume of
25l (22, 23). After incubation for 12 to 15 h, the samples were diluted with
phosphate-buffered saline and were added to HeLa cell monolayers (22, 23). Virus titers were determined by plaque assay, as described previously (22, 23).
Preinitiation RNA replication complexes.Preinitiation RNA replication com-plexes were prepared as described previously (3), except for some minor modi-fications. Translation-RNA replication reactions lacking initiation factors were incubated for 4 h at 34°C in the presence of 2 mM guanidine HCl. The complexes
were isolated by centrifugation, resuspended in 50l HeLa S10 translation-RNA
replication reaction mixture without viral RNA, and incubated for 11 h at 34°C.
Proteins.The following proteins with a C-terminal His tag were expressed in
Escherichia coliand purified by nickel column chromatography (QIAGEN): (1)
cellular protein PCBP2 (28); poliovirus proteins 3CDpro(3CproH40A) (33),
3Cpro
(C147G) (31), 3CDpro
(3Cpro
H40G; 3Dpol
D339A/S341A/D349A) (31), and
3ABC(3CproH40A) (A. Paul and E. Wimmer, unpublished results). The
expres-sion inE. coliof poliovirus 3BC(3Cpro
C147G) and 3BCD(3Cpro
C147G) with a
C-terminal His tag, and of untagged 3CDpro(C147G) will be described elsewhere
(H. B. Pathak and C. E. Cameron, unpublished results). The expression and
purification of 3CDpro(3CproH40G; 3DpolR455A, R456A) was described
previ-ously (31). Poliovirus protein 3Dpol
was expressed in E. colifrom plasmid
pT5T3D and was purified as described before (30).
Construction of plasmids.Poliovirus sequences were derived from plasmid pT7PVM, which contains the full-length (nucleotides 1 to 7525) plus strand poliovirus cDNA sequence (40). All constructs were sequenced to ensure their accuracy.
(i) pLOP315ser.Plasmid pLOP315ser contains the 3CDpro
coding sequence preceded by a translation start codon and the T7 promoter sequence (41). It was digested with EcoRI prior to transcription by T7 RNA polymerase.
(ii) pLOP315ser(3CproR84S/I86A).An EcoRI-to-BglII fragment of pT7PVM
(3Cpro
R84S/I86A) was ligated into similarly restricted pLOP315ser. The plasmid was digested with EcoRI and was transcribed by T7 RNA polymerase.
Transcription and in vitro translation.All plasmids were linearized with EcoRI prior to transcription by T7 RNA polymerase. The transcript RNAs were purified by phenol-chloroform extraction and ethanol precipitation. Translation
reactions (25l) containing 8.8Ci of35S-TransLabel (ICN Biochemicals) were
incubated for 12 h at 34°C (22, 23). The samples were analyzed by electrophore-sis on sodium dodecyl sulfate-12% polyacrylamide gels, followed by autoradiog-raphy.
RESULTS
We have previously shown that the addition of 3CDpro
mRNA to translation-RNA replication assays, programmed with viral RNA, results in about 100-fold stimulation of virus production (41). The aim of the experiments presented here was to extend those studies and to obtain further information about the mechanism by which 3CDproexerts its function.
3CDpro
mRNA stimulates virus production in the in vitro
translation-RNA replication system. In this study, we
con-firmed our previous observation (41) that the addition of 3CDpromRNA along with viral RNA at the time translation
commences increases the virus yield about 100-fold from 6⫻
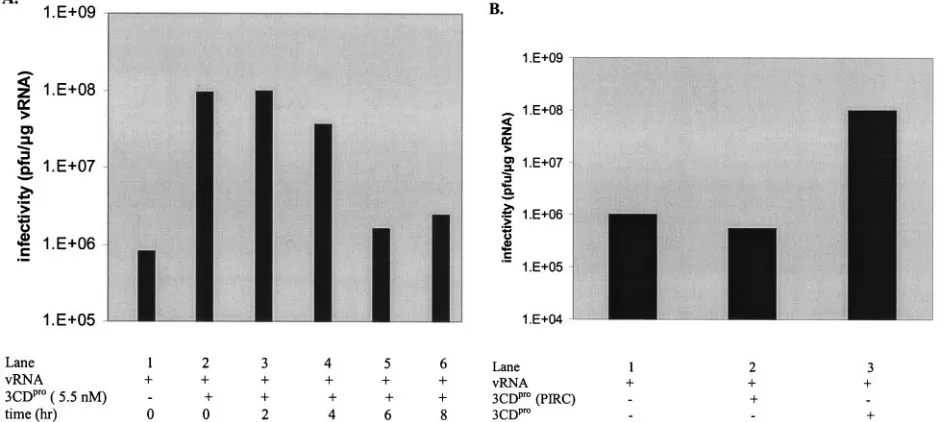
FIG. 2. Stimulation of virus production in the in vitro translation-RNA replication system by 3CDpro. In vitro translation-RNA replica-tion reacreplica-tions and plaque assays were carried out as described in Materials and Methods. Where indicated, 3CDpromRNA or purified 3CDpro(3CproH40A) protein was added to the translation reactions. (A) Effect of 3CDpromRNA concentration. The amount of 3CDpro mRNA added to the translation reactions att⫽0 h is indicated in the figure. (B) Effect of purified 3CDpro protein concentration. The amount of purified 3CDpro(3CproH40A) protein added to the transla-tion reactransla-tions att⫽0 h was varied as indicated in the figure. From three different experiments, the average value for the stimulation of virus synthesis by 3CDprowas 130-fold. (C) Comparison of the stimu-latory activities of 3CDpromRNA and purified 3CDpro(3CproH40A) protein. Virus production was measured with optimal concentrations of either 3CDpromRNA (1.4g/ml) or 3CDpro(3CproH40A) protein (5.5 nM) added to the translation-RNA replication reactions.
6360 FRANCO ET AL. J. VIROL.
on November 8, 2019 by guest
http://jvi.asm.org/
105PFU/g RNA (1.2⫻107PFU/ml) (Fig. 2A, column 1) to
1⫻108PFU/g RNA (2⫻ 109PFU/ml) (Fig. 2A, columns
4 to 7). The extent of stimulation is strongly dependent on the concentration of 3CDpromRNA used, with an optimal range
between 1.4 to 11.2g RNA/ml of reaction. As the RNA con-centration is increased further, first the extent of stimulation is reduced and then the virus production is inhibited to a level below the value obtained in the absence of 3CDpro(Fig. 2A,
compare column 1 with columns 8 and 9).
Purified 3CDpro
protein enhances the virus yield in
trans-lation-RNA replication reactions.The enhancement of virus
synthesis in the in vitro translation-RNA replication assay can be achieved not only by the cotranslation of 3CDpro
from an mRNA but also by the addition of purified 3CDpro
protein, regardless of whether it carries a C-terminal His tag (3CDpro3CproH40A) (Fig. 2B) or is untagged (3CDpro
3CproC147G) (data not shown). In both cases, the proteolytic
activity of 3CDpro is inactivated through a mutation, as
indi-cated. The extent of stimulation is dependent on protein con-centration, and optimal stimulation is reached at 300 to 400 ng/ml (4.5 to 5.5 nM) 3CDpro (Fig. 2B). At their optimal
concentrations, purified 3CDpro(3CproH40A), just like 3CDpro
mRNA, leads to a 100-fold increase in virus yield (Fig. 2C, compare column 1 with columns 2 and 3). These results con-firm our previous finding that translation of the 3CDpromRNA
to yield the corresponding polypeptide is required for its en-hancing activity (41). The 3CDpro(3CproH40A) protein used in
these experiments had no effect on the overall translation or processing of the viral polyprotein (see Fig. 7, lane 2).
It should be noted that, with a typical HeLa cell extract, the yield of virus varies between different experiments from 5⫻ 105to 5⫻106PFU/g RNA, which corresponds to 1⫻107to
1⫻108PFU/ml. In reactions supplemented with optimal
con-centrations of either 3CDpro mRNA or purified protein, the
fluctuation in virus yield is less pronounced. It is consistently in the range of 8⫻107to 1.2⫻108PFU/g RNA (1.6⫻109to
2.4 ⫻ 109 PFU/ml). Therefore the extent of stimulation by
3CDprovaries from 20- to 200-fold. Because of the relatively
large variation in virus yield in reactions lacking extra 3CDpro,
we have included such a control in all of our experiments. The data shown on all figures represent an average of two or more experiments.
3CDpro
has to be added early to retain its optimal enhancing
activity in virus synthesis.In an effort to determine the time at
which 3CDpro has to be added to the reactions to exert its
stimulatory function during the growth cycle of the virus, we have tested the effect of adding purified 3CDpro(3CproH40A)
protein at various times after the reactions were incubated. As shown in Fig. 3A, 3CDpro(3CproH40A) can be added either at
0 h or 2 h of incubation to retain its optimal enhancing activity in virus production (compare column 1 with columns 2 and 3). If added at 4 h, the stimulatory effect of 3CDpro is already
somewhat diminished (Fig. 3A, compare columns 2 and 3 with column 4), and if added at 6 or 8 h of incubation, the enhance-ment is nearly completely abolished (Fig. 3A, compare col-umns 2 and 3 with colcol-umns 5 and 6).
3CDpro
does not stimulate virus synthesis when added to
isolated preinitiation replication complexes.Addition of
[image:4.585.57.526.74.285.2]gua-nidine HCl, a reversible inhibitor of poliovirus replication, to in vitro translation-RNA replication reactions completely in-hibits RNA synthesis (22). By supplementing such reactions with this drug, it is possible to isolate preinitiation replica-tion complexes (3, 4). This drug blocks the initiareplica-tion of minus-strand RNA synthesis but has no effect on poliovirus
FIG. 3. 3CDprohas to be added early to the RNA replication reactions to exert optimal stimulatory activity. In vitro translation-RNA replication reactions and plaque assays were carried out as described in Materials and Methods. Purified 3CDpro(3CproH40A) protein was added to the reactions at the indicated times. (A) Effect of time of 3CDproaddition. The time at which 3CDpro(H40A) protein (5.5 nM) was added to the translation reactions was varied as indicated. (B) Addition of purified 3CDpro(3CproH40A) protein to preinintiation replication complexes. Preinitiation replication complexes (PIRC) were made as described in Materials and Methods. The complexes were resuspended in either the absence (column 1) or presence (column 2) of purified 3CDpro(3CproH40A) (5.5 nM). In column 3, 3CDpro(3CproH40A) (5.5 nM) was added at
t⫽0 h before the formation of the PIRC.
VOL. 79, 2005 STIMULATION OF PV SYNTHESIS BY VIRAL PROTEIN 3CDpro 6361
on November 8, 2019 by guest
http://jvi.asm.org/
polyprotein translation and processing. Upon the removal of guanidine, the synthesis of negative-strand RNA is initiated in a synchronous manner followed by the production of plus-strand RNA (3, 4). To evaluate the effect of 3CDproon virus
synthesis by preinitiation replication complexes, we have car-ried out the translation of vRNA in the presence of 2 mM guanidine HCl for 4 h. The complexes were isolated by cen-trifugation, the pellets were resuspended in HeLa extracts, and the incubation was continued for 11 h. When purified 3CDpro
(3CproH40A) protein was added at the beginning of the
incu-bation, the yield of virus was 100-fold higher than in reactions which contained no extra 3CDpro(Fig. 3B, compare column 1
with column 3). On the other hand, the addition of 3CDpro
(3CproH40A) to the resuspended preinitiation complexes had
no effect on the production of infectious virions (Fig. 3B, compare column 1 with column 2). These results, as well as those presented in Fig. 3A, suggest that 3CDpro has to be
present in the reaction at an early time point in the viral life cycle so that it can be incorporated into replication complexes that are subsequently required for RNA replication and/or encapsidation.
RNA binding by 3CDpro
is required for its stimulatory
ac-tivity in virus synthesis.The 3CDpro polypeptide is the
pre-cursor of both proteinase 3Cpro and the RNA polymerase
3Dpol. Although both the proteinase and RNA binding
se-quences of 3CDproreside in the 3Cprodomain, the two
pro-teins differ greatly with respect to these activities. In function-ing as a proteinase, only 3CDproand not 3Cprohas the ability
to cleave the structural precursor P1 (19, 46). When function-ing as an RNA bindfunction-ing protein at the cloverleaf, in concert with PCBP2 (13, 28), or with 3AB (16), 3CDpro has an
en-hanced ability to form a functional ribonucleoprotein complex over 3Cpro. The stimulatory activity of 3CDproin virus
produc-tion in the in vitro translaproduc-tion-RNA replicaproduc-tion reacproduc-tions is not
due to its proteolytic activity. As pointed out before, the puri-fied protein used in our experiments is inactive as a proteinase due to a mutation (3CproH40A) in its active site. To test the
possibility that the RNA binding activity of 3CDprois required
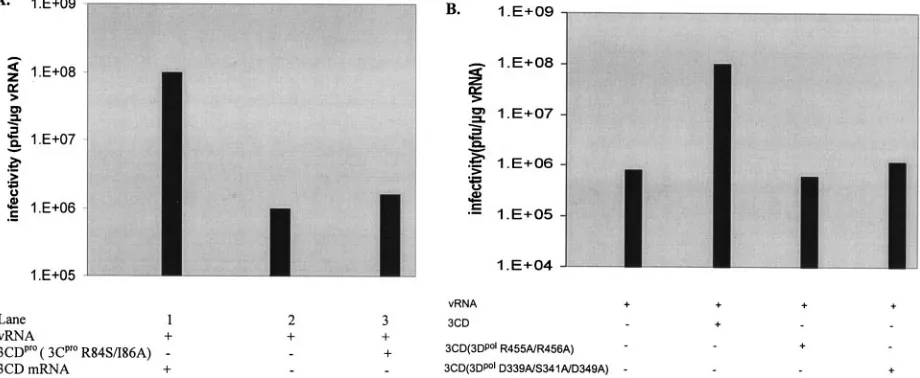
for its enhancing function, we mutated the RNA binding do-main of the protein (3CproR84A/I86A) (5, 14). As shown in
Fig. 4A, translation of 3CDpro(3CproR84A/I86A) mRNA along
with the viral RNA template had no significant stimulatory effect on virus production (compare column 2 with column 3). This indicates that the RNA binding domain of 3Cpro, in the
context of 3CDpro, is required for the enhancing function of
the protein.
The 3Dpol
domain of 3CDpro
is also important for its
stim-ulatory activity in virus synthesis.It has been previously shown
that the 3Dpoldomain of 3CDpromodulates both the
protein-ase and RNA binding activities of the protein (6, 29). The RNA polymerase itself is a multifunctional protein, which has two types of synthetic activities. It has the ability to elongate RNA primers on suitable templates (10) and to covalently link UMP to the hydroxyl group of a tyrosine in VPg (33). The crystal structure for 3Dpolrevealed that this enzyme possess a
typical nucleic acid polymerase structure containing a palm, thumb, and finger subdomains (15). A considerable amount of evidence has accumulated thus far suggesting that 3Dpol
oli-gomerizes and that the oligomeric forms of the protein are important for function (18, 30). Two charged amino acids, R455 and R456 (8), located in the thumb domain along inter-face I interact with D339/S341/D349 in the palm subdomain of another polymerase molecule, and this interaction is essential for stability along interface I (31). It was recently shown that the R455A/R456A mutations in the thumb domain of 3Dpol
strongly reduce the ability of the polymerase to catalyze 3CDpro-stimulated uridylylation of VPg on the cre(2C)
[image:5.585.63.523.75.264.2]ele-ment but the 3Dpolpalm mutations have no detrimental effect
FIG. 4. Mutations both in the 3Cproand 3Dpoldomains of 3CDproaffect the stimulation of virus synthesis. In vitro translation-RNA replication reactions and plaque assays were carried out as described in Materials and Methods. (A) Either wild-type or mutant 3Cpro(R84S/I86A) 3CDpro mRNAs (1.4g/ml) were added to the reactions att⫽0 h as indicated. From three different experiments, the average value for the stimulation of virus synthesis by 3CDpro(3CproR84S/I86A) was 1.3-fold. (B) CDpro(3CproH40A), 3CDpro(3CproH40G; 3DpolR455A/R456A), or 3CDpro (3CproH40G; 3DpolD339A/S341A/D349A) purified proteins (5.5 nM) were added to the reactions att⫽0 h as indicated. From two different ex-periments, the average value for the stimulation of virus synthesis by 3CDpro(3CproH40G/3DpolR455A/R456A) protein was 1.0-fold and by 3CDpro (3CproH40G; 3DpolD339A/S341A/D349A) it was 1.4-fold.
6362 FRANCO ET AL. J. VIROL.
on November 8, 2019 by guest
http://jvi.asm.org/
on the reaction (31). Interestingly, in the context of 3CDpro,
neither of these groups of mutations in 3Dpolaltered the ability
of the protein to stimulate the uridylylation reaction in vitro (31). However, about 2-fold-higher concentrations of the mu-tant proteins were required to achieve optimal RNA binding of acre(2C) RNA probe in vitro than what was observed with the wild-type 3CDproprotein (Pathak and Cameron, unpublished
results). In addition, the thumb mutant 3CDproproteinase
ex-hibited a modest reduction in the processing of the VP0/VP3 precursor in in vitro translation reactions of mutant genomic transcript RNAs (31). To determine whether the 3CDpro
pro-tein, carrying the 3DpolR455A/R456A mutations, possesses
normal stimulating activity of virus production in the in vitro system, we have compared its enhancing activity to that of 3CDpro(3CproH40A). As shown in Fig. 4B, there is no
stim-ulation of virus production in the presence of the mutant 3CDpro(3DpolR455A/R456A) protein (compare column 1 with
column 3), while there is a 100-fold enhancement of virus yield when 3CDpro(3CproH40A) is added to the reaction (compare
column 1 with column 2). These results suggest that the integ-rity of interface I in 3Dpol, in the context of 3CDpro, is
impor-tant for the protein’s stimulatory activity. If this is true, then one would expect that 3CDprocarrying the palm mutations
D339A/S341A/D349A in the 3Dpol domain, would exhibit
the same phenotype as the thumb mutant protein 3CDpro
(3DpolR455A/R456A). This hypothesis was confirmed by the
finding that 3CDprocarrying the 3Dpolpalm mutations D339A/
S341A/D349A also lacks stimulatory activity in virus
produc-tion (Fig. 4B, compare column 1 with column 4). It should be noted that increasing the concentration of the mutant 3CDpro
polypeptides from the standard concentration (5.5 nM) to 11 nM and 22 nM did not enhance virus synthesis (data not shown). Since not only 3CDpro(3CproH40A) but also both
mu-tant 3Dpol-3CDpropolypeptides contain a mutation (H40G) in
the proteinase 3Cproactive site, the observed differences
can-not be due to the altered proteolytic properties of the proteins. Effect of other viral and cellular proteins on the enhance-ment of virus production in the in vitro translation-RNA
rep-lication system.Protein 3CDprois the precursor of both
pro-teinase 3Cproand the RNA polymerase 3Dpol. Our finding
that the RNA binding activity of 3CDprois required for its
enhancing properties and the fact that its RNA binding site is located in the 3Cprodomain suggested to us the possibility
that 3Cpro or some of its precursors (3ABC, 3BC, 3BCD)
might also stimulate virus synthesis in the in vitro transla-tion-RNA replication assay. Surprisingly, the addition of com-parable amounts of purified 3Cpro(C147G), 3BC (3CproC147G),
3BCD(3CproC147G), or 3ABC(3CproH40A) proteins to the in
vitro translation-RNA replication reactions did not lead to an increase in viral yield (Fig. 5A). None of the proteins had any effect on the translation of the viral RNA template or process-ing of the polyprotein (data not shown).
[image:6.585.49.540.77.308.2]Cellular protein PCBP2 plays a role in both PV translation initiation and in viral RNA synthesis (42). This protein specif-ically interacts with two domains of the poliovirus 5⬘NTR, the cloverleaf structure, and domain IV of the internal ribosome
FIG. 5. Effect of 3ABC, 3BC, 3BCD, 3Cpro, and PCBP2 on virus production in the RNA replication system. In vitro translation-RNA replication reactions and plaque assays were carried out as described in Materials and Methods. Purified viral protein was added (t⫽0 h) to the translation-RNA replication reactions (40 ng/ml and 400 ng/ml). The effect of each protein on virus synthesis was tested at least twice, and the data shown are an average of the two experiments. (A) The effect of 3ABC, 3BC, 3BCD, or 3Cproon virus production. Column 2, 3CDpro (3CproH40A), 5.5 nM; columns 3 and 4, 3ABC(3CproH40A), 2.5 nM and 12.5 nM, respectively; columns 5 and 6, 3Cpro(C147G), 2 nM and 20 nM, respectively; columns 7 and 8, 3BC(3CproC147G), 1.8 nM and 18 nM, respectively; columns 9 and10, 3BCD(3CproC147G), 0.54 nM and 5.4 nM, respectively. (B) Effect of adding PCBP2 with or without 3CDproon virus production. Purified PCBP2 and/or purified 3CDpro(3CproH40A) was added as indicated in the figure.
VOL. 79, 2005 STIMULATION OF PV SYNTHESIS BY VIRAL PROTEIN 3CDpro 6363
on November 8, 2019 by guest
http://jvi.asm.org/
entry site (13, 28). Since PCBP2 is known to bind to the cloverleaf structure as a complex with 3CDpro (2), we have
tested the possibility that the addition of both proteins to-gether to the in vitro translation-RNA replication reactions would result in even further stimulation of virus production. We observed that when PCBP2 was added att⫽0 h to the translation-RNA replication reactions alone, at concentrations of 1 nM to 100 nM, it had no effect on virus yield (Fig. 5B, compare column 1 with column 4, and data not shown). How-ever, when added together with 3CDproat 100 nM (⬃20-fold
molar excess over 3CDpro), PCBP2 totally blocked the
stimu-latory activity of 3CDpro (Fig. 5B, compare column 1 with
columns 2 and 3). Exogenously added PCBP2 at the same concentrations had no effect on the translation of the viral RNA or processing of the polyprotein (data not shown). A possible explanation of these observations is that the added 3CDprois sequestered with PCBP2 either at the 5⬘cloverleaf in
a ternary complex (13, 28) or in a complex with poly(A) bind-ing protein (PABP) (17) and that one or both of these com-plexes lack stimulatory activity in virus production.
Protein 3Cpro
inhibits the enhancing activity of 3CDpro
in
virus production.Since 3CDprois the precursor of both 3Cpro
and 3Dpol, we have tested the possibility that supplying the
translation-RNA replication reactions with the two mature cleavage products, instead of the precursor, would also en-hance virus production. However, no stimulation of virus syn-thesis can be achieved by adding to the in vitro reactions purified 3Dpoland 3Cpro together (Fig. 6, compare column 1
with column 6). Neither of the two proteins when added alone to the reactions has an effect on virus yield (Fig. 6, compare column 1 with columns 7 and 8). Surprisingly, when protein 3Cprois included in the reactions along with 3CDpro, there is a
striking inhibition of virus production (Fig. 6, compare column 1 with column 4). Indeed, the yield of infectious virions in the reactions is reduced about 100-fold when compared to reac-tions to which no 3CDpro was added. The reason for the
in-hibitory activity of 3Cprois not yet understood, but it is likely
related to a competition between the two proteins for some RNA sequence/structure that is required for replication and/or encapsidation. The fact that 3Cpro by itself has no effect on
virus yield suggests that it is not able to compete for that RNA sequence/structure with 3CDpro that is made incisfrom the
input viral RNA. Neither 3Cpro alone, nor a combination of
3Cpro with 3CDpro(3CproH40A), has any detectable effect on
the translation and processing of the viral polyprotein (Fig. 7, compare lane 1 with lanes 5 and 6). The translation reactions shown in Fig. 7 were incubated for 12 h at 34°C, but we also obtained the same results at earlier time points of incubation (data not shown).
We have already shown in Fig. 3A that 3CDpro(H40A)
pro-tein has to be added to the translation-RNA replication reac-tions early in the incubation to retain its optimal stimulatory activity. To determine whether 3Cpro also has to be added to
the reactions early in the incubation period to inhibit the stim-ulatory function of 3CDpro, we used preinitiation replication
complexes. Table 1 shows that when 3Cpro and 3CDpro are
added together att⫽0 h in the presence of guanidine HCl, followed by the isolation of preinitiation replication complexes and resuspension in HeLa extracts, virus yield is strongly in-hibited. Note that in this case the polypeptides were added
while the viral RNA was translated in the presence of guani-dine HCl. On the other hand, if 3CDprois added att⫽0 h but
3Cprois added at the time the isolated preinitiation complexes
are resuspended, it has no effect on the stimulatory activity of 3CDpro on virus production (Table 1). These results confirm
our previous finding that, for exogenously added 3CDpro to
possess the ability to stimulate virus production, it already has to be present in the reactions at the time the replication com-plexes are assembled.
DISCUSSION
In this study, we have confirmed and extended our previous work on the stimulation of virus production by 3CDpromRNA
in the in vitro cell-free translation-RNA replication system, programmed by viral RNA. Our finding that purified 3CDpro
stimulates virus yield just as well as the addition of its mRNA proves that the function of 3CDprooccurs at the level of
pro-tein rather than RNA. Since the addition of 3CDproto the in
vitro reactions has no effect on the translation of viral proteins from the input RNA and on the processing of the polyprotein, we conclude that the enhancing activity of this protein is in-volved at a later stage of the viral growth cycle, such as RNA replication, encapsidation, or both. It should be noted, how-ever, that the protein has to be added to the reactions during the first 2 to 4 h postincubation, which is the time of optimal translation, to retain its maximal stimulatory activity. In addi-tion, after the replication complexes have been formed from the newly made viral proteins in the presence of 2 mM guani-dine HCl, 3CDproloses its ability to stimulate virus production.
These observations suggest that to be fully active the
exog-FIG. 6. Effect of adding 3CDprotogether with 3Cproand/or 3Dpol on virus production in translation-RNA replication reactions. In vitro translation-RNA replication reactions and plaque assays were carried out as described in Materials and Methods. Purified 3CDpro (3CproH40A), 3Dpol, or 3Cpro(C147G), each at 400 ng/ml, was added to the reactions att⫽0 h as indicated in the figure. The molar concentration of 3CDpro(3CproH40A) was 5.5 nM, that of 3Cpro(C147G) was 20 nM, and that of 3Dpolwas 7.7 nM. The average value obtained from three different experiments for the effect of 3Cpro on virus synthesis was 1.0-fold; the inhibitory effect of 3Cproand 3CDproadded together was 60-fold.
6364 FRANCO ET AL. J. VIROL.
on November 8, 2019 by guest
http://jvi.asm.org/
[image:7.585.304.541.70.253.2]enously added 3CDprohas to be present in the reactions at the
time the replication complexes are assembled.
3CDprofunctions in the viral growth cycle both as a
protein-ase and as an RNA binding protein. In an effort to elucidate the mechanism by which 3CDprostimulates virus synthesis,
we have mutated the RNA binding site of the protein (3Cpro
R84S/I86A) (5, 14) and showed that this abolished its stimu-latory activity. Since the purified 3CDpro we use in these in
vitro reactions is proteolytically inactive, its stimulatory activity is likely to be, at least in part, due to its RNA binding prop-erties. Interaction of 3CDprowith RNA is known to be
impor-tant for RNA replication, minimally at two different locations within the poliovirus genome. These are the cloverleaf (1, 2, 13, 28, 35) and thecre(2C) element (33, 45). 3CDproalso binds
to the 3⬘ NTR-poly(A) region of the RNA genome, but the relevance of this interaction for RNA replication has not yet been demonstrated (16). Whether the binding of any of these RNA sequences/structures by 3CDprois a prerequisite for the
stimulation of virus synthesis in the in vitro system is not yet known. The observation that 3Cpro is inactive in the
stimula-tion but together with 3CDprostrongly inhibits virus
produc-tion might be explained by a competiproduc-tion between these two proteins for the same essential RNA sequence/structure either alone or in a complex with other proteins during RNA repli-cation and/or encapsidation. The fact that both 3Cpro and
3CDproform a ribonucleoprotein complex at the cloverleaf (2),
but only the latter is biologically relevant, suggests the possi-bility that interaction with the cloverleaf is at least one of the steps involved in the stimulatory activity of 3CDpro in virus
production. In contrast to the 5⬘cloverleaf, thecre(2C) RNA element binds 3Cpro and 3CDpro equally well (45) and both
proteins stimulate VPg uridylylation in vitro (31), suggesting that this reaction is not likely a process involved in the en-hancement of virus production by 3CDpro.
The observation that 3Dpol and 3Cpro individually cannot
replace 3CDproin its stimulatory properties indicates that the
3Dpoldomain of 3CDprois also required for its function. This
conclusion was confirmed by the observation that two 3CDpro
proteins, each containing mutations in the 3Dpol domain
(R455A/R456A or D339A/S341A/D349A), possess very little, if any, enhancing activity in virus production in the in vitro system. Interestingly, about 2-fold-higher concentrations of the mutant 3CDpro proteins were required to achieve optimal
RNA binding in vitro than with the wild-type protein. How-ever, in our experiments a similar increase in mutant 3CDpro
protein concentration did not enhance the stimulation of virus synthesis in vitro, suggesting that the inability of mutant pro-teins to stimulate virus synthesis is not simply due to their reduced RNA binding activities. It should be noted that these two mutations disrupt oligomerization of 3Dpolalong interface
I; hence, it is possible that an interaction of 3CDpromolecules
or of 3CDprowith 3Dpolis required for the stimulatory activity
of the protein. However, in the yeast two-hybrid system, 3CDpro homopairs or 3CDpro and 3Dpol exhibit only weak
interactions (44). The observation that neither the palm nor the thumb 3Dpolmutant, in the context of 3CDpro, is defective
in enhancing VPg uridylylation in vitro (31), but they lack stimulating activity in virus synthesis, further confirms our con-clusion that thecre(2C) templated uridylylation reaction is not affected by the exogenously added 3CDpro.
The primary processing of the P3 domain of the polyprotein yields 3AB and 3CDpro, while alternate minor processing
cas-cades produce 3ABC and 3Dpolor 3A and 3BCD (20). None of
the other viral proteins tested, which are potential precursors of 3Cpro(3BC, 3BCD, 3ABC), possess the ability to stimulate
virus production in the in vitro translation-RNA replication reactions. It should be noted that 3ABC and 3BC are normally not detectable in PV-infected HeLa cell lysates (26) or in vitro translation reactions (22), but the possibility cannot be ex-cluded that the reason for this is their short half-life. 3BCD can
[image:8.585.56.276.71.325.2]FIG. 7. In vitro translation of vRNA is not affected by the addition of 3CDproor 3Cpro. In vitro translation reactions of viral RNA were incubated for 12 h at 34°C, and the products were analyzed (see Materials and Methods). Purified proteins (400 ng/ml) or mRNA (1.4 g/ml) was added to the reactions att⫽0 h. Lane 1, vRNA; lane 2, 5.5 nM 3CDpro(3CproH40A) protein; lane 3, 3CDpro(3CproR84S/I86A) mRNA; lane 4, 5.5 nM 3CDpro(3CproH40G;3DpolR455A/R456A) pro-tein; lane 5, 20 nM 3Cpro(C147G) protein; lane 6, 5.5 nM 3CDpro (3CproH40A) and 20 nM 3Cpro(C147G) proteins.
TABLE 1. Effect of adding 3Cpro(C147G) to preinitiation replication complexesa
Effect on vRNA at time shown
Infectivity
(PFU/g vRNA)
3CDpro
(0 h)
3Cpro
0 h 6 h
⫺ ⫹ ⫺ 2⫻106
⫺ ⫺ ⫺ 1⫻106
⫹ ⫺ ⫺ 1⫻108
⫹ ⫹ ⫺ 1⫻104
⫹ ⫺ ⫹ 9⫻107
aPreinitiation complexes were made from reactions incubated in either the
absence or presence of 3CDpro(3CproH40A) (400 ng/ml, 5.5 nM) for 4 h (see
Materials and Methods). 3Cpro(C147G) (400 ng/ml, 20 nM) was added either at
t⫽0 h or at two hours after resuspension of the complexes (t⫽6 h).
VOL. 79, 2005 STIMULATION OF PV SYNTHESIS BY VIRAL PROTEIN 3CDpro 6365
on November 8, 2019 by guest
http://jvi.asm.org/
be observed both in vivo and in vitro in small amounts, partic-ularly during the early hours of translation of poliovirus RNA (32). In other picornaviruses, such as encephalomyocarditis virus (EMCV) or hepatitis A virus (HAV), 3ABC is one of the major processing products (27, 37). The exact functions, if any, of these large precursors are not yet known. Recent observa-tions, however, suggest that 3BC can substitute for VPg as a substrate for 3Dpolin the uridylylation reaction (C. Cameron
et al., unpublished results). Cellular protein PCBP2, which is known to form a complex with 3CDproand bind to the
clover-leaf (2, 13, 28), also has no effect on virus yield in the in vitro translation-RNA replication system, but at high concentra-tions, it inhibits the stimulatory activity of the exogenously added 3CDpro. This result might be related to the sequestering
of the exogenously added 3CDprointo a complex with PCBP2
at the cloverleaf (13, 28) or with PABP (17) that possesses no stimulatory activity in virus synthesis.
It has been previously reported that poliovirus RNA repli-cation in vivo requires protein translation in cisthrough an internal region of the genome (25). Other studies, in addition, showed that the formation of the poliovirus replication com-plex in vivo requires coupled viral translation, vesicle produc-tion, and viral RNA synthesis (9). These results can be inter-preted to mean that during virus infection the proteins translated from the input viral RNA are essentially the only ones that assemble and form the replication complex. This conclusion is in agreement with other mutational studies that showed that mutations in nonstructural proteins couldn’t be efficiently complemented intrans, or if they were, they repre-sented only certain activities of a multifunctional protein (38, 39, 43). The reason for this might be the formation of a tightly enclosed replication complex incis, which sequesters its com-ponents and prevents their exchange with proteins located on the outside (9). Our studies suggest that, in the vitro transla-tion-RNA replication system, one or more functions of 3CDpro
can be provided intrans.
The HeLa cell-free translation-RNA replication system of-fers an easy way to investigate those steps in the life cycle of poliovirus, which include the synthesis and processing of the polyprotein, RNA replication, and encapsidation. In these ex-periments, we have analyzed the factors that affect the stimu-latory properties of 3CDpro in virus production. We are now
extending these studies to determine the effect extra 3CDpro
directly on minus- and plus-strand RNA synthesis and on en-capsidation (unpublished data).
ACKNOWLEDGMENTS
We thank K., Kirkegaard for plasmid pT5T3D and B. L. Semler for the PCBP2 expression clone. We are grateful to D. W. Kim for his help in the preparation of HeLa cell extracts and for helpful discussions.
This work was supported by two grants from the National Institute of Allergy and Infectious Diseases (E. Wimmer, R37 AI015122-30; and C. Cameron, AI053531).
REFERENCES
1.Andino, R., G. E. Rieckhof, and D. Baltimore.1990. A functional
ribonucleo-protein complex forms around the 5⬘end of poliovirus RNA. Cell63:369–
380.
2.Andino, R., E. Rieckhof, P. L. Achacoso, and D. Baltimore.1993. Poliovirus
RNA synthesis utilizes an RNP complex formed around the 5⬘end of viral
RNA. EMBO J.12:3587–3598.
3.Barton, D. J., E. P. Black, and J. B. Flanegan.1995. Complete replication of poliovirus in vitro: preinitiation RNA replication complexes require soluble
cellular factors for the synthesis of VPg-linked RNA. J. Virol.69:5516–5527.
4.Barton, D. J., and J. B. Flanegan.1997. Synchronous replication of poliovi-rus RNA: initiation of negative-strand RNA synthesis requires the
guani-dine-inhibited activity of protein 2C. J. Virol.71:8482–8489.
5.Blair, W. S., T. B. Parsley, H. P. Bogerd, J. S. Towner, B. L. Semler, and B. R. Cullen.1998. Utilization of a mammalian cell-based RNA binding assay to characterize the RNA binding properties of picornavirus 3C proteinases.
RNA4:215–225.
6.Cornell, C. T., and B. L. Semler.2002. Subdomain specific functions of the
RNA polymerase region of poliovirus 3CD polypeptide. Virology298:200–
213.
7.Cuconati, A., A. Molla, and E. Wimmer.1998. Brefeldin A inhibits cell-free,
de novo synthesis of poliovirus. J. Virol.72:6456–6464.
8.Diamond, S. E., and K. Kirkegaard. 1994. Clustered charged-to-alanine mutagenesis of poliovirus RNA-dependent RNA polymerase yields multiple
temperature-sensitive mutants defective in RNA synthesis. J. Virol.68:863–
876.
9.Egger, D., N. Teterina, E. Ehrenfeld, and K. Bienz.2000. Formation of the poliovirus replication complex requires coupled viral translation, vesicle
pro-duction, and viral RNA synthesis. J. Virol.74:6570–6580.
10.Flanegan, J. B., and D. Baltimore.1977. Poliovirus-specific primer-depen-dent RNA polymerase able to copy poly(A). Proc. Natl. Acad. Sci. USA
74:3677–3680.
11.Fogg, M. H., N. L. Teterina, and E. Ehrenfeld.2003. Membrane require-ments for uridylylation of the poliovirus VPg protein and viral RNA
synthe-sis in vitro. J. Virol.77:11408–11416.
12.Gamarnik, A., and R. Andino.1998. Switch from translation to replication in
a positive strand RNA virus. Genes Dev.12:2293–2304.
13.Gamarnik, A. V., and R. Andino.2000. Interactions of viral protein 3CD and
poly(rC) binding protein with the 5⬘nontranslated region of the poliovirus
genome. J. Virol.74:2219–2226.
14.Hammerle, T., A. Molla, and E. Wimmer.1992. Mutational analysis of the proposed FG loop of poliovirus proteinase 3C identifies amino acids that are necessary for 3CD cleavage and might be determinants of a function distinct
from proteolytic activity. J. Virol.66:6028–6034.
15.Hansen, J. L., A. M. Long, and S. C. Schultz.1997. Structure of the
RNA-dependent RNA polymerase. Structure5:1109–1122.
16.Harris, K., W. Xiang, L. Alexander, W. S. Lane, A. V. Paul, and E. Wimmer.
1994. Interaction of the poliovirus polypeptide 3CDpro
with the 5⬘and 3⬘
termini of the poliovirus genome: identification of viral and cellular cofactors
needed for efficient binding. J. Biol. Chem.269:27004–27014.
17.Herold, J., and R. Andino.2001. Poliovirus RNA replication requires
ge-nome circularization through a protein-protein bridge. Mol. Cell7:581–591.
18.Hobson, S. D., E. S. Rosenblum, O. C. Richards, K. Richmond, K. Kirke-gaard, and S. C. Schultz.2001. Oligomeric structures of poliovirus
polymer-ase are important for function. EMBO J.20:1153–1163.
19.Jore, J., B. de Geus, R. J. Jackson, P. Pouwels, and B. Walk.1988. Poliovirus protein 3CD is the active protease for processing of the precursor protein P1
in vitro. J. Gen. Virol.69:1627–1636.
20.Lawson, M. A., and B. L. Semler.1992. Alternate poliovirus nonstructural processing cascades generated by primary sites of 3C proteinase cleavage.
Virology191:309–320.
21.Lu, H.-H., C.-F. Yang, A. D. Murdin, M. H. Klein, J. J. Harber, O. M. Kew, and E. Wimmer.1994. Mouse neurovirulence determinants of poliovirus type 1 strain LS-a map to the coding regions of capsid protein VP1 and
proteinase 2Apro. J. Virol.68:7507–7515.
22.Molla, A., A. V. Paul, and E. Wimmer.1991. Cell-free de novo synthesis of
poliovirus. Science254:1647–1651.
23.Molla, A., A. V. Paul, and E. Wimmer.1993. Effects of temperature and lipophilic agents on poliovirus formation and RNA synthesis in a cell-free
system. J. Virol.67:5932–5938.
24.Murray, K. E., and D. J. Barton.2003. Poliovirus CRE-dependent VPg uridylylation is required for positive-strand RNA synthesis but not for
neg-ative-strand RNA synthesis. J. Virol.77:4739–4750.
25.Novak, J. E., and K. Kirkegaard.1994. Coupling between genome
transla-tion and replicatransla-tion in an RNA virus. Genes Dev.8:1726–1737.
26.Pallansch, M. A., O. M. Kew, B. L. Semler, D. R. Omilianowski, C. W. Anderson, E. Wimmer, and R. R. Rueckert.1984. Protein processing map of
poliovirus. J. Virol.49:873–880.
27.Parks, G. D., J. C. Baker, and A. C. Palmenberg.1989. Proteolytic cleavage of encephalomyocarditis virus capsid region substrates by precursors to the
3C enzyme. J. Virol.63:1054–1058.
28.Parsley, T. B., J. S. Towner, L. B. Blyn, E. Ehrenfeld, and B. L. Semler.1997.
Poly((rC) binding protein 2 forms a ternary complex with the 5⬘-terminal
sequences of poliovirus RNA and the viral 3CD proteinase. RNA3:1124–
1134.
29.Parsley, T. B., C. T. Cornell, and B. L. Semler.1999. Modulation of the RNA binding and protein processing activities of poliovirus polypeptide 3CD by
the viral RNA polymerase domain. J. Biol. Chem.274:12867–12876.
30.Pata, J. D., S. C. Schultz, and K. Kirkegaard.1995. Functional
oligomer-ization of poliovirus RNA dependent RNA polymerase. RNA1:466–477.
31.Pathak, H. B., S. K. Ghosh, A. W. Roberts, S. D. Sharma, J. D. Yoder, J. J. Arnold, D. W. Gohara, D. J. Barton, A. V. Paul, and C. E. Cameron.2002.
6366 FRANCO ET AL. J. VIROL.
on November 8, 2019 by guest
http://jvi.asm.org/
Structure-function relationships of the RNA-dependent RNA polymerase
from poliovirus (3Dpol). A surface of the primary oligomerization domain
functions in capsid precursor processing and VPg uridylylation. J. Biol.
Chem.277:31551–31562.
32.Paul, A. V., J. Mugavero, A. Molla, and E. Wimmer.1998. Internal ribosomal entry site scanning of the poliovirus polyprotein: implications for proteolytic
processing. Virology250:241–253.
33.Paul, A. V., E. Rieder, D. W. Kim, J. H. van Boom, and E. Wimmer.2000. Identification of an RNA hairpin in poliovirus RNA that serves as the
primary template in the in vitro uridylylation of VPg. J. Virol.74:10359–
10370.
34.Paul, A. V.2002. Possible unifying mechanism of picornavirus genome
rep-lication, p. 227–246.InB. L. Semler and E. Wimmer (ed.), Molecular biology
of picornaviruses. ASM Press, Washington, D.C.
35.Rieder, E., W. Xiang, A. Paul, and E. Wimmer.2003. Analysis of the clo-verleaf element in a human rhinovirus type 14/poliovirus chimera: correla-tion of subdomain D structure, ternary complex formacorrela-tion and virus
repli-cation. J. Gen. Virol.84:2203–2216.
36.Roehl, H. H., T. B. Parsley, T. V. Ho, and B. L. Semler.1997. Processing of a cellular polypeptide by 3CD proteinase is required for poliovirus
ribonu-cleoprotein complex formation. J. Virol.71:578–585.
37.Tesar, M., I. Pak, X.-Y. Jia, O. C. Richards, D. F. Summers, and E. Ehren-feld.1994. Expression of hepatitis A virus precursor protein P3 in vivo and
in vitro: polyprotein processing of the 3CD cleavage site. Virology198:524–
533.
38.Teterina, N. L., W. D. Zhou, M. W. Cho, and E. Ehrenfeld.1995. Inefficient complementation activity of poliovirus 2C and 3D proteins for rescue of
lethal mutations. J. Virol.69:4245–42564.
39.Towner, J. S., M. M. Mazanet, and B. L. Semler.1998. Rescue of defective poliovirus RNA replication by 3AB-containing precursor polypeptides. J.
Vi-rol.72:7191–7200.
40.Van der Werf, S. J., J. Bradley, E. Wimmer, F. W. Studier, and J. J. Dunn.
1986. Synthesis of infectious poliovirus RNA by purified T7 RNA
polymer-ase. Proc. Natl. Acad. Sci. USA83:2330–2334.
41.Verlinden, Y., A. Cuconati, E. Wimmer, and B. Rombaut.2001. The viral protein 3CD induces an equilibrium between the viral protein and RNA
synthesis in a cell-free system for poliovirus replication. Arch. Virol.147:1–
14.
42.Walter, B. L., T. B. Parsley, E. Ehrenfeld, and B. L. Semler.2002. Distinct poly(rC) binding protein KH domain determinants for poliovirus translation
initiation and viral RNA replication. J. Virol.76:12008–12022.
43.Wimmer, E., C. U. Hellen, and X. Cao.1993. Genetics of poliovirus. Annu.
Rev. Genet.27:353–436.
44.Xiang, W., A. Cuconati, D. Hope, K. Kirkegaard, and E. Wimmer.1998. Complete protein linkage map of poliovirus P3 proteins: interaction of
polymerase 3Dpol
with VPg and with genetic variants of 3AB. J. Virol.
72:6732–6741.
45.Yin, J., A. V. Paul, E. Wimmer, and E. Rieder.2003. Functional dissection of
a polioviruscis-acting replication element [PV-cre(2C)]: analysis of
single-and dual-creviral genomes and proteins that bind specifically to PV-cre
RNA. J. Virol.77:5152–5166.
46.Ypma-Wong, M. F., P. G. Dewalt, V. H. Johnson, J. G. Lamb, and B. L. Semler.1988. Protein 3CD is the major poliovirus proteinase responsible for
cleavage of the P1 capsid precursor. Virology166:265–270.
VOL. 79, 2005 STIMULATION OF PV SYNTHESIS BY VIRAL PROTEIN 3CDpro 6367