0022-538X/05/$08.00⫹0 doi:10.1128/JVI.79.15.9410–9418.2005
Copyright © 2005, American Society for Microbiology. All Rights Reserved.
An Envelope-Determined, pH-Independent Endocytic Route of Viral
Entry Determines the Susceptibility of Human Immunodeficiency
Virus Type 1 (HIV-1) and HIV-2 to Lv2 Restriction
David Marchant,† Stuart J. D. Neil,† Keith Aubin, Christian Schmitz, and A
´ ine McKnight*
Wohl Virion Centre, Windeyer Institute of Medical Sciences, UCL, London, United Kingdom
Received 6 December 2004/Accepted 21 April 2005
We identified a postentry restriction, termed Lv2, which determines the cellular tropism of two related human immunodeficiency virus type 2 (HIV-2) isolates and is dependent on the sequence of the capsid (CA) and envelope (Env) proteins. To explain the reliance on both CA and Env, we proposed that restrictive Envs deliver susceptible capsids to a compartment where Lv2 is active whereas nonrestrictive Envs deliver capsids into a compartment where Lv2 is either absent or less active. To test this model, we used compounds that affect endocytic pathways (ammonium chloride, bafilomycin A1, hypertonic sucrose) or lipid rafts (methyl- -cyclo-dextrin) to treat restrictive cells and show that restricted virus can be rescued from Lv2 if a lipid-raft-dependent, pH-independent endocytic pathway is inhibited. Furthermore, viral entry into HeLa/CD4 cells containing a tailless CD4 receptor, located outside lipid rafts, was fully permissive. Finally, we show that a variety of primary HIV-1 and HIV-2 viruses are susceptible to Lv2. Thus, we show that the route of entry, determined by the viral envelope, can influence cellular tropism by avoiding intracellular blocks to infection.
Intracellular factors can restrict viral replication after entry into the cell and could represent an arm of the innate immune system that deals with intracellular parasites (2). In this paper, we examine whether the susceptibility of human immunodefi-ciency virus types 1 and 2 (HIV-1/2) to the Lv2 restriction factor is dependent on the route of virus delivery into the cell as previously postulated by us (33).
To date, members of two genes families that encode posten-try restriction factors that target HIV-1/2 have been identified. APOBEC3G (CEM15), a cytidine deaminase, induces hyper-mutation of newly synthesized viral DNA (13, 34, 41) and is overcome by the HIV-1 viral infectivity factor (Vif). Members of the APOBEC family with antiviral activity include 3B and 3F (14). A second, TRIM5␣(tripartite family of proteins 5␣), acts prior to reverse transcription (38). HIV-1 can partially avoid TRIM5␣by amino acid substitutions in the capsid pro-tein (CA) (16, 25). TRIM5␣ encodes the antiviral activities Ref1 (human) and Lv1 (monkey) (2, 10).
We have reported a distinct postentry, post-reverse tran-scription restriction to HIV-1/2 infection in some human cells termed Lv2 (10, 22, 33). Like Lv1, the determinant for Lv2 restriction maps to CA. Unlike Lv1/Ref1, viruses pseudotyped with vesicular stomatitis virus envelope glycoprotein (VSV-G), which enters cells via an endocytic route, bypass Lv2. In addi-tion to CA, we showed that changes in the envelope protein (Env) also overcome Lv2 (33). The Env contribution to Lv2 can be accounted for by a single amino acid change in the V1 domain (29). So far, viral Envs have not been implicated in the Lv1/Ref1 restriction (33).
To explain the reliance on both CA and Env for Lv2, we
proposed that a restrictive Env delivers to a compartment where Lv2 is potent and results in abortive infection. However, a permissive CA avoids Lv2 or reroutes to a permissive path-way where Lv2 is absent or less potent. The permissive Env delivers directly via a nonrestricted pathway or one where Lv2 is less potent. Here, we test this model.
MATERIALS AND METHODS
Cells.HeLa/CD4, HeLa/CD4H399(H399), and U87/CD4/CXCR4 were main-tained in Dulbecco’s minimal Eagle’s medium (DMEM)–5% (vol/vol) fetal calf serum (FCS)–60g/ml of penicillin–100g/ml of streptomycin–1 mg/ml of G418. U87/CD4/CXCR4 cells were also supplemented with 1g/ml of puromy-cin.
Viruses, virus clones, and vectors. Generation of HIV-2 molecular clone restricted (MCR) and molecular clone nonrestricted (MCN) full-length molec-ular clones and construction of recombinant MCNmcrgagand MCNmcrenv pro-viruses were described previously (33). VSV-G pseudotypes of HIV-2 and HIV-1 were produced by cotransfection of pMDG with an Env-deleted backbone plas-mid. Molecular clones of MCR and MCN with premature Env termination codons, MCR⌬env and MCN⌬env, respectively, were used for the optimal pro-duction of mixed Env pseudotypes, as described previously (33). HIV-1 strains were derived from molecular clones pNL4-3 and p89.6 (National Institutes of Health AIDS research and reference reagent program). Primary HIV-1 and HIV-2 isolates were propagated in activated human peripheral blood mononu-clear cells (37). HIV-1-based vectors were derived from the packaging construct p8.91 (42) and the enhanced green fluorescent protein (eGFP) encoding vector genome pCSGW (7) in conjuction with pMP11 encoding either MCN or MCR Env (33). Molecular clones, pseudotyped viruses, and vectors were produced by transient transfection of 293T cells, as described previously (33).
qPCR.U87/CD4/CXCR4, H399, and HeLa/CD4 cells in 24-well trays were treated with 5,000 focus-forming units (FFU, determined on U87/CD4/CXCR4) of either MCN or MCR pretreated with DNaseI (Roche) for 1 h at 37°C. DNA was prepared with the QIAGEN DNA-blood minikit. Next, 500 ng of total DNA was used for quantitative PCR (qPCR) with primers as follows: for HIV-2 strong-stop, 900 nM forward primer (5⬘-AGCTGCCAGTTAGAAGCAAGTTA AGT-3⬘), 300 nM reverse primer (5⬘-TGTTATTCAGATGAACACCGAATG A-3⬘), and 150 nMTaqManprobe (5⬘-6-carboxyfluorescein-TTCCCATCTCTC CTAGTCGCCGCCT-6-carboxytetramethyl-rhodamine-3⬘), and for HIV-1 packaging signal (⌿), 300 nM forward primer (TGGGCAAGCAGGGAGCTA), 300 nM reverse primer (TCCTGTCTGAAGGGATGGTTGT), and 150 nM TaqManprobe (5⬘-6-carboxyfluorescein-AACGATTCGCAGTTAATCCTGGC CTGTT-6-carboxytetramethyl-rhodamine-3⬘). The PCR conditions consisted of
* Corresponding author. Mailing address: Wohl Virion Centre, Windeyer Institute of Medical Sciences, UCL, 46 Cleveland St, Lon-don W1T 4JF, United Kingdom. Phone: 44 207 6799581. Fax: 44 207 679 9555. E-mail: a.mcknight@ucl.ac.uk.
† D.M. and S.J.D.N. contributed equally to this study.
9410
on November 8, 2019 by guest
http://jvi.asm.org/
one cycle of denaturation (95°C for 10 min) followed by 45 cycles of amplification (95°C for 15 s, 60°C for 1 min). The amplification, data acquisition, and analysis were performed using the ABI PRISM 7000 sequence detection system.
Infectivity assays.Virus infections were performed as described in reference 33. Briefly, cold methanol-acetone (1:1)-fixed cells infected with HIV-2 or HIV-1 were immunostained with HIV-2 human serum diluted 1/4,000 or anti-HIV-1 p24 monoclonal antibody diluted 1/40 (1:1 mix of EVA 365 and 366 from the Medical Research Council AIDS Reagent Program, Potters Bar, United King-dom). Second-layer-galactosidase conjugates of goat anti-human immunoglob-ulin G (IgG) (for HIV-2) or goat anti-mouse IgG (HIV-1) were used to detect first-layer antibodies at a dilution of 1:400 (Southern Biotechnology Associates, Inc., Birmingham, Ala.). Infected cells were stained blue with X-Gal (5-bromo-4-chloro-3-indoyl--galactopyranoside) in phosphate-buffered saline containing 3 mM potassium ferricyanide, 3 mM potassium ferrocyanide, and 1 mM mag-nesium chloride. Foci of infection, which stained blue, were counted, and virus infectivity was estimated as FFU per milliliter. The restriction (n-fold) was calculated as the ratio of infectivity of unrestricted to restricted cells. For HIV-based vectors encoding GFP, transduced cells were enumerated by flow cytom-etry (FACScan; Becton Dickinson) and results analyzed using the Cellquest software package.
NH4Cl and BFLA treatment.Cells were treated with 0.1 to 0.01M bafilo-mycin A1 (BFLA) or 50 mM ammonium chloride (NH4Cl) for 1 h prior to infection and then for 2 h during virus incubation. Lysosomal-tropic treatments were replaced with normal growth media and fixed and stained for HIV infection 48 h later.
Inhibition of endocytosis with hypertonic sucrose.Forty-eight-well plates were pretreated for 1 h with 1% gelatin in DMEM. Cells were then plated and washed once before adding virus at 4°C for 1 h. Inoculum was removed and was replaced with 0.45 M sucrose–DMEM–5% FCS for 45 min at 37°C. The sucrose medium was then removed and replaced with normal growth medium.
Cholesterol depletion.Cells were washed with DMEM, and 10 mM
methyl--cyclodextrin (MCD) was added for 30 min at 37°C. Cells were rinsed with DMEM, and virus was incubated with cells on ice for 1 h. Inoculum was replaced with prewarmed DMEM⫾0.2M water-soluble cholesterol. After 2 h at 37°C, DMEM was replaced with DMEM–5% FCS and cells were fixed and stained 3 days later (infectivity assays).
Subcellular fractionation.Cell lysates were prepared on ice. Briefly, 5.0⫻107 cells were harvested with EDTA at 1:5,000 and washed twice (phosphate-buff-ered saline). The pellet was resuspended in 1 ml hypotonic HEPES-h buffer and centrifuged for 5 min (800⫻g, 4°C). Next, 300l 1% (vol/vol) Triton X-100 HEPES-h buffer was added to the pellet (30 min on ice) and then the lysate was homogenized with 10 strokes of a Dounce homogenizer. To remove nuclei, the lysate was spun at 300⫻gfor 3 min and 4°C and the supernatant (cell mem-branes) was harvested and loaded onto a sucrose gradient.
Sucrose gradient centrifugation.Four hundred microliters of cell membrane lysate was mixed with 800l of 60% (wt/vol) sucrose in HEPES-h buffer and layered under sucrose gradients (3 ml and 6 ml of 5 and 35% [wt/vol] sucrose, respectively, in HEPES-h buffer). Samples were spun at 100,000⫻gfor 20 h at 4°C. One-milliliter fractions were collected from the top of the tube and stored at⫺20°C for Western and slot blotting.
Immunofluorescence microscopy.Cells were visualized after treatment with either fluorescein isothiocyanate-conjugated transferrin (trf-FITC) or FITC-con-jugated cholera toxin B subunit (CtxB-FITC). Cells were mounted with immu-nofluorescence mounting medium (Dako, Carpinteria, CA) and observed by confocal microscopy (MRC 1024; Bio-Rad, Hercules, CA) equipped with a krypton-argon laser. Pictures were acquired using Kalman averaging and ana-lyzed with Lasersharp software (Bio-Rad).
RESULTS
An endocytic pathway of entry results in viral susceptibility to Lv2 restriction and relies on Env.Enveloped viruses can fuse with cells either at the plasma membrane (PM) or from within an endocytic vesicular route that is often, but not ex-clusively, pH dependent (20). Since HIV susceptibility to Lv2 restriction maps to both Env and CA, we postulated that it was dependent on the Env-mediated delivery of susceptible CAs into a compartment where Lv2 is active (33). MCN and MCR are molecularly cloned HIV-2 viruses (33). MCN is derived from a T-cell-line-adapted isolate, CBL-23, and is not sensitive to Lv2, whereas MCR is derived from prCBL-23, a primary
isolate from the same patient (22), and is Lv2 sensitive. To determine if an endocytic or pH-dependent route of entry is involved in the Lv2 restriction, we first determined the effect of NH4Cl treatment on the infectivity of the MCR virus and the
MCN virus. NH4Cl is taken up by the cell and becomes
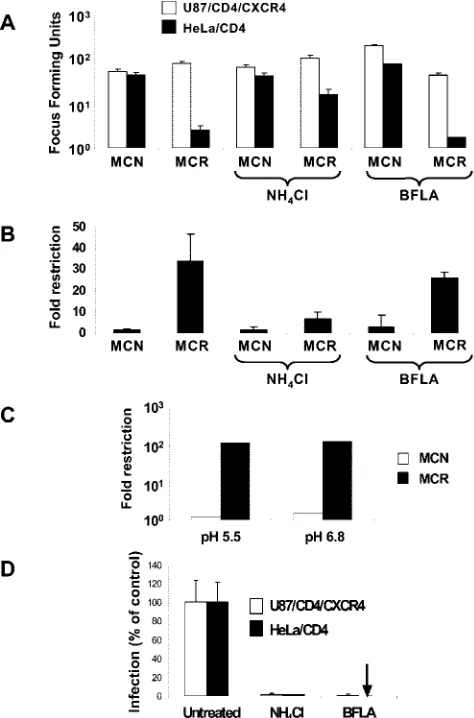
[image:2.585.301.538.70.429.2]pro-tonated in acidic compartments, resulting in pH neutralization (5). Figure 1A and B shows the relative infectivity of two HIV-2 molecular clones. As expected, MCR is about 40-fold less infectious (Fig. 1B) than MCN for HeLa/CD4 cells (33). Both viruses infect permissive U87/CD4/CXCR4 equivalently. The treatment of U87/CD4/CXCR4 and HeLa/CD4 with 50 mM NH4Cl resulted in 90 to 98% inhibition of pH-dependent
FIG. 1. Ammonium chloride treatment of restrictive cells rescues MCR from Lv2 restriction; the viral envelope fusion is pH indepen-dent. (A) 200 FFU (on permissive U87/CD4/CXCR4) of either MCN or MCR HIV-2 virus were plated on restrictive HeLa/CD4 cells and the resulting FFU calculated (see Materials and Methods) in the presence or absence of 50 mM NH4Cl and 0.10M BFLA. (B) The
fold restriction of each virus on HeLa/CD4 cells relative to that on U87/CD4/CXCR4 cells was calculated (see Materials and Methods). (C) MCR and MCN were titrated in acid medium pH 5.5 or physio-logical pH 6.8. (D) Both NH4Cl and BFLA treatments inhibit
VSV-G-pseudotyped HIV-1-based vector. Vectors were titrated with or without NH4Cl or BFLA and GFP pplus vector cells quantitated by
flow cytometry. Data is expressed as the number of GFP plus vector cells as a percentage of the untreated control. Error bars represent the standard errors of the mean from three independent experiments.
VOL. 79, 2005 SUSCEPTIBILITY OF HIV-1/2 TO Lv2 RESTRICTION 9411
on November 8, 2019 by guest
http://jvi.asm.org/
VSV-G pseudotypes of HIV-1 (Fig. 1D). NH4Cl rescued the
restriction of MCR on HeLa/CD4 cells from 40- to 8-fold. However, NH4Cl had no effect on the infectivity of MCN for
HeLa/CD4 or of either MCR or MCN on permissive U87/ CD4/CXCR4 cells (Fig. 1A and B). These results suggest that Lv2 occurs along a pH-dependent route. HIV-1/2-mediated fusion is pH independent (21). We next determined if MCR has a pH-dependent Env for entry into restrictive cells. We
titrated MCR and MCN on restrictive and permissive cells in acidified medium to mimic the effect of a drop in endosomal pH. The infectivity of MCR and MCN was unaffected on either restrictive or permissive cells (Fig. 1C).
[image:3.585.142.467.69.579.2]To determine whether an acidified endosome is responsible for the Lv2 restriction, we treated cells with the vacuolar H⫹ ATPase inhibitor BFLA. This drug is more specific in its action than NH4Cl and counteracts vacuolar acidification (7). BFLA
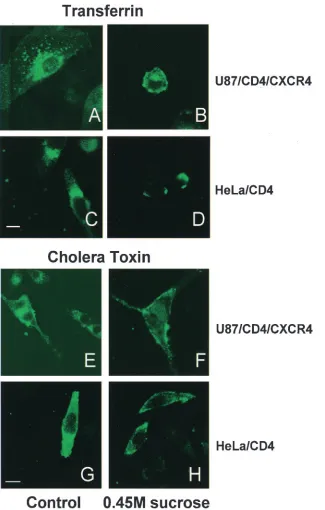
FIG. 2. Sucrose inhibits transferrin and cholera toxin B uptake. trf-FITC (A through D) or CtxB-FITC (E through H) was bound to the permissive U87/CD4/CXCR4 or restrictive HeLa/CD4 cells with or without 0.45 M sucrose and examined by confocal microscopy. Inhibition is shown by trf-FITC or CtxB-FITC capping at the cell surface with simultaneous uptake into perinuclear (endosomal) regions of the cell. White bars indicate 10-m magnification.
on November 8, 2019 by guest
http://jvi.asm.org/
had no effect on MCR or MCN infection on restrictive HeLa/ CD4 or on MCN on unrestrictive U87/CD4/CXCR4 cells (Fig. 1A). There was only a small effect (a twofold drop) on MCR infection on U87/CD4/CXCR4 cells (Fig. 1A).
Although NH4Cl primarily increases the pH of endocytic
routes, it may also affect the recycling of endosomes and other
non-pH-dependent routes of entry because it is used at slightly hypertonic concentrations (16). So we tested the effect of hy-pertonic sucrose on Lv2. Hyhy-pertonic sucrose inhibits clathrin-coated pit formation and other endocytic pathways without affecting pH (4, 17). As a positive control for the inhibition of endocytosis with hypertonic sucrose, we used the uptake of trf-FITC, which is internalized by clathrin-coated pits (11), and CtxB-FITC, which binds the GM1 glycolipid and is endocy-tosed in a lipid-raft-dependent pathway (40). Both Trf-FITC and CTxB-FITC were blocked by hypertonic sucrose (Fig. 2A through H).
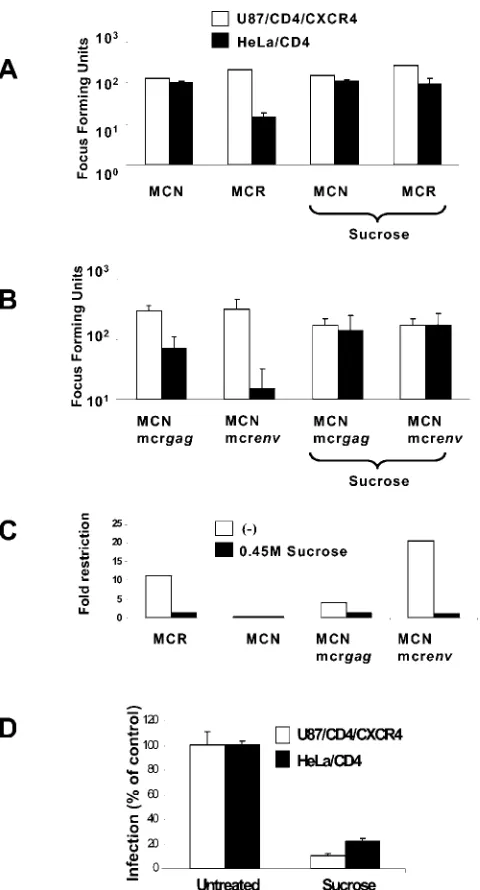
Figure 3A shows the result of hypertonic sucrose treatment on Lv2 restriction. To carry this out, 200 FFU (determined on U87/CD4/CXCR4) of MCR or MCN was plated on sucrose-treated HeLa/CD4 or U87/CD4/CXCR4 cells. The restriction of MCR was reduced from 40- to 5.2-fold, and there was no effect on infection of the permissive U87/CD4/CXCR4 by MCR or the infectivity of MCN on both cell lines (Fig. 3A). In contrast, an HIV-1 core pseudotyped with an endocytosis-de-pendent VSV-G envelope was inhibited on both cell types (90% on U87/CD4/CXCR4 and 82% on HeLa/CD4) (Fig. 3D). Thus, treatment of cells with either hypertonic sucrose or NH4Cl resulted in partial rescue of MCR from Lv2 in
restric-tive cells.
We have previously reported that the Lv2 restriction was dependent on viral Env or CA (33). We next tested whether Env- or CA-mediated restriction (or both) is relieved by block-ing endocytosis with hypertonic sucrose. Figure 3C shows that viruses containing a restrictive Env (MCNmcrenv) were res-cued from 21- to 1-fold on restrictive cells. The molecular clone with a permissive Env but a restrictive Gag (MCNmcr
gag) was less restricted (fourfold), and this restriction was rescued also to onefold. Thus, both the Env- and CA-mediated restriction of Lv2 is rescued by the prevention of endocytosis.
Membrane cholesterol is an important component in the pathway that leads to Lv2 restriction.Cholesterol is important for lipid-raft-dependent endocytic pathways (30). We thus de-termined the effect of the removal of membrane cholesterol on Lv2 restriction using MCD. We followed MCD disruption of lipid rafts by monitoring the uptake of CtxB-FITC and trf-FITC into cells. Cholera toxin enters cells via a lipid-raft-dependent endocytic pathway and is disrupted by the depletion of membrane cholesterol (9, 24, 30, 31). Figure 4D through F shows that CtxB-FITC uptake in HeLa/CD4 is inhibited by MCD that is reversed by the addition of cholesterol. Surpris-ingly, internalization of CtxB-FITC on U87/CD4/CXCR4 is unaffected by the same MCD treatment (Fig. 4A through C), suggesting that uptake of CtxB-FITC by these cells is lipid raft independent, and so there may be differences between HeLa/ CD4 and U87/CD4/CXCR4 regarding the trafficking of viral receptors. MCD treatment had no effect on the uptake of clathrin-dependent trf-FITC (Fig. 4G through J). Further-more, MCD treatment had no effect on the titer of VSV-G-pseudotyped MCN and MCR cores (data not shown) (28), confirming that the VSV-G pathway that bypasses Lv2 (22, 33) is distinct from that taken by MCR.
[image:4.585.44.284.65.510.2]We challenged HeLa/CD4 with MCR and MCN after MCD treatment. Figure 4K shows that MCD treatment of nonpermissive HeLa/CD4 cells prior to challenge with 100 FFU (determined on permissive U87/CD4/CXCR4) of MCR FIG. 3. Endocytosis inhibition by hypertonic sucrose rescues MCR
infection on HeLa/CD4. An equivalent input of 200 FFU (on U87/ CD4/CXCR4 cells) of MCR and MCN (A) or MCNmcrgagand MC-Nmcrenvgene swaps (B) was plated on restrictive HeLa/CD4 and nonrestrictive U87/CD4/CXCR4 cells with or without hypertonic su-crose. (C) The fold restriction was determined (from panels A and B, above) by dividing the infectious titer on U87/CD4/CXCR4 cells by the titer on HeLa/CD4 cells. (D) Sucrose treatment inhibits VSV-G-pseudotyped HIV-1-based vectors. Vector was titrated with or without sucrose and GFP plus vector cells were quantitated by flow cytometry. Data are expressed as the numbers of GFP plus vector cells as per-centages of the untreated control. Error bars represent the standard errors of the mean from three independent experiments.
VOL. 79, 2005 SUSCEPTIBILITY OF HIV-1/2 TO Lv2 RESTRICTION 9413
on November 8, 2019 by guest
http://jvi.asm.org/
9414
on November 8, 2019 by guest
results in eightfold recovery from restriction, as seen with NH4Cl and hypertonic sucrose. There was little effect on the
infectivity of MCN on the restrictive HeLa/CD4 cells. Also, 0.2
M cholesterol replenishment partially reconstituted the Lv2 restriction for MCR, further confirming the dependence of Lv2 on membrane cholesterol.
Lv2 can be bypassed if CD4 is localized outside lipid rafts.
The experiments described above using MCD to disrupt lipid rafts need to be interpreted with caution. Cholesterol itself may be a requirement for HIV fusion regardless of whether or not it is associated with lipid rafts or endocytosis (12). We determined if localizing the CD4 receptor outside lipid rafts, thus forcing fusion to occur outside lipid rafts but still in the presence of physiological cholesterol, overcomes the Lv2 re-striction.
Full-length CD4 localizes mainly within lipid rafts on the PM (28), whereas C-terminally truncated CD4 localizes outside lipid rafts (27). We used H399, a HeLa cell line expressing a variant of CD4 with a stop codon at amino acid position 399 in the cytoplasmic domain (26). This amino acid is upstream of amino acids 419 through 427, the polyarginine tract, shown to direct CD4 into lipid rafts (27). First, we verified the location of this tailless CD4 compared to the wild type CD4 within lipid rafts. Lipid rafts were prepared from HeLa/CD4, H399, and U87/CD4/CXCR4 cells (see Materials and Methods). Densi-tometry on the fractions determined the precise quantity of CD4 localized. A lipid raft marker, placental alkaline phospha-tase (PLAP), was tested in parallel for each fraction. Figure 5A shows that the majority of CD4 receptors in HeLa and U87 cells was localized in the lower-density lipid raft fractions 3, 4, and 5, as was the lipid raft marker PLAP (Fig. 5B). In contrast, the majority of tailless CD4 receptors in H399 cells localized to the higher-density fractions 9 and 10 and were thus excluded from lipid rafts (Fig. 5A). Figure 5C shows that the restriction to MCR on HeLa/CD4 cells is recovered on H399 cells (from 30-fold to 2.2-fold). There was little effect on the infectivity of the MCN on H399 compared to HeLa/CD4.
Lv2 restriction results in aborted HIV infection post-reverse transcription in HeLa/CD4 cells (33). We also determined the reverse transcription activity on H399 compared to HeLa/CD4. Figure 5D shows that while reverse transcription in nonrestric-tive U87/CD4/CXCR4 is more rapid (reaching a maximum at 6 h), equal numbers of strong-stop reverse transcripts were detected in both U87/CD4/CXCR4 and HeLa/CD4 cells by 18 h, confirming that restriction is postentry (post-reverse tran-scription initiation). Furthermore, since there was no increase in transcripts in the H399 compared to HeLa/CD4, the recov-ery of infectivity for MCR was not due to increased viral entry. The Lv2 restriction phenotype can be conferred on an HIV-1 core by pseudotyping with a MCR Env (33). Figure 5E con-firms this observation and further shows that the MCR Env mediates the H399 cell rescue.
HIV-1 and -2 isolates are commonly susceptible to Lv2 re-striction. We tested a range of primary- and laboratory-adapted HIV-1 (NL4.3, RF, IIIB, MN, SF-2, 2044, 2005, 2028, 2076, M13, HAN2, and 89.6) and HIV-2 (Mil, ETP, JAU, ACR23, MIR, AND, SAB, and prCBL-20) viruses on HeLa/ CD4 and U87/CD4/CXCR4 cells to determine the prevalence of Lv2-like restrictions. We also determined the ratios of in-fection on H399 to that on HeLa/CD4. Figure 6A and B showed the relative restriction and demonstrated that almost 40% of the HIV-1/2 isolates were restricted in an Lv2-like manner. In general, the HIV-1 isolates were more susceptible to Lv2 than HIV-2. Of the HIV-1 isolates, 2076, M13, HAN2, and 89.6 showed a similar phenotype to the MCR (Fig. 6B). Of the HIV-2 isolates, ETP, prCBL20, AND, and SAB were re-stricted in an Lv2-like manner (Fig. 6A).
The Lv2 restriction (22, 33), unlike Lv1 (15), is overcome by rerouting the virus to an endocytic pathway using a VSV-G envelope. Similar to MCR, the restricted HIV-1 isolate 89.6 was rescued by VSV-G Env on HeLa/CD4 (Fig. 6C). To de-termine whether the restriction of HIV-1 isolates was post-reverse transcription as previously described (33), we deter-mined the number of reverse transcripts for the unrestricted NL4.3(HIV-1) and the restricted 89.6(HIV-1) in U87/CD4/ CXCR4, HeLa/CD4, and H399 cells. Figure 6D shows that the levels of reverse transcripts (first-strand synthesis) for these HIV-1 isolates were similar in both restrictive and permissive cells. The restricted 89.6 showed threefold lessgag-LTR prod-uct at 24 h than the unrestricted NL4.3 in restricted HeLa/CD4 cells.
DISCUSSION
We have found that the susceptibility of HIV-1 and HIV-2 to Lv2 depends on the Env-mediated delivery of susceptible viral capsids into the cell, after trafficking into a pH-indepen-dent, endosomal route. We demonstrate that HIV Env can influence the route of entry, affecting viral tropism by avoiding intracellular Lv2 restriction.
Viruses can fuse with cells either at the plasma membrane or via an endocytic route that is often, but not exclusively, pH dependent (20). Here, we have shown that either of two treat-ments, hypertonic sucrose or MCD, rescues Lv2 restriction and we conclude that Lv2 is active after delivery via a lipid-raft-dependent endocytic route. In addition, Lv2 was rescued by treatment with NH4Cl. Recently, Reuter et al. reported that
Lv2 restriction can be partially overcome with NH4Cl
treat-ment (29). These authors conclude the involvetreat-ment of a pH-dependent route. While NH4Cl primarily affects the pH of
endosomes (5), it may also affect endocytic trafficking. Two further experiments support our conclusion that endosomal acidification is not involved. We show that treatment of the virus itself with acid pH does not enhance viral infectivity. Further, BFLA treatment prior to or during challenge had no
FIG. 4. Methyl--cyclodextrin differentially inhibits cholera toxin B uptake in restricted and nonrestricted cells. Permissive U87/CD4/CXCR4 or restrictive HeLa/CD4 cells were treated with MCD. CtxB-FITC (A through F) and trf-FITC (G through J) were bound to treated or untreated cells for 1 h with or without water-soluble cholesterol and examined by confocal microscopy. Capping of CtxB-FITC at the cell surface shows inhibition. (K) MCN and MCR on HeLa/CD4 cells with or without MCD treatment, with or without cholesterol replenishment. White bars denote no treatment, black bars denote MCD, and grey bars denote replenishment with cholesterol.
VOL. 79, 2005 SUSCEPTIBILITY OF HIV-1/2 TO Lv2 RESTRICTION 9415
on November 8, 2019 by guest
http://jvi.asm.org/
effect on Lv2 restriction. BFLA treatment inhibits the acidifi-cation of endosomes without affecting endosome trafficking (8). We conclude that the rescue by NH4Cl is likely to be due
to the inhibition of endocytic trafficking and that the Lv2 en-docytic route is pH independent.
As expected, MCD treatment disrupted cholera toxin en-FIG. 5. CD4 membrane localization in permissive and restrictive
[image:7.585.304.540.65.498.2]cell lines: H399 CD4 localizes outside of lipid rafts. Lipid rafts were prepared from membrane lysates of U87/CD4/CXCR4, HeLa/CD4, and H399 and analyzed for CD4 (A) and PLAP localization (B) as a lipid raft marker. (C) An equivalent input of 200 FFU (on U87/CD4/ CXCR4) of MCR and MCN was plated on U87/CD4/CXCR4, HeLa/ CD4, and H399 and the FFUs calculated. (D) Time course of qPCR (strong-stop) of MCR on permissive and restrictive cell lines. (E) Vec-tor pseudotypes were prepared from the MCN and MCR envelopes with HIV-1gag-pol(p8.91) and titrated on permissive and restrictive cell lines. Transduced eGFP was measured by fluorescence-activated cell sorter and the number of eGFP-positive cells per ml calculated.
FIG. 6. HIV-1 and HIV-2 isolates are susceptible to Lv2, rescued by the VSV-G envelope, and blocked post-reverse transcription. An array of HIV-2 (A) and HIV-1 (B) isolates was titrated on permissive U87/CD4/CXCR4, H399, and restrictive HeLa/CD4 cells and the FFUs calculated. Fold restriction was calculated by dividing the titer on permissive U87/CD4/CXCR4 cells by the titer on restrictive HeLa/ CD4 cells or by permissive H399 cells. (C) VSV-G envelope pseudotypes were prepared with restricted HIV-1 89.6 or nonrestricted NL4.3 gag-poland titrated on permissive and restrictive cell lines. Transduced eGFP was measured with a fluorescence-activated cell sorter and eGFP-positive cells per ml calculated. (D) Time course of qPCR, first-strand synthesis (), of restricted HIV-1 89.6 and unre-stricted HIV-1 NL4.3 on permissive and restrictive cell lines. Zidovu-dine (AZT) was added at 100M as a negative control for reverse transcription.
on November 8, 2019 by guest
http://jvi.asm.org/
[image:7.585.51.349.76.598.2]docytosis by depletion of cholesterol from the plasma mem-brane (30, 40) but did not inhibit transferrin uptake, suggesting a limited effect on clathrin-mediated endocytosis (11). Further-more, it did not inhibit VSV-G-mediated viral entry. MCD treatment did, however, rescue Lv2 restriction, further sup-porting our model that Lv2 restriction results from entry via an endosomal route. Although MCD may have other effects on the cell, the reconstitution of the Lv2 phenotype by adding cholesterol back to treated cells demonstrates a requirement for membrane cholesterol as well as endocytosis in restrictive infection. Furthermore, the same result was observed using filipin III, which chelates PM cholesterol (data not shown).
It is possible that the fusion process itself may be disrupted by MCD treatment because HIV-1 fusion depends on cho-lesterol (12). We therefore determined the effect of relocating CD4 out of lipid rafts by using a clone of restrictive HeLa/CD4 cells expressing tailless CD4 (H399). Thus, we predicted that viral fusion would occur outside lipid rafts where membrane cholesterol is present. Lv2 restriction was almost completely abolished on H399 cells, supporting the idea that fusion out-side a lipid raft pathway leads to escape from Lv2. Interest-ingly, the internalization of CD4 in the H399 cells is two- to threefold slower than in HeLa/CD4 (M. Marsh, personal com-munication), again suggesting a link between receptor traffick-ing and postentry events in HIV replication. Interesttraffick-ingly, oth-ers have reported that the enhancement of infectivity by Nef depends on the pathway of entry (1).
All treatments used in this work had only minor effects on the unrestricted HIV-2 virus MCN, indicating either that this endocytic route of entry may be a normal HIV entry pathway for viruses with unsusceptible cores or that nonrestricted Env avoids this route. Further studies will determine whether per-missive Env also delivers the viral capsid through this endocytic route and whether this pathway can, in some circumstances, be used to infect permissive cells. It will also be of interest to test whether these routes of delivery can be used to saturate Lv2 restriction in a similar way to Ref1 (TRIM5␣) saturation (39). We cannot rule out that the restrictive Lv2 factor is also ex-pressed in permissive cells but that the endocytic pathway delivering to it is absent. Various endocytic processes, includ-ing caveolar uptake, have been shown to require membrane cholesterol, depending on the cell type (23). Further informa-tion on the precise pathway using dominant negative or active mutants to components of specific endocytic pathways will allow identification of the precise pathway(s) that leads to Lv2 restriction.
We determined the prevalence of sensitivity to Lv2 among HIV-1 and HIV-2 primary viruses. Interestingly, HIV-1 ap-pears to be more frequently, and potently, restricted than HIV-2. Further, we observed a threefold effect on the levels of reverse transcription for HIV-1 but this does not account for the observed 40-fold restriction. We are currently investigating the mechanism of HIV-1 escape from Lv2. Thus, we confirm that the original isolate prCBL-23 used to demonstrate this restriction is not unusual (22) and that Lv2 restricts several primary- and laboratory-adapted HIV-1 and HIV-2 viruses.
Rescue of viral infectivity for either HIV-1 or HIV-2 on restrictive HeLa/CD4 cells did not result in altered levels of reverse transcription, supporting our previous observation that
the Lv2 restriction (at least in HeLa/CD4 cells) operates after reverse transcription (33).
The role of lipid rafts and CD4/coreceptor localization in HIV-1 entry is contentious (6, 18, 19). The results presented here, in which the effects of lipid raft depletion depend on the pathway of viral entry, the cell type, and the virus strain itself, could explain such contradictory results.
Our results support the previously suggested role for HIV Env (18, 33), which is to ensure that fusion occurs at specific sites on the plasma membrane. In this light, it is interesting to note that CXCR4-tropic isolates of HIV-1 poorly infect mac-rophages, despite being competent for fusion (35, 36), with the infection being restricted during reverse transcription (32). We speculate that this may result from virus trafficking into a restrictive Lv2-like pathway, a notion that we are currently investigating.
We conclude that HIV Env is a major determinant of cel-lular tropism not only because of its ability to bind CD4 and either CXCR4 or CCR5 (3) but also because it can avoid antiviral factors active postentry.
ACKNOWLEDGMENTS
We thank Mark Marsh for invaluable discussions and Marlen Aasa-Chapman and Greg Towers for critically reading the manuscript.
A´ .M. and K.A. were supported by a Wellcome Trust RCDF (no. 060758), and this work is currently funded by a WT project grant.
We confirm that we have no competing financial interests.
REFERENCES
1.Aiken, C.1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol.71:5871–5877.
2.Bieniasz, P. D.2004. Intrinsic immunity: a front-line defense against viral attack. Nat. Immunol.5:1109–1115.
3.Clapham, P. R., and A. McKnight.2002. Cell surface receptors, virus entry and tropism of primate lentiviruses. J. Gen. Virol.83:1809–1829. 4.Daukas, G., and S. H. Zigmond.1985. Inhibition of receptor-mediated but
not fluid-phase endocytosis in polymorphonuclear leukocytes. J. Cell Biol. 101:1673–1679.
5.de Duve, C., T. de Barsy, B. Poole, A. Trouet, P. Tulkens, and F. Van Hoof. 1974. Commentary. Lysosomotropic agents. Biochem. Pharmacol.23:2495– 2531.
6.Del Real, G., S. Jimenez-Baranda, R. A. Lacalle, E. Mira, P. Lucas, C. Gomez-Mouton, A. C. Carrera, A. C. Martinez, and S. Manes.2002. Block-ing of HIV-1 infection by targetBlock-ing CD4 to nonraft membrane domains. J. Exp. Med.196:293–301.
7.Demaison, C., K. Parsley, G. Brouns, M. Scherr, K. Battmer, C. Kinnon, M. Grez, and A. J. Thrasher.2002. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [cor-rection of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum. Gene Ther.13:803–813. 8.Drose, S., K. U. Bindseil, E. J. Bowman, A. Siebers, A. Zeeck, and K. Altendorf.1993. Inhibitory effect of modified bafilomycins and concanamy-cins on P- and V-type adenosinetriphosphatases. Biochemistry 32:3902– 3906.
9.Fujinaga, Y., A. A. Wolf, C. Rodighiero, H. Wheeler, B. Tsai, L. Allen, M. G. Jobling, T. Rapoport, R. K. Holmes, and W. I. Lencer.2003. Gangliosides that associate with lipid rafts mediate transport of cholera and related toxins from the plasma membrane to endoplasmic reticulum. Mol. Biol. Cell14: 4783–4793.
10.Goff, S. P.2004. Genetic control of retrovirus susceptibility in mammalian cells. Annu. Rev. Genet.38:61–85.
11.Gruenberg, J.2001. The endocytic pathway: a mosaic of domains. Nat. Rev. Mol. Cell. Biol.2:721–730.
12.Guyader, M., E. Kiyokawa, L. Abrami, P. Turelli, and D. Trono.2002. Role for human immunodeficiency virus type 1 membrane cholesterol in viral internalization. J. Virol.76:10356–10364.
13.Harris, R. S., K. N. Bishop, A. M. Sheehy, H. M. Craig, S. K. Petersen-Mahrt, I. N. Watt, M. S. Neuberger, and M. H. Malim.2003. DNA deami-nation mediates innate immunity to retroviral infection. Cell113:803–809. 14.Harris, R. S., and M. T. Liddament.2004. Retroviral restriction by APOBEC
proteins. Nat. Rev. Immunol.4:868–877.
VOL. 79, 2005 SUSCEPTIBILITY OF HIV-1/2 TO Lv2 RESTRICTION 9417
on November 8, 2019 by guest
http://jvi.asm.org/
15.Hatziioannou, T., S. Cowan, S. P. Goff, P. D. Bieniasz, and G. J. Towers. 2003. Restriction of multiple divergent retroviruses by Lv1 and Ref1. EMBO J.22:385–394.
16.Hatziioannou, T., S. Cowan, U. K. Von Schwedler, W. I. Sundquist, and P. D. Bieniasz.2004. Species-specific tropism determinants in the human immu-nodeficiency virus type 1 capsid. J. Virol.78:6005–6012.
17.Heuser, J. E., and R. G. Anderson.1989. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108:389–400.
18.Kozak, S. L., J. M. Heard, and D. Kabat.2002. Segregation of CD4 and CXCR4 into distinct lipid microdomains in T lymphocytes suggests a mech-anism for membrane destabilization by human immunodeficiency virus. J. Virol.76:1802–1815.
19.Manes, S., G. del Real, R. A. Lacalle, P. Lucas, C. Gomez-Mouton, S. Sanchez-Palomino, R. Delgado, J. Alcami, E. Mira, and A. C. Martinez. 2000. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep.1:190–196.
20.Marsh, M., and A. Pelchen-Matthews.2000. Endocytosis in viral replication. Traffic1:525–532.
21.McClure, M. O., M. Marsh, and R. A. Weiss.1988. Human immunodefi-ciency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J.7:513–518.
22.McKnight, A´., D. J. Griffiths, M. Dittmar, P. Clapham, and E. Thomas. 2001. Characterization of a late entry event in the replication cycle of human immunodeficiency virus type 2. J. Virol.75:6914–6922.
23.Nichols, B.2003. Caveosomes and endocytosis of lipid rafts. J. Cell Sci. 116:4707–4714.
24.Nichols, B. J.2002. A distinct class of endosome mediates clathrin-indepen-dent endocytosis to the Golgi complex. Nat. Cell. Biol.4:374–378. 25.Owens, C. M., B. Song, M. J. Perron, P. C. Yang, M. Stremlau, and J.
Sodroski.2004. Binding and susceptibility to postentry restriction factors in monkey cells are specified by distinct regions of the human immunodefi-ciency virus type 1 capsid. J. Virol.78:5423–5437.
26.Pelchen-Matthews, A., I. Boulet, D. R. Littman, R. Fagard, and M. Marsh. 1992. The protein tyrosine kinase p56lck inhibits CD4 endocytosis by pre-venting entry of CD4 into coated pits. J. Cell Biol.117:279–290. 27.Popik, W., and T. M. Alce.2004. CD4 receptor localized to non-raft
mem-brane microdomains supports HIV-1 entry: identification of a novel raft localization marker in CD4. J. Biol. Chem.279:704–712.
28.Popik, W., T. M. Alce, and W. C. Au.2002. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for produc-tive entry into CD4⫹T cells. J. Virol.76:4709–4722.
29.Reuter, S., P. Kaumanns, S. B. Buschhorn, and M. T. Dittmar.2005. Role of HIV-2 envelope in Lv2-mediated restriction. Virology332:347–358.
30.Sandvig, K., B. Spilsberg, S. U. Lauvrak, M. L. Torgersen, T. G. Iversen, and B. van Deurs.2004. Pathways followed by protein toxins into cells. Int. J. Med. Microbiol.293:483–490.
31.Sandvig, K., and B. van Deurs.2002. Transport of protein toxins into cells: pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett.529:49–53. 32.Schmidtmayerova, H., M. Alfano, G. Nuovo, and M. Bukrinsky.1998. Hu-man immunodeficiency virus type 1 T-lymphotropic strains enter macro-phages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J. Virol.72:4633–4642.
33.Schmitz, C., D. Marchant, S. J. D. Neil, K. Aubin, S. Reuter, M. T. Dittmar, and A´. McKnight.2004. Lv2, a novel postentry restriction, is mediated by both capsid and envelope. J. Virol.78:2006–2016.
34.Sheehy, A. M., N. C. Gaddis, J. D. Choi, and M. H. Malim.2002. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature418:646–650.
35.Simmons, G., A. McKnight, Y. Takeuchi, H. Hoshino, and P. R. Clapham. 1995. Cell-to-cell fusion, but not virus entry in macrophages by T-cell line tropic HIV-1 strains: a V3 loop-determined restriction. Virology209:696– 700.
36.Simmons, G., J. D. Reeves, A´. McKnight, N. Dejucq, S. Hibbitts, C. A. Power, E. Aarons, D. Schols, E. De Clercq, A. E. I. Proudfoot, and P. R. Clapham. 1998. CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. J. Virol.72:8453–8457. 37.Simmons, G., D. Wilkinson, J. D. Reeves, M. T. Dittmar, S. Beddows, J.
Weber, G. Carnegie, U. Desselberger, P. W. Gray, R. A. Weiss, and P. R. Clapham.1996. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J. Virol.70:8355–8360.
38.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski.2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature427:848–853.
39.Towers, G., M. Collins, and Y. Takeuchi.2002. Abrogation of Ref1 retrovi-rus restriction in human cells. J. Virol.76:2548–2550.
40.Wolf, A. A., Y. Fujinaga, and W. I. Lencer.2002. Uncoupling of the cholera toxin-G(M1) ganglioside receptor complex from endocytosis, retrograde Golgi trafficking, and downstream signal transduction by depletion of mem-brane cholesterol. J. Biol. Chem.277:16249–16256.
41.Zheng, Y. H., D. Irwin, T. Kurosu, K. Tokunaga, T. Sata, and B. M. Peterlin. 2004. Human APOBEC3F is another host factor that blocks human immu-nodeficiency virus type 1 replication. J. Virol.78:6073–6076.
42.Zufferey, R., D. Nagy, R. J. Mandel, L. Naldini, and D. Trono.1997. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol.15:871–875.