Copyright( 1973 AmericanSociety forMicrobiology Printed inU.S.A.
RNA
Synthesis by Vesicular Stomatitis
Virus
and
a
Small Plaque
Mutant:
Effects of
Cycloheximide
GAIL W. WERTZ ANDMYRON LEVINE
Department of Human Genetics, University of Michigan Medical School, Ann Arbor, Michigan48104 Received forpublication15March1973
The synthesis of viral RNA by wild-type vesicular stomatitis virus
(L,VSV)
and a small, plaque-size mutant (S2VSV) was studied in vitro and in chicken embryo (CE) andmouseL-cellcultures.Virus-specific RNA synthesized in CEor Lcells infected with either
L,
orS2VSVatlowmultiplicitywasof thesamesize classes, 12to15S, 28S, and38S. Themajor differenceswerein theproportion ofRNAproduced of each size class.
L,VSV
alwayssynthesized largerproportionsof 38SRNA, and S2VSVproduced larger proportionsof 12 to15SRNA.BothS2andL,VSV
exhibited RNA transcriptase activity in vitro and in cell culture. The products of the in vitro reactionwerethesame, 12to15S for both. The products ofthe virion-associated transcriptase in CEorL-cell cultures inthepresenceofcycloheximide werealso thesameforbothviruses but differed from theinvitro products in that 28Sand 12to 15SRNAwere made. Theeffects ofaddition of
cycloheximide at various times after infection demonstrated that new protein synthesis isrequired early (0-2 h) for both S2 and
L,VSV
toinitiate andmaintain the normal rate of viral RNA synthesis. However, the overall rate of RNA synthesis inL,VSV
infections becameindependent of protein synthesis after 2 h whereas therateinS2VSVinfections didnot. With eithervirus, synthesis of 38S RNA didnotoccurin the absenceofprotein synthesis. Moreover, continuous38S RNAproduction required continuous protein synthesis. Production of38SRNA ceased within 30min after addition ofcycloheximide toS2- orL,VSV-infected
CE or L cells that had already begun to synthesize the 38S form. The cycloheximide-induced cessation of38SRNA synthesis was accompanied bya marked increaseinproduction of 12to15Sand28SRNA inL,VSV-infected
cells, butno increase insynthesis of small RNA species occurred in S2VSV-infected cells.Previously, we reported the isolation of a
large plaque strain ofthe Indiana serotype of vesicular stomatitis virus, called
L,VSV
(wild type) and a small plaque-size mutant, S2VSV (23). Both viruses elicited equivalent titers of circulating interferoninmice at 12h, although the wild-type virus was approximately 1,000timesmorelethal than thesmall plaquemutant
(23). In both chicken embryo (CE) andmouse L-cell cultures, the
L,
and S2mutantsdiffered in ability to shut off host RNA and proteinsynthesis, in ability to stimulate synthesis of interferon, andinefficiency of virusproduction
(20, 21).
L,
produced an average yield of 150PFU/cell
in L cells, whereas S2 yielded anaverage of 6 PFU/cell. Both viruses replicated moreefficiently in CEcells, giving an average
yield
of 850PFU/cell
forL,
and130PFU/cell
forS2. The relative
yield
ofthetwoviruseswasnot related to the effects of interferon produced during the infection.The kinetics of RNA
synthesis during
L,
and S2 infections of L cells alsowere foundtodiffer (20). Therate ofRNAsynthesis
directedby
S2
in Lcells reached a maximum at 2 to 3h after infection and declinedgraduallyoverthenext 4 h.Very little viral RNA wassynthesizedby8 h after infection. In contrast, RNAsynthesis di-rectedby
L,
didnotreach amaximalrateuntil6 to 8 h postinfection, although there was an
early peakat2h.InCEcells, therewas asteady
increase in viral RNAsynthesiswith bothviral
strains, until a maximum rate was reached at
approximately3to5h after infection. Progeny 253
on November 10, 2019 by guest
http://jvi.asm.org/
virus particles first appeared at about 2 h in
both infections.
In view of these observations, a detailed
examination of
L,
and S2viral RNA synthesis was undertaken in an attempt to explain thedifferences in virus yields. Since VSV has an
RNA-dependent RNA transcriptase that func-tions in vitro (1, 2, 3) and incell culturein the presence ofcycloheximide (9, 12), it was possi-ble to examine transcription apart from other viralreplicative events.
This paper reportsthe examination of RNA
synthesis directed by S2 and
L,VSV
both in vitroandinCE and L-cellcultures. Evidenceis presented indicating that the reduced viral yield of S2VSV is not due to a defect in thevirion-associated RNA transcriptase butis the resultof some defect in subsequent viral RNA
synthetic events. In addition, we show that
continuous protein synthesis is necessary for
production of 38S viral RNA.
MATERIALS ANDMETHODS
Media and cell cultures. Eagleminimal essential mediumsupplemented with 10% heat-inactivated calf serum wasused for growth and maintenance of all cell cultures.
Primary cultures ofCE cells were prepared from 11-day-old embryos. Theembryos were minced and treatedwith trypsin, and after centrifugation 1 ml of packed cells was suspended in 650 ml of growth medium. Asample (4 ml) of this material was used to seed 60-mm petri dishes. These cultures were used in experiments after 48 h of incubation at 37 C in a humidifiedatmosphere of 5% Co2. At this time each plate contained approximately 2 x 106cells.
A continuous line of mouse Lcells(clone 929)was maintainedasmonolayersandwassubcultured every 7dayswithonechangeofmedium. Culturesinpetri dishes were prepared by scrapingthe cells from the glass with a rubber policeman, suspending them in growthmedium, andseeding2 x 10f cellsper60-mm petriplate.These cultureswereused after incubation for12h inahumidifiedatmosphereof 5%CO2at37C. A continuousline ofbaby hamsterkidney (BHK)
cells was grown in monolayercultures and used for propagationofvirus.
Virus. The Indianastrain ofVSVwas propagated
in CE or BHK cell monolayers. Cell cultures were inoculated withvirus at alowmultiplicityof infection (0.001)toavoid formation ofincomplete, autointerfer-ing T particles (6, 8). Afteranadsorption periodof 60 minat37C, mediawereadded and the cultureswere incubated for 18 h. Infected culture fluids were harvested, pooled, and centrifugedat1,000 x gfor 15 min to remove cellular debris. The clarified fluids werecentrifuged at 54,450 x gfor 90minat5C.Virus pellets were resuspended in EDTA to 1/100 the volume of thestarting material and treatedfor 30 s in a250-W,10-kcycleRaytheonsonicoscillatorto
disag-gregate clumps of virus. Concentratedvirus suspen-sions werefurtherpurifiedbytreatmentwith
fluoro-carbon by the method of Hackett et al. (6). The
partially purified virus concentrates were layered on preformed, linear 15 to 45% sucrose gradients and centrifuged at 20,000 rpm for 105 min at 10 C in a Spinco model L-2 rotor (SW25.1). Fractions were collected from the bottom of the gradient with
moni-toringat280nminacontinuousflow cell of aGilford recordingspectrophotometer. A single peak of absorb-ance at 280nm and a corresponding single visible band indicated the presence of only infectious B particles.
Chemicals. Cycloheximide, purchased from Nu-tritional Biochemicals Corp., was diluted in medium and used at a final concentration of 50
Ag/ml.
Ac-tinomycin D was purchased fromSchwarz/Mann and used at afinal concentration of2 gg/ml. Uridine-2-"C (specific activity, 58 mCi/mmol), uridine-5-6-3H (specific activity, 43 Ci/mmol), and 3H-UTP (specific activity, 15 Ci/mmol) were from Schwarz/ Mann.Incorporation of labeled precursors. Viral RNA synthesis was studied inmonolayer cultures contain-ing2 x 106CEorL cells perdish. Cells were infected atthe desiredmultiplicity of virus in the presence of 2
,ug
ofactinomycin
D per ml. This concentration ofactinomycin inhibits 98% of host cell RNA synthesis within 30 min (20). Uninfected control cultures were prepared simultaneously in all experiments. Aftera 30-minabsorption period at 37 C, the fluids contain-ing the inoculum were drawn off and replaced with2 ml of fresh medium containing actinomycin D. A sample (2ml) of culture medium containing radioac-tive uridine (2-5 gCi/ml) was added to each cell monolayer at indicated intervals. Total cellular incor-poration oflabel was measured as follows. After the labeling period, the fluid containing the label was discarded and the cells were washed twice with phosphate-buffered saline (PBS), resuspended by scraping in PBS, and mixed withanequal volume of cold10%trichloracetic acid. Thetrichloroacetic acid-precipitable fraction was collected by suction filtra-tion on Whatman GF/c filters. Thedry filters were treated with 0.5 ml ofNCS solubilizer(Amersham/
SearleCorp.) for 30minat22 C. Asample (10 ml) of toluene-based scintillation mixturewasaddedtoeach solubilized sample. The samples were counted in a Packardliquidscintillationspectrometer.
Sucrose gradient analysisofcytoplasmicRNA. Cells exposed to radioactive uridine as described above were washed and suspended in reticulocyte
standard buffer(RSB: 0.01 M Tris [pH
7.41-0.01
M NaCl-0.0015 MMgCl2).The cellsweredisrupted by15 to 20 strokes of a motorized Duallhomogenizer.
Efficiency ofdisruption was >95% and wasalways
monitored by phase-contrast microscopy. Intact nu-clei wereremovedbycentrifugation at750 xgfor 2 min. The supernatant fluid(cytoplasmicextract)was
made 1% with respect to sodium dodecyl sulfate
(SDS). These solubilized extracts wereanalyzed by
velocity sedimentation in 15 to 30% (wt/wt) linear sucrose gradients containing 0.01% SDS in buffer (0.01 MTris, pH 7.5;0.1 MNaCl;0.001 MEDTA). Centrifugation wascarried out at20C for 15.5 h at 23,000 rpmintheSW25.1rotorinaSpincomodelL-2. After centrifugation, the gradients were
on November 10, 2019 by guest
http://jvi.asm.org/
tionated and monitored for absorbanceat260nmby
pumping from the bottom of the tube through the continuousflow cell ofaGilfordrecording
spectropho-tometer. Radioactivity present in the acid-insoluble portion of each fraction was assayed as described
above. Ribosomal 18S and 28S RNA present inthe cytoplasmic extracts and detected by absorbanceat 260nmwereusedasmarkers inallgradients.
In vitro RNA polymerase assay.
Virion-associated RNApolymeraseactivitywasassayed by
incubating purified virus particles in a standard
reaction mixtureconsisting of: 15
Amol
of Tris-hydro-chloridebuffer, pH 7.5; 1.5 Mmoleach of theunlabeled ribonucleoside triphosphates, ATP, GTP, and CTP; 0.02 ;smol of3H-UTP(100 MCi/Mmol);and 0.25mgof Triton N-101 in atotalvolume of 300gliters. Reac-tionswereallowedtoproceedinsealedtesttubesand were terminated by addition of 0.5 ml of 0.08 Msodium pyrophosphate. Carrier yeast RNA and cold 10%trichloroacetic acidwereadded, and
acid-precipi-table material was collected on GF/c filters and assayed forradioactivityasdescribed above.
RESULTS
Sedimentationproperties of RNA from
pu-rifiedS2and L1virions.No differences inrate
ofsedimentation insucrose orinparticle mor-phology, asdeterminedby electronmicroscopy, have been detected between
L,
and S2 virions (Youngner and Wertz, unpublished data). In addition, the sedimentation coefficients of RNA from infectious S2orL,
virions were examined and foundtobe similar.%2
andL,
werepropa-gated in BHK cells infectedatlowmultiplicity (0.001) in the presence of actinomycin D and
3H-uridine. The respective virus pools were harvested and purified by banding in sucrose
gradients. The single peak of virus presentwas collected and dialyzed against RSB toremove
sucrose. Samples of purified S2 and
L,
virus were made 1% with respect to SDS and the released labeled RNAwasanalyzed byvelocity sedimentation insucrose. Asingle peak of RNAwhich sedimented at38Swaspresentin bothS2 and
L,
preparations. This finding indicatedno difference in the size ofthe RNAreleased from infectious S2 and L1 virions. This observed sedimentation coefficient is in agreement with previous estimates (36-43S) for RNAmolecules released fromVSV Bparticles (4, 10, 14, 16,18). Comparison of L1- and S2-virus-specificRNA synthesis in cell culture. Virus-specific RNAwasexamined in cellsinfected with
L,
orS2todetectanyvariations insizeoramountof RNA synthesized. Actinomycin D-treated CE orLcellswereinfected withaninput multiplic-ity of three. At various times after infection cells were exposed to 3H-uridine. At the end of the labeling period the cells were harvested and cytoplasmic extracts were prepared and
solubi-lized by addition of SDS. RNA synthesized during intervals from0to2, 2to4, and 4to6 h
postinfection was examined on sucrose
gradi-ents. In all cases, uninfected, control cultures
were prepared simultaneously. The radioactiv-ity present in the gradients from control
cul-tures was background level except for the 4S
area, indicating that the actinomycin D treat-ment used was effective in inhibiting cellular
RNAsynthesis. The labelpresentinthe4Sarea
of the control gradients was apparently due to
terminal labeling of transfer RNA which is
I
0
10 20 30 40 50
FRACTION NUMBER
FIG. 1. Velocity sedimentation analysis of RNA synthesized inactinomycin D-treated CEcellsafter infection with S2VSV(0)orL,VSV (0). Cellswere
exposedto4ACiof3H-uridinefor designated intervals after infection with S2 or L, ata multiplicity of3.
Afterthe labeling period, cytoplasmicextracts were
prepared, made 1% with SDS, and analyzed by centrifugation insucrose-SDSgradients for radioac-tivityincorporated intoacid-insoluble material0to2
hpostinfection (a),2to4hpostinfection (b),and 4to
6hpostinfection (c).
A ~~~~~28S s
28 S IeSsI
6 B It~~~~~2
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.498.259.449.218.566.2]resistant to the presenceofactinomycin D (5). Control counts have been subtracted from
ex-perimental values in allfigures. Figure 1 shows
the profile of virus-specific RNA species
ex-tracted from
S2,
or L1-infected CEcells. The RNA species synthesizedin cellsinfected withL,
at low multiplicity during each time interval fall into three major size classes: (i) small, 12to15S; (ii) intermediate, 28S;and(iii)large, 38S. The total amount ofRNA
synthe-sizedduringeach of the intervals increasedfrom
early to late times. Table 1 shows the relative
amounts ofRNA in thethree majorsizeclasses synthesizedduringthe observed time intervals. These values were obtained by summing the areas under the peaks ofthe profiles in Fig. 1, but are representative of at least three other
separateexperiments.In
L,-infected
cells the12to15Sspecies constituted thelargestfractionof
total RNAsynthesizedduringalltimeintervals,
and the proportion represented by 12 to 15S
materialwasapproximatelyconstantwithtime.
Intermediate 28S RNA was synthesized at a
fairly constant levelat alltimes. Previouswork (7, 13, 22)suggeststhat 12 to 15Sand28SRNA
can function as mRNA. The 38S RNA, which we presumecorrespondsto maturevirionRNA,
was synthesized in constant orslightly
increas-ing amounts from early to late times. These results for wild-type,
L,
RNA synthesis are in basic agreement with those reported by others (7, 13, 15, 16, 17, 18, 19, 22) with variationsprobably due to cell type, strain of virus, multiplicityofinfection, andexperimental
pro-cedure.
RNA synthesis in S2-infected cells was char-acterizedby synthesisof thesamesize classesof
RNA seen in L, infections. Differences were
evident, however, in the amount and time of
synthesis of these RNA classes. As withL1, the
total amountof RNAsynthesized inS2-infected
cells
during
each of the observed intervals increased fromearly
to late times(Fig.
1).
However,
the accumulation of radioactiveuri-dineinto totalviralRNAwasgreater inCE cells
infected with
S2
than inthose infected withLl.
In contrast to
L, infections,
the proportions of12 to 15S RNA
steadily
increased while theproportions
of28Sand38S RNAdecreased. Thelargest
proportion
ofS2
38S RNA wassynthe-sized
early
andthendecreasedtoless than3%ofthe total
during
the 4 to 6-h interval. Theproportions
of each of the three main sizeclassesofRNA
synthesized
duringeachintervalby
L,
andS2
werethesameinCEand L cells. Insummary, the
major difference
betweenL,
andS2
infections was in the proportions of RNAproduced
of each size class.L,
synthesizedlarger
proportions of38SRNAand, in contrast,S2 produced
greater proportions of 12 to 15Smaterial. The reducedamounts of
intracellular
38S RNA in
S2
infections compared withL,
infectionswasnotduetomorerapid maturation and release of
S2
virions as revealed by exami-nation of extracellular fluids for released, la-beled 38SRNA.In vitro RNA synthesis. To
determine
whetherthealteredpatternof
S2
RNAsynthesiswasduetoanalteredordefective transcriptase
(1,
2, 3),
S2
andL,
virionswereexamined forinvitro RNA
polymerase
activity.Equal amountsof virion
protein
containingequivalent titers ofplaque-forming
units were incubated in thestandard reaction mixture described above.
Both
S2
andL,
particles
have an enzymaticactivity
thatstimulates incorporationoflabeledribonucleotides
into RNA. The incorporationwas linear with time and with
increasing
amounts of viral protein for both viruses and
resulted in approximately equal amounts of
acid-insoluble
product.
Adifference inoptimal conditionsfor
expres-TABLE 1. Distributionoflabeled RNA among three majorsizeclasses
Small(12-15S) Intermediate(28S) Large(38S) Total
3H-uridine
Hourspost
~~~~~~~~~~~~~~~~~~~incorporated
infection Mutant Counts/ Counts/ Counts/ during
each
min% min % min %
~~~~~~~~~~interval
min min min (counts/min)
0-2 L1 4,208 58 2,292 31 794 11 7,294
S2 14,219 62 6,640 29 1,827 8 22,686
2-4 L, 23,066 55 13,383 32 5,224 13 41,673
S2 56,541 70 19,906 24 4,896 6 81,339
4-6 43,276 57 25,631 34 6,621 9 75,528
S2 773,888 76 20,249 21 2,791 3 96,925
J.-VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
sion of in vitro enzymatic activity wasobserved.
15-Equal amounts of virus (15
,ug
of protein) wereLI
L VSVincubated in the standard reaction mixture for
14_
60 min at the temperatures indicated in Fig. 2 F and then assayed for total radioactivity incor-
13-porated
intoacid-precipitable
material. TheL1
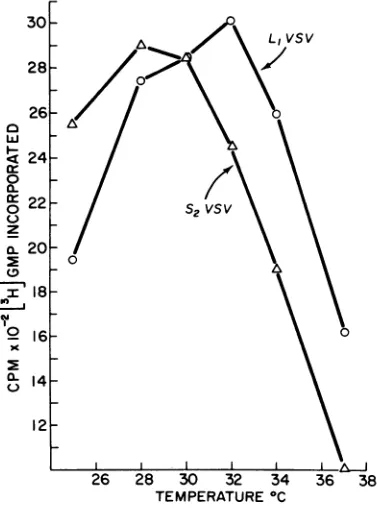
a polymerase activity displayed a temperature 12-optimumof32C,whereasS2 polymerase exhib- g / s2vsv
ited
optimal
activity
at 28 C. Inaddition,
a cr~
/series ofsamples was assayed inwhich the pH of I
the reaction mixtures was varied but the tem-
C I01-
0perature
was held constant(Fig.
3).
Both vi-3
/0
ruses
displayed
a rather broadoptimum
of9L
/ 0activity,
with a
peak
at
pH
7.6 for
L,
and
peaks
N
at
7.2 and 7.6 for
S2.
Thesedifferences are not
8
surprising, perhaps,
in apreparation
ofwhole
/
virus particles. For the present, we were satis-
7-fied
tolearn
that bothL,
andS2
virions did havetranscriptase activity.
6
examinedonsucrose-SDS gradients.The
stand-ard
reaction mixture was incubated for 60
mmin
l
with 15,ug of either
L1
orS2.
The reaction was 7.0 7.2 7.4 7.6 7.8 8.0 8.2 8.4terminated by addition of SDS to a final con- pH
FIG.
3.
S2VSV
and
L1VSV
RNA
polymerase
activ-ity
as afunction of pH.
Equal
quantities
ofS2
orL,
(15 ,g
of
protein)
wereincubatedfor60min
at30C in30
-
f'
the standard reaction mixture containing 15Mlmol
of~
7S
/ \ L,
VSV
Tris
buffer
at the different
pH
values
indicated.28 V Reactions were terminated and
assayed
for
radioac-a
o~ \
\
tivity incorporated
into acid-insoluble
product.26
/
centration of 1%
and
the
solubilized
mixturesw were immediately layered on 15 to 30%
sucrose-!
24- SDS gradients and processed as described in0 - Materials and Methods. Controls containingno
2s
virus wereprocessed
simultaneously.
The only22 S2 VSV synthesized by S2 L1
X 20/ conditions was approximately
2 0 results concerning the size of the in vitro
prod-0-
\ uct are in agreement with those of previousX: 18 workers (2, 3), who also demonstrated that the
products
were
complementary
to virion
RNA°
16
\
°
0and that therefore
the
enzyme
could
properly
be termed a transcriptase., 14-
Virion-directed
RNA synthesis in absenceof
protein
synthesis.
Having
determined that12 -
L,
andS2 particles
both possess an RNAtran-scriptase activity
that functions in vitro, the,
,
,
,
,
^j
following
experiments
were designed
to
examine
26
28
30
32
34
36
38
the activity
of
the
L,
and
S2
virion-associated
TEMPERATURE
OC
transcriptase
in
CE
and
L cells.
Actinomycin
FIG.
2.
S2VSV
and L1VSV RNA
polymerase
activ-
D-treated
cells
were infected
with
equal
multi-ity
as afunction of temperature. Equal quantities
of
plicities
of
L,
and
S2
in
the presence
or absenceL,
orS2
(15
ug of protein)
were incubatedfor
60min
in. .
,thestandard reaction mixtureat each of theindicated
ofcycoexidide
(gin
anden
os
totemperatures.
Atthe
endof
the incubationperiod,
radloactive
uridine.
This
concentration
of
cy-reactions were
terminated and
assayed for
radioactiv-
cloheximide
inhibits 96% of CE or L-cell
protein
ityincorporatedin acid-insoluble product. synthesis within 20
min.
At intervals afterVOL.12,1973
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.498.257.445.68.313.2] [image:5.498.53.242.333.587.2]infection, cells were harvested and assayed for
incorporation of radioactive uridine into
acid-insoluble product. It is presumed that theonly
RNAsynthesisin thepresenceofcycloheximide wouldbe due to thevirion-associated
transcrip-tase (9, 12). Figure 4a shows that cumulative RNA synthesis in Lcells infectedwith
L,
orS2in the presenceof cycloheximide was
approxi-LIVSV
S2vsv
2 3
[image:6.498.77.220.172.585.2]Hours after Infection
FIG. 4. CumulativeRNA synthesisinL (a)orCE
(b) cells in the absence (upper curves) orpresence
(lower curves) ofcycloheximideadded atthetimeof infection. Actinomycin D-treated cells were infected
withanequal multiplicity (17) of L,orS2 andexposed
to "4C-uridine. At indicated intervals cellswere
har-vestedandassayed for radioactivityincorporatedinto
acid-insoluble material.
*0,
a.
o 0x
mately 8% of that in the absence of the drug. Here, as in all subsequent experiments, unin-fected control cultures were prepared simul-taneously, and control values have been
sub-tractedfromexperimental valuesinallfigures.
As observed previously (Fig. 1), in CE cells
infected with equal multiplicities of S2 or
Ll,
the total amount of RNA synthesized was
greater in
S2-infected
cells than inL,-infected
cells. This is true also for the amount ofRNA synthesized in the presence of cycloheximide;
L,
andS2
RNA production was 5 and 10%,respectively, ofthe total amount of viral RNA
synthesizedinthe absenceofthedrug(Fig.4b). The RNA
produced
intheabsenceofprotein synthesis was analyzed by velocitysedimenta-tion in
sucrose-SDS
gradients. The purpose ofthisanalysiswastocompare thespeciesofRNA made by
S2
andL,
underthese conditions andto see whether these species of RNA
corre-spondedinanyway to viral RNAtranscribedin vitroor toviral RNAsynthesizedincellsinthe
presenceofprotein synthesis.
Figure 5 shows thatpredominately 12to 15S and 28S RNA molecules weresynthesized dur-ingthefirst 3.5 h after
L,
andS2
infection ofCEorL cellsinthe presenceofactinomycin Dand cycloheximide. No38S RNAwasformed. These resultsconfirm theprevious observationof Mar-cus et al. (12), that VSV RNA
synthesis
doesoccur in cells in the absenceofprotein
synthe-sis, and alsothe report ofHuang and Manders (9), that intracellular RNAtranscription results
inproduction ofboth 28S and 12to 15Sspecies
ofRNA, whereas in vitro synthesis results only
in the production of RNA smaller than 15S. Additionally,thesereports (9, 12) demonstrated
that the RNA
produced
in the presence ofcycloheximide
was complementary to virionRNA.
Viral RNA
synthesis
in the presence ofcycloheximide
added at intervals afterinfection. The following
experiments
wereper-formed to determine the effect ofinhibition of
protein synthesis on the rate of viral RNA
synthesis that is in progress. Actinomycin
D-treated L and CE cells were infected with
L,
orS2RNA
at amultiplicityof10.Atintervalsstart-ing at the end of theadsorption period,
dupli-cate samples of each of the infections were
labeled with 3H-uridine for 20min. The control rates ofviral RNA synthesis in the four
infec-tions are shown in Fig. 6
(solid
lines). At the times indicated by arrows,cycloheximide
(50,g/ml)
was added to a series ofduplicatecul-tures which were subsequently examined at
intervals for the rate of uptake oflabeled uri-dine. The results presented in Fig. 6 (broken 258
on November 10, 2019 by guest
http://jvi.asm.org/
2 o ,
u 500
2 6 10 14 18 22 26 30 34 38 42 46 50 54 FRACTION NUMBER
FIG. 5. Velocity sedimentation analyses of RNA made inS2VSV- orL1VSV-infected CE or L cells in the presence of cycloheximide. Actinomycin D-treated cells were infected in the presence of cycloheximide with an equal multiplicity (17) of
L,
orS2. 14C-uridine was added and thecultures were incubated at 37 C for 3.5 h.Cytoplasmic
extracts were made 1% with SDS and analyzed on sucrose-SDS gradients for radioactivity incorporated into acid-insoluble material.lines) show the effect of
cycloheximide
on therate ofLlorS2
synthesis.
Ineither celltypein-fected with
S:,
cyclohemixide prevented
anyfurther increase in the rate of RNA
synthesis.
With continued incubation with
cycloheximide
the rate either remained constant or declined.
Similarly,
addition ofcycloheximide during
thefirst 1.5 to 2 h after infection of CE orLcells with
L,
prevented the subsequent increase inthe rate of RNA synthesis that was observed
in untreated control cultures.
Surprisingly,
however, addition of cycloheximide to
L,-in-fected cells after1.5 to 2hpostinfection hadno
inhibitory effect on the subsequent rate of
RNA synthesis. Rather, the rate of
L,
RNA synthesis increased compared with untreated controls when the drug was added after 1.5 to 2 h. Multiplicity of infection had no effect onthis phenomenon. S2or
L,
infections ofCE orL cells carried out at multiplicities ranging
from1to 100gaveessentially similarresults.
In view of these results it was of interest to determine the species of RNA synthesized
undertheseconditions. Because wehave
previ-ously demonstrated that no 38S RNA is made when protein synthesis is inhibited from the start of infection, the following experiments weredesignedto examine RNA synthesis in the presence ofcycloheximideadded at a time after
synthesisof38SRNA had already begun.
ActinomycinD-treated CE cellswereinfected
with
L1
orS2
at amultiplicity
of 3. At 2 hpostinfection, onesetofcultureswas
exposed
to 3H-uridine for 1h.Thisgroupofcellswasthen harvested, andcytoplasmic
extracts wereanalyzed
on sucrosegradients
forspecies
oflabeled RNA. At 3 and 4 h
postinfection,
cycloheximide
wasadded to one setofcultureswhile anothersetremained untreated. Bothsets of cultures were
exposed
tolabeled uridine 30min after the addition of
cycloheximide.
One hour after addition of3H-uridine(4.5 and5.5hpostinfection),
thecultures
were harvested andcytoplasmic
extracts wereexamined in sucrosegradients.
The
profile
of RNA species synthesized ininfected cells labeled2 to3h after infectionwas
identical tothe results shown in
Fig.
1B. BothL,
andS2
weresynthesizing
38S RNA at thetime
cycloheximide
was added. Figure 7com-paresRNA
synthesized
inL,-infected
CE cellsin the presence or absence of cycloheximide.
Similar resultswere obtained in L cells. In the
absence ofcycloheximide, 12 to 15S, 28S, and 38S RNA species were synthesized.Total
inhi-bitionof38SRNAsynthesisoccurredwithin 30 minafteradditionofcycloheximide.The cessa-tion of38S RNA synthesiswasaccompaniedby increasedproductionof 12to15S and 28S RNA.
Synthesis
of 12 to 15S material inon November 10, 2019 by guest
http://jvi.asm.org/
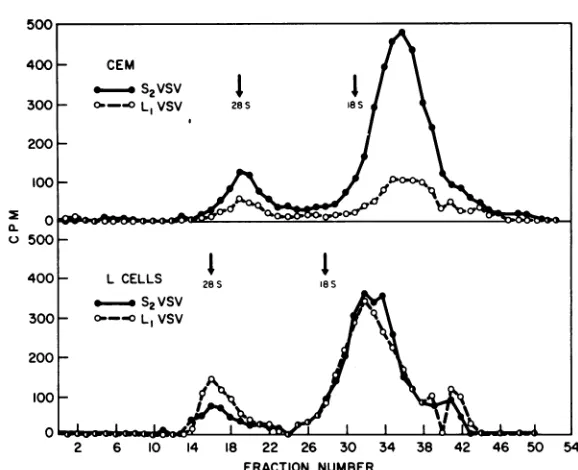
[image:7.498.104.393.62.297.2]WERTZ AND LEVINE
Li
VSV in L CELLSHOURS AFTERINFECTION S2VSV in LCELLS
° C3hr
E700C
O600 C4hr
2
400 /
wIQ20 CIhr/ r/
4 C0t\
2
z~300~ 4
HOURS AFTER INFECTION
[image:8.498.98.418.63.460.2]Hours after Infection
FIG. 6. Effect of cycloheximideon the rateofS,VSVorL,VSVRNA synthesis. CE- orL-cell monolayers
wereinfectedwith S2orL,atamultiplicity of 10. At intervals after infection cellswereexposedfor 20minto
3H-uridine and thenassayed for incorporation of radioactivity into acid-insoluble material. Starting from time
zero,at times indicated byarrows, cycloheximide (50 iig/ml) wasaddedtoduplicatesetsof cultures. At the indicated intervalsafter addition of cycloheximide these cultureswereexposed for20minto3H-uridine in the
presenceof the drug and subsequently assayed for radioactivitypresentinacid-insoluble material. Therateof viral RNAsynthesis in the absence(@-) orpresence(0--0) of cycloheximide is shown.
mide-treated cellswasincreasedby110to130% of that in the absence of thedrug. Synthesisof
28Smaterial increasedby approximately20% of that in untreated cells.
Similarly, addition of cycloheximide to
S2-infected CE cells (Fig. 8) resulted in shutoff of 38S RNAsynthesis. However, incontrast tothe results observed in
L,
infections noincrease insynthesis of 12 to 15S or 28S RNA species
occurred. In fact, a decrease of 5 to 20% was
observed for both classes of RNA. Here again,
similar results wereobtainedin L cells.
It should be noted that in both the S2 and
L,
infectionsachangein theshapeof the28Speak
occurred. In the absence ofcycloheximide this
peakwasbroad, indicating heterogeneity. RNA
synthesized after addition of cycloheximide, however, sedimented in this area in a sharp, homogeneous peak.
DISCUSSION
The results presented here suggest that the reduced particle yield of S2VSV compared to
L,VSV
is not traceable to a defect in the260
J. VIROL.800r
LI VSV In CEM
w
z
a
w
4
on November 10, 2019 by guest
http://jvi.asm.org/
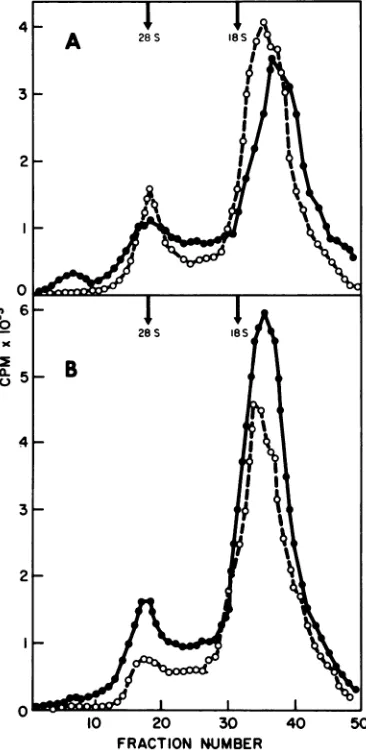
VSV RNA SYNTHESIS: NEED FORPROTEINSYNTHESIS
4 that would account for the reduced yield of S2
virions.
A 28 S 18S When total
viral-specific
RNA synthesiswasexamined inCEorL-cell
cultures,
itwasfoundthatS2 and L1 direct synthesis of the same size
I § classes ofRNA, i.e., 12 to 15S, 28S, and 38S.
The
major
difference betweenL,
orS2
infections2_ inthis
regard
wasintheproportion
of each sizeclass
of RNAproduced.
L,
always synthesized
AoLI1 28S18
0 3
3
-~~~~~
L) 4 - 2818-k1l <
32--
I
do
~~~~~~~I
0~~~~I2IZ I X t SA1 §\10 20 30 40 50 4_-t
fRACTION NUMBER
FIG. 7. Velocitysedimentation analysis ofL1VSV It
RNAsynthesized in CE cells in the absence (0)or 3
presence (O)ofcycloheximide(50
1gg/ml).
Duplicate 3_ 1setsofactinomycinD-treated CE cellswereinfected h h with L, at a multiplicity of2. At 3 (a) or4 (b) h r postinfection cycloheximidewas addedto onesetof 2- f
cultures. Thirtyminutes afteradditionof
cyclohexi-mide bothsetsofcultureswereexposed for60minto4n
'UCi of 3H-uridine.At the end ofthe labeling period t\ fE
cytoplasmic extracts were prepared, made i% with I _f_1
SDS,
andanalyzed by centrifugation
insucrose-SDSrgradientsforradioactivityinacid-insoluble material. _ I~~~~~
~~I
II 6virion-associated RNA
transcriptase.
Both S50° lo 20 30 40 and LFexhibittranscriptase activity
in vitro fRACTION NUMBERandincell culture.
Further,
theproducts
SLVSV
ofthe FIG.8.
Velocity sedimentation analysis of in vitro reaction have similar sedimentation(0) orRNA synthesized in CE cells in the absence coefficients(0) of(12
to15S)
for bothS,
andlAg/ml).
L,
The presence cycloheximide (50 DuplicateRNA
products synthesized by
thetranscriptase
setsof actinomycinD-treated CE cells wereinfectedin CESLor L-cell cultures in the presence of with at a multiplicity of 3. At 3 (a) or 4 (b)
cycloheximide
arealso thesameforbothS,
and hpostinfection cycloheximidewasadded tooneset ofL,
but differfromtheinvitroproducts
inthat cultures. Thirty minutes after addition of cyclohexi-mito15S and 28S RNAare made.Therefore,by mide bothsetsof cultureswereexposed for 60 min to41the
pof8H-uridine. At theand
we have measu red , y Ci end of the labeling period,the parameters we have measured, S2 viriotl- cytoplasmic extracts were prepared, made 1% with associated
transcriptase
functions at least as SDS, and analyzed by centrifugation in sucrose gradi-wellLdasthatofand,
apparently,
hasnodefect ents forradioactivity in acid-insoluble material.VOL.12,1973
261
on November 10, 2019 by guest
http://jvi.asm.org/
[image:9.498.260.443.170.545.2]larger proportions of 38S RNA, and, in contrast, S2 produced greater proportions of 12 to 15S material (Table 1 and Fig. 1). Because the RNA inS2 and
L,
particles is38S, these findings may indicate that the poor viral yield ofS2 compared withL,
is due to the failure of S2 to produce adequate amounts of this class of RNA.Our results confirm the finding (9) that 38S
RNA is not produced unless protein synthesis occurs afterVSVinfection. The enzymatic
ma-chinery brought into the cell by the virion is sufficient to produce only 12 to 15S and 28S
RNA (Fig. 5), whichiscomplementarytovirion RNA (9). Production of 38S RNA appears to require the synthesis of new protein(s) or at least themodification of pre-existing molecules. The data reported here extend these findings.
First, theresultsoftheexperiments showing the effect ofcycloheximide on rate ofRNA synthe-sis suggest that there are two phases in the synthesis of viral RNA, an early and a late
phase. The early phase extends through the
first 1.5 to 2h
postinfection. During
thisperiodthere seems to be little difference in
L,
or S2infections, especiallywithregardtothe effect of
cycloheximide on rate of RNA synthesis. Cy-cloheximide prevented any further increase in therate ofRNA synthesis asseen in untreated
controls. At times after 2h, however, there are markeddifferences in effect of proteinsynthesis inhibitionon
L,
and S2 RNAsynthesis.Therateof RNAsynthesisin
L,
infectionswasenhancedby
cycloheximide-induced
inhibition ofprotein
synthesis, whereas in S2 infections the rate of RNA synthesis remained constant or declined
after addition ofthe drug.
Secondly,
our data demonstrate that production of 38S RNA re-quires continuousproteinsynthesis.
Addition ofcycloheximidetoS2-or
L,-infected
CEorLcells that had alreadybegunto synthesize 38S RNA resulted in cessation of 38S RNA production within 30 min after addition of thedrug.
Con-comitant with the shutoff of 38S RNA forma-tion inL,-infected
cellswas astriking
increase insynthesis of12 to 15S and 28SRNA.Inhibi-tion of protein synthesis in S2 infections also caused cessation of38SRNA production; how-ever, in contrast to the findings with
Li,
noincrease insynthesis of 12 to 15S or 28S RNA occurred. These observations will be discussed in reference to several hypotheses concerning
the mechanism of VSV RNAreplication.
One hypothesis is
that,
afterinfection,
the input 38S RNAistranscribed to 12to15S and 28S messenger RNA. Theinput38S RNA would also serve as template for the synthesis of acomplementary 38S
molecule,
which would inturnserve asthetemplate forthereplication of progeny RNA. This mechanism of replication might require, at the least, the alteration of the pre-existing virion transcriptase to allow syn-thesis of acomplementary 38S templateand/or
the synthesis of a new enzyme, an RNA
repli-case, capable ofreplicatingprogenyvirion RNA from thistemplate.
A second class of models would suppose, as above, that the input38S RNA is transcribed to complementary 12 to 15S and 28S RNA but that no complementary 38S RNA is made by usingthe input 38S RNA as template. Rather, a
38S complementary template could be formed by ligation of small RNA molecules which would then serve as template for 38S RNA replication. A second form of this model sug-gests that progeny 38S RNA would result from replication of the 28S and smaller RNA species by using the small complementary molecules as template. These products would then be ligated
to form progeny 38S particle RNA.
Our presentdata cannot distinguish between the models described above for 38S RNA forma-tion.
However,
our observations confirm that protein synthesis must occur after infection in order to have any 38S RNA production, andthey also show that there must be continued proteinsynthesis in order to have continued 38S RNA formation. The increased production (or
perhaps accumulation) of 12 to 15S and 28S RNA when protein and, concomitantly, 38S RNAsynthesisisinhibitedin
L,
infections maybe interpretedin a number of ways.
(i) If, as suggested in the second model, 38S RNA formation occurs via a ligation step (to make either template or virion nucleic acid),
then shutoff of protein synthesis might cause shutoff ofligaseproduction, and therefore small
moleculeswouldbeexpected toaccumulate.An extension of this supposition which is also
applicable to the first model arises from the finding that38SRNA formation stopswithin 30 min after protein synthesis inhibition. This
observation indicates that the new protein
re-quiredfor38SRNA formation is eitherunstable
oris used upsoon after itssynthesis. This may suggest that the putative ligase or replicase
becomes a part ofthe VS virion, which could accountfor its short activity span. The protein mayfunction, bepackaged, and leave the cellas apart ofthevirion,orit may functiononlyafter it has become a part ofthe virion.
(ii) An alternative explanation for the
in-creased production of 28S and 12 to 15S RNA after inhibition ofprotein synthesismay be the elimination ofcompetition fortemplate. When
262 J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
the protein(s) necessary for 38S RNA produc-tion isno
longer
being
made,
template
would be available only fortranscription,
which would thenoccur in the presenceofcycloheximide
(9,12; Fig. 5) at an increasedrate.This
possibility
mightoccurunder either model forreplication.
Kiley and Wagner (11) have
suggested
that formationof38S RNA mayoccurvia aprocessinvolving RNA ligation.
They
envisionedliga-tion of the 23S and 28S RNAs present in nucleocapsids. We,
however,
do not observe apeak of RNA in the 23S
region
ofgradients
of cell extracts infectedatthe lowmultiplicity
ofinfectionused. Onlyin infectionsat
multiplici-ties ofapproximately35or
higher
doweobservea definite peak of RNA
sedimenting
at 21S. This is the approximate sedimentation coeffi-cient ofTparticle
RNA in our system (Wertz and Levine,unpublished
data).
Finally, there is the observation in S2
infec-tions that although 38S formation halts after
cycloheximide
additionjust
asinL,
infections,
no increase in 28S and 12 to 15S production
occurs.
Rather,
28S and 12 to 15Ssynthesis
proceeds attherateachievedbefore additionof
the drug, or declines
slightly.
Because it isapparent that
S2
can make 38S RNA and indeed cansynthesize greatertotal amountsofRNA thancan
L1,
wemustconclude that thereis no absolute defect in the
S2 replication
process. As previously
noted,
there appears to bean early and alatephase
inS2
and L1RNAsynthesis. S2 and L1 behave
similarly
in the early phase butnotthelate.Ifwesupposethatthe
early phase
involvespredominantly
tran-scriptive functions and the late phase
encom-passes a switch to RNA
replication,
then itcould be that
S2
has a greateraffinity
for the transcriptive process than thereplicative
proc-ess.Clearly,
the mechanismofVSVreplication
isnotyetresolved. Weare
currently investigating
(i) the
complementarity
of the RNA speciesmadeinthe absence andpresence of
cyclohexi-mide, (ii) the
possibility
that the 28S and smaller RNA species are precursors to 38S RNA, and (iii) the appearance ofnew proteins which correlate with the effects ofcyclohexi-mide treatment. We hope that more informa-tion on these points will
help
delineate themechanismofVSV replication.
ADDENDUM INPROOF
Wehaveexamined the labeled RNA, which is syn-thesizedinincreased amountsafteradditionof cyclo-heximide, by annealingto excessunlabeled 30S viral RNA. Both the 28S and 12 to 15S RNA species are
almost completely complementary to VSV 38S (-)
strand RNA. This finding indicates that the increase in 28S and 12 to 15S RNA species after addition of cycloheximide late in infection isanincrease in tran-scription, probably due to availability of additional
newtemplate.
ACKNOWLEDGMENTS
ThisinvestigationwassupportedbyPublic Health Service grantGM-15419-06 from the National Institute for General Medical Sciences.
We thank Patricia G. Voss for her competent technical assistance.
LITERATURE CITED
1. Baltimore, D., A. S. Huang, and M. Stampfer. 1970. Ribonucleic acid synthesis of vesicular stomatitis virus. II. AnRNApolymerase in the virion. Proc. Nat. Acad. Sci. U.S.A. 66:572-576.
2. Bishop, D. H. L. 1971. Complete transcription by the transcriptase of vesicular stomatitis virus. J. Virol. 7:486-490.
3. Bishop, D. H. L., and P. Roy. 1971. Kinetics of RNA synthesis by vesicular stomatitis virus particles. J. Mol. Biol. 57:513-527.
4. Brown, F., S. J.Martin, and B. Cartwright. 1967. The
ribonucleicacids of the infective and interfering com-ponents ofvesicular stomatitis virus. J. Gen. Virol. 1:479-486.
5. Franklin, R. M. 1963. The inhibition of ribonucleic acid synthesis in mammalian cells by actinomycin D. Bio-chim.Biophys. Acta 72:555-565.
6. Hackett, A. J., F. L. Schaffer, and S. H. Madin. 1967. The separation of infectiousand autointerfering parti-cles in vesicular stomatitis viruspreparations. Virology 31:114-119.
7. Huang, A. S., D. Baltimore, and M. Stampfer. 1970. Ribonucleic acid synthesis of vesicular stomatitis virus. III. Multiple complementary messenger RNA mole-cules.Virology 42:946-957.
8. Huang, A. S., J. W.Greenawalt,andR. R. Wagner. 1966.
Defective T particles of vesicular stomatitis virus. I. Preparation, morphology and some biological proper-ties.Virology 30:161-172.
9. Huang, A. S., and E. K. Manders. 1972.Ribonucleicacid synthesisof vesicularstomatitisvirus. IV. Transcrip-tion by standard virus in the presence of defective interfering particles. J. Virol. 9:909-916.
10. Huang, A. S., and R. R. Wagner. 1966. Comparative sedimentation coefficients of RNA extracted from
plaque-forming and defective particles of vesicular stomatitis virus. J.Mol. Biol. 22:380-384.
11. Kiley, M. P., and R. R. Wagner. 1972. Ribonucleic acid species of intracellular nucleocapsids and released vions of vesicular stomatitus virus. J. Virol. 10:244-255.
12. Marcus, P. I., D. L.Engelhardt. J. M. Hunt, and M. J. Sekellick. 1971.Interferon action:inhibition of vesicu-larstomatitis virusRNA synthesis induced by virion-boundpolymerase. Science 174:593-598.
13. Mudd, J. A., and D. F. Summers. 1970. Polysomal ribonucleic acid of vesicular stomatitis virus-infected HeLacells.Virology42:95-968.
14. Petric, M., and L. Prevec. 1970. Vesicular stomatitis virus-a new interfering particle, intracellular struc-tures, and virus-specific RNA. Virology41:615-630. 15. Schaffer, F. L., A. J. Hackett, and M. E. Soergel. 1968.
Vesicular stomatitis virus: complementarity between infected cell RNA and RNA's from infectious and
263
on November 10, 2019 by guest
http://jvi.asm.org/
autointerfering viralfractions.Biochem.Biophys. Res. Commun.31:685-692.
16. Schincariol,A. L.,and A. F. Howatson.1970.Replication ofvesicular stomatitis virus. I. Viral specific RNAand nucleoprotein in infected L cells. Virology42:732-743. 17. Schincariol,A.L., and A. F. Howatson.1972.Replication ofvesicular stomatitis virus.II.Separation and
charac-terization of virus-specific RNA species. Virology 49:766-783.
18. Stampfer, M., D. Baltimore, and A. S. Huang. 1969. Ribonucleic acidsynthesis of vesicular stomatitis virus.
I.Species of ribonucleic acid foundinChinese hamster
ovarycells infected with plaque-forming andefective
particles.J. Virol.4:154-161.
19. Wagner, R. R., M. P. Kiley, R. M. Snyder, and C. A. Schnaitman.1972.Cytoplasmic compartmentalization
ofthe proteinandribonucleicacidspeciesof vesicular stomatitis virus. J.Virol. 9:672-683.
20. Wertz, G. W., and J. S. Youngner. 1970. Interferon production and inhibition of host synthesis in cells infected with vesicular stomatis virus. J. Virol. 6:476-484.
21. Wertz, G. W., and J. S. Youngner. 1972. Inhibition of protein synthesis in L cells infected with vesicular stomatitis virus. J. Virol. 9:85-89.
22. Wild, T. F. 1971. Replication of vesicular stomatitis: characterization of the virus-induced RNA. J. Gen. Virol.13:295-310.
23. Youngner,J. S., and G. Wertz.1968.Interferon produc-tion in mice by vesicular stomatitis virus. J. Virol. 2:1360-1361.