JOURNAL OF VIROLOGY, Nov. 1973,p. 1034-1042 Copyright 01973 AmericanSociety forMicrobiology
Vol.12,No.5 Printed in U.S.A.
Antigenic Components
of
Group
A
Arbovirus
Virions
J. M. DALRYMPLE, S. N. VOGEL, A. Y. TERAMOTO,1 AND P. K. RUSSELL Departmentof Virus Diseases, Walter ReedArmyInstitute ofResearch, Washington, D.C.20012
Received forpublication 11June1973
ThreegroupAarboviruses, Sindbis (SIN),western (WEE) andeasternequine encephalitis (EEE), were selectively degraded with a nonionic detergent
toyieldacoreparticle andasolubleenvelopecomponent.Antigenic analysisby using radioimmuneprecipitation techniquesrevealed markedantigenic
similar-ityamongthecoreparticlesof the three viruses. The soluble envelopecomponent
exhibitedantigenic specificitysimilarto that ofintact virions. A close relation-ship between SIN and WEE envelopes was shown, whereas EEE envelope antigen appeared antigenicallyspecific. These data indicate that nucleocapsids ofgroup Aarboviruses contain anantigenic determinant commontothegroup; the envelope contains virus-specific antigens as well as antigens which relate
members ofasubgroup.
Antigenic relationships among the group A
arboviruses havebeen established primarilyon
the basis ofcomplement fixation,
hemaggluti-nation inhibition, and neutralization test re-sults by usingunpurified antigen preparations
derived from infectedsuckling mouse brain (7,
8, 10, 11, 28). These tests have exhibitedvarying degreesof both groupandtype specificity. The
individual antigenic determinants contributing
totheobservedreactionshave notbeenclearly defined, although bothgroup-andtype-specific determinants mustreside onthe virionsurface because type-specific as well as cross-reactions have been observed by using purified virion
preparations (12, 13, 21, 22, 26).Group-specific nucleoprotein antigens have beendescribed for
other RNA viruses (3, 14, 18, 19, 23), but only limited information (12) has been obtained for
arboviruses.
Group
A arboviruses have been disrupted by a variety of methods to yield envelope and nucleoprotein antigens (1, 2, 4, 15, 16). Radioimmune precipitation (RIP) tech-niques allowed precise determination ofanti-genic relationships between subunits of
de-graded virions of three group A arboviruses.
This investigation describes theantigenic
rela-tionships of the RNA-rich "core" particle and the detergent-solubilized "envelope" compo-nents.
MATERIALS ANDMETHODS
Viruspropagation.Virusesselectedforthese stud-ies were Sindbis (SIN) strain AR339 (29), westem
'Present address:DepartmentofPathology, Universityof
CaliforniaMedicalSchool, Davis, California95616.
equine encephalitis (WEE), MacMillan strain (17), and theCambridgestrain of easternequine encepha-litis (EEE) virus (6). Viruses were propagated in primary chicken embryo cell cultures infected with suckling mouse brain seed virus at a multiplicity of infection ofapproximately 10. Procedures for protein radiolabeling with3H-aminoacid mixtures and virion
purification by ammonium sulfate precipitation and sucrosegradient centrifugation have been previously described (13). RNA radiolabeling included the addi-tion of "4C-uridine (New England Nuclear Corp., Boston, Mass.) to infected cell cultures in conjunction with the 3H-amino acid mixture.
Preparation of virus subunit antigens. Purified virion preparations were treated with the nonionic
detergent Nonidet P-40 (Shell Chemical Co., N.Y., N.Y.) atafinal concentration of 0.1% and incubated at 4C for10minwithfrequent mixing.Separation of virion components was accomplished by rate-zonal centrifugationon10 to 40% sucrosedensity gradients. Gradients were prepared by using a programmed gradient pump(InstrumentationSpecialtiesCo.,
Lin-coln,Neb.) with RNase-freesucrose(Schwarz/Mann, Div. ofBecton, Dickinson & Co., Orangeburg, N.Y.) in a buffer consisting of0.05 M Tris-hydrochloride,
pH 7.4, 0.1 MNaCl, and0.3%heat-inactivated fetal bovineserum.Centrifugationat200,000xginanSW 50 L rotor (Spinco) for 50 min resulted in two
separable radioactive components.
Polyacrylamide gel electrophoresis (PAGE).
Separation and identification of virion polypeptides
was performed on 7.5%acrylamide gels according to themethodofMaizel(20).SamplesforPAGEanalysis
wereheatedat 100Cfor 10 min in 1%sodiumlauryl
sulfate and 1% B-2-mercaptoethanol prior to gel
analysis. Gel sliceswere incubated ina scintillation cocktailfor 12hat37Cpriortocounting. Theliquid
scintillation counting cocktail consistedof 3% Proto-soland4.6%Liquifluor (NewEnglandNuclearCorp., 1034
on November 10, 2019 by guest
http://jvi.asm.org/
plication of radioimmuneprecipitation proceduresto
the study ofgroupAarboviruses has beenpreviously
described (13). Antiseratothesevirusesconsisted of
mousehyperimmune ascitic fluids (MHAF) prepared
according to the method of Brandt et al. (5) and modified by the use ofSarcoma 180 cells to induce ascites (24). Antisera to degraded virus components were prepared asabove with the exception that all
immunogenpreparationsweretreated with0.1%
For-malin for 12 h at 37C, followed by 96 h at 4C to
insure complete inactivation of infectivity. Residual infectious viruscouldnotbe detected by either plaque
assayinprimary chicken embryo cellsorintracerebral
inoculation of suckling mice.
RESULTS
Antigenic specificity of intact virions. Puri-fied virion preparations of each of the three viruses were examined by RIP tests by using antisera (MHAF) from animals immunized withinfected sucklingmousebrain suspensions (Fig. 1). Virionswereprecipitated maximally by theirhomologous antiserumoverawiderangeof antibody dilutions; however, reciprocal cross-reactions were demonstrable between SIN and WEE viruses at somewhat lower antiserum dilutions.Precipitation of EEE virus appeared specific by comparison in that very little EEE viruswasprecipitated by SINorWEEantisera. Conversely, EEE antisera didnot react appre-ciably with SINorWEEviruses. These results agree with the previously reported close rela-tionship betweenWEE andSIN viruses and the serologicallyseparateEEEvirus (9). To
investi-gatethe hypothesis that antibodies of differing specificities resulted fromseparateantigen moi-eties in the immunizing antigen preparation, virion components were prepared for antigenic analysis.
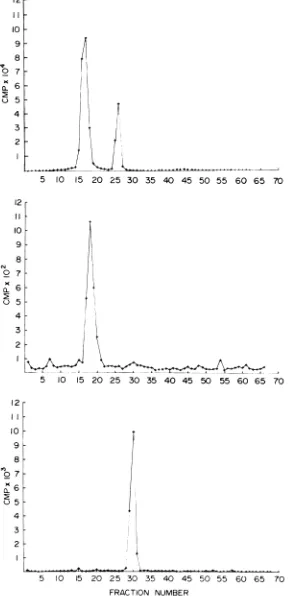
Characterization of subunit antigens. Treatmentof3H-aminoacid-labeledSIN virion preparations with the nonionic detergent NP-40 and subsequent rate-zonal centrifugation in a sucrose gradient resulted in twoseparable pro-tein peaks (Fig. 2A). No remaining intact vi-rionswereevident intreated preparations, indi-cating complete disruption by the detergenttoa particle of approximately 140S and a soluble fraction. Untreated virions centrifuged on an identical gradient resulted in a single peak of radioactivity near the bottom of the gradient. Similar NP-40 treatment of preparations la-beled with 8H-uridine yieldeda single peak of radioactivity(Fig. 2B), indicating that all of the virus RNA was contained in theparticle sedi-mentingat140S.Detergenttreatmentof virions labeled with 3H-glucosamine resulted in a
ra-glucosamine label in the sedimenting particle indicatedahigh degreeofpurity ofeach prepa-ration.
Intact SIN virionsand the products of NP-40 disruption (Fig. 2A) were analyzed by PAGE. SIN virion preparations exhibited the
charac-teristic polypeptide composition previously de-scribed (27): the 53,000 mol wt membrane glycopeptide and the smaller30,000molwt core
associated polypeptide (Fig. 3). The RNA-rich, non-glycosylated, 140S particle contained only the 30,000 mol wt polypeptide, whereas the nonsedimenting glycosylated fraction
con-sisted entirely of the larger 53,000 mol wt protein. Purified virion preparations of WEE and EEE virus were similarly treated with essentially identical results. PAGE analysis of
core and envelope preparations indicated that each was essentially free of contamination by theother.
Serological reactivity of envelope andcore
antigens. The RIPreaction of corepreparations
from each ofthe threeviruseswithhomologous and heterologous antisera is shown in Fig. 4.
Nucleocapsid antigens were cross-reactive to
the extent that differentiation of the
homolo-gous reaction was
impossible. Although
end point titerswere not asgreat as thoseobserved with these antisera and virion antigens,cross-reactivity was increased, especially with EEE nucleocapsid antigen.
The SIN and EEEantiserahadhightiters of anti-core antibody and effectively demon-strated the extent of the cross-reactivity
be-tween core antigens. The WEE antiserum, on
the otherhand, exhibited somespecificity, but extensive cross-reactions with SIN and EEE
nucleocapsids
were evident.Soluble
envelope
antigens were examined inthesamemanner,and theresulting RIPprofiles
are
presented
inFig. 5.Envelope
antigens were more specific than core antigens and strongly resembled intactvirions inRIP
reactivity. Ho-mologous reactions were most prominent; theexpected
cross-reaction was observed with SIN and WEE viruses, and EEE virus envelope antigen was relatively specific. Again, asob-served with nucleocapsid antigens, the max-imum precipitation and antibody titers were
lower than observed when using intact virions.
Reactivity of antisera to virion, envelope, and nucleocapsid antigens. SIN virions, as well as nucleocapsid and envelope antigens,
were used as separate immunogens for the preparation ofspecific antibody. Reactions of
MHAF prepared against each ofthe SIN
on November 10, 2019 by guest
http://jvi.asm.org/
1.0 2.0 3.0 4.0 5.0 6.0
1.0 2.0 3.0 4.0 5.0 60
1.0 2.0 3.0 4.0 5.0
LOG I/ANTISERUM DILUTION
6.0
FIG. 1. Radioimmuneprecipitation profiles of purified radioactive (0) SIN, (0) WEE, and (A) EEE virions
with mouse hyperimmuneasciticfluids. Virusantisera wereanti-SIN(top panel), anti-WEE(middle), and
anti-EEE (lower panel).Rabbit antimouseserum was usedasthesecondaryprecipitation antibody. Percent precipitation isplottedaspercentageofRIPversus thelog of the reciprocal of the virus antibody dilution.
10:36 100
%RIP
40
20
100
80
60
% RIP
40
20
100
80
60
% RIP
40
20
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.501.127.385.28.654.2]I0 20 30 40 50 60 70 80 90 100
B
10 20 30 40 50 60 70 80 90 100
I
C*Wsis wos"toi it 1 I I I I
10 20 30 40 50 60 70 80 90 100
FRACTION NUMBER
FIG. 2. Sedimentationof detergent-disruptedSIN virus.PurifiedSIN virionsintrinsicallylabeledwith (A) 3H-aminoacids, (B) 3H-uridine,and(C)3H-glucosamineweretreatedwith 0.1% NP-40at4C for20min prior
tocentrifugation. Centrifugationwasperformedina4.8-ml10to40%o(wt/vol)sucrosegradienton a0.1-ml70%o
sucrosecushionat200,000xgfor50min.Fractions(0.05 ml)werecollectedvolumetrically fromthebottomof
the tube.
10:37 0
0t 0x L
6r
5H
4
0
x
23
2
6
5
4
N
0
23
2
I, .
i
I I
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.501.103.396.39.619.2]12
II
10
079j8_
4
3
2
5 10 15 20 25 30 35 40 45 50 55 60 65 70
12
I I
011
10
9
8
2 7
0 5
4 3
2
5 10 15 20 25 30 35 40 45 50 55 60 65 70
i12
I0
9
8
to7
057
4 3
2
5 10 IS 20 25 30 35 40 45 50 55 60 65 70
[image:5.501.113.404.34.631.2]FRACTION NUMBER
FIG. 3. Polyacrylamide gel electrophoresis of 3H-amino acid-labeled SIN virion (top), soluble envelope (middle), andnucleocapsidcomponents(bottom). Priortoelectrophoresis, sampleswereheatedat100Cfor10 min in 1%SLS and1%2-ME.Electrophoresis wasperformedin8%gelsinabuffercontaining0.1%SLSat 60V untila bromophenol bluedyemarker hadmigratedthroughtheentiregellength. Migrationwasfrom leftto right. Gels werefrozenand sliced into 1-mmfractions.
1038
on November 10, 2019 by guest
http://jvi.asm.org/
1.0 2.0 3.0 4.0 5.0 6.0
1.0 2.0 3.0 0.4 5.0 6.0
1.0 2.0 3.0 4.0 5.0
LOG I/ANTISERUM DILUTION
6.0
FIG. 4. Radioimmune precipitation profiles of purifiedradioactivenucleocapsid preparations from (0)SIN, (0) WEE,and(A)EEEvirus,with(A) SIN, (B) WEE,and(C)EEEvirusmousehyperimmuneasciticfluids. Antigenconcentrationswereadjustedtoapproximately250countsperminper0.05 ml.
1(39
60-%RIP
40-20
0
100
80
60-% RIP
40-
20-
100-%RIP
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.501.126.374.30.623.2]100
% RIP
40
20-0
_
1.0 2.0 30 4.0 5.0 6.0
100
B
80-- 60-% RIP
40-20-_
1.0 20 3.0 4.0 5.0 6.0
00_ c
80
60 % RIP
40
20
0 T
1.0 20 3.0 4.0 5.0 6.0
LOG
l/ANTISERUM
DILUTIONFIG. 5. Radioimmuneprecipitation profiles of purifiedradioactive solubleenvelope components of(0)SIN, (0) WEE,and(A)EEEdetergentdisruptedvirus with(A)SIN,(B) WEE,and(C)EEEmousehyperimmune
asciticfluids.Equivalent antigenconcentrationwasestimatedby radioactivityatapproximately250 countsper minper0.05ml.
1040
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.501.137.383.29.625.2]Antisera
Antigen Anti- Anti- Anti- Anti-infective virion envelope nucleo-virus (%) (%) (%) capsid
Sindbis virion 98a 70 80 50
Sindbis nucleo- 94 60 0 90
capsid
Sindbisenvelope 96 25 40 0
WEEvirion 92 40 0
aMaximum precipitation occurring at optimal antibody concentration in the standard RIP test.
gens areshown inTable 1.ASIN virus MHAF preparedin the conventionalmanner, byusing
infected suckling mousebrain asthe immuno-gen, has been included for comparison. This
antiserum reacted with all antigens tested, including the cross-reactive WEE virion, and precipitated all antigens nearly100%. Antisera to Formalin-inactivated SIN virions
(antivi-rion) likewise reacted with all antigens, but
maximum RIP was lower, especially with the envelope antigen. Antisera to envelope antigen reacted with both SIN virion and envelope antigens and even exhibited a cross-reaction with WEE virion, but did not react with
nu-cleocapsid
antigen. Antisera to SIN virusnu-cleocapsid
gave maximum RIP withhomolo-gous
nucleocapsid
antigen and did not react with either envelope antigen or WEE virion;however, it did unexpectedly react with SIN virion.
DISCUSSION
Detergent disruption of purified group A
arbovirus virions allowed the resolution oftwo
virus components which differed
markedly
intheirantigenic specificity. Core particles, which contained all ofthe virus RNA as well as the 30,000 mol wt
polypeptide,
were broadly cross-reactive when using antisera prepared withinfected suckling mouse brain immunogens. Thecross-reactions ofantisera (MHAF)to SIN and EEE viruses with heterologous core anti-gens were ofnearly thesame magnitudeas the homologous reactions, although no cross-reac-tions between these two agents occurred with intact virions orenvelope antigens. The WEE antiseraalso cross-reactedextensivelywith core
antigen; however, the homologous titers were
significantly higher than heterologous titers. This group-reactive nucleocapsid antigen ap-pears analogous to the common
ribonucleo-tained only the larger53,000 mol wt glycopep-tide,exhibited antigenic relationships similar to
thoseofthe intact virion.SIN and WEEviruses were closely related and did not cross-react
appreciably withEEE virus antisera. Although
all radioactive envelope protein migrated as a
single peak when using this method of polya-crylamide gel electrophoresis, convincing evi-dence has been presented which indicatesthat twoseparate virus glycopeptides are present in
the53,000molwtcomponent (25).It is
reasona-bleto suggest thatonce separated, one orboth of these glycoproteins may be virus-specific antigen(s).
The RIP reactionsofantisera to envelope and
nucleocapsid showed no antigenic relationship
between envelope and core antigens.
Anti-envelope reacted with both homologous SIN
and closely related WEE virions as expected;
however, anti-SIN nucleocapsid also reacted
with intact SIN virion. It seems unlikely that
SIN nucleocapsid
antigen is exposed on the surface of theintact virionbecause WEEviriondid not react, even though this antiserum
re-acted strongly with cores prepared from all
threeviruses.
LITERATURE CITED
1. Acheson, N. H., and I. Tamm. 1970. Purification and properties ofSemlikiForest virusnucleocapsids. Virol-ogy 41:306-320.
2.Appleyard, G., J. D. Oram, and J. L. Stanley. 1970. Dissociation of Semliki Forest virus intobiologically
activecomponents. J.Gen. Virol. 9:179-189. 3. Bauer,H.,and W. Schafer.1966.Originofgroup-specific
antigen of chicken leukosis virus.Virology29:494-497. 4.Bose, H. R., and B. P. Sakik. 1970. Immunological
activity associatedwith thenucleocapsidandenvelope
components of anarbovirus. J. Virol. 5:410-412. 5.Brandt,W.E.,E. L.Buescher,and F. M. Hetrick.1967.
Production and characterization of arbovirusantibody
in mouse ascitic fluid. Amer. J. Trop. Med. Hyg.
16:339-347.
6. Byrne, R. J., G. R. French, F. S. Yancey, W. S.
Gochenour, P. K. Russell, H. H. Ramsburg, 0. A.
Brand, F. G. Scheider, and E. L. Buescher. 1964. Clinical and immunologic interrelationship among
Venezuelan, eastem and westem equine enceph-alomyelitisviruses. Amer.J. Vet. Res. 25:24-31. 7. Casals,J. 1944.Immunological relationshipsamong
cen-tralnervoussystemviruses.J.Exp.Med. 79:341-359. 8. Casals, J. 1957. Viruses: the versatileparasites. I. The
arthropod-bomegroup of animalviruses.Trans.N. Y. Acad. Sci. 19:219-235.
9. Casals, J.,and D. H.Clarke. 1965.Arboviruses;Group
A., p.583-605.In F. L. HorsfallandI.Tamm,(ed.),
Viral and rickettsial infections of man, 4th ed. J. B. LippincottCo., Philadelphia.
10. Clarke,D.H.,and J.Casals.1955.Improvedmethodsfor
hemagglutination studies with arthropod-bome vi-ruses.Proc.Soc.Exp.Biol. Med. 88:96-99.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.501.52.247.66.202.2]DALRYMPLE ET AL.
11. Clarke, D. H., and J. Casals. 1958. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-bome viruses. Amer. J. Trop. Med. Hyg. 7:561-573.
12. Dalrymple, J. M. 1972. Biochemical and biophysical characteristics of Venezuelan equine encephalitis virus. Venezuelanencephalitis. PanAmer. HealthOrg. Sci. Publ. No. 243:56-4.
13. Dalrymple, J. M., A. Y. Teramoto, R.D.Cardiff,and P. K.Russell. 1972. Radioimmune precipitation ofgroup Aarboviruses. J. Immunol. 109:426-433.
14. Geering, G., L. J. Old, andE.A.Boyse. 1966. Antigensof leukemias induced by naturally occurringmurine leu-kemia virus:their relationtotheantigensofGross virus and other murine leukemia viruses. J. Exp. Med. 124:753-772.
15. Goldblum,N., A. Ravid, Z.Ben-Tshai, and Y.Becker. 1970.Immunologicalproperties of subviralcomponents ofarboviruses. Proc. Tut. Conf.: Appl. ofVaccines against Viral, Rickettsial and Bacterial Diseases of Man. Pan Amer. HealthOrg. Sci.Publ. No.226:48-52. 16. Kaariainen, L., K. Simons, and C. H. Von Bonsdorff.
1969.Studies in subviralcomponentsofSemliki Forest virus. Ann. Med.Exp. Biol.Fenn.47:235-248. 17. Karabatsos, N., A. T. C. Bourke, and J. R. Henderson.
1963. Antigenic variation among strains of western equineencephalomyelitisvirus. Amer. J. Trop.Med. Hyg. 12:408-412.
18. Lief, F. S., A. Fabiyi, and W. Henle. 1958. Antigenic analyses of influenzavirusesby complementfixation. 1.Theproduction of antibodiestothe solubleantigen inguinea pigs. J. Immunol. 80:53-65.
19. Lief, F. S., and W. Henle. 1956. Studiesonthesoluble antigen of influenzavirus. 1.The releaseofSantigen from elementarybodiesbytreatmentwith ether.
Virol-ogy2:753-771.
20. Maizel, J. W. 1969. Acrylamide gel electrophoresisof proteinsand nucleic acids,p.35-48.In K. Habel and N. P. Salzman (ed.), Fundamental techniques in virology.Academic Press Inc., New York.
21. Mussgay, M., and M. Horzinek.1966.Investigationson
complement-fixing subunits ofa groupA arbovirus (Sindbis). Virology 29:199-204.
22. Mussgay,M., and R. Rott.1964.Studiesonthestructure ofahemagglutinating component ofa groupAarbo
virus(Sindbis). Virology 23:573-581.
23. Nowinski, R. C., L. J. Old, D. H. Moore, G. Geering, and E. A.Boyse. 1967. Asoluble antigen of themammary
tumorvirus.Virology31:1-14.
24. Russell, P. K., D. Chiewsilp, and W. E. Brandt. 1970. Immunoprecipitationanalysis of soluble complement-fixing antigens of dengue viruses. J. Immunol. 105:838-845.
25. Schlesinger, M. J., S. Schlesinger, and B. W. Burge. 1972. Identification ofasecond glycoproteininSindbis virus. Virology 47:539-541.
26. Stinski, M. F., and J. Gruber. 1971. Distribution of arbovirusantigens in density gradients. Proc. Soc. Exp. Biol. Med. 136:1340-1346.
27. Strauss, J. H., B. W. Burge, E. R. Pfefferkorn, and J. E. Damell. 1968. Identification of the membraneprotein and "core"protein of Sindbis virus. Proc. Nat. Acad. Sci. U.S.A. 59:533-537.
28. Taylor, R. M. 1967. Catalogue of arthropod-borne viruses of the world. Public HealthService Publ. No. 1760. GovernmentPrintingOffice, Washington, D.C. 29. Taylor R. M., H. S. Hurlbut, T. H. Work, J. R. Kingston,
and T. E. Frothingham. 1955. Sindbis virus:anewly
recognized arthropod-transmitted virus. Amer. J. Trop. Med.Hyg. 4:844-862.