JOURNAL OFVIROLOGY,JUlY1975,P.168-178 Copyrighti 1975 AmericanSocietyforMicrobiology
Vol. 16,No. 1
Printed in U.SA.
Regulation
of Tumor
Antigen Synthesis by Simian
Virus 40
Gene
A
PETER TEGTMEYER,'* MICHAEL SCHWARTZ, JOHN K. COLLINS, AND KATHLEEN RUNDELL
DepartmentsofPharmacology,* Microbiology,andAnatomy, Case WesternReserveUniversity School of
Medicine, Cleveland, Ohio44106
Received forpublication 28February1975
Simianvirus 40geneA haspreviously been showntopromotethereplication of viral DNAand thetranscription of late viral RNA in productive infection andto
maintain the growthcharacteristics ofsometransformed cells. The presentstudy
examines the effect of the A functiononproteins synthesizedduring productive
andtransforming infections. Under restrictive conditions, temperature-sensitive A mutants induce the overproduction of a 100,000-dalton protein both in
productively infected monkey cells and in transformed rabbit cells. Immuno-precipitation of theinduced protein with antisera, prepared against simian virus 40-induced tumors in hamsters, was used to identify the induced protein as
tumor antigen. The same protein can be precipitated from extracts of cells infected by wild-type virus but not from uninfected cells. Furthermore, the mutant-induced protein is more rapidly degraded in vivo and is less tightly
boundto intranuclear components than theprotein induced bywild-type virus. The presenceofthesame virus-induced protein in infected cells from different
species and the altered behavior of that protein in mutant infection strongly
suggest that the protein is virus coded. Because the protein islarge enough to accountforthe entirecoding capacityintheearly region of the simian virus 40
genome, the 100,000-dalton proteinmaywell be the primary product of the only
earlygene identifiedby complementation studies, the Agene.Ifthe
100,000-dal-tonprotein that isoverproduced inmutantinfection is the Aprotein and the only early protein, then functionalwild-typeAproteinmustregulate itsownsynthesis
inboth productive and transforming infections.
In productive infection by simian virus 40
(SV40),
gene A functioncontinuously regulatesthe initiation ofviral DNA replication (5, 21,
34) and transiently controls the synthesis of late
viralRNA(6, 21).Inrestrictive infection, theA
function is
required
to establish the stabletransformation ofcellsand alsotomaintain the
growth characteristicsof sometransformed cell
lines (3, 16, 22, 25, 35). Thus, it seems quite
possible that the A protein may directly interactwithspecific recognitionsites on either viral or cellular DNA to regulatethe replication or transcription of either DNA. A satisfactory
testingofthis hypothesiswill require the
iden-tification, isolation, and characterization of the
A protein and a study of its interaction with
different DNA molecules. These studies could
eventually lead to a better understanding of
growth control mechanisms in mammalian cells
atthe molecular level.
' Present address: Department of Microbiology, State Universityof New York atStony Brook, StonyBrook,N.Y. 11794.
168
A major obstacle to these studies has long
been the
difficulty
encountered in identifyingthe A protein within the large
background
ofcellular proteins (1, 15, 38, 39). We reasoned
that the synthesis,
location,
orprocessing oftheA protein could be perturbed in infection by
temperature-sensitive A mutants and that the
resultant alteration might be useful in
identify-ing the A protein. Thus a study of in vivo
protein synthesis in productive and transform-ing infections by the A mutants was under-taken.
Each of the A mutants tested induced the
overproduction of a 100,000-dalton protein at
the restrictivetemperature inboth monkeyand
rabbit cells. The induced protein was
specifi-cally and efficiently precipitated from extracts
ofproductivelyinfected or transformed cellsby
antiserapreparedagainstSV40-inducedtumors
in hamsters. A similar protein could not be
identified in uninfected cells. This and other
studies (2, 9, 13, 20, 26, 27, 29, 30, 33) suggest
thatSV40-induced tumorantigen is virus
on November 10, 2019 by guest
http://jvi.asm.org/
ed. Weproposethat theoverproduced protein is
the A protein and that functional A protein
regulates itsown synthesis.
MATERIALS AND METHODS
Cellcultures.TheTC7clone(31)oftheCV-1 line of monkey kidney cells was grown in Eagle basal
medium containing 2 to 5% fetal bovine serum. Transformed lines were derived from New Zealand White rabbit kidney cells (Flow Laboratories) as previously described (35) and were cultivated in
mediumwith 10% fetal bovine serum.
Virus. The origin of the parental wild-type clone
(WT)ofSV40and mutants A7, A28, and A30 has been previouslydescribed(36, 37).Mutants A40, A47, A57, and A58 were recently isolated from stocks of WT virus exposed tohydroxylamine.A58 fails to produce any detectable progeny at 39C. All of the other A mutants are leaky at 39C but produce little or no progeny at 41 C. Virus stocks were grown at 33 C after inoculation ofTC7 monolayers at 0.1 PFU/cell. Vi-rions were assayed at 33 and 40 C as previously
described (36).
Productive infection. ConfluentTC7 monolayers were inoculated with input multiplicities of 10PFU/
cell. Mockinfection wascarried out in the same way withlysates from uninfected cells. After a 2-h adsorp-tion period at room temperature, the inoculum was replaced with medium containing 2% fetal bovine serum.
Radioactive labeling of proteins. Productively
infected or transformedcells,grown in 8-ounce(about 240-ml) prescription bottles (45-cm2 cell growing area), were radiolabeled with ['"SJmethionine (New
England Nuclear Corp.; 40 to 60 Ci/mmol). Short pulses ofradioisotope (1 h) were carried out in
me-thionine-free mediuminasmuchaspreliminary stud-ies had shown that protein synthesis continued at a constant ratefor 3h under these conditions. Chases
wereperformedwithmedium containing a200-fold ex-cessofunlabeled methionine. Long pulsesof radioiso-tope(24 h) were performedwith ["S
]methionine
incompletemedium to providean adequate supply of aminoacids to maintain proteinsynthesis.The precise conditions for the radioactivelabelingofproteins are indicated in each figure legend.
Fractionation and extraction of radiolabeled
proteins. Cells were extracted with 0.5% Nonidet P-40(NP-40)inphosphate-buffered saline at pH 6 or in Tris-buffered saline at pH 7 and 8 as previously
described(38). Thedisruptedcells were spun at 2,000 xg for 10 minat 4Ctoseparatethecytoplasmfrom the nuclearpellet.All extraction buffers contained 0.3 mg ofphenylmethylsulfonyl fluoride per ml to inhibit proteaseactivityand0.001Mdithioerythritol.
Gelelectrophoresis. Samples were heated for 10
minat100Cinelectrophoresissample buffer contain-ing 0.075 MTris-sulfate, 2% sodium dodecyl sulfate
(SDS), 2% 2-mercaptoethanol, 15% glycerol, and
0.001% bromophenol blue, pH 8.4, and were then dialyzed against sample buffer containing 0.2% SDS
overnight at 4C. Sampleswere analyzed by
discon-tinuous polyacrylamide gel electrophoresis using a
modification of the method described by Maurer and
Allen (23). The separating gel contained 0.375 M Tris-sulfate, 20% acrylamide, and 0.1% bisacrylam-ide. The well gel contained 0.075 M Tris-sulfate, 5% acrylamide, and 0.12% bisacrylamide. The 0.065 M Tris-borate tank buffer contained 0.2% SDS. The gels were prepared and run on a slab gel apparatus (Hoefer, San Francisco, Calif.). Electrophoresis was carriedout at room temperature for 7 h at 25 mA/gel. The gels were fixed and stained with Coomassie blue according to Fairbanks et al. (8), vacuumdried, and autoradiographed on Kodak Royal X-omat medical X-ray film. Stained gels or autoradiograms were scannedwith aJoyce-Loebl densitometer to estimate the relative quantities of protein or radiolabel in individual bands in the gel.
Immunoprecipitation. Cytoplasmic fractions of cellular extracts were spun at100,000 x g for 30 min. The supernatantfluids(0.3ml)wereincubated with either preimmunization control serum or hamster antitumor serum(0.01ml)preparedagainst virus-free
SV40-transformed hamster cells. After 90 min at 30C, rabbit anti-hamster globulin (0.05 to 0.15 ml) was added at equivalence for another 90 min. The samples were centrifuged at 2,000 x g for 10 min at 4C, and the pellet was washed three times with extraction buffer. The immunoprecipitate and
re-imainingsupernatant fractions were heatedin electro-phoresis sample buffer for 10 min at 100 C and
analyzedby gel electrophoresis.
Immunofluorescentassay. The presence of
SV40-induced tumor antigen in infected cells was deter-mined by the indirect immunofluorescence tech-nique (27) with the same hamster antiserum used for theimmunoprecipitation studies.
RESULTS
Overproduction of a specific protein in
infection by A mutants. The patterns of pro-teinsynthesis in cells infected by WTor mutant
virus were compared to detect any significant perturbance that might provide clues to the function of theA gene orthe identity of the A
protein. The cells were separated into nuclear
andcytoplasmic fractions primarily to increase
resolution but also to comparethe distribution
ofproteinswithin cells. Thepatternsof protein
synthesis in uninfected cells varied depending
onthetemperature ofincubation, the lengthof
theperiodofradiolabeling, and thegrowthstate
of the cells. Thus, extracts of mock-infected cells were included in every experiment.
Figure 1 compares the patterns of proteins
labeled byashort pulse(1 h)with [35S
]methio-nine in cellsinfectedbyWTvirus or A30 at the permissive temperature (33 C), at the restric-tivetemperature (41 C),orafterashift from 33 to 41 C. WT virus induced the synthesis of virion
piroteins
VP1 (46,000 daltons), VP2(40,000 daltons), and VP3 (28,000 daltons)
equally well under each set of temperature
on November 10, 2019 by guest
http://jvi.asm.org/
100K-
*
.t,
s 4
; 4 e,
fi _ :!
I w
VP1-
Ita
b
c
t i t. 4; $
* X* #- - &
f
If
If
I
de e
d
e
f
f
t
100K
w. la ,. .10 e
41p 4 dip... . -...
* 9 t '
'f
g
hif
-VPi
g
h
i
VPI-
j41
vpt.
: '. y
L ;
-t
I
I
2 l- a
i1
4*x-oet- f-
-*w
-VP3
170
100K-
-100K
VP3-4 ...-._.m&
00
::,. .VplI jqf. 11
on November 10, 2019 by guest
http://jvi.asm.org/
conditions. Most of the capsid proteins were
found in the nuclear fraction ofthe cells even
though approximately 80% ofthe total labeled
protein was present in the cytoplasmic fraction
of the cells. Cells infected by A30 produced
virion proteins during continuous incubation at
33C but failed to produce capsid proteins
during continuous incubation at 41 C. In
con-trast,when cellsinfected byA30 for 72 h at 33 C were shifted to 41 C for 24 h and then labeled
with [35S]methionine for 1 h, capsid proteins
were synthesized at the same rate as in cells
infected by WT virus under the same
condi-tions. These findings support previous studies
(6, 21) showing that the A function is only
transiently requiredforthe transcription of late
viral RNA.
The most significant finding in infection by A30 at 41 C was the overproduction of a
100,000-dalton (1OOK)protein.This proteinhad
noapparentcounterpart inuninfectedcellsand
wasdifficult toidentify in cells infected by WT
virus. After extraction at pH 7, most of the
A-induced proteinwasfoundinthecytoplasmic
fraction of cells, but smaller quantities were
present in the nuclear fraction as well. The
overproduction ofthe.100KproteinbyA30 was
not the resultof a high multiplicityof infection
because the same protein was not present in
increased quantities in infection by the same
stockofvirus at 33 C. Nor wasthe
overproduc-tion of the protein caused by an absence of
capsid proteins incells infected byA mutants,
since the protein is also overproduced in cells
synthesizing capsid proteins after a shift from
33 to41 C (Fig. 1).Further,the excess synthesis
ofthe A-induced protein is a general
phenome-nonininfectionbyA mutants at 41 C inasmuch as each of seven independently isolated mu-tants (A7, A28, A30, A40, A47, A57, A58)
induced the
synthesis
ofthesame protein to asimilar extent. The excess
synthesis
is not theresult of a nonspecific inhibition ofviral DNA
synthesis because inhibition ofthe replication
of DNA
by
150,ug ofcytosinearabinosidepermlin cells infected by WT virus does not cause
overproduction
oftheprotein. The 100K proteinisalso madein excess 24and72haswellas48h
after infection by theA mutants at 41 C. This
finding excludes the possibility that the lOOK
protein isproduced in similar quantities in WT
and mutant infection but with analtered tem-poral sequence.
Identification of the A-induced protein as
tumor antigen. Cellular extracts, known to
contain the lOOK protein induced by the A
mutants, were exposed either to serum from hamsters bearing SV40-induced tumors or to serum taken from the same hamsters before immunization. Antigen-antibody complexes
werethenprecipitated by the additionofrabbit
immunoglobulin prepared against hamster
globulin. Theprecipitated andnon-precipitated
proteins were analyzed by SDS-gel electropho-resis (Fig. 2).TheA-inducedprotein was specif-ically and efficientlyprecipitated byantitumor
antibody but not to a significant extent
by
preimmunization control serum. Further, the
same protein could also be identified
unam-biguously in immunoprecipitates of cells
in-fected byWT virus butnot inuninfected cells.
The amount ofradioactivityinthe lOOKbands ingels ofthe supernatantand pellet fractionsof
the immunoprecipitation mixture was
com-pared byexcision oftheappropriate bands and
liquid scintillation counting. More than80% of
the lOOK proteinwas
precipitated by
antitumorserumwhereasless than2% was
precipitated
by
the control
preimmunization
serum. A similarquantitation can be seen indensitometer
trac-ings of an autoradiogram of the same gel (Fig.
3). Several other proteins with molecular
weights ranging from 66,000 to 88,000 daltons
were also immunoprecipitated in small
quan-tities from extracts of WT or
mutant-infected
cellsbutnotfrom extractsofcontrol cells.
Intracellular localization of the A-induced
protein. Carrolletal. have recently shown that
"T"antigen bindstoDNA in vitroatpH 6but
elutesatpH8(4). On thebasis of this
informa-tion, the intracellular localization and in vivo
binding
characteristics of tumor antigen andthe 100K proteinwerecomparedininfection
by
WT or mutant virus. The location of tumor antigen wasdetermined by immunofluorescent
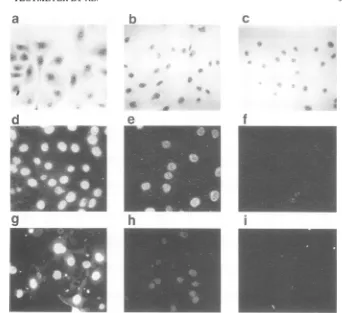
staining (Fig. 4). Intact cells showed a more
FIG. 1. Alteredpatternsofprotein synthesisin cells infected bywild-type (WT) virus andA30at33C,at 41 C, and afterashift from33 to 41 C. The cultures were labeled with50uCiof [35S]methionineper mlof
methionine-free medium70 to 72hafterinfectionat33C,47 to 48hafterinfectionat 41 C,and23to24hafter ashift from33C(72-hpreincubation) to 41 C.SDS-polyacrylamide
(20%o)
gelautoradiogramsoffractionatedcellproteinsare shown. Thesample order is: (a) control cells,33 C; (b) control cells,41 C; (c) controlcells,
temperatureshift; (d)WT-infected cells,33C; (e)WT-infectedcells,41C; (f) WT-infected cells,temperature shift; (g) A30-infected cells,33C; (h) A30-infected cells,41 C; and (i) A30-infected cells, temperatureshift.The upper and lowerpanels show thecytoplasmicand nuclear extracts, respectively.
on November 10, 2019 by guest
http://jvi.asm.org/
172 TEGTMEYER ETAL.
Supernate
a
b
c
d
Precipitate
a
b
cd
e
f
e
t
A58
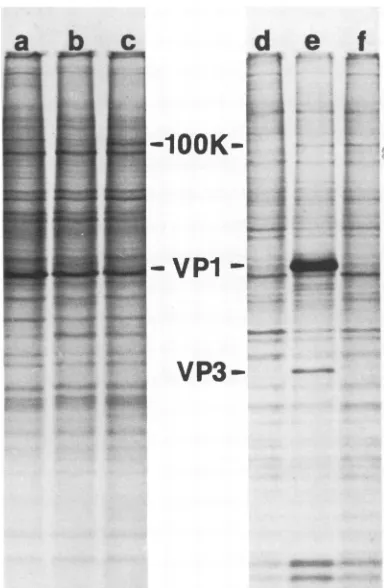
FIG. 2. Virus-inducedproteinsspecifically precipitated byantitumorserum. Cultureswerelabeled with 30
'UCiOf [35SjImethioninepermlof methionine-freemedium 47to48hafterinfection.Solubleproteinsextracted fromcells with 0.5%NP-40atpH7wereprecipitatedwithserumfromhamstersbearingS V40-inducedtumors
orwithpreimmunizationcontrolserumusingtheindirectimmunoprecipitation techniquedescribed in thetext. The precipitated proteins were identified by SDS-polyacrylamide (20%) gel electrophoresis. Both the
supernatant and the pelleted fractions of the immunoprecipitation reactions are shown in the slab gel autoradiogram. Thesample order is:(a) controlcells, controlserum;(b) WT-infected cells, controlserum;(c) A58-infected cells, controlserum; (d)controlcells, antitumorserum;(e) WVT-infectedcells, antitumorserum; and (f) A58-infected cells, antitumor serum. The side wells are whole cytoplasmic extracts of WT- and A58-infected cells. Thearrows indicate theposition oftheoverproduced 100,000-daltonprotein.
uniformstainingofnucleiinWTinfectionthan
in rnrlant infection. Further, the cytoplasm of
cellsinfectedby A58, butnotbyWTvirus, was
distinctly immunofluroescent in most but not allinfectedcells. Extraction with NP-40atpH6
removed most of the tumor antigen from the nuclei ofcells infectedby A58 butnotfrom the nuclei infectedby WT virus. Extraction at pH 8, however, efficiently removed tumor antigen from the nuclei infected by either WT or
mu-tantvirus.
The location of the lOOK protein was
deter-mineddirectly by the electrophoresis of proteins from cellularextracts (Fig. 5). After extraction atpH 6,mostof the lOOK proteinwaspresentin thecytoplasmicextractofcells infectedby A58
but inthe nuclei ofcells infected by WT virus. AfterextractionatpH 8, the lOOK protein could
no longer be identified in the nuclei of cells
infected by either virus. The dataareconsistent
with the interpretation that the WT-induced protein binds to intranuclear DNA with a greateraffinitythan the A-induced protein.
Temperature-sensitive degradation of the
lOOK protein. The lOOK proteins induced by WTvirusorA58andradiolabeledbya1-hpulse
with [35S ]methionine were compared in the same slab gel both by staining with Coomassie
blue andautoradiography (Fig. 6).Although the lOOK protein ofthe Amutantwas moreheavily
radiolabeled than the corresponding protein induced by WT virus in immunoprecipitated
WT
J.VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.498.58.459.68.402.2]-T
D
E
F
MIGRATI ON
FIG. 3. Densitometertracings ofselectedsamples
of thegel autoradiogram shown inFig.2tocompare the relative quantities of proteins precipitated by
antitumor serum. The sample order is: (A)
A58-infected cell extracts without immunoprecipitation; (B)A58precipitate,antitumorserum;(C) WTprecip-itate, antitumor serum; (D) control cell precipitate, antitumorserum;(E)A58precipitate, controlserum;
(F) WTprecipitate, control serum; and (G) control
cellprecipitate, controlserum.
material, theprotein from cells infected by WT
virus was moredensely stainedwith Coomassie
blue.
The stained gels and autoradiograms of the same gels were traced with adensitometerand the relative amounts of accumulated,
stained protein and newly synthesized,
pulse-labeledprotein were quantitated bymeasuring
the appropriate areas in the tracings. The
mutant-induced protein contained 8.2 times as
much radiolabel but only 0.6 times as much
stain as the WT-induced protein. Thus, the
mutant-induced proteinhad a specific activity
(radioactivity/stain)
more than 10-fold greater than the WT-induced protein. Theseobserva-tionsindicate that the A-induced proteinis not
only more rapidly synthesized than the
WT-inducedproteinbut alsomorerapidly degraded.
To confirm these findings, the stability of the
lOOK protein was determined by pulse-chase
radiolabeling techniques. After the lOOK
pro-teinwaslabeled with [35S ]methionine for 1hat
41 C, infected cultures were incubated in
me-dium containinga 200-fold excess ofunlabeled
methionine for 12 h at 41 C. The 100K protein
was extracted with 0.5% NP-40 at pH 8.0,
precipitated with antitumor serum, and
quan-titated by SDS-gel electrophoresis (Fig. 7).
After a short pulse, radiolabeled,
mutant-induced 100K protein was degraded more
rap-idly than the WT-inducedproteinduringa12-h
chase at 41 C. A 24-h pulse with
[35S]methio-nine without a subsequent chase resulted in
approximately
the samedegree
oflabeling
ofthe 100K protein ininfection
by
WTormutantvirus. Thesefindings strongly supportthe
con-clusion that the induced protein is both
over-produced and more rapidly turned over in
infection by theA mutants and that the 100K
protein istemperaturesensitivein its behavior
andstability invivo.
Tumor antigen in transformed cells. After
stable transformation ofrabbit cells by WT or
mutantvirus at 33C, transformed and
untrans-formed control cultureswereshiftedto 41 Cfor
24 h and thenradiolabeled foreither 1or 24h.
When proteins were extracted at pH 8 and
examined
directly
by electrophoresis,
no 100Kprotein could be identified in the transformed
cells.
After immunoprecipitation, however, the100K
protein could be easily identified intrans-formed cells butnotincontrolrabbit cells (Fig.
8). As in productive infection, the
mutant-induced protein was more heavilylabeled after
a short but not after a long pulse with
[35S]methionine.
These findings indicate that the same protein is induced by SV40 in two distinct species of host cells and that the A function regulates the production of tumor bgT
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.498.50.233.55.592.2]174 TEGTMEYER ET AL.
a
b
C.A
f
h
FIG. 4. Intracellular localization ofSV40-induced tumor antigen. Intact cells or nuclei of cells were stained either with toluidine blue or with antitumor serum using the indirect immunofluorescent technique. The cytoplasmwasremoved from the nuclei with 0.5% NP-40 at either pH 6.0 or 8.0. During extraction with NP-40 without agitation, most of the nuclei remained adherent to the glass surface so that morphological and
fluorescentantibodystudiesonextracted nuclei could beperformed in situoncoverslips.Thesampleorder is: (a)intactuninfectedcells, toluidine blue; (b) uninfected nuclei extracted at pH 6, toluidine blue; (c) uninfected nuclei extracted atpH 8, toluidine blue; (d) intact WT-infected cells,immunofluorescence; (e) WT-infected nucleiextractedatpH 6.0, immunofluorescence;
(f)
WT-infectednuclei extractedatpH 8.0, immunofluores-cence; (g) intact A58-infected cells, immunofluorescence; (h) A58-infected nuclei extracted at pH 6.0, immunofluorescence;and(i) A58-infected nuclei extracted at pH 8, immunofluorescence.antigen in
transforming
aswellasinproductive
infection.
DISCUSSION
At the restrictive temperature, the tempera-ture-sensitive A mutants induce the
overpro-ductionofatemperature-sensitive protein with
an altered intracellular distribution. Further-more, theproteinextractedfromeitherinfected
monkey ortransformed rabbit cellsreacts
effi-ciently with antisera obtained from hamsters
bearing SV40-induced tumors. These findings
strongly suggestthat the 100Kprotein isaviral
protein. Recent directevidenceto bepresented
elsewhere confirms that the structure of 100K
tumor antigen is determined by the viral ge-nome. The induced protein isasingle
polypep-tide chain that cannot be converted into
sub-units by reduction and alkylation. The protein
is, therefore, large enough to represent the
entire
coding
potential of the early region ofSV40 DNA (14). This observation is also
con-sistentwithaccumulating genetic evidence that
SV40 hasasingle earlygene, the A gene.
Ifthe 100K protein is the A
protein
and theonly earlyprotein, then it mustregulate its own
synthesis directly or indirectly. The rate of
synthesisofthe mutant-inducedprotein is5- to
10-fold greater than that of the WT-induced protein. This overproduction is striking when
cnnsidered in terms ofgene dosage. In
produc-tiveinfection, the replicationof mutantDNA is
completely blocked atthe restrictive
tempera-ture, whereas thousands ofcopies of WTDNA
d
.J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.498.86.428.65.378.2]per cell are present late in infection. Although
d e f these findings provide the first evidence for
t I or autogenous regulation in animal virus systems,
examples of this kind of regulation have been identified in bacteriophage systems (10). The f
"^F lambdarepressor acts asapositive regulator of
1OOK-1
!.
its own structural gene(11, 17).
Functionalproductsofgenes32 and 43 ofbacteriophageT4
inhibit expression of theirrespective structural
MM -
i+
genes(18, 32).
In thecase ofSV40,self-regula-tion may represent the only possibility for the
control ofgene function in view of the limited
VPi
-4,
C
"
content
of its
genome.
Thus, theA function initiates DNA
replica-tion and late transcription but apparently
in-hibits the synthesis of early protein. Further
studies willbe required to determine the
molec-VP3
- ular mechanism for theAregulatory
function,
but the mechanism probably requires binding of
the A protein to viral DNA at a specific site.
Presentevidence is consistent with the idea that
viral DNA replication and early and late
tran-scriptionare initiatedinthe same region of the
viralgenome (14, 24). By binding to a single site
FIG. 5. Intracellular localization of the
100,000-dalton virus-induced protein. The cultures were la-beled with 30 uCi of [35S]methionine per ml of
methionine-freemedium47to48 hafter infectionat
*B_ ~~~S
a
b
41 C. Nuclear and cytoplasmic proteins were ex-tracted with 0.5% NP-40 at pH6. SDS-polyacryla-mide (20%) gel autoradiograms of fractionated cell
proteins are shown. The sample orderis: (a)
unin-fected cells, cytoplasm; (b) WT-inunin-fected cells,
cyto-plasm; (c) A58-infected cells, cytoplasm; (d)
unin-fected cells, nuclei; (e) WT-inunin-fected cells, nuclei;and (f)A58-infected cells,nuclei.
[image:8.498.44.236.81.375.2]c
d
FIG. 6. Comparison of the synthesisandaccumulation of the100Kprotein in infectionby WT virus and A58. Cultures werelabeledwith 30gCiof [35S]methionine47to48h afterinfection at41 C. Afterextraction with 0.5% NP-40 at pH 8.0, the soluble proteins were precipitated with antitumor serum and analyzed by
SDS-polyacrylamide (20%) gel electrophoresis. Theupperpanelshowsanautoradiogram of thegel; thelower
panelisaCoomassie bluestain of thesamegel. The sampleorderis: (a) control cell immunoprecipitate; (b)
WT-infected cell immunoprecipitate; (c) A58-infected cell immunoprecipitate; and (d) whole cytoplasmic
extractof A58-infectedcells. Theareaof the gel containing the 100K protein is shown by thearrow.
*.Or. 4
*,#z
r J; i
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.498.100.386.433.594.2]176 TEGTMEYER ET AL.
a
b
cd
e
f
-*
1OOK
FIG. 7. Temperature lability of the virus-induced 100,000-dalton protein as determined by pulse chase
studies. Cultureswerelabeled with30/Ci of[3S6S]methioninepermlofmethionine-free medium47to48hafter infectionat41C(shortpulse). Some cultureswerecollectedafter the shortpulse;otherswerechased for12hat 41 C in thepresence ofa 200-fold excess of unlabeled methionine. Alternatively cultureswere labeled with
[ 5S]methionine from 48to72h afterinfection (long pulse). Thesampleswereextractedwith 0.5%NP-40at
pH8.Solubleproteinswereprecipitated with antitumorserumandanalyzed bySDS-polyacrylamide (20%) gel
electrophoresis and autoradiography. Thesample orderis:(a) control cells, shortpulse; (b) WT-infected cells,
shortpulse; (c) A58-infected cells, short pulse; (d) control cells, pulse chase; (e) WT-infected cells,pulse chase; (f) A58-infected cells, pulse chase; (g) control cells, long pulse; (h) WT-infected cells, long pulse; and (i) A58-infected cells, long pulse.
onviral DNA, theA proteincould bothrepress
earlytranscription and induce DNA replication
orlatetranscription.
Antitumor sera precipitated at least four proteins from infected cells in addition to the
virus-induced lOOK protein.The sameproteins were not precipitated from extracts of unin-fected cells. These proteins, with molecular
weights ranging from 66,000 to 88,000 daltons,
could be cellular proteins complexed with the 100K protein, independent tumor antigens, or
products of the lOOK protein. Characterization
ofeachprotein with monospecific antisera
pre-pared against pure proteinsandby
fingerprint-ing of tryptic digests should determine the origin and interrelation of these proteins. Whether these proteins are viral or cellular in
origin, theymaybeoffunctional importance in
infection by SV40. Clearly, the findings
re-ported here and by other laboratories (4, 7, 17, 19, 28) indicate that the isolation, purification, andcharacterization of SV40tumorantigen and associatedproteinsare nowpossible.
Finally, it isespecially important to note the implications of the overproduction of the A-induced protein in cells transformed by the A
100K
g
h
i
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:9.498.92.427.82.411.2]SIMIAN VIRUS TUMORANTIGEN 177
a
b
c
d
tSw ,. __ _.. $1 .w vl .1 "
e
_. _-- ..
f
g
h
[image:10.498.76.409.81.445.2]ML 40
FIG. 8. Overproduction of the 100 K protein in rabbit cells transformed by A mutants. After stable transformation of the rabbit cells by WTormutant virusat33 C, transformedand untransformed control
cultures were shifted to41 C. After 24 h ofincubation at 41 C, the cultures were labeled with 30 uCiof ['5Sjmethioninepermlforeither1h(short pulse)or24h(long pulse). Afterextractionwith 0.5%NP-40atpH 8, the soluble proteinswereprecipitatedwithantitumorserumandanalyzed bySDS-polyacrylamide(20%) gel
electrophoresis andautoradiography. Thesampleorder is: (a) control cells,shortpulse; (b) WT-transformed
cells, short pulse; (c)A28-transformedcells,shortpulse; (d)A58-transformedcells,shortpulse; (e)controlcells, long pulse; (f) WT-transformed cells, long pulse; (g) A28-transformed cells, long pulse; and (h)
A58-trans-formedcells, long pulse.
mutants. First, these studies show that the A
protein can regulate the production of tumor
antigen when the viral genome is in the
inte-grated state. Second, they strongly confirm previous findings showing that the A protein
may have a direct effect on the physiological
stateoftransformed cells (3, 16, 22, 25, 35).
ACKNOWLEDGMENTS
ThisinvestigationwassupportedbygrantPRA-113 from theAmerican Cancer Society, grant 1256 from the Damon
Runyon Fund, and Public Health Service grantCA 16497 from theNational Cancer Institute.
We are grateful for the skillful assistance of Judith Kohout.
LITERATURE CITED
1. Anderson,C.W., andR. F. Gesteland. 1972. Pattem of protein synthesis in monkey cellsinfected by simian virus40.J.Virol.9:758-765.
2. Black, P. H., W. P. Rowe, H. C. Turner, and R. J. Huebner. 1963. Aspecific complement-fixing antigen
present in SV40 tumor and transformed cells. Proc. Natl.Acad.Sci.U.S.A.50:1148-1156.
lOOK-
-100K
VOL.
m
._-I
t. I 11 il
a...A
on November 10, 2019 by guest
http://jvi.asm.org/
178 TEGTMEYER ET AL.
3. Brugge,J. S., and J. S. Butel. 1975.Involvement ofthe simianvirus 40geneAfunctioninthemaintenanceof transformation. J. Virol. 15:619-635.
4. Carroll, R. B., L. Hager, and R. Dulbecco.1974.SV40 T antigen bindstoDNA. Proc. Natl. Acad. Sci. U.S.A. 71:3754-3757.
5. Chou, J. Y., J. Avila, andR.G.Martin. 1974.ViralDNA synthesis in cells infected by temperature-sensitive
mutantsof simian virus40.J.Virol. 14:116-124. 6. Cowan, K., P. Tegtmeyer, and D. D. Anthony. 1973.
Relationship of replication and transcription of simian virus 40 DNA. Proc. Natl. Acad. Sci. U.S.A. 70: 1927-1930.
7. Del Villano, B. C., and V. Defendi. 1973. Characteriza-tionofSV40 T antigen. Virology 51:34-46.
8. Fairbanks, G.,T. L.Steck,and D. F.H.Wallach. 1971. Electrophoretic analysis of the polypeptides of the human erythrocyte membrane. Biochemistry 10:2606-2617.
9. Gilden, R. V., R. I. Carp, F.Taguchi, and V. Defendi.
1965.Thenatureand localization of theSV40-induced complement-fixing antigen. Proc. Natl. Acad. Sci. U.S.A.53:684-692.
10. Goldberger, R. F. 1974. Autogenousregulation ofgene
expression.Science 183:810-816.
11. Heinemann, S. F.,and W. G.Spiegelman. 1970. Control oftranscription of therepressor geneinbacteriophage lambda. Proc. Natl. Acad. Sci. U.S.A. 67:1122-1129. 12. Henderson, I. C., and D. M. Livingston. 1974. Partial
purification and characterization of the SV40 T
anti-gen.Cell 3:65-70.
13. Hoggan, M. D., W. P. Rowe, P. H. Black, and R. J. Huebner. 1965. Production of "tumor-specific"
anti-gensbyoncogenicvirusesduringacutecytolytic infec-tions. Proc.Natl. Acad. Sci. U.S.A. 53:12-19. 14. Khoury, G., P. Howley, D. Nathans, and M. Martin.
1975. Post-transcriptional selection of simian virus 40-specific RNA. J. Virol. 15:433-437.
15. Kiehn, E. D. 1973. Protein metabolism inSV40-infected cells.Virology 56:313-333.
16. Kimura, G., and A. Itagaki. 1975. Initiation and
mainte-nanceofcelltransformation by simianvirus 40:aviral genetic property. Proc. Natl. Acad. Sci. U.S.A. 72:673-677.
17. Kourilsky, O., M. F. Bourginon, M. Bouquet, F. Gros. 1970. Early transcription controls after induction of prophage lambda. Cold Spring Harbor Symp. Quant. Biol.35:305-314.
18. Krisch, H. M., A. Bolle, and R. H. Epstein. 1974. Regulation of the synthesis of bacteriophage T4gene32 protein. J. Mol. Biol. 88:89-104.
19.Lazarus, H. M., M. B. Sporn, J. M. Smith, and W. R. Henderson. 1967.Purificationof Tantigenfromnuclei of simian virus40-induced hamstertumors. J. Virol. 1:1093-1095.
20. Lewis,A.M., andW. P.Rowe.1971.Studieson
nondefec-tive adenovirus-simian 40hybrid viruses. I. A newly characterized simianvirus 40antigen induced by the
Ad2+ND,virus.J. Virol.7:189-197.
21. Manteuil, S., and M. Girard. 1974. Inhibitors ofDNA
synthesis: their influenceonthereplication and
tran-scription of simianvirus 40DNA.Virology 60:438-454. 22. Martin, R. G., and J. Y. Chou. 1975. Simian virus 40
functions required for the establishment and
mainte-nance of malignant transformation. J. Virol.
15:599-612.
23. Maurer, H.R., and R. C. Allen.1972.Useful bufferandgel
systemsforpolyacrylamide gel electrophoresis.Z. Klin. Chem.Klin. Biochem.10:220-225.
24. Nathans, D., and K. J. Danna. 1972. Specificoriginin SV40 DNA replication. Nature (London) New Biol. 236:200-202.
25. Osborn, M., and K. Weber.1975.Simian virus40geneA function and maintenance of transformation. J. Virol.
15:636-644.
26. Oxman, M. N., S. Baron, P. H. Black, K. K. Takemoto, K. Habel, and W. P. Rowe. 1967. The effect of interferon on SV40 T antigen production in SV40
transformed cells. Virology32:122-127.
27. Pope, J. H., and W. P. Rowe. 1964.Detection ofspecific antigen inSV40 transformed cells by
immunofluores-cence.J.Exp. Med. 120:121-128.
28. Potter, C. W., B. C. McLaughlin, and J. S. Oxford.1969. Simian virus 40-induced T and tumor antigens. J. Virol. 4:574-579.
29. Rapp, F., J. S. Butel, and J. L. Melnick. 1964. Virus-induced intranuclear antigen in cellstransformed by papovavirus SV40. Proc. Soc. Exp. Biol. Med. 116: 1131-1142
30. Rapp, F., T. Kitahara, J. S.Butel, and J. L. Melnick. 1964. Synthesis ofSV40tumorantigenduring replica-tion of simian papovavirus(SV40). Proc. Natl. Acad. Sci.U.S.A.52:1138-1142.
31. Robb, J. A., and K. Huebner. 1973. Effect of cell chromosome number on simian virus 40 replication
Exp. Cell. Res. 81:120-126.
32. Russel, M. 1973. Control of bacteriophage T4 polymerase synthesis. J. Mol. Biol. 79:83-94.
33. Sabin, A. B., and M. A. Koch. 1964. Source of genetic information forspecific complement-fixing antigens in SV40 virus-induced tumors. Proc. Natl. Acad. Sci. U.S.A.52:1131-1138.
34.Tegtmeyer, P. 1972. Simian virus 40 deoxyribonucleic acidsynthesis: the viral replicon. J. Virol. 10:591-598. 35. Tegtmeyer,P. 1975.Function of simianvirus 40geneA in
transforming infection. J. Virol. 15:613-618.
36. Tegtmeyer, P., C. Dohan, and C. Reznikoff. 1970. Inac-tivating and mutagenic effects ofnitrosoguanidineon
simian virus 40. Proc. Natl. Acad. Sci. U.S.A. 66:745-752.
37. Tegtmeyer, P., and H.L.Ozer.1971. Temperature-sensi-tive mutants of simian virus 40: infection of
per-missive cells. J. Virol. 8:516-524.
38. Tegtmeyer, P., J.A.Robb, C. Widmer, and H. L. Ozer. 1974. Altered protein metabolism in infection by the late tsBll mutant of simian virus 40. J. Virol. 14:997-1007.
39. Walter, G., R. Roblin, and R. Dulbecco. 1972.Protein synthesis in simian virus 40-infected monkey cells. Proc. Natl.Acad. Sci.U.S.A.69:921-924.
J.VIROL.


![FIG. 7.pHA58-infectedshortstudies.41electrophoresisinfection(f)[ 5S]methionine C A58-infected Temperature lability of the virus-induced 100,000-dalton protein as determined by pulse chase Cultures were labeled with 30 /Ci of [3S6S]methionine per ml of meth](https://thumb-us.123doks.com/thumbv2/123dok_us/1571664.109758/9.498.92.427.82.411/infectedshortstudies-electrophoresisinfection-methionine-infected-temperature-determined-cultures-methionine.webp)
