Copyright X 1992, American Society for Microbiology
Herpes Simplex Virus IE63
Acts
at
the Posttranscriptional
Level To Stimulate Viral
mRNA
3' Processing
J. McLAUCHLAN,' A. PHELAN,1 C. LONEY,1 R. M. SANDRI-GOLDIN,2 ANDJ. B.
CLEMENTS"*
Medical Research Council Virology Unit, Instituteof Virology, University of Glasgow, Church Street,
Glasgow Gil SJR, Scotland,1andDepartmentofMicrobiology and MolecularGenetics,
College of Medicine, University of California, Irvine, California 927172
Received 9 July 1992/Accepted 21August 1992
We have shownpreviouslythatanovel herpes simplex virus-induced activity, LPF, selectivelyincreasesRNA 3'-endprocessingatthepoly(A)site ofalate virusgene(J. McLauchlan, S. Simpson, and J. B. Clements, Cell
59:1093-1105, 1989). Here,ourin vivo and in vitroanalysesboth demonstrate that LPF is induced duringearly stages ofvirus infection. Studies of virusmutantsindicate that expression ofthe immediate-earlyIE63geneis
required for induction ofthis activity. The selective effectson3' processing displayed inthepresenceofIE63
provide direct evidence that IE63 caninfluence this posttranscription process.This extends previous studies
whichreported increases inreportergeneactivity with certainpoly(A)sites byIE63 (R. M. Sandri-Goldinand G. E. Mendoza, Genes Dev. 6:848-863, 1992).
Transcription of herpes simplex virus (HSV) DNA can be
separatedinto three broadtemporal phases, immediate early
(IE), early, and late (L). To achieve regulated expression, HSV encodes polypeptides with transactivating functions which mediate control through sequences present within virus gene promoters (reviewed in reference 9). However,
there isno reason to assumethat suchregulationoccursonly
at the level oftranscription initiation, since modulation of
gene expression could be mediated also by
posttranscrip-tionalevents. Such regulation operates inanumberof virus systems through various mechanisms such as differential
splicing and polyadenylation (21), RNA transport (6), and
RNAstability (12).
Recent transfection studies on the function of the HSV type 1 (HSV-1) IE63 (UL54) geneproduct suggest that this
polypeptide influences the efficiency of mRNA 3'-end
for-mation at selectedpoly(A) sites and reducesexpression of
splicedgenes (30, 32).Virus recombinants containing
alter-ationsto IE63 display a
variety
ofphenotypes; however, a common feature is thedependence
on IE63expression
forthesynthesisof late geneproducts
(16,
19, 26,29).
Instudiesofonetemperature-sensitive (ts)mutant, lack of correlation
between levels of transcription and accumulation of gC
mRNA, a late-gene product
(UL44),
suggested that IE63mayaffect
posttranscriptional processing
ofRNA(33).
Mu-tational
analysis
hasrevealed thatIE63canbothrepressandactivate geneexpression,and thesefunctional domains have
been mapped to the C-terminal
portion
of theprotein
(11,
19). IE63 also stimulates
expression
fromregulatory
se-quencesof retroviral
origin;
this stimulationwasdependent
onsequencesattheretroviruspoly(A)
site butwasindepen-dent of the retroviral promoter
(5).
Fromin vitro
polyadenylation studies,
wehave identifieda virus-induced factor, LPF, which
selectively
increasesprocessingatthe
poly(A)
site of the HSV-2 UL38 gene(18),
a late gene
encoding
acapsid protein
(27). By
contrast, a secondpoly(A)
sitecommon totheUS10, US11,
andUS12genes failedto respond toLPF. In accord withour invitro
results,analysisof mRNAs
produced by
virusrecombinants* Correspondingauthor.
provided evidence that LPF exerted this selective effect during lytic growth in tissue culture cells (18).
Here, we extend our in vitro analysis of LPF and show that LPFactivitycanbedetected not only ininfected HeLa cells but also in infected BHK cells. Moreover, during productive infection, selective increases in poly(A) site usage can be detected from early stages of lytic growth.
Usingconditions selective for theexpression of certain virus
genes, our results indicate that the IE63 gene product is required for the increase in utilization of the UL38 poly(A) site. Data derived from transfection studies reinforce the view that IE63 is a component of the LPF-mediated effect.
MATERIALSANDMETHODS
Cells andviruses. BHK-21C13 cellsweregrownas mono-layers in Glasgow minimum essential medium supplemented with 10% newborn calf serum. Spinner cultures of HeLa cellsweremaintained in Dulbecco's mediumsupplemented
with5% newborn calf serum. Vero 2-2cells, which express
IE63 (33), and Vero cells were maintained in Glasgow
minimumessential medium supplemented with5%newborn calf serum and 5% fetal calfserum. Monolayers of HeLa
(WS)cellsweregrowninDulbecco's mediumsupplemented with5%newborn calfserumand5%fetal calfserum.
Stocks of wild-type (wt) HSV-1 17, vFJ7, and vSAU3 weregrownat37°C,whileHSV-1ts mutants weregrownat
31°C.Virus271acZ,inwhich the IE63 gene is inactivatedby
insertion of lacZ(33),wasgrownonVero 2-2cells. Viruses
d11403,
which contains a 2-kbp deletion in both copies ofIE110(34),and
HSV-1(F)A325,
which containsadeletion in IE68 (23; a gift from I. Halliburton) were grown on BHKcells.
UV inactivation of viruswasperformed by
treating
viruspreparationswith 240,000 ,uJ ofUVlight at 260nm with a
Stratalinker model 1800
(Stratagene).
Construction of virus recombinants. Details of the con-struction ofvFJ7,which contains the promoter for the small subunit of theribonucleotide reductase gene
(R2),
have been described previously (28). To generatevSAU3,
plasmid
pSAU3Cwasconstructed. pSAU3Cwasmade
by
replacing
a 100-bp DNA fragment
containing
the HSV-2 mRNA6939
on November 9, 2019 by guest
http://jvi.asm.org/
6940 McLAUCHLAN ET AL.
poly(A)sitesequences inplasmid pFJ7 (28)with a HindlIl-SmaIfragmentfrompSAU3which carries the HSV-2 UL38 poly(A) site sequences. pSAU3C was digested withXbaI,
and the DNA fragment containing the chloramphenicol acetyltransferase (CAT) and lacZ gene cassetteswas
puri-fied and ligated into the unique XbaI site in HSV-1 1802 DNA (28). Ligation products were transfected into BHK cells and overlaid with 2 ml of 0.5% agarin medium. After incubation at 37°C for 3 days, cells were overlaid with a
further 2 ml of 0.5% agar in medium containing 750 ,ug of 5-bromo-4-chloro-3-indolyl-,-D-galactopyranoside per ml. Blue plaqueswere isolated and purifiedto homogeneity by furtherplaque selection. One isolatewaschosen and named
vSAU3. Stocksof vSAU3weregrownand storedat -70°C. In vitro polyadenylation reactions. Spinner cultures of HeLa cells(1x 109)ormonolayersof BHKorHeLa cells(6
x 107) were infected for up to 16 h at a multiplicity of
infection of 10attheappropriatetemperature. For infections carried out in the presence of cycloheximide, cells were
incubatedin mediumcontaining50 ,ugofcycloheximideper mlfor 40 min priortovirus inoculation, andcycloheximide
was maintained atthis concentration in the medium for the durationofthe infection.
Nuclearextractswerepreparedeitherasdescribed
previ-ously (18)orby usingasmall-scale extraction method (14).
For the small-scale method,cells grownto 70% confluence
wereharvestedinphosphate-bufferedsaline (PBS) and
pel-leted by centrifugation at 1,400 x g at 4°C. Cells were
washed with 30 packed-cell volumes of PBS and pelleted. Aftersuspensionin 1packed-cellvolume of buffer A(10mM HEPES [N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid; pH 8.0],1.5 mMMgCl2, 10 mMKCl,1 mM dithiothre-itol),cellswereswollenonice for 15minand thenlysed by passing them through a narrow-gauge needle five to eight times. A crude nuclear pellet wasrecovered by
centrifuga-tionat12,000x gfor 20s.Nucleiweresuspendedin2/3vol
of theoriginal packed-cellvolumeby usingbufferC(20mM HEPES[pH 8.0], 1.5 mMMgCl2,25%[vol/vol] glycerol,420 mM NaCl, 0.2 mM EDTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride) and incubated on ice for 30 minwith continuous agitation. Following centrifugation at
12,000 x g for 5 min at 4°C, the supernatant, designated nuclearextract,was storedat -70°C.
SAU5 precursor RNA was transcribed in vitro as
de-scribed previously (18). In vitro polyadenylation reactions
wereperformedwith 1x 104to5x 104cpmof RNA in 1 mM
3' dATP-5 mM creatine phosphate-264 mM KCl-0.7 mM MgCl2-8.8% glycerol-8.8 mM HEPES (pH 7.6)-0.1 mM EDTA-0.2 mM dithiothreitol-2.5% polyethylene glycol at
30°Cfor 2 h(18). Inreaction mixturescontaining amixture
ofnuclearextracts, 7,ul of HeLa cellextractwasmixed with 4 ,ulof BHKcellextract. Reactionproductswere analyzed
asdescribed previously (20).
CATassays.Atappropriatetimes after eithertransfection
orinfection, cellswerewashed with PBS andsuspended in
250 mM Tris-HCl (pH 8.0). Cellswere frozen and thawed
threetimes, andcell debriswasremoved by centrifugation.
ExtractswereassayedforCAT activity by using the solvent
extraction procedure (31), and protein concentrations were
determinedby Bradford assays
(3).
CAT activities are ex-pressed as the percentage of [1 C]chloramphenicolcon-vertedtothebutyrylated formpermicrogram of proteinper
hour.Forsampleswithmorethan30% substrate conversion,
activities were determined from sample dilutions. Thus,
activities could bemorethan 100%.
Transfections. Transfections were performed on
50-mm-diameterpetridishescontainingsubconfluentmonolayersof HeLa cells with DOTAP transfection reagent (Boehringer
Mannheim) accordingto the manufacturer's instructions. A
3-p,g
portionofplasmid carryingthe CAT reporter gene andtheappropriateamountof effectorplasmidweretransfected
foreach dish. At 24 h aftertransfection,cellswereincubated infreshmedium; theywereharvested afterafurther 24 h.
Plasmids. The construction of plasmids pLW2 (10),
pSAU5
(18),
andpSG130B/S (32)
have been describedpreviously. pSAU2was constructed by inserting a 265-bp
Sau3A1 fragment
containing
the HSV-2 UL38poly(A)
siteinto pUC9,generating plasmid pSAU1.HindIII-XmnI
frag-ments from
pSAUl
[containing
the HSV-2 UL38poly(A)
site] and pLW1
(containing
the HSV-2IE-4/-5
promoterlinkedto the CATcoding sequences;
10)
were isolated andligatedtogive plasmid pSAU2.
RESULTS
LPF is active from
early
stages of virus infection.Previ-ously, we showed thatan
activity
which alterspoly(A)
siteusage could be detected in nuclear extracts made from
HSV-1-infected HeLa cells. This
activity,
termedLPF,
selectivelyincreased
processing
in vitroatthe HSV-2 UL38poly(A)
site(termed
the Lsite),
whereaspoly(A)
sitese-quencesfromHSV-2US10-US11-US12
(termed
theIEsite)
failed to respond. To detect LPF
activity
in extracts, weconstructed a
plasmid,
pSAU5, in which the L site waslinked to the IE site
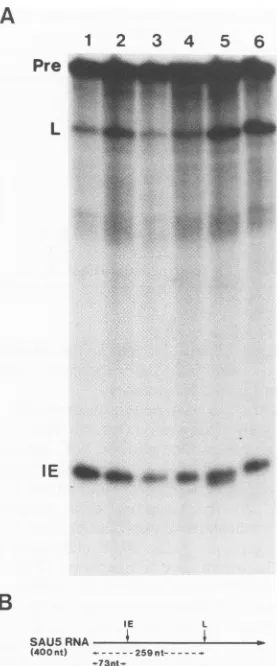
(Fig. 1B). Using
precursor RNAsynthesized from pSAU5, we
developed
an assay systemwhereby increased levels of LPF arereflected by a rise in
processing at the L site. As shown in
Fig.
1A, nuclearextractsfrommock-infected HeLacellsprocess
efficiently
attheIEsite,while theamountof RNA
processed
atthe L siteis either low
(Fig.
1A, lane1)
or notdetected(Fig. 3,
lane2).
By contrast, infected-cell extracts process considerably
more efficiently at the L site (Fig. 1A, lane
2),
while noincrease in processing at the IE site is routinely detected
(18).
Although
ourdata hadindicatedthat LPFcouldinfluenceprocessingatthepoly(A)site ofalate virus gene, thephase
of the virus
lytic cycle
at which theactivity
could be detected was not known. Therefore, HeLa cells were in-fected with wt HSV-1 virus at 37°C, and nuclear extractswere prepared at 2-h intervals
following
infection. Thepresenceof LPF activity in theseextracts was determined
by comparingthe relative levels ofprocessingat theIE and
Lsites in SAU5 precursor RNA.
Analysis
of the levels ofprocessingateach timepointrevealed that increasedrelative
amounts of L-site product can be detected by 2 h after infection comparedwith the amounts in extracts of
mock-infected cells
(Fig.
1A, lane3).
Later, the relative levels ofprocessing at the two sites remained essentially the same
(Fig.
1A,lanes 4 through 6).Parallel studies with virus recombinantshad indicated that
LPFwascapableofincreasingusageof the Lpoly(A) sitein
vivo
(18).
To investigate whether LPF activity could bedetected in vivo atearly times, assuggested by the in vitro
data, the levels of CAT activity produced by two virus
recombinants,vFJ7andvSAU3,weremeasured.Virus vFJ7
contains the CAT polypeptide coding sequences linked to the R2 promoter and has the IE site downstream from the CAT gene (Fig. 2B). Virus vSAU3 alsocontains the CAT
coding sequences linked to the R2 promoter; however,the
IE site isreplaced bythe L site(Fig. 2B). Spinnercultures of HeLacellswereinfected with eithervirusat amultiplicityof
J. VIROL.
on November 9, 2019 by guest
http://jvi.asm.org/
A
1 2 3 4 5 6
Pre
L
I-0
C.)
co
IE *4
_.
_B
IE L
nt) 259nt
-73nt-FIG. 1. Analysisof LPFactivity atvarious times after HSV-1 infection. (A) SAU5 RNAreactionproducts generated by nuclear extracts prepared at different times afterinfection. Reaction mix-tures contained nuclear extracts prepared at the following times postinfection:lane2, 16h;lane3,2h;lane4,4h;lane5, 6 h; lane 6,8 h. Reactionproductsin lane 1weregenerated bymock-infected
nuclearextract.BandscorrespondingtounreactedprecursorRNA
(Pre)and the IE and Lcleavage productsareindicated.(B)Sizes of SAU5precursorRNA andproducts generated by cleavageatthe IE and Lpoly(A)sites. The size of SAU5 RNA is shown in
parenthe-ses,and the sizes ofcleavage products (in nucleotides)withrespect
tothe RNA 5' terminusareindicated.
infection of 5at37°C, and CATactivitywasdetermined for each virusatdifferent times after infection.
The CAT activitiesproduced byvFJ7inspinner cultures of HeLa cellsatvarious timesduringinfection(Fig. 2A)are
comparabletothose measured in BHK cells(28). However, CATactivityissignificantly higherin vSAU3-infected cells 3 h after infectioncomparedwith that invFJ7-infectedcells, andby6h,the level of CATactivity produced byvSAU3 is
approximately 10-fold greater than that produced byvFJ7.
At 9 and 12 hpostinfection, CATactivityinvSAU3-infected cells remains significantly higherthan that in vFJ7-infected cells. Since the promoter sequences linked to the CAT
codingsequences areidentical in vFJ7 andvSAU3,the data
suggestthat CATmRNA ismoreefficiently processedatthe
L site thanattheIEsitefromearlystagesof virusinfection.
Similar increasesin the amount of CAT mRNAprocessedat
the L sitecomparedwith thatprocessed atthe IE sitewere
observed whenBHK and HeLa cellmonolayerswere used
Prom CAT IE Prom CAT L
FIG. 2. CAT activities produced by virus recombinantsvFJ7 and
vSAU3. (A) HeLa cellswereinfectedat37°C, and CAT activities
weredeterminedat3-h intervals following infection. Activitieswere
expressedasthepercentageof["4C]chloramphenicolconvertedper
microgram of protein per hour. *, vFJ7 activities; 0, vSAU3
activities. (B) Description of CAT mRNAs produced by vFJ7 and vSAU3. Prom, R2promotersequencesusedtoinitiate synthesisof
CAT mRNAs.
(data not shown). These results are consistent with our in vitro results and indicate that LPF is active fromearlystages of infection. Thus, agene productmadeearly during infec-tionapparently induces the selective increase inprocessing
at the L site.
Expression ofIEgenes is sufficient for detection ofLPFin
vitro. Furtheranalysis of the temporalclass ofgene which mediates LPF activity was done in vitro by performing
infections under conditions that limited virusgene expres-sion. In vitro analysis of LPFwas facilitatedby the devel-opment ofanassay method which allowed detection of the
activity in BHK cell monolayers. This was necessary
be-cause the phenotypes of the ts mutants had been fully characterized in these cells. Moreover, for technical
rea-sons, the ability to detect LPF in extracts made from cell monolayers would permit experiments to be performed undermoretightlycontrolledconditions. Anexampleofthe assay method is shown in Fig. 3. BHK cells grown on
90-mm-diameter petri disheswere infected with HSV-1 at 37°C for 16h, and the abilities of thenuclear extracts from these cellsto process SAU5 RNAweretested. Analysis of the reaction products indicated that the extract from BHK cells failedtoprocessateither the IEorthe L sites(datanot shown);this failureis duetothe inherentinabilityto prepare
active processing extracts from this cell type. However,
A
120
B
Time(h)
vFJ7 vSAU3
All-1-11
9%, 1?1..
M ,,:-,-.z.
4.0",W-1,
on November 9, 2019 by guest
http://jvi.asm.org/
[image:3.612.109.248.73.406.2] [image:3.612.322.557.81.455.2]6942 McLAUCHLAN ET AL.
1 2 3 4 5 6 7 Pre5i. ....
L
IE * Aw
FIG. 3. LPF activity produced in BHK cells infected under conditions which limit virus gene expression. SAU5 RNA was
incubated with the following quantities of HeLa and BHK cell nuclear extracts;lane2, 11 p.lofmock-infectedHeLa cell extract; lane 3, 11 p.1 of infected HeLa cell extract; lane 4, 7 ,ul of mock-infected HeLa cellextractplus4p.1ofextractfromBHKcells infected with wt HSV-1 for 16 h at 37°C; lane 5, 7 ,ul of mock-infected HeLa cell extract plus 4 p.1 of extract from BHK cells infected with wtHSV-1in the presence of50
pLg
ofcycloheximide perml; lane 6,7p.lofmock-infected HeLa cellextractplus4pl. of extract fromBHKcells infectedatNPTwithtsK;lane 7, 7p.l
of mock-infected HeLa cellextractplus4p.1ofextractfromBHK cells infected atPTwithtsK. Lane 1contains unreacted SAU5 RNA. Bandscorrespondingtounreacted precursor RNA(Pre)andtheIE and Lcleavage productsareindicated.combinationof theextract frominfected BHK cells withan
extract from mock-infected HeLa cells generatedproducts
processed at both the IE and the L sites (Fig. 3, lane 4). Incubation of SAU5 RNA with onlythe HeLa cell extract
gaveriseto aproductprocessed atthe IE site butfailed to
generate anyL-site product (Fig. 3, lane 2). Therefore, in this assay,the components for RNAprocessingaresupplied
bythe HeLa cell nuclear extract. This allows detection of LPF activity in the extract from infectedBHKcells,which stimulates processing at the L site. Nuclear extracts from
mock-infected BHK cells also failed to process RNA at
eitherpoly(A) site and, on combination with extracts from mock-infected HeLa cells, did not stimulate processing at the L site (data not shown).
Withthis assay method, the presence of LPF in extracts prepared from cells infected under various conditions was examined.Todetermine whether de novo synthesis of virus
polypeptides was necessary for LPF induction, BHK cells
were infected with HSV-1 in the presence of 50
p.g
ofcycloheximide perml, and a nuclear extract was prepared 8
h after infection. Combination of this extract with
mock-infected HeLa cell nuclear extract did not produce RNA
processed at the L site; however, efficient processing did occur at the IE site (Fig. 3, lane 5). Similar results were obtained with extracts from BHK cells infected with
UV-TABLE
1.
Correlation ofLPFactivitywithvirus geneexpressionVirus Virusgene(s) expressed LPFactivity
wtHSV-1 IE, E,L +
wtHSV-1 + None
cycloheximide
UV-inactivated wt None HSV-1
ts1204 None
tsK IE +
ts1207 IE, E +
d11403 IE(exceptIEllO), E, L +
271acZ IE(except IE63), E, L
HSV-1(F)A325 IE(truncatedIE68), E, L +
inactivated virus (Table 1). Thus, the inability of virion components to stimulate LPF indicates that virus gene expression is required for activity.
Thetemporal class of gene which generates LPFactivity
was analyzed by using a series of virus mutants with
restrictedphenotypes.One mutant,tsK,hasanalteration in Vmwl75,aprotein which iscontinuously requiredfor prog-ressof virus infectiontotheearlyandlatephasesof thelytic
cycle (7, 35). At the nonpermissive temperature (NPT;
38.5°C),tsKsynthesizesIEpolypeptidesbut failstoproduce
earlyand lategene products. BHK cells were infected with
tsKat 31°C,thepermissivetemperature (PT), andat38.5°C
for 16 h, at which point nuclear extracts were prepared. Addition of either of these extracts to mock-infected HeLa cell nuclearextractstimulatedprocessingatthe L site(Fig. 3, lanes 6 and 7). Indeed, processing at the L site was
consistently greater with extracts prepared from cells
in-fected at the NPT than with those infected at the PT. Another ts mutant, ts1207, which contains a mutation in
UL39 and synthesizes IE and early but not late virus
products(22, 24),also stimulated LPFactivityatthe PT and
NPT (Table 1). By contrast, a third ts mutant, ts1204,
generates LPFatthe PT butnot atthe NPT(Table 1).ts1204
virionsbind to cells but donotrelease their contents because
of an alteration in gene UL25 (1, 17); hence, they fail to express anyvirus genes. These results suggest that an IE component is involved ininduction of LPF activity.
Inactivation of IE63 reduces LPFactivity. Since IE175 is not functional in tsK-infected cells at the NPT, it appears reasonable toconclude thateitheroneofthe four other IE
products or a combination of them is involved in LPF
activity.tsmutations ineachof these four IE genesare not
available. However, recombinants which fail to express
IE110 (d11403; 34), IE68 [HSV-1(F)A325; 23], and IE63
(271acZ; 33) have been constructed. Extracts made from
HeLacellsinfected with these recombinantsweretested for
LPF activity. Figure 4 shows that extracts made from
d11403-infectedcells contain LPFactivity(lane4), whereas
extracts from 271acZ-infected cells fail to induce any com-parable increase inprocessingatthe Lsite(compare lanes 1 and 2with lane3).The apparent smallincreaseinprocessing at the L site between lanes 1 and3 representsvariability in the intrinsic processing activities in individual extracts. In commonwithd11403,extractsfromHSV-1(F)A325-infected
cells produced LPF activity (Table 1). These data identify IE63 as acomponentrequired for the induction of LPF.
Expression of IE63 intransient assays
selectively
increases processingattheLpoly(A) site.Previous results from trans-fections had indicated that plasmids expressing IE63 were J. VIROL.on November 9, 2019 by guest
http://jvi.asm.org/
[image:4.612.134.227.77.320.2]1 2 3 4
Pre
IE
FIG. 4. LPF activity produced by virus recombinants with inac-tivatedIEgenes.HeLa cellswereeithermockinfectedorinfected with different virusesat37°C for 8 h,atwhich pointnuclearextracts
were prepared. SAU5 RNA was incubated with the following
extracts: lane 1, mockinfected; lane2,wtHSV-1; lane 3,271acZ; lane 4, d11403. Bands corresponding tounreactedprecursor RNA (Pre) and the IE and L cleavage products areindicated.
A
0)
(U C1) U
o
LL.
1 2 3 4 5
Molar
equivalents
IE63
B
pLW2
capableofstimulatingCAT reportergeneactivity(5, 30, 32). This stimulation was dependent on the polyadenylation signalsused butindependentofthepromotersequences.To determine whether IE63 could increase expression from plasmids containing the Lpoly(A) site, transfections were
performedwithpLW2 andpSAU2. Both oftheseplasmids contain the CAT coding sequences linked to promoter
sequences from the HSV-2 IE-4/-5 genes; however, pLW2 contains the IE site downstream from the CATsequences,
whilepSAU2possessesthe L site(Fig.5B). HeLa cellswere
transfected with either of theseplasmids along with increas-ingamounts ofpSG130B/S,aplasmid whichproduces IE63
(32). Results revealed that CAT activity from pSAU2 was
stimulated up to fivefold by increasing amounts of pSG130B/S (Fig. 5A).In contrast, the level ofstimulationof CAT activity from pLW2 was maximally twofold at the highest ratio of pSG130B/S to pLW2. In parallel experi-ments, aplasmid expressing HSV-1 IE175 stimulated CAT activity from both plasmids to the same extent (data not
shown). Therefore, in agreement with previous results ob-tained by using other poly(A) sites, expression of IE63 is reflected in increased reportergeneactivitywhich ispoly(A) sitedependent.Takentogether, ourin vitro andtransfection data indicate that IE63 isrequired for increases in 3'
proc-essing atthe L site.
DISCUSSION
Our studies indicate that the selective increase in
process-ingatthe UL38poly(A)site is mediatedbyexpressionofat least one HSV IE component. This conclusion is derived from both in vivo studies with virus recombinants and in vitro analysis of nuclear extracts made from cells infected with virusmutants. Moreover, the increaseinprocessingat the UL38 poly(A) site is not dependenton either the pro-moterwhich drivestranscriptionorthetemporalclass of the
pSAU2 Prom CAT IE Prom CAT L
FIG. 5. CATactivities producedin cells transfectedwith CAT reporter plasmids together with a plasmid expressing IE63. (A) HeLacellsweretransfected withplasmid pLW2orplasmid pSAU2. Quantities of plasmid pSG130B/S which gave molarratiosin the rangeof1 to5ofIE63 gene to CAT reporter gene were cotrans-fected withpLW2 and pSAU2.At48haftertransfection,cells were harvested and CAT activitiesweredeterminedfor cells transfected with pLW2
(@)
and pSAU2(0). CATactivities are expressed as fold increase relative to those obtained in the absence of pSG130B/S. (B) Description of CATmRNAsproduced by plasmids pLW2 and pSAU2. Prom, promoter sequences derived from the HSV-2IE-4/-5genewhichwereused toinitiate transcription.promoter. In wild-type DNA, the UL38 poly(A) site is
predominantly usedatlate times ininfection (2, 17a);
how-ever, we have shown that this poly(A) site can respond to
LPFatearly times. Thus, unlike latepromoters, the ability
toincreaseprocessingatviruspoly(A)late sites isnotlinked to additional structural features such as replicating DNA. This conclusion is alsoborneoutby in vitro studies in which
processingis uncoupledfrom activetranscription.
By using in vitro analysis (summarized in Table 1) and
transfection data (Fig. 5), we show that the HSV-1 IE63
polypeptide contributes to the increased processing
ob-servedattheUL38poly(A)site.Expressionof HSV-1IE63
is essential forlyticvirus growth; however,the function of
this polypeptide in virus infection has been unclear. In
transfection
experiments, coexpression
of IE63 with otherIEproducts did enhance transcription fromcertain but not
all HSV promoters (8, 25). More recently, IE63 has been shownto reduce
expression
of genes which contain introns(30, 32). Moreover,thedata also indicated that the levels of
reporter gene expression from constructs that contained a
synthetic
poly(A) site,
which functionedinefficiently,
andon November 9, 2019 by guest
http://jvi.asm.org/
[image:5.612.140.220.79.285.2] [image:5.612.320.559.79.499.2]6944 McLAUCHLAN ET AL.
the IE63 poly(A) site were stimulated in the presence of
IE63; this level of stimulation was similar to that for the UL38poly(A) site used inourstudies. Bycontrast, expres-sion was not increased from constructs containing simian virus early or late poly(A) signals. Thus, the stimulatory effectsweredependent onthe 3' processing signals.
It isnot apparenthow the effect ofIE63onRNA
process-ingis relatedtothephenotypesof virus recombinants which
carry mutations in IE63. These mutants display a range of
restrictions in gene expression, although levels of certain late polypeptides generally arereduced for all mutants (16, 19, 26, 29). Itmaybe thatIE63 isamultifunctionalprotein which could mediate effects on cellular events other than RNAprocessing. Notably,domains whichnotonlyactivate butalsorepress geneexpressionhave been identified within theIE63 gene (11, 19).
The mechanismbywhichIE63influencesRNAprocessing is unknown. HSVinduces alterations in the pattern of small nuclear ribonucleoproteins (snRNPs) in infected cells (15). Our recent analysis has revealed that virus recombinants with mutations in IE63 fail to alter snRNP patterns (22a). Moreover, IE63colocalizes with U2snRNP,which binds to the splicingbranch pointin introns(4, 13).Theseproperties indicate thatatleastonefunction ofIE63istoinfluence the behavior of cellular components which perform posttran-scriptional processing of RNA. The ability of invitro
sys-tems to reproduce changes in levels of processing should facilitate analysis of the mode of action ofIE63.
REFERENCES
1. Addison, C., F. J. Rixon, J.W. Palfreyman, M. O'Hara, and
V. G.Preston. 1984. Characterisation ofaherpes simplexvirus type 1 mutant which has a temperature-sensitive defect in penetrationof cells andassemblyofcapsids. Virology
138:246-259.
2. Anderson, K. P., R. J. Frink, G.DeviRao,B.Gaylord,R.Costa,
and E. K.Wagner.1981. Detailed characterization of the mRNA
mappingin theHindlllfragmentKregionof theherpes simplex
virus type 1genome.J. Virol. 37:1011-1027.
3. Bradford, M. M. 1976. Arapid and sensitive method for the
quantitation of microgram quantities of protein utilizing the
principleofprotein-dye binding.Anal. Biochem. 72:248-254. 4. Chabot, B.,andJ.A. Steitz. 1987.Multipleinteractions between
the splicing substrate and small ribonucleoproteins in
splice-somes. Mol. Cell Biol. 7:281-293.
5. Chapman,C.J., J.D.Harris,M. A.Hardwicke,R. M.
Sandri-Goldin,M. K. L.Collins,and D. S. Latchman. 1992. Promoter independentactivation ofheterologousgeneexpression bythe
herpes simplexvirusimmediate-early protein ICP27. Virology
186:573-578.
6. Cullen,B. R., and W. C. Greene. 1989. Regulatory pathways governingHIV-1regulation. Cell 58:423-426.
7. Davison, M.-J., V. G. Preston, and D. J. McGeoch. 1984. Determination of the sequence alteration in the DNA of the
herpes simplex virus type 1 temperature-sensitive mutant
tsK.J. Gen.Virol.65:859-863.
8. Everett,R. D. 1986. Theproductsofherpes simplexvirus type 1(HSV-1)immediateearlygenes 1,2 and 3canactivate HSV-1 geneexpressionintrans.J. Gen. Virol. 67:2507-2513. 9. Everett,R. D. 1987. Theregulationoftranscriptionof viral and
cellulargenesby herpesvirus immediate-early products. Anti-cancerRes. 7:589-604.
10. Gaffney, D. F., J. McLauchlan, J. L. Whitton, and J. B. Clements. 1985. Amodular system for theassayoftranscription regulatory signals: the sequenceTAATGARATisrequiredfor
herpes simplexvirus immediateearlygeneactivation. Nucleic
Acids Res. 13:7847-7863.
11. Hardwicke, M. A., P. J. Vaughan, R. E. Sekulovich,R.
O'Con-ner,and R. M. Sandri-Goldin. 1989. Theregions importantfor the activator and repressor functions ofherpes simplexvirus
type 1 ot protein ICP27 map to the C-terminal half of the molecule.J.Virol. 63:4590-4602.
12. Kennedy, I. M.,J. K. Haddow, andJ. B. Clements. 1991. A negativeregulatoryelementin the human papillomavirustype 16 genome acts at thelevel of late mRNA
stability.
J. Virol. 65:2093-2097.13. Lamond, A. I., B. Sproat, U. Ryder, and J. Hamm. 1989. Probingthestructureandfunction of U2snRNP with anti-sense oligonucleotidesmadeof 2'-OMeRNA.Cell 58:383-390. 14. Lee,K.A.W.,and M. R.Green.1990. Small scalepreparation
ofextractsfrom radiolabelled cellsefficient inpre-mRNA
splic-ing.MethodsEnzymol. 181:20-30.
15. Martin,T. E.,S. C. Barghusen,G. P.Leser,and P.G.Spear. 1987.Redistribution of nuclearribonucleoproteinantigens dur-ing herpes simplexvirusinfection.J. Cell Biol. 105:2069-2082. 16. McCarthy, A. M., L. McMahan, and P. A. Schaffer. 1989. Herpes simplex virus type 1 ICP27 deletion mutants exhibit alteredpatternsoftranscriptionandareDNAdeficient.J.Virol. 63:18-27.
17. McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M.C.Frame,D.McNab,L.J.Perry,J.E.Scott,and P.Taylor. 1988.ThecompleteDNA sequenceofthelong uniqueregionin the genome of herpes simplex virus type 1. J. Gen. Virol. 69:1531-1574.
17a.McLauchlan, J.,andJ.B.Clements.Unpublisheddata. 18. McLauchlan, J.,S. Simpson, andJ.B. Clements.1989.Herpes
simplex virus induces a processing factor that stimulates poly(A)siteusage.Cell 59:1093-1105.
19. McMahan, L., and P. A. Schaffer. 1990. The repressing and
enhancing functions of the herpes simplex virus
regulatory
protein ICP27 map to C-terminalregions and are required to modulate viralgeneexpressionveryearlyininfection. J. Virol. 64:3471-3485.
20. Moore, C. L., and P. A. Sharp. 1985. Accurate cleavage and
polyadenylationofexogenousRNAsubstrate. Cell 41:845-855.
21. Nevins,J. R.,andM.C. Wilson. 1981. Regulationof adenovi-rus-2 geneexpressionatthelevel oftranscriptionaltermination
andRNAprocessing. Nature(London)290:113-118.
22. Nikas, I.,A. J. Darling, H. M. Lankinen,A. M. Cross, H. S.
Marsden,andJ.B.Clements. 1990. Asingleamino acid substi-tution in the large subunit of herpes simplex virus type 1 ribonucleotidereductase whichprevents subunitassociation.J. Gen. Virol. 71:2369-2376.
22a.Phelan, A.,A.I. Lamond,J.McLauchlan,andJ.B.Clements.
Unpublisheddata.
23. Post,L.E.,andB. Roizman.1981. Ageneralized techniquefor deletionofspecificgenesinlargegenomes:(a)gene22ofherpes
simplexvirus 1 isnotessential forgrowth.Cell 25:227-232. 24. Preston, V. G., J. W. Palfreyman, and B. M. Dutia. 1984.
Identification of a herpes simplex virus type 1 polypeptide
whichis a component of the virus-induced ribonucleotide
re-ductase. J. Gen. Virol. 65:1457-1466.
25. Rice, S.A., and D. M.Knipe. 1988.Gene-specific transactiva-tion by herpes simplex virus type 1 alpha protein ICP27. J. Virol. 62:3814-3823.
26. Rice,S.A., and D. M. Knipe. 1990. Genetic evidence fortwo distinct transactivation functions oftheherpes simplexvirusa
protein ICP27.J. Virol. 64:1704-1715.
27. Rixon,F.J.,M. D.Davison,and A.J.Davison. 1990. Identifi-cation of the genes encoding two capsid proteins of herpes
simplexvirus type 1bydirect amino acidsequencing.J. Gen. Virol. 71:1211-1214.
28. Rixon, F. J., and J. McLauchlan. 1990. Insertion of DNA sequences at a unique restriction enzyme site engineered for vectorpurposesinto the genome ofherpes simplexvirus type 1. J. Gen. Virol. 71:2931-2939.
29. Sacks,W.R.,C. C.Greene,D. P.Aschman,and P.A.Schaffer. 1985.Herpessimplexvirus type 1ICP27isanessential regula-toryprotein. J.Virol. 55:796-805.
30. Sandri-Goldin,R.M.,andG.E.Mendoza. 1992. Aherpesvirus
regulatory proteinappears to act post-transcriptionally by af-fectingmRNAprocessing. Genes Dev. 6:848-863.
31. Seed, B., and J.-Y. Sheen. 1988. A simple phase-extraction
J. VIROL.
on November 9, 2019 by guest
http://jvi.asm.org/
assayforchloramphenicol acetyltransferase activity. Gene 67:
271-277.
32. Sekulovich, R. E., K.Leary,and R. M.Sandri-Goldin. 1988. The herpes simplex virustype 1 alpha protein ICP27can act as a
trans-repressor or atrans-activator in combination with ICP4
andICPO. J. Virol. 62:4510-4522.
33. Smith,I.L.,M. A.Hardwicke,and R. M.Sandri-Goldin. 1992.
Evidencethat the herpes simplex virusimmediate early protein ICP27 acts post-transcriptionally during infection to regulate
geneexpression. Virology 186:74-86.
34. Stow,N. D.,and E. C. Stow. 1986. Isolation and characterisation ofa herpes simplex virus type1mutant containinga deletion within the gene encoding the immediate early polypeptide
Vmw1lO.J. Gen. Virol. 67:2571-2585.
35. Watson, R. J., and J. B. Clements. 1980.Aherpes simplex virus type 1 functioncontinuously requiredfor early and late virus
RNAsynthesis.Nature(London) 285:329-330.