Enhanced Thermal Characteristics of NG Based Acetamide Composites
Full text
Figure

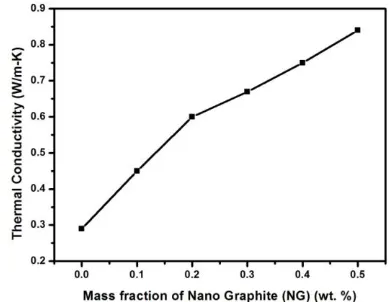
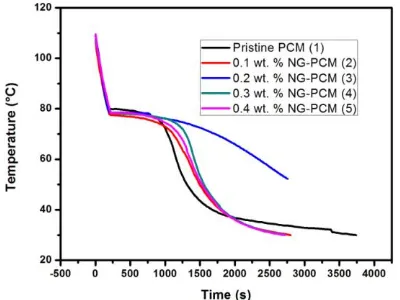
Related documents
• Follow up with your employer each reporting period to ensure your hours are reported on a regular basis?. • Discuss your progress with
As with other rapidly reconfigurable devices, optically reconfigurable gate arrays (ORGAs) have been developed, which combine a holographic memory and an optically programmable
Even though previous L1 studies have reported strong impact of interest on reading comprehension by young learners, which has sometimes been mediated by other individual
It was possible to improve the production process by applying the simulation technique, which shows that the general objective was achieved. Also, it was possible to
on deeply held misconceptions and fears of people who hold different beliefs, strategies of conceptual change theory, most often found in science education, were
To the finest of our information, this is the first report of the use of PEG-400 and DMAP, DIPEA catalyst for the protection of secondary amine of pyrazole nucleus using Boc..
Furthermore, while symbolic execution systems often avoid reasoning precisely about symbolic memory accesses (e.g., access- ing a symbolic offset in an array), C OMMUTER ’s test