The synthesis and separation properties of organic cage compounds
Full text
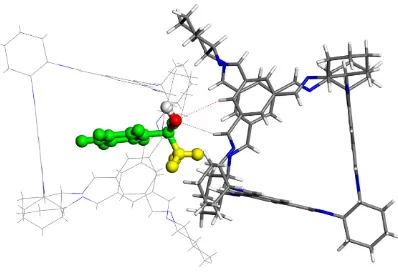
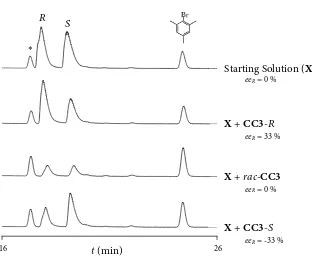
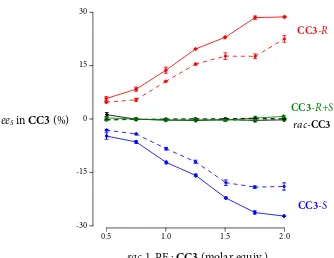
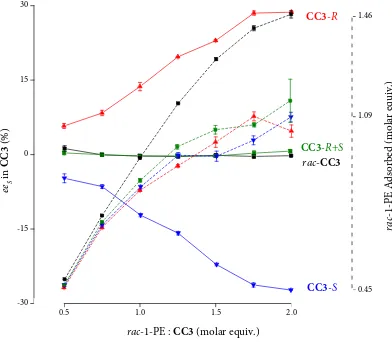
Figure
![Figure 2-3trialdehydenergy mmental Secombinati[2+3] (c), 3,4-diamin3 | Models ofdes were builtinimisation](https://thumb-us.123doks.com/thumbv2/123dok_us/8071879.227063/45.595.120.515.253.621/figure-trialdehydenergy-mmental-secombinati-diamin-models-ofdes-builtinimisation.webp)
Related documents
AMS: Aging Male Symptoms; CI: Confidence interval; DGU: German Society of Urology; ECOG : East cooperative oncology group (Score); EORTC-QLQ- C30: European Organization for Research
associated ASD symptoms, such as behavioral problems, hyperactivity, sleep disorders,
the Siberian stonechats, whereas the growth rate towards adult wing length is higher in the European stonechats (note, however, that in the European birds the wing
For this analysis they used the maximum compressive load which was measured experimentally, and the maximum tensile load which is essentially the inertia load of the piston
There is a large variety of Imidazole ring containing derivatives which exhibit different kinds of pharmacological and biological activities and are being developed for
Qualitative evaluation of the choroidal vessels and retina The three histologically defined layers of choroidal vas- culature, the choriocapillaris, medium choroidal vessels
Guangming Shi, School of Electronic Engineering at Xidian University of China with support from Xian Hi- Tech Industries Development Zone and “MPEG-CHINA: Prof.. Wen Gao