ELECTRON AND SPECIES
POPULATIONS BEHIND HIGH
ENTHALPY SHOCK WAVES IN LOW
DENSITY AIR
By
Paul Anthony Taloni
A thesis submitted for the degree of Doctor of Philosophy
of The Australian National University
Statement of Authorship
The contents of this thesis, except where indicated by references, are entirely my own work.
A ck n ow led gem en ts
M any people hav e a ssiste d m e over th e course of m y P hD re se a rc h . To
th a n k th e m all in d iv id u ally w ould ta k e as m an y pages as I h av e u sed to
describe m y ex p erim en ts. T hose I do n o t m en tio n p erso n ally know th a t
I am sin cerely g rate fu l. A t some tim e or a n o th e r, every m em b er of th e
D e p a rtm e n t o f P h y sics h a s h e lp e d m e in som e w ay sh a p e or form .
S p ecifically I w o u ld lik e to th a n k m y s u p e rv is o rs P ro fe s s o r J o h n
S a n d e m a n a n d D r F r a n k H ouw ing. M an y th a n k s to J o h n S a n d e m a n
who w ould offer advice on som e a sp e c t of th e e x p e rim e n ta l tec h n iq u e
t h a t w o u ld in v a r ia b ly o v erco m e a n a p p a r e n t l y in s u r m o u n ta b le
p ro b lem . I am also in d e b te d to F r a n k H o u w in g w hose c o n tin u a l
e n c o u ra g e m e n t a n d ad v ice, n o t to m e n tio n h is e n d le s s su p p ly of
e n th u s ia s m , m ad e th e r e s e a r c h p ro g ra m a s p ro d u c tiv e a s i t w as.
T h a n k s a re due also to D r D on B one for th e coaching he gave m e in
s q u a s h a s w ell a s th e m a n y d is c u s s io n s we h a d o n a to m ic
spectroscopy. U n fo rtu n a te ly m y s q u a s h g am e did n o t s u b s ta n tia lly
im p ro v e, b u t m y u n d e rs ta n d in g of sp e ctro p h y sic s a n d spectroscopic
tech n iq u e c e rta in ly did. T h a n k s to D r H ans-A B ach o r w hose ex p ertise
in th is a re a w as often u tilize d . I m u s t also th a n k m y fellow s tu d e n ts
w ith w hom I e n d u re d th e rig o rs of e x p e rim e n ta l physics. T h a n k s to
J im T ay lo r, w ith w hom I h a v e s p e n t th e l a s t e le v en y e a rs of m y
schooling, for h is a ssista n c e in th e lab a n d h is good h u m o u r d u rin g th e
f r u s tr a tin g tim e s. To T im M c In ty re (now D r) a n d P h ilip R y an (still
M r) for th e m a n y f ru itfu l d isc u ssio n s c o n ce rn in g th e a r e a s of o u r
r e s e a r c h a s w ell a s th e le s s f r u itf u l, b u t e q u a lly e n te r ta in in g ,
d isp u ta tio n s over e x p e rim e n ta l tec h n iq u e . I w ould also lik e to th a n k
Abstract
E x p e rim e n ts w ere co n d u cted to te s t th e v a lid ity of th e o re tic a l m odels
w h ic h d e sc rib e th e b e h a v io u r of c h em ic al a n d th e r m a l re la x a tio n
p ro cesses a t h ig h te m p e r a tu re s a n d low d e n s itie s . In te rfe ro m e tric ,
em issio n a n d a b so rp tio n e x p e rim e n ts w ere p erfo rm ed to m e a s u re th e
e le c tr o n d e n s itie s , r o t a t io n a l t e m p e r a t u r e , a n d e m is s io n a n d
ab so rp tio n profiles for com parison w ith th eo ry .
E le c tro n p o p u la tio n s b e h in d h ig h e n th a lp y sh o ck w a v e s in low
p re s s u re a ir w ere m e a s u re d w ith s p a tia l a n d te m p o ra l re s o lu tio n a t
c o n d itio n s a p p lic a b le to th e flig h t re g im e s of th e NASA p ro p o se d
a e ro a s sis te d o rb ita l tr a n s f e r vehicle u s in g a n in fra re d in te rfe ro m e tric
te c h n iq u e . M e a s u re m e n ts u s in g th is te c h n iq u e w ere c o n firm ed by
o b serv in g th e S ta r k b ro a d e n in g of th e h y d ro g en ß tra n s itio n . I t w as
o b se rv ed t h a t th e e le c tro n p o p u la tio n s p la te a u to a lev e l t h a t is
sig n ifican tly less th a n t h a t p red ic te d by a one—te m p e ra tu re m odel, b u t
are in ex cellen t a g re e m e n t w ith v alu es p red ic te d by a two—te m p e ra tu re
m odel. T he m e a su re d ra te of io n isatio n a g reed fav o u rab ly w ith th e ra te
p re d ic te d by th e tw o - te m p e r a tu r e m odel, b u t w as a g a in sig n ific an tly
less th a n t h a t p red icted by th e o n e -te m p e ra tu re m odel.
T he b ro a d b a n d te m p o ra l em issio n c h a ra c te ris tic s of th e p la s m a w ere
s tu d ie d in o rd e r to d e te rm in e c h a ra c te ris tic re la x a tio n tim e s. T he
em issio n p ro files w ere in ex cellen t accord w ith th e p re d ic tio n s of th e
two—te m p e ra tu re m odel. No m e a su re of th e em issive pow er w as m ad e
how ever, a n d th e re fo re no com m ent is m ad e a s to th e re lia b ility of th e
T e m p o ra lly re s o lv e d e m is s io n m e a s u r e m e n ts w ere c o n d u c te d on
specific ro ta tio n a l tra n s itio n s in th e N 2+ m olecule in th e reg io n b e h in d
th e shock to d e te rm in e th e ro ta tio n a l te m p e ra tu re . T h is te m p e r a tu re
w as fo u n d to be co rrectly p re d ic te d by th e tw o—te m p e r a tu re m odel,
w hile th e one—te m p e ra tu re m odel, u n d e re s tim a te d th e te m p e ra tu re by
a factor of ab o u t 50%.
T he re la tiv e in te n s ity of tw o tr a n s itio n s in ato m ic a n d ionic oxygen
w ere m e a s u re d a n d co m p ared w ith th e o re tic a l p re d ic tio n s. I t w as
o b s e rv e d t h a t th e t h e o r e tic a l io n -to -a to m e m is s io n r a t i o w a s
su b s ta n tia lly la rg e r w hen described by a single te m p e ra tu re th a n w h en
d escrib ed by tw o te m p e ra tu re s . T he e x p e rim e n ta l r e s u lts su p p o rt th e
two—te m p e ra tu re d escrip tio n a n d len d su p p o rt to th e conclusion of th e
e le c tro n n u m b e r d e n s ity s tu d ie s , w h ich s u g g e s t t h a t th e o b se rv ed
d e crea se in th e s e p o p u la tio n s w as d u e to a lo w e rin g of th e e le ctro n
te m p e ra tu re .
An in fra re d CW diode la s e r a b so rp tio n e x p e rim e n t w as c a rrie d o u t in
oxygen on th e sam e t r a n s itio n a s in th e e m iss io n s tu d ie s . T h e
m e a s u r e d a b s o rp tio n w as c o m p a re d w ith t h a t p r e d ic te d by th e
two—te m p e r a tu re m odel, a n d found to be in fa ir a g re e m e n t, a lth o u g h
th e u n c e rta in ty in th e m e a su re m e n ts is high.
T he e x p e rim e n ta l r e s u lts le n d s u p p o rt to c u r r e n t th e o re tic a l w ork,
w hich proposes t h a t it is n e ce ssa ry to c h arac terize th e flow by d ifferen t
te m p e r a tu r e s to a c c u ra te ly m odel c o n d itio n s a t th e h ig h sh o ck
TABLE OF CONTENTS
Chapter 1. INTRODUCTION Page
1.1 Background
26
1.2 A review of relevant literature
30
1.3 An overview of the current experiments
44
1.4 The structure of this thesis
45
Chapter 2. IONISATION PROCESSES AND THE EMISSIVE
PROPERTIES OF SHOCK HEATED AIR
2.1 Introduction
47
2.2 Ionisation processes
48
2.2.1
Ionisation by atomic and molecular collision
48
2.2.2
Electron and ion impact ionisation
53
2.2.3
Photoionisation
56
2.2.4
Charge exchange
59
2.2.5 Electron attachment
60
2.3 The emissive properties of shock heated air
62
Chapter 3. THE TWO-TEMPERATURE KINETIC MODEL
3.1 Introduction
67
3.2 The need for a multi-temperature kinetic model
67
3.3 A three-temperature description
71
3.4 The two-temperature kinetic model
77
3.5 Vibrational excitation in the TTV model
81
3.5.1
Correction to the vibrational excitation cross
83
section
3.5.3 Preferential dissociation from excited states
87
3.6
The reaction kinetics in the TTV model
88
3.6.1
Reactions and rate controlling temperatures
88
3.7
Predictions of the TTV model
93
Chapter 4. THE EXPERIMENTS
4.1 The experimental objectives
95
4.2 The shock tube facilities
96
4.2.1
Construction and operation
96
4.2.2
Driver conditions
100
4.2.3
Timing and triggering
102
4.2.4
The test section
103
4.3 Ionisation diagnostics and their relative merits
104
4.4 Infrared Interferometry
109
4.4.1
The experimental arrangement
109
4.4.2
The Michelson interferometer
111
4.4.3
The tunable CW C02 laser
112
4.4.4
The HgCdTe infrared detectors
113
4.4.5 Data acquisition
116
4.5 Stark broadening of the Hß line
117
4.5.1
Introduction
117
4.5.2
The experimental arrangement
118
4.5.3
The Optical Multichannel Analyser
120
4.5.4
The photomultiplier
121
4.5.5
Triggering and timing
122
4.6 Emission from molecular nitrogen
123
4.6.1
The experimental arrangement
123
4.7
Emission from atomic and ionic oxygen
124
4.7.1
The experimental arrangement
124
4.7.2
The oxygen transitions
125
4.8
Infrared CW diode laser absorption by atomic oxygen
126
4.8.1
The experimental arrangement
126
4.8.2
The infrared CW diode laser
128
C h a p te r 5. D A T A R E D U C T IO N
5.1 Introduction
129
5.2 Infrared interferometric fringe analysis
129
5.3 Stark broadened Hß profile analysis
134
5.4 Emission from molecular nitrogen
135
5.4.1
Introduction
135
5.4.2
Molecular spectroscopy
136
5.4.3
The thermal distribution of the rotational levels
145
5.5 Atomic and ionic oxygen emission analysis
149
5.5.1
Atomic transitions as temperature indicators
150
5.6 Infrared CW diode laser absorption by atomic oxygen
153
C h a p te r 6. RESULTS
6.1 The experimental limitations
160
6.1.1
Test gas contamination
163
6.2 Infrared interferometry
165
6.2.1
Argon results
165
6.2.2
Nitrogen and air results
166
6.3 The Stark Broadened Hß Profile
172
6.4 Emission from molecular nitrogen
174
6.5 Atomic and ionic oxygen emission
179
Chapter 7. DISCUSSION
7.1 Electron number density measurements in air
7.2 Em ission from molecular nitrogen
7.3 Atomic and ionic oxygen emission
7.4 Infrared CW diode laser absorption by atomic oxygen
183 189
192
193
Chapter 8. CONCLUSIONS 196
REFERENCED LITERATURE
APPENDICES
A ppendix A
Appendix B
Appendix C
Appendix D
Appendix E
Reaction sequences for ionising atomic and
molecular collisions
Reaction sequences for the charge transfer
reactions
Reaction sequences for the electron attachm ent
reactions
Park's model - reactions and rate parameters
Reduction o f output in Species Mole Fractions to
LIST OF FIGURES AND TABLES
C h a p te r 2 A f t e r p a g e
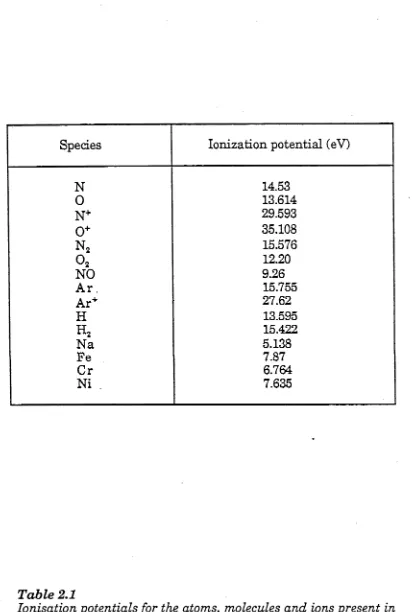
T a b le 2.1 I o n is a tio n p o t e n t i a ls o f th e e x p e r im e n ta l sp e c ie s 58
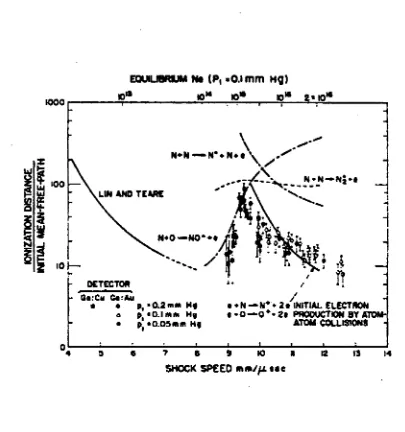
F ig u r e 2.1 T h e o r e tic a l a n d o b s e r v e d io n is a tio n d is ta n c e s
b e h in d sh o c k w a v e s in a i r 62
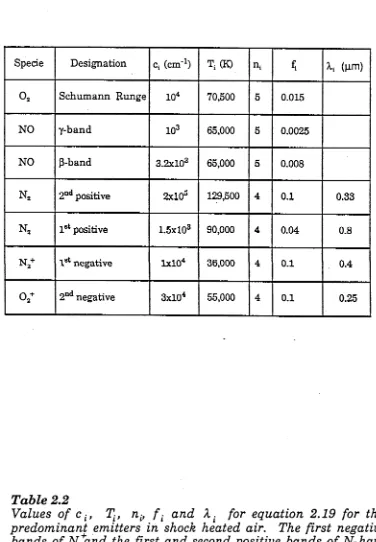
T a b le 2.2 P a r a m e te r s f o r th e th e o r e tic a l e s tim a te s o f a i r
e m is s iv itie s r e p r o d u c e d f r o m P e n n e r (1 9 5 9 ) 64
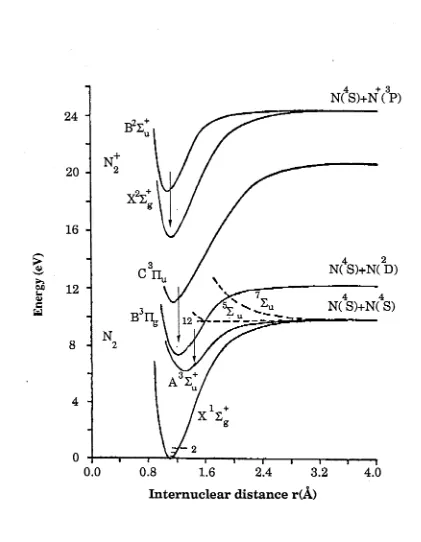
F ig u r e 2 .2 P o te n tia l w e ll f o r N 2 a n d N 2+ 65
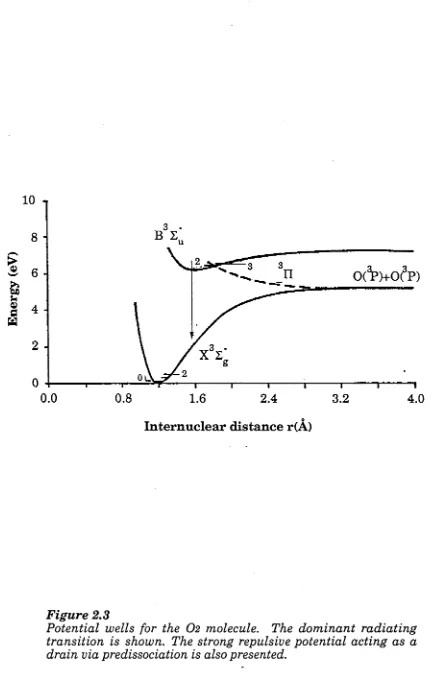
F ig u r e 2 .3 P o te n tia l w e ll fo r 0 2 65
F ig u r e 2 .4 P o te n tia l w e ll f o r N O 65
C h a p te r 3
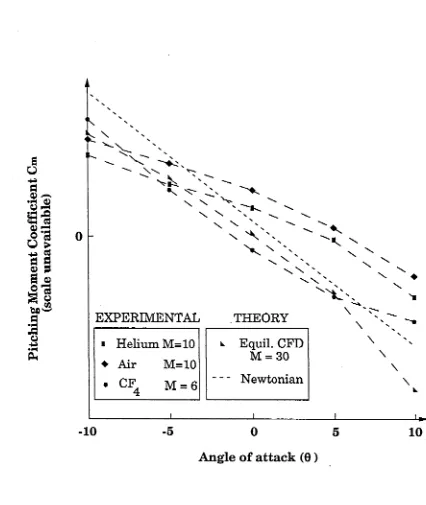
F ig u r e 3.1 P it c h i n g m o m e n t c h a r a c te r is tic s o f th e
a e r o a s s is t f l i g h t e x p e r im e n t 68
F ig u r e 3 .2 E x p e r i m e n ta l a n d th e o r e tic a l s h o c k la y e r
th ic k n e s s o v e r a 45? co n e 68
F ig u r e 3 .3 S p e c ie s m o le fr a c tio n p r e d ic tio n s o f th e T T V
m o d e l 93
F ig u r e 3 .4 T e m p e r a tu r e a n d e m is s io n p r e d i c tio n s
o f th e T T V m o d e l 93
F ig u r e 3 .5 C h a r a c te r is tic r e la x a tio n t im e p a r a m e t e r
v ’s sh o c k v elo city 93
T a b le 3.1 T y p ic a l s h o c k tu b e te s t c o n d itio n s p r e d ic te d
Chapter 4
Figure 4.1 Schematic diagram o f the free piston shock tube 97
Figure 4.2 Relative movements o f the shock/ contact surface 99
interfaces.
Table 4.1 Driver conditions for DDT 102
Table 4.2 Driver conditions for T3 and T2 102
Figure 4.3 Exit of tube detailing Prandtl-Meyer expansion
fan lim iting steady flow to a Mach cone 104
Figure 4.4 Schematic diagram detailing the infrared
interferometric experiment 109
Figure 4.5 The electronics system used in the infrared
interferometric experiment 110
Figure 4.6 The Infrared detector and its hardware 115
Figure 4.7 Schematic detailing the Stark broadening,
molecular and atomic emission experiments 119
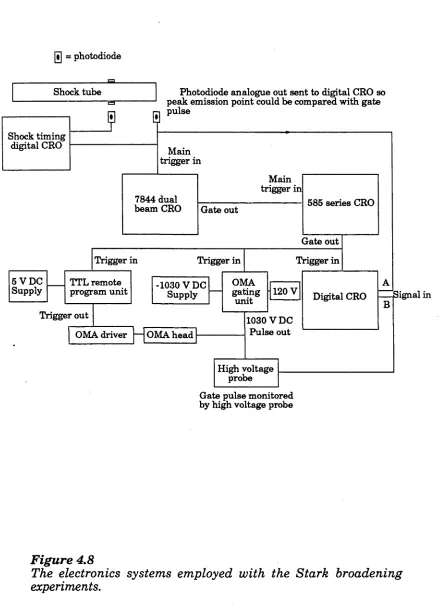
Figure 4.8 The electronics system used in the
aforementioned experiments 121
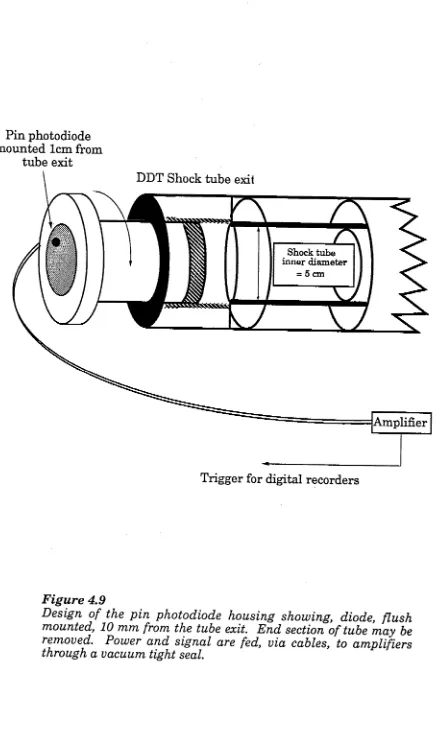
Figure 4.9 The flush mounted photodiode trigger housing 123
Figure 4.10 Energy level diagram o f the N 2 and N 2+ molecule 124
Figure 4.11 The energy level diagram o f the O atom 126
Figure 4.12 Schematic diagram detailing the infrared CW
laser absorption spectroscopy experiment 126
Chapter 5
Figure 5.1 Calculated FWHM for the H-Beta transition
as a function o f the electron number density 134
Table 5.1 Molecular constants used to determine the
Figure 5.2 H u n d ’s coupling cases (a) and (b) 141
Figure 5.3 Thermal distribution o f the rotational levels 146
Figure 5.4 Atom I ion level scheme 151
Chapter 6
Photographic plate #1 Observed im purity emission 164
Figure 6.1(a) Contaminated fringe shift 164
Figure 6.1(b) Un-contaminated fringe shift 164
Figure 6.2 Contaminated OMA emission spectrum 164
Figure 6.3 M ultiple fringe shift resulting from large
ionisation fraction in 133 Pa o f argon 171
Figure 6.4 M ultiple fringe shift resulting from large
ionisation fraction in 1333 Pa o f argon 171
Figure 6.5(a) Electron population v ’s distance behind shock
front for 133 Pa of argon 171
Figure 6.5(b) Electron population v ’s distance behind shock
front for 1333 Pa o f argon 171
Figure 6.6 Observed fringe shift v=10.2 kms-1 , 13 Pa o f air 171
Figure 6.7 Observed fringe shift v=10.9 km s'1 , 13 Pa o f air 171
Figure 6.8 Observed fringe shift v=10.8 km s'1 , 13 Pa o f N 2 171
Figure 6.9 Observed fringe shift v=10.2 km s'1 , 13 Pa o f air 171
Figure 6.10 Observed fringe shift v=10.5 km s'1 , 13 Pa o f N 2 171
Figure 6.11 Observed fringe shift v=10.5 kms-1 , 13 Pa o f air 171
Figure 6.12 Observed fringe shift v=10.6 km s'1 , 13 Pa o f air 171
Figure 6.13-26 Electron population v’s distance behind shock,
experiment plus theory at the varying conditions 171
Figure 6.27 Time resolved emission of Hß transition at
various detunings from line centre 173
Figure
6.29Experimental
Hßtransition together with
the theoretical profile for Ne=1015 cm-3
173Figure
6.30Reduced
Hßtransition together with the
theoretical profile for
Ne=1015and
1016 cm-3 173Figure
6.31Experimental time resolved broadband
emission labelled with the relaxation
parameters
178Figure 6.32
Experimental time resolved broadband
emission (convoluted) together with the
emissive predictions of the TTV model
178Figure
6.33OMA spectrum of driver contaminants
178Figure
6.34OMA spectrum of driver contaminants
and some ro-vibrational structure
178
Figure 6.35
OMA spectrum of pure ro-vibrational
structure about band heads of N2+
178
Figure 6.36
OMA spectrum of three oxygen transitions
180
Figure 6.37
OMA spectrum of oxygen transitions
detailing the decrease in the emissive
power with decreasing pressure
180
Figure 6.38
Emissive power per particle v’s pressure
180
Figure 6.39
OMA spectrum of O and O
+transitions
after several OMA accumulation cycles
180
Figure 6.40 Theoretical ion-to atom intensity ratio for O
180
Figure 6.41
Observed and predicted diode laser absorption
at for the experimental parameters at
ACRONYMS AND NOTATIONS
This thesis is written in, and adheres to, the SI units system. On
occasion however, certain equations contain dimensioned constants.
In these instances, the equations are detailing either cross sectional or
spectroscopic parameters. It is common practice to employ the cgs
units system in these cases. For example, practically all spectroscopic
data is given in cgs units. The thesis therefore follows this convention
for those specific equations, namely, equations 2.3, 2.6, 2.7, 2.17, 2.19,
3.12, 3.16, 3.23, 4.7, 5.48 and 5.49.
The following acronyms and notations are presented in the order in
which they appear in the given chapter.
AOTV:
CW
:
FWHM:
HEO :
LEO
:
OTV :
TTV
:
aeroassisted orbital transfer vehicle
continuous wave
full-width-half-maximum
high earth orbit
low earth orbit
orbital transfer vehicle
pertaining to the two-temperature kinetic model of Park
(1989)
Chapter 2
a0
:
first Bohr radius
A^T)
:
forward rate constant for collisional ionisation
c
:
speed of light
Cx
d AE AE® E E* Ex E0 f fie) fi g h I k L m e n(v) n e % 2 nX QQ
Qe Q& Q1m ean therm al velocity of species X
distance between the atomic or molecular fields of two particles
total internal energy of a system energy of reaction
im pact energy
energy difference between two electronic states of a molecule
activation energy for ionisation
lowest vibrational level of an upper state oscillator strength
local electron energy distribution function
characteristic oscillator strength of the ith band system ratio of the statistical weights
Plank's constant
total radiation intensity Boltzmann's constant
effective thickness of the reaction region electron mass
num ber density of photons with frequency v local electron num ber density
averaged num ber density of N2 molecules local density of species X (atom or molecule)
Total cross section for electron impact with atoms or ions velocity averaged cross section
cross section for ionising collisions
Q ix (e )
Q o Q s
R
Rxa0 T
t
rp*
Te
Ti
V
V e
W ix
X
cross section for electro n collisional io n isatio n
e lastic cross section
e x citatio n cross section
in te r n a l se p a ra tio n for collisional io n isatio n
c en tre of m ass d istan c e
tr a n s la tio n a l te m p e ra tu re
tim e
c h a ra c te ristic te m p e ra tu re for th e electronic s ta te
electro n te m p e ra tu re
c h a ra c te ristic te m p e ra tu re for th e ith b a n d sy stem
rela tiv e velocity of two p articles
electron velocity
th re s h o ld energy for species X
species type (atom or m olecule)
G reek sym bols
8 p la s m a th ic k n e s s
8 electro n energy
e e f f effective em m isivity
fo rw ard r a te coefficient for collisional io n isatio n
c h a ra c te ristic w av elen g th of th e ith b a n d sy stem
M- red u ced m ass of a sy stem
V ra d ia tio n frequency
P d en sity
Po den sity a t STP
* A I a u to io n isatio n lifetim e
T m ea n lifetim e before a u to io n isatio n occurs
collisional lifetim e
number of NO molecules
C h a p te r 3
A
C
c
C ™m
Cp
Cy
Dx
e
E
E,
e e
Ev
i=l, 2,.., 5 rate controlling constants
parameter dependent on the molecule type
reaction rate constant for vibrational excitation
average molecular speed
pitching moment coefficient
specific heat at constant pressure
specific heat at constant volume
dissociation energy of molecule X
average vibrational energy per unit mass of molecule s
effective diffusive coefficient for species s
electron charge
total energy per unit mass
sum of the electronic excitation energy and kinetic energy
of the electrons
activation energy of the reaction
vibrational energy per unit mass of species s at
temperature Te
vibrational energy at the electron temperature
vibrational energy per unit mass of species s at
temperature T
total electronic energy per unit mass of molecule s
vibrational energy
®v,s
Evib
E Vib,Eq :
flea
n v
Nx
P
Pe
Or
Qrad
R T
v ib ra tio n a l en erg y p e r u n it m ass of species s
av erag e v ib ra tio n a l ex citatio n en erg y p e r m olecule
av erag e v ib ra tio n a l ex citatio n en erg y p e r m olecule a t
e q u ilib riu m
electro n gas h e a t tra n s fe r
to ta l e n th a lp y p e r u n it m ass
electronic e n th a lp y p e r u n it m ass of species s
v ib ra tio n a l e n th a lp y p e r u n it m ass of species s
firs t io n isatio n p o te n tia l for species s
B o ltz m an n 's c o n sta n t
th e rm a l conductivity by th e electron gas
e q u ilib riu m c o n sta n t for th e rea ctio n
fo rw ard rea ctio n ra te for v ib ratio n a l ex citatio n
r a te coefficient for a tra n s itio n from s ta te v to v'
m ach n u m b e r
m o lecu lar w eig h t of species s
n u m b e r d en sity of th e Xth s ta te
n u m b e r d e n sity of free electrons
m o la r r a te of p ro d u ctio n of species s by ele ctro n im p a c t
io n isa tio n
n u m b e r d en sity of th e vth v ib ratio n a l s ta te
n u m b e r d en sity of th e xth s ta te
p re s s u re
e lectro n p re s s u re
ra d ia tiv e pow er loss
ra d ia tiv e en erg y tra n s fe r ra te
u n iv e rs a l gas c o n sta n t
t
time
T
A VU 1, u J , u k
Vi
:
w
:
wg
:
X\ xj, xk
ys *•
atom temperature
= VTTv = geometrically averaged temperature
electron-electronic excitation temperature
rotational temperature
reaction dependent rate controlling temperature
heavy particle translational temperature immediately
behind the shock front
Vibrational-electron-electronic temperature
:
velocity vector in 3-D space i, j, k=l, 2, 3
diffusion velocity for species i
flow velocity
mass rate of production of species s
:
vector in 3-D space i, j, k=l, 2, 3
mole fraction of species s
Greek Symbols
A
5Ü
evib Diss
£vE
^ei
£ v
y
moment of energy transfer
Kronecker delta
average vibrational excitation energy
average vibrational excitation energy per molecule at
equilibrium
average vibrational excitation energy per particle i
average vibrational excitation energy per molecule
ratio of specific heats (Cp/Cy) .
frozen thermal conductivity for
translational-rotational energy of heavy particles
K
P
Pm
Vi
P Pe Ps av
T
%
%
TVib
0
frozen th e rm a l co n d u ctiv ity for v ib ra tio n a l e n erg y due to
m o le cu la r collisions
e q u iv a le n t th e rm a l conductivity
to ta l viscosity
red u ced m o lecu lar m ass of th e colliding species
effective collision frequency for electro n s a n d h eav y
p a rtic le s
collision frequency of species i
to tal den sity
electro n d en sity
d en sity of species s
lim itin g cross sectio n for v ib ra tio n a l e x cita tio n a t in fin ite
te m p e ra tu re
v ib ra tio n a l re la x a tio n tim e corrected for th e lim itin g cross
section
v ib ratio n a l ex citatio n tim e by h eav y p article im p a ct
v ib ratio n a l re la x a tio n tim e for ex citatio n by electron
co llisio n s
electronic v ib ra tio n a l re la x a tio n tim e for species s
tra n s la tio n a l v ib ratio n a l re la x a tio n tim e for species s
v ib ra tio n a l re la x a tio n tim e for ex citatio n by h eav y p a rticle
collisions
trim angle of a tta c k
C hapter 4
a : speed of sound
C(Ne>T): S ta rk b ro ad e n in g coefficient
Als : Stark width
Io : initial radiation in ten sity
k1 V : absorption coefficient for the inverse B rem sstrahlung
process
k : spectral absorption coefficient
L : optical path length
M : m ach num ber
m : m olecular m ass
N e : electron num ber density
P : pressure
R : universal gas constant
r : distance from shock tube axis to the Mach cone
r' : distance from the Mach cone to the expansion fan
T : tem perature
u : shock velocity
z : distance from the shock tube exit
Subscripts
1 : undisturbed test gas
5 : com pressed driver gas
D : undisturbed driver gas
s : shock wave
R : reservoir
Greek Symbols
a : microwave attenuation factor
Y : ratio of specific heats (Cp/Cy)
A \ s : S tark broadened wavelength
H : Mach angle
C0r> p : plasm a frequency
C hapter 5
A : constant times the inverse moment of inertia of the electrons
4 i m E instein A coefficient
A
1 '■max maximum fringe am plitude
- ^ m in m inim um fringe am plitude
B : rotational constant
C : constant depending on the change of dipole m oment of a molecule
c : speed of light
C a b s & C e m : constants depending on the change of dipole moment
D y : centrifugal distortion constant
d : line shift
Ag : FWHM
e : electric charge E
^ e , v , r • energies of electronic, vibration and rotation respectively
F : term values of rotational transitions for the nonrigid rotator
f i : oscillator strength
G : term values of vibrational transitions
S i : degeneracy of level i
Gs : Gladstone dale coefficient for species s H(<x,v) : Voigt profile
h : Planks constant
a x is
m o m en t of in e rtia of th e m olecule ab o u t a n axis
p e rp e n d ic u la r to th e in te rn u c le a r axis
tra n s itio n in te n s ity of th e j th level of a n atom
tra n s itio n in te n s ity of th e k th level of a n ion
in itia l in te n s ity
ro ta tio n a l q u a n tu m n u m b e r
to ta l a n g u la r m o m e n tu m vector
to ta l a n g u la r m o m e n tu m a p a r t from sp in
freq u en cy d e p en d e n t a b so rp tio n coefficient
a n g u la r m o m e n tu m of th e electrons
optical p a th le n g th
atom ic m ass
electro n m ass
refra ctiv e index
a n g u la r m o m e n tu m of n u c le a r ro ta tio n
electro n n u m b e r d e n sity
p o p u latio n of th e j th level
fringe o rd er n u m b e r
lin e profile as a fu nction of d e tu n in g
p h a s e
p a rtitio n function
p a rtitio n fu n ctio n
sp in v ecto r of th e electrons
H onl-L ondon fo rm u lae
te rm v a lu e s for th e electronic sta te s
v ib ra tio n a l q u a n tu m n u m b e r
y : v e rtica l fringe p osition a t th e im age p lan e
Z : effective n u c le a r charge
G reek Sym bols
a a
aE
A^d
Y
angle of two b eam s a t th e sp ectro m eter
ion b ro a d e n in g p a ra m e te r
io n isa tio n fractio n a t e q u ilib riu m
D oppler w idth
FW H M
Av A e o Kv X K ^nm V Vo P Ps Z 0) COiXe Q e l Pi
d e tu n in g from lin e cen tre
a n g u la r m o m en tu m of th e electro n s along th e in te rn u c le a r
a x is
p e rm ittiv ity of free space
sp ectral a b so rp tio n coefficient
ra d ia tio n w av elen g th
C om pton w av elen g th = h/mc
lin e cen tre frequency of th e tra n s itio n from s ta te n to m
ra d ia tio n freq u en cy
b a n d orig in
d en sity
d en sity of species s
projection of th e spin vector S along th e in te rn u c le a r axis
lifetim e of level i
w av en u m b er = 1/A,
m e a su re of th e a n h arm o n ic ity of th e oscillator
vector su m of A an d Z
io n isatio n te m p e ra tu re (0j=I/k)
Chapter 1 : Introduction 2 6
C h ap ter 1
IN T R O D U C T IO N
1 .1 B ack grou n d
In re c e n t y e ars w ith th e a d v e n t of th e space sh u ttle , m u ch a ctiv ity h a s
ta k e n p lace in th e low e a r th o rb it (LEO). T h is a ctiv ity h a s in clu d ed
scientific e x p lo ra tio n a s w ell a s com m ercial e n te rp ris e . M u ch of th e
co m m ercial a c tiv ity h a s in c lu d e d th e d e p lo y m e n t o f s a te llite s in to
v a rio u s o rb its ra n g in g from L EO 's, u p to 400 km , to h ig h e a r th orbits,
H E O 's, w ith d e p lo y m e n t a ltitu d e s a s h ig h a s 35,900 k m for th e
g eo sy n ch ro n o u s sites.
To d a te , th e s a te llite d ep lo y m en t vehicles h av e b een m u ltis ta g e ro ck et
sy stem s, co n sistin g of a low er stag e w hich tra n s p o rts th e p ay lo ad from
g ro u n d to a LEO , a n d a n u p p e r stag e b o o ster c o n tin u in g from th e LEO
to th e H E O . T h e s e u p p e r s ta g e ro c k e ts , w h ic h a re o f c o u rse
e x p e n d a b le , a re e x p e n siv e a n d of re la tiv e ly low re lia b ility . T he
s e rv ic in g o f b o th g e o s ta tio n a r y a n d e q u a to r ia l s a t e l li t e s , th e
e s ta b lis h m e n t of p e r m a n e n tly m a n n e d sp ace s ta tio n s a n d sp ace
m a n u fa c tu rin g , all re q u ire a tr a n s p o rta tio n sy stem m ore flexible th a n
th e s h u ttle , or u p p e r sta g e rocket, capable of tra v e l b e tw ee n th e LEO
an d th e HEO.
A co n sid erab le com m ercial a d v a n ta g e w ould be ach iev ed if th e u p p e r
Chapter 1 : Introduction 27
of a n o rb ital tra n s fe r vehicle, OTV, w hich em ploys rockets for its ascen t
a n d d escen t stag es. A h ig h price is p a id in fuel how ever, for th e ab ility
to re u se th e vehicle, as th e fuel re q u ire d for th e d escen t b u rn is c arried
a t th e ex p en se of cargo. To overcom e th is p ro b lem th e a e ro a s s is te d
o rb ita l tr a n s f e r vehicle, AOTV, h a s b een proposed. (F or a rev iew see
P a r k 1985a a n d 1987a). S u ch a vehicle h a s a n a ero d y n am ic su rface,
cap ab le of p ro d u cin g d ra g a n d a sm all a m o u n t of lift. I t is en v isag ed
t h a t su ch a vehicle, on r e tu r n to th e LEO from a H E O w ould c a rry out
a e ro b ra k in g a n d a e ro m a n e u v e rin g p ro ced u res a n d d e ce le rate by drag,
rem oving th e n eed for a d e ce le ratin g ro ck et b u m .
T he AOTV h a s a well defined flig h t regim e. T his vehicle is expected to
hav e a perigee a ltitu d e of ap p ro x im ately 80 km , w h ere th e a ir p re s su re
is a p p ro x im a te ly 13 P a , a n d , a t th is p o in t, tr a v e l a t n e a r escap e
velocities of a p p ro x im ate ly 10 k m s '1. C e rta in ly , a t th e s e a ltitu d e s , th e
a tm o sp h e re is v ery ten u o u s b u t proves to be h ig h ly reactiv e. T he shock
w aves a sso c ia te d w ith su ch a n e n v iro n m e n t a re ex p ected to produce
flow fields t h a t a re in a s ta te of chem ical a n d th e rm a l n o n eq u ilib riu m .
T h e a ir in th e s e flow fie ld s w ill u n d e rg o v ib r a tio n a l e x c ita tio n ,
disso ciatio n a n d sig n ifican t io n isatio n . T he stu d y of re e n try physics is
becom ing of sig n ific a n t im p o rta n c e , a n d th e d e v elo p m e n t o f su c h a
vehicle is s e t to becom e a m ajo r technological ch allen g e in th e com ing
d ecad es.
In o rd e r to develop th e AOTV, it w ill be n e c e s s a ry to o b ta in a n
u n d e rs ta n d in g of th e ch em ical k in e tic s a t th e flig h t re g im e s w h ere
th e se processes a re im p o rta n t. T he n o n e q u ilib riu m c h e m istry affects
Chapter 1 : Introduction 28
c ap a b ilitie s . U n lik e ch em ical k in e tic m odels a p p lie d to th e s tu d y of
shock h e a te d m on ato m ic g ases such as argon, m odelling th e c h em istry
of shock h e a te d a ir is in h e re n tly difficu lt due to th e la rg e n u m b e r of
species in th e p la s m a , th e ir ty p e a n d th e n u m b e r of possible rea ctio n s
in w hich th e y m ay p a rtic ip a te . E le v en species, n am ely , N 2, 0 2, N , 0 ,
N O , N +, 0 +, N 2+, 0 2+, N 0 + a n d e- m a y be in v o lv ed in som e fifty
re a c tio n s , m a n y o f w h ic h h a v e r a t e c o n s ta n ts t h a t a re c u rr e n tly
u n k n o w n . F u r t h e r m o r e , m o le c u la r s p e c ie s i n tr o d u c e o th e r
t e m p e r a t u r e s w ith w h ic h th e flow m a y be c h a r a c te r iz e d . A ny
chem ical k in e tic m odel th e re fo re , m u s t a d d re s s th e p ro b lem s o f larg e
species n u m b e rs , m u ltip le re a c tio n s a n d be cap ab le of a c c o u n tin g for
v ib ra tio n a l, ro ta tio n a l, electronic ex citatio n , electro n tr a n s la tio n a l an d
h eav y p a rtic le tr a n s la tio n a l te m p e ra tu re s ; n o t a triv ia l exercise. T hese
a re problem s faced by th e th e o rist. T he e x p e rim e n ta lis t too h a s c e rta in
lim ita tio n s t h a t h a m p e r th e stu d y of shock h e a te d a ir a t h ig h e n th a lp y
c o n d itio n s, a c o n se q u e n c e of th e n a tu r e of th e p la s m a a n d th e
d iffic u ltie s a s s o c ia te d w ith th e g e n e r a tio n o f s u c h flow s in th e
lab o rato ry .
S tu d y in g th e chem ical k in etics involves a n in v e s tig a tio n of th e v ario u s
species, th e ir p o p u la tio n h isto rie s a n d th e ir role in rea ctio n sequences.
L a s e r a b s o r p tio n a n d e m is s io n sp e c tro s c o p y a r e n o n - in tr u s iv e
e x p e rim e n ta l tec h n iq u e s t h a t len d th em se lv e s to su ch a stu d y . T h ere
a re p ro b lem s h o w ev er a sso c iated w ith spectroscopy in a n a ir p lasm a .
E m is s io n m e a s u r e m e n ts m a y be m a d e , for e x a m p le , in o rd e r to
d e te rm in e ro ta tio n a l a n d v ib ra tio n a l te m p e ra tu re s of excited s ta te s of
N 2+. H o w ev e r th is does n o t r e s u l t in a n y in fo rm a tio n on th e
p o p u latio n s of th e g ro u n d electronic sta te s. A bsorption te c h n iq u e s can
Chapter 1 : Introduction 2 9
N a rro w b a n d a b s o rp tio n is d iffic u lt to re d u c e h o w ev er, b e c a u s e a
know ledge of th e w id th s, sh ifts a n d sh a p es of ab so rb in g tr a n s itio n s is
e s s e n tia l a n d th e s e lin e sh a p e p a ra m e te rs a re p re s e n tly u n k n o w n for
m an y species in th e en v iro n m en ts of in te re s t.
U sefu l a b s o rp tio n m e a s u re m e n ts for m o le c u la r n itro g e n m u s t ta k e
place in th e u ltra v io le t. T he s tro n g e s t a b s o rp tio n b a n d s in th e 0 2
m olecule a re also in th e u ltra v io le t (th e S ch u m an n -R u n g e tra n s itio n s ).
T he ß a n d y tr a n s itio n s in th e NO m olecule lie in th e ra n g e 200 to 400 nm . S tro n g ato m ic a b so rb e rs in a n a ir p la s m a h a v e tr a n s itio n s
a p p ro a c h in g th e in f r a r e d . T h e s p e c tra l p o s itio n s of th e s e s tro n g
tra n s itio n s place severe re s tric tio n s on th e e x p e rim e n ta l d iagnostic.
D ue to th e la rg e n u m b e r of species of b o th th e m o le cu la r a n d atom ic
form to account for, m a n y possible tra n s itio n s of v a ry in g w a v ele n g th s
from th e v a c u u m u ltra v io le t to th e fa r-in fra re d a re p o ssib le, m a k in g
th e selectio n of a s u ita b le lig h t source or em issio n d e te cto r, difficult.
T u n a b le dye la s e r s a re re q u ire d for CW a b s o rp tio n in th e v isib le ,
ex cim er la s e r s m ay be u s e d as lig h t so u rces in th e u ltr a v io le t a n d
tu n a b le diode la s e rs a re n eed ed in th e in fra re d . B ro ad b an d c o n tin u u m
sources m u s t have a n effective blackbody te m p e ra tu re g re a te r th a n th e
p la s m a u n d e r stu d y . T h ese lig h t sources a n d th e ir d e te cto rs m u s t of
course be coupled w ith th e a p p ro p ria te optics.
A g r e a t d eal of th e o re tic a l w ork in th e a re a of io n ised a ir h a s b e en
c a rrie d o u t in th e la s t th ir ty y e ars. M ore recen tly , a tw o -te m p e ra tu re
k in etic m odel for io n isin g a ir h a s b een developed by P a r k (1989). H is
Chapter 1 : Introduction 30
th e flig h t regim e of th e AOTV a n d h a s m ade p red ictio n s on:
i) th e p o p u la tio n h is to rie s of th e elev en m o le cu la r, a to m ic an d
ionic species,
ii) th e ro ta tio n a l a n d v ib ra tio n a l te m p e ra tu re s c h a ra c te riz in g th e
flow a n d
iii) th e n o n e q u ilib riu m ra d ia tiv e em ission of th e p lasm a.
T h is code r e p r e s e n ts p e rh a p s th e m o st a c c u ra te a tte m p t to m odel
conditions b e h in d th e shock fro n ts asso ciated w ith th e AOTV.
O n th e e x p e rim e n ta l fro n t, v e ry little d a ta e x is ts for th e species
p o p u la tio n s a t th e s e conditions. T h ere a re d ifficu lties (to be d e ta ile d
la te r) a sso ciated w ith th e p ro d u ctio n of such h ig h e n th a lp y shock fro n ts
in th e lab o rato ry . I t is obvious t h a t no single body of ex p erim e n tal w ork
could possibly hope to m e a su re all of th e p a ra m e te rs p re d ic te d by any
k in e tic m odel for io n is in g a ir. R eco g n izin g th is fac t, th e p r e s e n t
in v e s tig a tio n looks specifically a t a few p a ra m e te r s in a n a tte m p t to
len d su p p o rt to th e th e o re tic a l w ork. In p a rtic u la r, th e io n isa tio n th a t
occurs b e h in d th e h ig h e n th a lp y shock w aves in low p re s s u re a ir a t
specific conditions is stu d ied in d etail. T herefore, th e lite ra tu r e review
t h a t follows, is devoted to th e e x p e rim e n ta l a n d th e o re tic a l w ork t h a t
h a s b een c a rrie d out in th e field of io n isatio n b e h in d shock w aves in air.
1.2 A r ev iew o f relev a n t litera tu re
W hile stu d y in g th e electrical conductivity of io n ised a ir, L am b a n d Lin
(1957) m ad e th e o b serv atio n t h a t th e io n isatio n r a te w as co n sid erab ly
fa s te r b e h in d th e shock w ave th a n h a d b een expected. T he io n isatio n
Chapter 1 : Introduction 31
co m p arab le s tre n g th in argon, w as sev eral o rd ers of m a g n itu d e slow er
th a n L am b a n d L in h a d observed; even allow ing for th e difference in
th e io n is a tio n p o te n tia ls b e tw e e n th e sp ecies o f a rg o n a n d th o se
c o n s titu e n ts of a ir. D u rin g th e ir in v e s tig a tio n , P e ts c h e k a n d B yron
em p lo y ed a sh o ck re fle c tio n te c h n iq u e t h a t d e te r m in e d th e local
electro n n u m b e r d e n sity by c o rre la tin g i t to th e in te n s ity of th e visible
lig h t e m itte d by th e shock h e a te d gas.
N ib le tt a n d B lack m an (1958) trie d to em ploy th e tec h n iq u e of P etsch ek
a n d B y ro n to d e te rm in e th e io n is a tio n re la x a tio n tim e b e h in d shock
w aves in a ir. A h y d ro m ag n e tic shock tu b e w as em ployed to produce
shock w aves w ith M ach n u m b ers from 11 to 17 tra v e llin g in to 133 P a of
air. T he a ssu m p tio n w as m ad e t h a t th e lu m in o u s reg io n follow ing th e
shock fro n t w as due to p ro cesses in v o lv in g free ele ctro n s. H ow ever,
w hile th e v isib le ra d ia tio n in sh o ck -h eated a rg o n is d u e p rim a rily to
free -free a n d free -b o u n d c o n tin u u m ra d ia tio n , th e v isib le ra d ia tio n
from sh o c k -h e a te d a ir h a s b e e n found to be d u e to m o le c u la r b a n d
system s (P en n er, 1959). As p o in ted o u t by L in et al (1962), th e p o in t of
o n set of in te n se ra d ia tio n b e h in d th e shock w ave w as m o st likely due to
th e a r r iv a l o f th e c o n ta m in a te d d r iv e r g a s fro m th e e le c tric a l
d isch arg e, a n d w as n o t due to th e io n isatio n as N ib le tt a n d B lack m an
h a d a s s u m e d . T h ese lu m in o s ity m e a s u r e m e n ts , p e rh a p s w ro n g ly
in te r p r e te d , w ere re p o rte d as b e in g ab le to d e m o n s tr a te t h a t th e
io n is a tio n tim e d e crea se s w ith in c re a sin g M ach n u m b e r.
A m eth o d , b a sed on th e m e a su re m e n t of th e a tte n u a tio n of m icrow aves
to d e te rm in e th e electron d e n sities a n d io n isatio n ra te s in shock-heated
air, w as em ployed by M a n h eim er-T im n at an d Low (1959). Shock w aves
Chapter 1 : Introduction 3 2
n itro g e n /o x y g e n m ix tu re s (99.75% / 0.25% by v o lum e) in th e M ach
n u m b e r ra n g e from 7.4 to 8.8, b o th in p re s s u re s ra n g in g from 0.13 to
1.33 k P a, w ere stu d ied . T he a u th o rs show ed th e re w as good a g re e m e n t
b e tw ee n th e e x p e rim e n ta lly d e te rm in e d e le ctro n n u m b e r d e n s ity an d
th e o re tic a l calcu latio n s b a se d on a th erm o d y n am ic e q u ilib riu m m odel.
E ig h t r e a c tio n s e q u e n c e s w e re c o n s id e re d in t h e i r m o d el, tw o
d isso c ia tio n re a c tio n s , one fo rm a tio n re a c tio n ( th a t of N O ) a n d five
io n isin g reactio n s. M icrow ave tec h n iq u e s te n d to suffer a fu n d a m e n ta l
lim it, n am ely , poor s p a tia l re so lu tio n , w hich is a co n seq u en ce of th e
long w av ele n g th s. T h is te n d s to m ak e good s p a tia l re s o lu tio n w ith in
th e r e la x a tio n zone d iffic u lt. M a n h e im e r-T im n a t a n d Low h a d a
r a t h e r sm a ll (12.7 m m d ia m e te r) t e s t se ctio n a n d a s su c h , th e ir
m icrow ave a tte n u a tio n sig n al w as p ro b ab ly re la te d to th e p resen c e of
shock w aves in th e w aveguide th a n to an y io n isatio n re la x a tio n b eh in d
th e shock w ave in th e te s t section.
L in et a l (1962) u s e d m a g n e tic -in d u c tio n a n d m icro w av e re fle c tio n
p robes in a 600 m m d ia m e te r shock tu b e to in v e s tig a te th e io n is a tio n
profile b e h in d stro n g shock w aves in a ir w ith M ach n u m b e rs ra n g in g
from 14 to 20 a t in itia l p re s s u re s of 2.6 to 26 P a . U sin g te c h n iq u e s
sim ila r to th o se u se d by L in & Kivel (1959) a n d M a n h e im e r-T im n a t &
Low (1959), th e y o b se rv ed th e p o w er re fle c tio n co efficien t of th e
m icrow ave probe in o rd er to deduce th e electro n n u m b e r d e n sity (m ore
p re c ise ly , th e e le c tro n d e n s itie s d e d u ce d fro m p ro b e s of d iffe re n t
fre q u e n c ie s u n d e r s im ila r e x p e rim e n ta l co n d itio n s w ere co m p ared ).
In co njunction w ith th is e x p e rim e n ta l tech n iq u e, L in et al em ployed a
m ag n etic-in d u ctio n process sim ila r to th a t u sed by L am b a n d L in (1957)
Chapter 1 : Introduction 33
in a ir w ith a s p a tia l reso lu tio n com parable to th e effective w id th of th e
a p p lie d m a g n e tic field . T h e m a in r e s u lt o f th is w o rk w a s th e
o b s e rv a tio n t h a t fo r th e ra n g e of sh o ck s tr e n g th s co v ered (shock
v e lo c itie s fro m 4.5 k m s '1 to 7 kms*1), th e io n is a tio n d is ta n c e w as
ap p ro x im ately 10 to 40 tim es th e m ea n free p a th of th e u n d is tu rb e d gas
a h e a d of th e shock fro n t a n d th a t th e electron n u m b e r d en sity ap p ea re d
to o v ershoot th e e q u ilib riu m v alu e, by a facto r of b etw een 2 a n d 3, for
som e d is ta n c e b e h in d th e shock fro n t before re la x in g b a c k to w a rd s
e q u ilib riu m .
In a c o m p le m e n ta ry p a p e r to t h a t of L in, N e a l a n d Fyfe (1962), an
e x te n siv e th e o re tic a l in te r p r e ta tio n w as m ad e o f th e e x p e rim e n ta l
re s u lts o b tain ed in th e afo rem en tio n ed w ork by L in a n d T eare (1963). A
c ritic a l e x a m in a tio n w a s m a d e of th e v a rio u s io n is in g r e a c tio n
sequences. E le ctro n im p a c t a n d ion im p a c t io n isatio n , p h o to io n isatio n ,
ch arg e exchange, e le ctro n a tta c h m e n t a n d io n is a tio n by n e u tr a l ato m
a n d m o lecu lar im p a c t w ere th e io n is a tio n p ro cesses considered. R ate
e q u a tio n s w ere d eriv e d for th e s e re a c tio n s a n d solved b y n u m e ric a l
in te g ra tio n . S ince th e low io n is a tio n reg im e w as s tu d ie d , L in an d
T e a re u se a ‘one-w ay co u p lin g ’ a p p ro x im a tio n b e tw e e n d isso ciatio n ,
io n is a tio n a n d ra d ia tiv e e x cita tio n . O ne-w ay co u p lin g re fe rs to th e
tech n iq u e w h ereb y th e te m p e ra tu re , d e n sity a n d species co n cen tratio n s
a re i n itia lly c a lc u la te d in d e p e n d e n t of io n is a tio n a n d r a d ia tiv e
e x citatio n , a n d th e n u se d to d e te rm in e th e r a te s for th e s e processes.
U pon n u m e ric a l in te g ra tio n , th e y produced, as a fu n ctio n of d istan c e
b e h in d th e shock fro n t, a n in s ta n ta n e o u s electron p ro d u ctio n r a te plus
electron a n d positive ion d en sities. As a re su lt, a d e ta ile d b reak d o w n of
Chapter 1 : Introduction 34
com piled. T he d o m in a n t io n isa tio n reactio n s in th e ir a n aly sis w ere th e
a to m -a to m re a c tio n s follow ed by p h o to io n is a tio n , e le c tro n -im p a c t,
a to m -m o le c u le a n d m o le c u le -m o le c u le c o llis io n a l io n is a tio n ; th e
im p o rtan c e of each re a c tio n b ein g a fu n ctio n of shock velocity. L in an d
T eare stre s s e d t h a t th e electro n im p a ct reactio n s c o n trib u te little to the
io n isa tio n a t shock velocities below a b o u t 5 kms*1 b u t m ay becom e th e
d o m in a n t io n is in g re a c tio n a t v elo cities g r e a te r t h a t 9 kins*1. The
d o m in a n t ato m -ato m process in th e low velocity reg im e w as considered
to be th e associative io n isatio n reactio n
N + O <=» N O + + e~. ...(R l)
T he n e t en erg y re q u ire d for th is io n isin g re a c tio n is c o n sid erab ly less
th a n th e full io n isatio n p o te n tia l of NO, as chem ical en erg y is rele ased
by th e a sso c ia tio n of th e N a n d O. O n c o m p a rin g th e ir th e o re tic a l
m odel w ith th e e x p e rim e n ts of L in et a l, L in a n d T e a re d e m o n s tra te d
t h a t th e r e w as r e a s o n a b ly close a g re e m e n t b e tw e e n th e o r y a n d
e x p erim e n t. T he conclusion w as m ad e t h a t for shock velo cities up to
9 km s*1, th e d o m in a n t electro n p ro d u cin g re a c tio n seq u en ces w ere th e
a to m -ato m io n isin g collisions, w h e re a s electro n im p a c t p ro cesses m ay
becom e p r e d o m in a n t a t sh o c k v e lo c itie s in ex cess o f 10 km s*1.
F u r th e r m o r e , a cc o rd in g to t h e ir c a lc u la tio n s, th e o v e rsh o o t of th e
e le c tro n n u m b e r d e n s ity w ould be ex p ected to d is a p p e a r a t shock
velocities less th a n 4 kms*1 a n d g r e a te r th a n 9 kms*1. T h e ir d e sire to
e x te n d th e m odel to sh o ck v e lo c itie s g r e a te r t h a n 9 km s*1 w as
h a m p e re d on tw o fro n ts. F irstly , a g r e a te r know ledge of th e electro n
im p a ct io n isatio n r a te s a t h ig h e r velocities w as re q u ire d a n d secondly,
th e ‘one-w ay co upling’ m u s t give w ay to a ‘tw o-w ay coupling’ b e tw ee n
Chapter 1 : Introduction 35
io n is a tio n a n d ra d ia tiv e p ro cesses o c cu rred s im u lta n e o u s ly w ith th e
c h e m ic a l p ro c e s s e s a n d a s su c h , t h e i r i te r a t i v e te c h n iq u e w as
u n s a tis fa c to ry .
W ilso n (1966) e x te n d e d th e e x p e rim e n ta l w o rk of L in et al a n d th e
th e o r e tic a l w o rk o f L in a n d T e a re . F ir s tly , W ilso n c o n d u c te d
e x p e rim e n ts a t shock velo cities from 9 to 12 k m s-1 a n d o b serv ed th e
in fra re d em issio n of th e sh o ck -h eated a ir a t w a v ele n g th s g r e a te r th a n
5 pm . Above 5 pm th e c o n tin u u m ra d ia tio n consists m ain ly of free-free
B re m s s tra h lu n g ra d ia tio n , th e in te n s ity of w hich is p ro p o rtio n a l to th e
s q u a re of th e e le c tro n n u m b e r d e n sity . W ilson p r e s e n ts a ‘sim p le ’
m odel for a ir in w h ich ‘in fin ite ly fa s t d isso c iatio n ’ is a ssu m e d . H ere
W ilson m ak es th e a ssu m p tio n t h a t a t shock speeds g re a te r th a n 8 kms*
1 in a ir, d isso c ia tio n r a te s a re in fin ite a n d th e g a s in s ta n ta n e o u s ly
ju m p s to a s ta te of th erm o d y n am ic eq u ilib riu m across th e shock. T his
m odel differs from t h a t of L in a n d T eare in t h a t th e ir m odel assum ed"
fin ite d isso c ia tio n a n d one-w ay coupling. W ilso n 's in f r a r e d r e s u lts
show som e c o rre la tio n w ith b o th th e p ro p o sed sim p le m odel a n d th e
m odel of L in a n d T e a re . H o w ev er th e r a d ia tio n in te n s ity in th e
c o n tin u u m show ed a ten d en cy to be low er th a n t h a t p red icted by c e rta in
m odels. T he conclusion w as m ad e t h a t below velocities of 9 km s-1, th e
a s s o c ia tiv e io n is a tio n re a c tio n ( R l) w as th e d o m in a n t io n is a tio n
p ro cess (as su g g e ste d by L in a n d T e a re ) a n d fu rth e rm o re confirm ed
t h a t a t velocities g r e a te r th a n 9.5 km s-1, th e electro n im p a c t processes
b eg in to d o m in ate. T h e rea ctio n (R l) how ever, is still th o u g h t to be an
im p o rta n t io n isin g re a c tio n beyond 9.5 km s-1. T he reactio n s,
Chapter 1 : Introduction 36
a n d
0 + 0<^>Ö2+e~, ...(R 3)
a re also s u g g e ste d a s b eco m in g im p o r ta n t a t h ig h e r te m p e r a tu re s .
W ilson observed th a t, above 9.5 kms*1, io n isatio n le n g th s d ecrease w ith
shock velocity. T h is r e s u lt le n d s su p p o rt to th e a s s u m p tio n th a t, for
v elocities g r e a te r th a n 9.5 k m s '1, electro n im p a c t io n is a tio n becom es a
process t h a t w ill s t a r t to com pete w ith rea ctio n (R l). In th e th eo re tic al
a n a ly sis of L in a n d T ea re, i t w as a ssu m e d t h a t th e en erg y in v e s te d in
io n is a tio n w as sm all co m p ared to th e e n th a lp y of th e shock h e a te d a ir
a n d w as th u s n e g le cted in th e en erg y c o n serv atio n e q u atio n . W ilson,
w ish in g to ex ten d th e velocity regim e beyond 9.0 kms*1, w as n o t able to
m a k e th is a s s u m p tio n . A bove 9.0 km s*1, th e e n e rg y in v e s te d in
io n is a tio n w as no lo n g er sm all com pared to th e e n th a lp y of th e shock
h e a te d a ir a n d h a d to be in clu d ed in th e energy co n serv atio n e q u atio n s
w h en c a lc u la tin g th e te m p e ra tu re . H ence a coupling h a d to be m ade
b etw een th e ra te e q u atio n s for ionic a n d n e u tra l sp ecies1.
C a lc u la tio n s w ere m ad e a n d m o d ellin g te c h n iq u e s d isc u sse d , for th e
d e te r m in a tio n of th e e le c tro n n u m b e r d e n s ity in th e sh o ck la y e r
s u r r o u n d in g a n u p p e r a tm o s p h e r e r e e n t r y v e h ic le b y E v a n s ,
S c h e x n ay d e r a n d H u b e r (1970). T he a u th o rs co n sid ered 54 re a c tio n
se q u e n c e s in v o lv in g 11 r e a c ta n ts . H o w ev er a re d u c e d s e t o f 18
s e q u e n c e s a n d 7 r e a c t a n t s w a s sh o w n to p ro d u c e a lm o s t
in d is tin g u is h a b le p re d ic tio n s for in te r m e d ia te v e lo c itie s . I t w as
d e m o n s tra te d t h a t th e electro n n u m b e r d e n sity profiles a re ex trem ely
s e n s itiv e to f in ite r a t e c h e m is try a n d t h a t th e p ro file s c a n be
l As dissociation tends to proceed faster than ionisation at higher shock speeds, the ionisation rate
Chapter 1 : Introduction 37
sig n ifican tly ch anged by finely a d ju stin g th e rea ctio n r a te c o n sta n ts.
I t h a s b e en e x p e rim e n ta lly o b serv ed (L in et a l, 1962 a n d A llen et al,
1962) t h a t a t shock v elo cities below 9.5 kms*1, th e n o n e q u ilib riu m
r a d ia tio n b e h in d th e shock fro n t is e le v a te d c o n sid era b ly above th e
e q u ilib riu m v a lu e . Z h e le sn y a k et al (1970) u n d e rto o k a th e o re tic a l
a n a ly s is o f r e la x a tio n a n d n o n e q u ilib riu m r a d ia tio n b e h in d shock
w aves in air. A calcu latio n w as m ad e as to th e expected p o p u latio n s of
atom ic a n d m o lecu lar ra d ia to rs . T h e ir a n aly sis p red icted th e in te n s ity
of ra d ia tio n from c e rta in sp e c tra l reg io n s to p a s s th ro u g h a m ax im u m
e x c e e d in g a n e q u ilib riu m le v e l. T h is n o n e q u ilib riu m r a d ia tio n
o v ersh o o t w as e x p la in e d as follows. As h a d b e e n d e m o n s tra te d by
A ppleton et al (1968), a decrease in th e disso ciatio n of N 2 r e s u lts in an
in cre ase in th e p o p u latio n of N 2 to g e th e r w ith a su b se q u e n t in cre ase in
th e a to m te m p e ra tu re T a. In a co m p lem en tary p a p e r A ppleton (1967)
also show ed t h a t a n in c re a s e in th e v ib ra tio n a l re la x a tio n tim e xv
in c re a se s th e v ib ra tio n a l te m p e ra tu re T v a n d so Tv ra p id ly ap p ro ach es
T a . T h is o c c u rs a t th e b e g in n in g of th e r e la x a tio n p ro c e ss .
Z h e le s n y a k et al propose t h a t th e s itu a tio n will change as th e electron
p o p u latio n in c re a s e s, as th e re is now a stro n g in te ra c tio n b e tw ee n th e
v ib ra tio n a l tr a n s itio n s a n d th e free electro n s. As su ch th e v ib ra tio n s
a re in te n s e ly “cooled” by ele ctro n collisions w ith th e r e s u lt t h a t T v
d e p a rts from T a a n d a p p ro ach es T e. A t h ig h e r v a lu e s of T a a n d T v, th e
e lectro n s a re f u r th e r h e a te d by th e in te ra c tio n s w ith th e v ib ra tio n a l
levels of N 2 a n d T e co n sid era b ly exceeds its local e q u ilib riu m v alu e.
T h is local o v e rh e a tin g of th e ele ctro n s ta k e s p lace in th e re la x a tio n
zone c au sin g th e em issio n from th e m o lecu lar tra n s itio n s to overshoot