JOURNALOF VIROLOGY, June 1980, p.782-788 Vol.34,No. 3 0022-538X/80/06-0782/07$02.00/0
Cytoplasmic
T
Antigens of
Mouse and Human Cells
Transformed
by a
Simian
Virus 40 tsA Mutant
D.R. DUBBS,* H.OTSUKA, AND S.KIT
Divisionof Biochemical Virology, Baylor College of Medicine, Houston, Texas77030
Simian virus 40 T antigens accumulate in the cytoplasm of simian virus 40
tsA207 transformants ofprimary mouse kidney or human retinoblastoma cells
grownat40°C in 10%serum.
Usingserafromhamstersbearing largesimian
virus 40 (SV40) tumors, SV40tumor (T)
anti-gens can be demonstrated by
immunofluores-cence in the nuclei of cellslytically infected or
transformedby SV40(13, 14).AlthoughSV40 T
antigensareassumedtobesynthesizedon
poly-ribosomes in thecytoplasm, followedby
migra-tion to the nucleus, SV40 T antigens are not
usually visible by immunofluorescence in the cytoplasm of SV40-transformed cells. Even in
mouse,Syrian hamster, Chinese hamster, rabbit,
andratcells transformedby SV40 tsAmutants
(whicharedefective inanearly SV40 function) abnormallocalization of Tantigenshasnotbeen reported (2, 10, 11, 17). This paper describes a temperature-dependent SV40tsAtransformant, mKSA207 clone6 (Cl6), which under
appropri-ategrowth conditions accumulates SV40 T
an-tigens in the cytoplasm. The purposes of this
study were to: (i) define the growth conditions
under which T antigens accumulate in the
cy-toplasmofmKSA207 Cl6cells and otherSV40
tsA207 transformants; (ii) determine the effect
of the growth phenotype on localization of T
antigens, using serum-requiring and
tempera-ture-resistant (tr) variants derived from
mKSA207 Cl 6; and (iii) determine whether
in-duction ofcytoplasmicTantigens is an inherent
property of SV40 tsA207 in transforming and
lyticinfections.
The cell lines used in this study are
summa-rized inTable1.Wild-typemKS [mKS(wt)] and
ERSV40(wt) cells were grown at 37°C;
mKSA207 Cl 2, 3, 6, 9, and 10, S6/R1/R'5,and
ERA207 cells were grown at 33.5°C; and
mKSA207-CH, mKSA207-S4, and mKSA207-S6
cellsweregrown at40°C. Allcellswere grown in
Eagle minimal essential medium (Auto-Pow,
Flow Laboratories, Rockville, Md.)
supple-mented witheither 10% calf serum or 10% fetal
calfserum.SV40 T-antigen determinations were
carriedoutwithcellswhich were grown on glass
coverslips, fixed at-20°C in absolute ethanol, and placed for 15 min on dry ice. T antigens
were demonstrated by indirect
immunofluores-cence,usingascitic fluid from hamstersbearing
SV40 tumors and goat anti-hamster gamma globulinconjugatedwithfluorescein
isothiocya-nate (Antibodies, Inc., Davis, Calif.). The
ham-ster ascitic fluid was adsorbed with calfserum
and human [HeLa(BU25)] and mouse
[LM(TK-)] cells beforeuse.The hamster ascitic
fluid used in this study immunoprecipitated bothlargeTandsmalltpolypeptides.Theterms
"cytoplasmic T antigen" and "intranuclear T antigen"areusedtodefinespecific
immunoflu-orescentmaterial locatedin the
cytoplasm
andnucleus, respectively. Cells were observed and
photographed with a Leitz Ortholux 2
micro-scope equipped with incident UV illumination.
Theresultsareshown inFig. 1,2, and3andare
summarized in Table2.
ThemKS(wt) cells,like many otherSV40(wt)
transformants of rodent cells, are temperature
independentforgrowthinlow(0.5%)serumand
are anchorage
independent
at both 33.5 and 40°C (5, 12).Thesecells,
like mostSV40-trans-formedcells,expressedtheintranuclearT
anti-gen at both 37 and
40°C
in either 0.5 or 10%serum(Table2;Fig. 1A, B,andC).No cytoplas-mic T antigen was observed in mKS(wt) cells
under anygrowthcondition studied.
ThemKSA207 Cl6cellsaretemperature
de-pendentforexpression ofsome growth proper-ties(6, 12). At33.5°C, growthofmKSA207Cl6
cells resembled that ofmKS(wt), and the cells
contained only the intranuclear T antigen
whether grown in 10 or 0.5% serum (Table 2;
Fig.1D). However, mKSA207 Cl6cells failedto
sustaingrowthin 0.5%serum at40°C and,when
incubated for 3 or 4 days, lost intranuclear T
antigens (Fig. IF). About 70 to 80% of cells
fluoresced only faintlyornot atallby
immuno-fluorescence,andabout 70% of Tantigens were
lostby complement fixation (6). The addition of
10% serum to cultures ofmKSA207 Cl 6 cells
that had been partially depletedofTantigens
by incubationat40°C in lowserum for several
days stimulated the cellstoenterthe Sphase of
the growth cycle. Within24 hafter addition of
782
on November 10, 2019 by guest
http://jvi.asm.org/
TABLE 1. Origin and growth properties ofSV40-transformed cells
Cell line Source Growth properties Reference
mKS(wt) BALB/cmousekidneycells Anchorageindependent, tr 5
transformed
by
SV40(wt) forgrowth
inlowserummKSA207Cl6 Independent isolates of ts forgrowth in either low 6 mouse kidneycellstrans- serum or methylcellulose formed by SV40 tsA207 suspension culture
mKSA207Cl9 ts forgrowth in low serum Otsuka et al.
(unpub-mKSA207Cl10 ts forgrowth in low serum lishedexperiments)
mKSA207Cl2 tr forgrowth in low serum
mKSA207Cl3 tr forgrowth in low serum
mKSA207-CH IsolatedfrommKSA207Cl6 tr forgrowth in low serum 12
mKSA207-S4 ormethyl cellulose
sus-mKSA207-S6 pension culture
S6/R1/R'5 IsolatedfrommKSA207-S6 Serumrequirement for Otsuka et al. (unpub-growth; anchoragede- lishedexperiments) pendent
ERSV40(wt) Human retinoblastomacells' Not done transformedbySV40(wt)
ERA207 Human retinoblastomacells Not done
transformed by SV40 tsA207 whichhad been rescued from mKSA207Cl
6
aHuman retinoblastoma
cells
wereobtained from V. Riccardi, Baylor College of Medicine, Houston, Tex. Thesecells containone no. 13chromosome with an interstitial deletion.10%serum at40°C, 95% of thecells
incorporated
[3H]thymidineinto DNA(6).Within2or3days
afteradditionof 10%serumat
40°C,
Tantigens
accumulatedinthecytoplasm ofmany
cells,
yetmostofthenuclei remained
relatively
Tantigen
negative (Fig. 1E). If mKSA207 Cl 6cells,
ac-tively growing at
33.5°C
in 10% serum, wereshifted to
40°C
in 10% serum, the cells also accumulatedcytoplasmic
Tantigen,
but inthiscase they continuedto
display
the intranuclear Tantigen(data
notshown).
These results sug-gestthat:(i)
preexisting
nuclear Tantigen
is lost under conditions whichpreclude
cellgrowth
(i.e.,inlowserum at40°C);
(ii)
addition of 10%serum stimulates the cellsto grow,
resulting
in synthesis of new Tantigens;
and(iii)
newly
synthesized
Tantigens
accumulatein thecyto-plasmofmKSA207 Cl6cellsandarenot
effec-tively
transported
tothe nucleus.Theresults withmKSA207Cl6
cells,
indicat-ing that synthesis of T
antigens,
localization ofT antigens,and loss of T
antigens
depended
onculture conditions,
suggested
that thegrowth
properties of thecells
might
play
animportant
role. Variant cell lines withaltered
growth
phe-notypeshave beenderived frommKSA207Cl 6
(12). It was,
therefore,
ofinteresttostudy
thesevariantcells to assess the impact of the growth
phenotype on localization of T antigens.
Three variant lines, mKSA207-CH,
mKSA207-S4, and mKSA207-S6, are
tempera-ture resistant (tr) for growth, resembling
mKS(wt). The trvariants are anchorage
inde-pendent and growwellin either 0.5 or 10% serum
at either 33.5 or 40°C (12). A serum-requiring
variant line, S6/R1/R'5, was derived from
mKSA207-S6 bytwostagesof suicide selection
based on (i) the inability to grow in methyl
cellulosesuspension cultureat40°C and (ii) the
inability to grow in methylcellulosesuspension
at 33.5°C (unpublished experiments). This cell line, in addition to being anchorage dependent
atboth33.5and40°C, also failed to grow in low
serum ateither temperature.
The trvariants, like mKSA207 Cl6 parental
cells, accumulated Tantigensinthe cytoplasm
when growing at 40°C in 10% serum (Table 2;
Fig. 1H). Occasionally, mKSA207-S6 contained
some cytoplasmic T antigen even when grown in 10%serum at33.5°C (Fig. 1G). However, the
trvariants also
displayed bright
nuclearfluores-cence atboth temperatures in 10% serum (Fig.
IG andH). These variants, whichgrowwell in
0.5%serum at40°C, didnotlose intranuclearT 34,1980
on November 10, 2019 by guest
http://jvi.asm.org/
784 NOTES
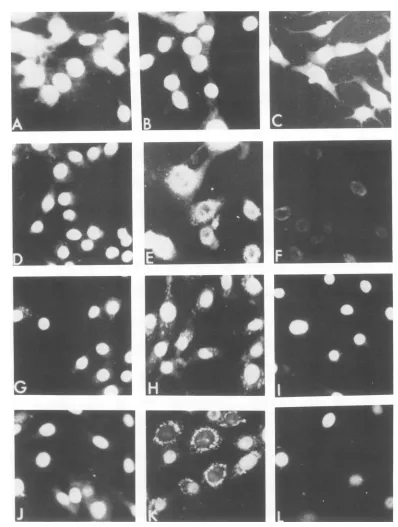
I
FIG. 1. Micrographsof mKS(wt), mKSA207 Cl 6, mKSA207-S6, andS6/Rl/R'5after immunofluorescent stainingforTantigens (X250). mKS(wt) cells grownfor2daysat(A)37°C in10%oserum, (B)40°C in 10% serum, and (C) 40°Cin 0.5%serum.mKSA207 Cl6cells(D) grownfor2daysat33.5°C in 10% serum, (E) grownfor2daysat33.5°C in 10%oserum, depleted of T antigens by incubationfor4days at40°C in0.5%
serum, and thenshiftedto10%serum at40°C for3days, and (F) incubatedat40°Cin0.5%serumfor3days. mKSA207-S6 cells grownfor3daysat(G)33.5°Cin10%oserum,(H) 40°C in 10% serum, and(I) 40°C in0.5% serum.S6/RJ/R'5cellsgrownfor2daysat(J) 33.5°Cin10%oserum(K),40°Cin10%oserum,and(L)40°Cin 0.5%serum.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
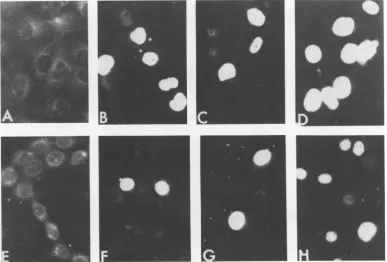
[image:3.504.53.451.66.593.2]FIG. 2. Micrographs of human retinoblastoma cells transformned by SV40(wt) and SV40 tsA207 after immunofluorescent staining for T antigens (x250). ERSV40(wt) cells grown for 2 days at (A) 36°C in 10% serum,(B)40°C in10% serum, (C) 36°C in 1% serum, and (D) 40°C in 1% serum.ERA207 grown for 3 days at (E)33.5°C in 10% serum, (F) 40°C in 10% serum, (G) 33.5°C in 1% serum, and (H) 40°C in 1% serum.
1
FIG. 3. Micrographsof CV-1 cellslytically infectedand 3T6 cellsabortivelyinfected with SV40 tsA207or
SV40(wt) after immunofluorescent staining for T antigens (x250). (A) Uninfected CV-1 cells; CV-1 cells
infectedwithSV40 tsA207for48hat(B)33.5°Cand(C)40°C. (D)CV-1 cellsinfectedwithSV40(wt)for48h
at40°C.(E) Uninfected3T6cells.3T6cellsinfectedwithSV40 tsA207for48 hat(F)33.5°C and(G)40°C.(H)
3T6 cellsinfectedwithSV40(wt)for48hat40°C.
785
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.504.51.438.354.616.2]786 NOTES
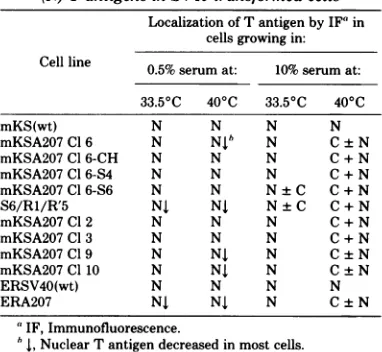
TABLE 2. Presenceof cytoplasmic (C)ornuclear (N) TantigensinSV40-transformed cells
Localization ofTantigenby IF' in cells growing in: Cell line 0.5% serumat: 10%serum at:
33.50C 400C 33.50C 400C
mKS(wt) N N N N
mKSA207Cl6 N Nib N C±N
mKSA207Cl 6-CH N N N C + N
mKSA207 C1 6-S4 N N N C+N
mKSA207 Cl 6-S6 N N N±C C+N
S6/R1/R'5 NJ NJ N±C C+N
mKSA207 Cl2 N N N C + N
mKSA207Cl3 N N N C+N
mKSA207 Cl9 N NJ N C±N
mKSA207Cl10 N NJ N C±N
ERSV40(wt) N N N N
ERA207 NJ NJ N C±N
'IF,Immunofluorescence.
b , Nuclear T antigendecreased in most cells.
antigenafter3 or 4days,asdidmKSA207Cl 6 cells, nor did they accumulate
cytoplasmic
T antigeninlowserum at400C(Fig.
1I).Thus,
thetrvariants
appeared
tobe somewhat lessdefec-tive in ability to transport T
antigen
to thenucleus than wasparental mKSA207Cl6.
Theserum-requiring variant S6/R1/R'5 also accumulatedcytoplasmicTantigenwhengrown at400Cin10%serum
(Fig.
1K).TheS6/R1/R'5
cells grew only to a low saturation
density
in10% serum ateithertemperature, and manycells
lost intranuclear T antigen as the cells
ap-proached confluence(Fig. 1Jand
K). S6/R1/R'5
cells failed togrow in 0.5%serum ateither 33.5 or 40°C, and many cells lost intranuclear T antigenin 2 or 3days (Fig.
1L).
Theseresults suggest that transportofT
an-tigens tothe nuclei wasdefective inmKSA207 Cl6cellsgrowingat40°Cin 10%serumand that
thisdefect was presentto somedegreeinall cell
lines derived from mKSA207 Cl6, irrespective
of thegrowthphenotype.In anattempt to learn
whether the altered localization of T antigens
wasdue to a tsdefect of thecell, localization of
SV40 T antigens was studied in other SV40
tsA207transformants.
Fourindependent lines of mouse kidneycells
transformed by the samestock of SV40 tsA207
that was used totransformmKSA207
Cl
6werestudied. Twoof theselines,mKSA207
Cl
9 andCl 10, were temperature dependent forgrowth
in lowserum. LikemKSA207Cl6 cells,Cl 9 and
Cl 10 cells lost T antigens when incubated at
40°C in low serum (Table 2). The other two
lines, mKSA207 Cl2andCl 3, resembled the tr
variants inabilityto grow at either temperature
in low serum and did not lose intranuclear T
antigen whenincubated at 40°C in 0.5% serum.
All fourtransformants accumulatedcytoplasmic
T antigen when grown at 40°C in 10% serum
(Table2).
Localization of T antigens was also examined
inSV40(wt)- andSV40tsA207-transformed
hu-man retinoblastoma cells [ERSV40(wt) and
ERA207, respectively]. For these studies, the
SV40 tsA207 used for transformation was
res-cued from mKSA207 Cl 6 cells by fusion with
CV-1 cells using UV-irradiated Sendai virus.
The rescued virus was plaque purified and
shown to be ts for both viralreplicationand viral
DNA synthesis before transformation ofhuman
retinoblastoma cells. The results inFig. 2A to D
show that T antigens were confined to the
nu-cleus in ERSV40(wt) at either 36 or 40°C in
either 10 or 1.0% serum. In contrast, when
ERA207 cells were incubated in 1.0% serum at
either 33.5 or 40°C, they tended to lose
intra-nuclear T antigen (Fig. 2G and H), and when
incubated at 40°C in 10% serum, T antigens
accumulated in thecytoplasm (Fig. 2F). These results indicated that aberrant localization of T antigens wasprobablynotdue to a tsdefectin
the cell transport system since it occurred in a
number of tsA207 transformants but did not
occur in wt transformants. Therefore, the
pos-sibility was considered that aberrant
cytoplas-micaccumulation of TantigensintsA207
trans-formantsmightbe due to theabnormal
proper-ties of the tsA gene products synthesized at
400C.
Previous studies by Tegtmeyer et al. (18)
showed that at41°CSV40 tsA mutantsinduced,
in both infectedmonkey cells and transformed
rabbit cells, the overproduction of a 100,000-daltonprotein (largeTantigen) immunoprecip-itableby hamster anti-SV40tumor sera. Immu-nofluorescent studies indicated that the nuclei of wild-type-infected cells stained more uni-formlythantsA-infected cells. Further, the cy-toplasmof monkey cellsinfected by tsA58, but not bywild-type virus, was distinctly immuno-fluorescent in most but not all infected cells. (The localization of T antigen in tsA-trans-formedcells was not reported.) Therefore,
local-ization ofT antigens wasstudied in CV-1 cells
lytically infected and 3T6 cells abortively
in-fected with SV40 tsA207. Forthese studies, virus
rescued frommKSA207Cl 6cellswasused. At
24 and 48 h after lytic infection with either
SV40(wt) orSV40tsA207 at either 33.5 or 40°C,
CV-1 cells displayed brightlyfluorescent,
T-an-tigen-positive nuclei (Fig. 3B, C and D). Very little,ifany, immunofluorescencewasobserved
inthecytoplasm. Similarly, 3T6cellsabortively
infected with SV40(wt) or SV40 tsA207
ex-hibited only intranuclear T antigen (Fig. 3F, G
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
NOTES 787
and H).In noinstanceweregranularaggregates characteristic of cytoplasmic T antigen of SV40 tsA207 transformants observed in infected cells. Whether or not SV40 tsA207 transformants
lose nuclear T antigen, synthesize new T
anti-gens, oraccumulate T antigens in the cytoplasm
apparently dependsonwhether or not the cells are proliferating. Hence, the localization of T antigens depends on the growth phenotype of thecellsaswellasonculture conditions. These conclusions are supported by thefollowing ob-servations. (i) Intranuclear T antigen was lost
fromSV40tsA207transformantswhich failed to
sustain growth in low serum at
400C
(i.e., mKSA207 C16,Cl9,andCl10,S6/R1/R'5,andERA207) (Table2). Tantigen was also lost, but
more slowly, from two transformants (S6/R1/ R'5 and ERA207) which grew poorly in low serum at
330C.
Apparently, preexisting intra-nuclearTantigenwasthermallyinactivated and synthesis ofnewantigenswascell cycle depend-ent (1, 19). Wild-type transformants [mKS(wt) andERSV40], tr variants (mKSA207-CH, -S4, and-S6), andSV40 tsA207 transformants whichgrew at 40°C in low serum all continued to
display intranuclear T antigen (Table 2). (ii) Addition of 10%serum to mKSA207 Cl 6 cells
which hadlost nuclearTantigenstimulated cell
proliferation and synthesis ofTantigens. (iii)T antigens accumulated in the cytoplasm ofall SV40 tsA207 transformants whenactively
grow-ing at
400C
in 10% serum, suggesting thatthetransportof newly synthesizedT antigens to the
nucleus might be defective in these cells. It shouldbepointedout,however, that considera-ble Tantigen, butnotall,wastransportedtothe nucleus in tr variants growing at 40°C in 10%
serum. Whetheror notthe
improved
abilitytotransport Tantigentothenucleusat
400C
isacontributing factorinthetemperature-resistant growth phenotype of thesecells isnotknown.
At the present time, it is not known why
aberrant accumulation ofT
antigens
ismanifest only in tsA207 transformants when grown at400C
in 10% serum and not in SV40 tsA207-infectedmonkey
or mousecells.Nordoweknow whether the antigens which accumulate in the cytoplasm aresmalltantigen, large
Tantigen,
truncated Tantigens,
or acombination of these (16).Cell
fractionationprocedures
with wild-typepolyoma virus-infectedNIH-3T3cells sug-gested thatlarge
Tantigen
waspredominantly
nuclear, whereas smalltantigen
was found inthe
cytosol
fraction(15).
Perhaps
at400C
inSV40 tsA207
transformants,
little tis overpro-ducedandaccumulatesinthecytoplasm.
Other possibilitiesare thataggregation
of Tantigens,
complexingof T
antigens
withcellularproteins
ormembranes, orcontrol oftransportof T an-tigens to the nuclei may be different in SV40 tsA207 transformants than in lytically infected cells.
Two other systems have been described in
whichSV40Tantigenoccursinthe cytoplasm.
Adefective SV40(PARA)mutant wasdescribed byButel and co-workers (3,4, 7) in which the SV40Tantigen occurred in the perinuclear re-gionin bothlytically infected and transformed cells.Lewis et al. (9) have described a nondefec-tive SV40-adenovirus hybrid, Ad2+ND1, which containsonlyasegmentof the earlySV40region inserted intotheadenovirustype 2 genome. This segment of SV40DNAcodes forthe U antigen
which canbe detected in the perinuclear region
of cellsafter lyticinfection (9).Unlikethe defec-tive SV40(PARA) mutant, however, hamster cells transformedby
Ad2+ND,
failed to express anySV40antigens (8).Thisinvestigation was aided by National Science Founda-tion grantPCM-7818901 and by Public Health Service grants 1-K6-Al-2352-17 and CA-06656-17 from the National Institute ofAllergy and Infectious Diseases and the National Cancer Institute, respectively.
We thank Judith Rotbein for technical assistance, Marion Hazen for photographic assistance, and Vincent Riccardi for the humanretinoblastoma cells.
LITERATURE CITED
1. Basilico, C., and D.Zouzias. 1976. Regulation of viral transcription and tumor antigen expression in cells transformedby simian virus 40. Proc. Natl. Acad. Sci. U.S.A.73:1931-1935.
2. Brugge, J.S., and J. S. Butel. 1975. Role of simian virus 40gene A function in maintenance of transformation. J. Virol. 15:619-635.
3. Butel, J.S.,M.J.Guentzel,and F. Rapp. 1969. Var-iants of defective simian papovavirus 40 (PARA) char-acterized by cytoplasmic localization of simian papo-vavirus 40 tumorantigen. J. Virol. 4:632-641. 4. Dottorini, S., and C. Tassi. 1975/76. Localization of
SV40 T antigen in hamstercellstransformed by PARA (3ct)-adenovirus7.Intervirology 6:343-349.
5. Dubbs,D. R.,and S. Kit. 1968.Isolation ofdefective lysogens from simian virus 40-transformed mouse kid-ney cultures. J. Virol.2:1272-1282.
6. Dubbs,D.R., D.Trkula,and S. Kit. 1978.Tantigen and initiation ofcellDNAsynthesis ina temperature-sensitive mouse linetransformedby anSV40tsA mutant and inheterokaryons of thetransformedcells and chick erythrocytes. SomaticCellGenet. 4:95-110.
7.Duff,R., F. Rapp, and J. S. Butel.1970.Transformation of hamstercells by variants of PARA-adenovirus7able toinduce SV40tumorantigeninthecytoplasm. Virol-ogy 42:273-275.
8.Lewis, A.M., Jr.,A. S.Rabson,and A. S. Levine. 1974.Studies of nondefective adenovirus 2simianvirus 40hybridviruses.X.Transformation of hamsterkidney cells by adenovirus2and thenondefectivehybrid vi-ruses.J. Virol.13:1291-1301.
9. Lewis,A.M., Jr.,and W. P. Rowe. 1971.Studieson
nondefectiveadenovirus-simianvirus 40hybridviruses.
I.Anewly characterized simianvirus 40antigeninduced bytheAd2+ND,virus.J.Virol. 7:189-197.
10. Martin,R.G.,and J. Y. Chou.1975. Simian virus40
on November 10, 2019 by guest
http://jvi.asm.org/
788 NOTES
functionsrequiredfor the establishment and
mainte-nance ofmalignant transformation. J. Virol.
15:599-612.
11. Osborn, M.,and K. Weber. 1975. Simian virus 40gene
A function and maintenanceoftransformation.J.Virol. 15:636-644.
12. Otauka, H.,D.R. Dubbs,and S. Kit. 1979. Variantlines ofmousekidneycellstransformedbyanSV40tsA
mu-tantwithgrowth properties of wildtypetransformed
cellsatnonpermissivetemperature.J.Gen.Virol.42:
373-386.
13. Rapp,F.,J. S.Butel,and J.L.Melnick. 1964. Virus induced intranuclearantigen incellstransfonned by papovavirus SV40. Proc. Soc. Exp. Biol. Med. 116: 1131-1135.
14.Rapp,F.,T.Kitahara,J. S.Butel,and J.L. Melnick. 1964.SynthesisofSV40tumorantigenduring
replica-tion of simianpapovavirus (SV40).Proc.Natl.Acad. Sci. U.S.A.52:1138-1142.
15. Silver, J., B.Schaffhausen, and T.Benjamin.1978. Tumor antigensinduced by nontransformingmutants
ofpolyoma virus. Cell 15:485-496.
16.Smith, A. E., R.Smith,andE.Paucha.1979. Charac-terization ofdifferenttumorantigenspresentincelis transformedby simian virus40.Cell18:335-346. 17.Tegtmeyer, P.1975.Function ofsimian virus 40geneA
intransforming infection. J. Virol.15:613-618. 18. Tegtmeyer, P., M. Schwartz, J. K. Collins,andK.
Rundell.1975.Regulation oftumorantigen synthesis by simian virus40geneA. J.Virol.16:168-178. 19.Zouzias, D., and C. Basilico.1979.T-antigenexpression
inproliferating andnon-proliferatingsimian virus 40-transformedmouse cells.J. Virol. 30:711-719.
J. VIROL.