Copyright© 1977 American Society forMicrobiology Printed in U.S.A.
Electron Microscopic Studies of Bacteriophage M13 DNA
Replication
D. P.ALLISON,* A. T.GANESANt A. C. OLSON, C. M.SNYDER, AND S. MITRA BiologyDivision, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37830
Received for publication13 June 1977
Intracellularforms of M13 phage DNA isolated after infection of Escherichia
coli with
wild-type
phage have been studied byelectron microscopy andultra-centrifugation. The data indicate the involvement ofrolling-circle intermediates insingle-stranded DNA synthesis. In additiontosingle-stranded,circular
DNA,
we observed covalently closed and nicked replicative-form (RF) DNAs, dimerRF DNAs, concatenated RF DNAs, RF DNAs with single-stranded tails (a,
rolling circles), and, occasionally, RF DNAs with 0 structures. The tails in a
moleculesare
always
single stranded andare neverlongerthan the DNA from mature phage; the proportion of a to other RF molecules does not changesignificantly
withtime after infection.Theoriginof single-strandedDNAsynthe-sishas beenmapped by electron microscopy at a unique location on RF DNA
byuse ofpartial denaturation mapping and restriction endonuclease digestion.
This location is between gene IV and gene II, and synthesis proceeds in a
counterclockwise directiononthe conventionalgenetic map.
M13 phage belongs to the group of
filamen-tous phage
specific
for male strains of Esche-richia coli and containscircular, single-stranded DNA (ssDNA) witha mass of2 x 106 daltons (26). Pratt and Erdahl (23) showed that thereare three basic stagesofM13 DNA
replication
after infection. These areanalogous tothose of ,X174, namely, (i) replication of
parental
ssDNAto
parental replicative-form (RF)
DNA; (ii)synthesis
of progeny RFDNA;
and(iii)
synthesis
of progeny ssDNA from RF DNA.Stageidoesnot
require
anyphage-induced
pro-teins; stage ii
requires
M13 gene 2protein;
andstage iii
requires
both gene 2 and 5proteins
(23). Thegene 5
product,
asmallssDNA-binding
protein,
isessential forprogenyssDNAsynthesis
(19). After infection with M13 gene 5 amber
mutant(am5)
phage,
progenyssDNAsynthesis
is absent. Dressler (6) showed that
4X174
ssDNA
synthesis
occursby
therolling-circle (a)
model(10);Ray (25) showed
by
ultracentrifugal
analysis of
pulse-labeled
intracellularM13 DNAthat the virus strandis
continuously displaced
from the RF DNA and chased into progeny ssDNA. Our examination of intracellular M13 DNAbyelectronmicroscopyand ultracentrifu-gation indicated that ssDNA
synthesis
occursbyaamechanism.This report includesa
partial
denaturation map of the a intermediates that
showsa
unique
origin
ofssDNAsynthesis.
The correlationof thepartial
denaturationmap andt Permanent address: Department ofGenetics, Stanford University Medical School, Stanford, CA94305.
the genetic map establishes that the origin of
M13 ssDNA synthesis isin or near gene II and
that there is a counterclockwise direction of
replicationinreferencetothegenetic map.
Dur-ingthepreparation ofthis manuscript,Horiuchi
and Zinder (12) and Suggs and Ray (31)
inde-pendently showed the uniqueorigin and
direc-tion ofprogeny ssDNAsynthesis. Our dataagree
with theirs. A preliminary report of this work
has been
published
(D. P. Allison,A. T.Gane-san,and S.Mitra, Fed. Proc.33:1491, 1974).
MATERIALS AND METHODS Bacteria andphage.E. coli K37 (fromD.Pratt)
wasusedasthewild-typehost. IsogenicE. coli K38 (su-), obtainedfrom P. Model,wasusedasthe
non-permissivehost.Wild-typeM13phageand M13am5,
obtained from D. Pratt, were further purified from
single-plaque isolates. The mutant phage stock had less than 0.1%revertants.
Growth media and buffers. Thegrowthand pu-rification ofphage and the extraction andpurification
ofphage DNA have been described(20).Thefollowing
buffers were used routinely: buffer A, 0.1 M NaCl,
0.05 M Tris-hydrochloride (pH 8.0), and 0.001 M EDTA; buffer B,1MNaCl,0.02MTris-hydrochloride
(pH 8.0), and0.002M EDTA;bufferC,0.01 M Tris-hydrochloride (pH8.0) and0.001MEDTA.
Chemicals andenzymes.Pronase(B grade), pan-creaticRNase, cytochrome c,and ethidiumbromide werepurchased fromCalbiochem,LaJolla,Calif. Be-fore use, Pronasesolution(10 mg/ml) inbufferAwas
self-digested at 37°C for 30 min, and RNase was heatedat100°Cfor10min. The[methyl-3H]thymidine
(6 to 20 Ci/mmol) was purchased from Amer-673
on November 10, 2019 by guest
http://jvi.asm.org/
sham/Searle, Arlington Heights, Ill.,orNewEngland
Nuclear Corp., Boston, Mass., and carrier-free H3
31pO4wasobtained fromAmersham/Searle. Sarkosyl
NL97was a gift of Ciba-Geigy, and sodiumdodecyl
sulfatewas aBDH Chemicalsproduct. All other chem-icalswereof reagentgrade.
The purification anddigestion withE. coli exonu-cleaseI andHaemophilus influenzaerestriction en-donuclease (HindII) have been reported earlier (4). H.aegypticusrestriction endonuclease(HaeII)was a giftof R. diLauro of the Public HealthService, Na-tional Cancer Institute, Bethesda,Md. This enzyme wasused accordingtothemethod ofvanden Hondel and Schoenmakers (35).
Isolation of intracellular phage DNA. E. coli cultures growntolog phase (2X108 to 4 x108cells/per ml) insupplemented M9medium (23) at 370C were infected (multiplicity ofinfection, 100 to 200) with wild-type M13 and maintained at 370C or infected withM13 am5and maintainedat34WC, unless other-wise stated. The cultureswerelabeled with20
jLCi
of [3H]dThd per ml 8or 45minpostinfection and, after specified times, weredumped into equal volumes of chilledpoisontostopbacterialsynthesis. Thepoison was either buffer A containing KCN (10-2 M) and pyridine (5%) (15) or 75% ethanol, 21 mM sodium acetate (pH 5.3), 2 mMEDTA, and 2%phenol (22). The cellswerethen harvestedbylow-speed centrifu-gation and washed twice with bufferAcontaining10-2MKCN. Sometimes the cellsuspensions were shaken in aVirTis homogenizer for 2min at low setting to removeunadsorbedphage particles.
Next, the cells were suspended in 1/10 to 1/20 volumeof bufferAandincubated in the presence of lysozyme (200
tg/ml)
at 0°C for 45 min (25). The cellswerethenlysed -with Sarkosyl NL97 (0.5%) for 15minat37°CanddigestedwithPronase (200 ,ug/ml) at 37°Cfor30to 60 min. In someexperiments lysis was accomplished by adding sodium dodecyl sulfate(0.8%)tocellssuspendedin50mMTris-hydrochloride (pH 8.0)containing5mM EDTA in 10%sucrose(11). Finally, the lysatesweregently layered on top of20%
sucrosein buffer Bin anSW27 swinging-bucket rotor tube and centrifuged at 25,000 rpm for 3 to 4 h at 15°C inaBeckman-Spinco ultracentrifuge. The liquid wastakenfrom thetopwithout disturbing the bacte-rialDNA,whichsedimnented at or near the bottom of the tube. Afterphenol extraction in the presence of 1% sodium dodecyl sulfate and subsequent alcohol
precipitation,thephage DNAwasdissolved in buffer C.Occasionally,the DNAsamplesweretreated with RNase (50,tg/ml, 37°C, 1 h) before electron micro-scopy.
Ultracentrifugalanalysis. Equilibrium ultracen-trifugation in CsCl containing ethidium bromide (1) (4.2 g of CsCl and0.4mlof 5-mg/ml ethidiumbromide per4.0ml ofDNA) was carriedout in polyallomer
tubes in a no. 40 rotor of aBeckman-Spinco ultracen-trifuge. After 40 to 50 h ofcentrifugation at 35,000 rpm and23°C, 30 to 40fractions were collected from thebottom, and portionswereassayed for radioactiv-ity. Ethidiumbromide fromthepooled fractions was removedby repeated extraction with isopropanol (sat-uratedwith 30% [wt/wt] CsClin 0.2 M Tris-hydro-chloride[pH 8.0])followedby dialysis against buffer C. Forequilibrium ultracentrifugation in alkaline CsCl
(8),7.65gof CsClwasaddedto5.5mlofDNAsolution in40mMK2PO4(pH12.1) andcentrifugedina Beck-manno.40 rotor at35,000 rpm for42hat23°C.
Bandsedimentation in neutral sucrose (5 to 20% sucrose in buffer B) and alkaline sucrose (5 to 20% sucrose in 0.8 M NaCl, 0.2 M NaOH, and 2 mM EDTA) gradients was accomplished in a Beckman SW41swinging-bucketrotor.Totrapthefast-moving, covalentlyclosed, supercoiledRFtype I (RFI) DNA,
wesometimes useda0.5-ml cushion of 60% CsCl and 60%sucrose(wt/wt)atthebottom.
Radioactivity measurement.Theportionswere eithercounteddirectlyin aqueous mediuminaTriton X-100-toluene scintillation solvent systemorspotted
onWhatman3MMdisks and washed with 5% trichlo-roacetic acid, alcohol, and ether, in succession, to
remove acid-soluble radioactivity. The dried disk-were counted in toluene containing
2,5-bis[2-(5-ter-butylbenzoxazolyl)]thiophene (BBOT) (20).
Electronmicroscopy. Samplesof DNAwere pre-pared for electron microscopy by the Inman and
Schnos(13) modification of theprotein filmtechnique (16). Fractions fromsucrose or CsCl gradients were
dialyzedagainstbufferC for3h atroomtemperature. A0.07-mlsamplewasmixed with0.03ml of buffer(4 ml of 37%formaldehyde,0.32ml of1MNa2CO3,and 0.4 mlof0.126 MEDTA) adjustedbeforemixing to eitherpH8.7 (neutral) or11.1 (denatured) with 1 N HClor 5NNaOH,respectively. Afterincubatingfor 10minat25°C, the DNA-buffer solutionswere trans-ferred to an ice bath, cooled for 5 min, and mixed with an equal volume of formamide (Mallinckrodt,
99%).Cytochromec wasthen addedto0.01%, and the solutionswereincubated for10min at room temper-aturebeforespreading0.005mlontothe surface ofa waterdroplet. The DNA waspickedup on nitrocel-lulose films supported on 400-mesh grids, rinsed in 95% ethanol, stained withuranyl acetate (5), rinsed inisopentane, and rotary shadowed withplatinumat a 60 angle. Electron micrographs were taken on a SiemensElmiskop 1A, with magnifications calibrated byusingadiffraction gratingreplica (Fullam,54,864 lines perinch). Themicrographswereprojectedonto paper and traced, and the tracings were measured withacurvimetertothenearest 0.05,um.Underour
conditions,therewas nostatisticallysignificant differ-ence inthe contourlengths ofM13 circularssDNA
andRFIDNA;therefore, itwasnotnecessaryto use a correction factor to correlate measurements of ssDNA and double-stranded DNA.
Alignment of partially denatured rolling cir-cles.Thepartiallydenatured circles werealigned for maximumoverlapofdenaturedregions bya correla-tionmethod similar to, but computationally simpler than, that ofYounget al. (36). Computations were carriedouton aPDP11/40 computer. Each molecule was divided into 100 segments; each segment was assignedavalue ofzeroifthesegment was more than half native,orofone ifitwas more than half dena-tured.A moleculewasalignedtoanother by shifting one relative to the other a segment at a time, flipping it, and again shifting. The measure of overlap for eachrelativealignmentwas
F
(X,+y,)2
i=1
on November 10, 2019 by guest
http://jvi.asm.org/
where X,and Yiare the values assigned to the ith segment of each of the two molecules. Before the alignment, themolecules were ordered by their extent ofdenaturation, from most to least. The alignment procedure considered the molecules in that order, aligning the second to overlap maximally with the first. The third was then aligned to the sum of the first two,the fourth to the sum of the first three, etc. When all of the molecules had been aligned in this way, asecond cycle, for refinement, was performed. In this cycle, each molecule was considered in the same order asbefore. Its contribution was subtracted fromthe sum; the moleculewasaligned with thesum of the remaining molecules and added back to the sumin thisnewposition. The differences between the results of the first and second cycleswerequite minor. Theresultingalignmentwasthen translated to bring it into correspondence with the picture of partially denaturedHind-cut, linear M13 RF DNA molecules.
RESULTS
Intracellular forms of M13DNA. The M13
DNA isolated from cells 8 min after infection with
wild-type
phage was spread directly for electronmicroscopyorsubjectedtoequilibrium centrifugation in CsCl containing ethidium bro-midetoseparatecovalently closedDNA(heavy band) from the rest (light band) (Fig. 1). Por-tionsfrom theheavy andlight
bandswerefreed ofdye,dialyzed,
andthensubjectedtoelectron microscopy. The different intracellular forms ofM13 DNA found in the
heavy
andlight
bandsare shown in Fig. 2 and 3, respectively. The
structuresin theheavy bandfrom DNAisolated
8 min postinfection include unit-length (2
,Am)
supercoiled RFI (Fig. 2A), dimer-lengthsuper-coiled RF
(<1%) (Fig. 2B),
and RFconcatenates(ca. 5%) (Fig. 2C). We have
previously
shown thatwhenRFIDNA isspread
withformamide-formaldehyde,
it contains asmall,
denatured region asdepicted
inFig.
2A(S.
Dasgupta etal., J. Biol.
Chem.,
inpress)anddoesnotappearsupercoiled.
Ithas beenpostulated
that thedi-mersandconcatenatesarise fromerrorsin
seg-regation of
daughter
molecules afteroneround ofreplication (9).
We have found veryfew (2/-1,000)
M13 molecules with a e structure likethose foundin the
replicating
intermediates of E.coli(3)
orcolicin El(14)
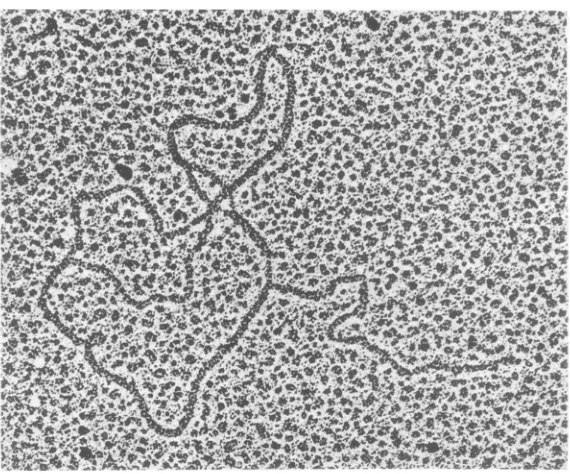
DNA. Themolecule shown inFig. 2D isacircle withtwoforks thatis 2
tum
whenonly
one branch of thedouble-stranded fork is included in the measurement. It appears possible that such structures, also observed by Ray (26),are intermediates in RF DNA
replication.
The light band from the CsCl gradient
por-trayed
inFig.
1 contains 70 to 80% RF type II(RFII) DNA molecules (relaxed RF DNAwith one or more discontinuities in one or both
strands) (Fig. 3A),ssDNA circles (Fig. 3B),and a structureswithanssDNA tailattached to RF
8
-
6-4
21
0 10 20 30
FRACTIONNO.
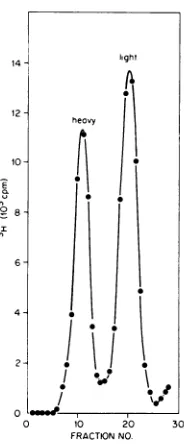
FIG. 1. Equilibriumcentrifugationof intracellular M13 DNA in CsClcontainingethidium bromide. A 100-ml log-phase culture ofE. coli K37 grown in
supplementedM9 mediumat370Cwasinfectedwith
wild-typeM13for8min and thenlabeled with RH]-dThdfor90s.Afterisolationofthe totalphage DNA,
itwassubjectedtoequilibriumcentrifugationinCsCl containing ethidium bromide (both procedures as
describedin thetext).Fractionswerecollectedfrom
the bottom andmonitoredforradioactivity. Sedimen-tationwasfrom righttoleft.Thedensityoftheheavy
bandwas 1.60g/mlandthatofthelight bandwas
1.56g/ml.
DNA (Fig. 3C). The tailed molecules are
ex-pected intermediates for ssDNA synthesis on
circular, double-stranded DNA templates (10),
and the tails
generally
appear distinctly single stranded in ourelectron micrographs.Dresser
(6) observed similar structures
during
4X174
DNAsynthesis,
andRay
(26) also detected such molecules in intracellular M13 DNA. Becausewe never, in
examining
more than 200 amole-cules,
foundtailslonger
than2Am
(unit length),
weconclude that thesingle
strands of therolling
circlearecleaved as soon as
they
reachmature virus DNAlength. Figure 3Dshowsa molecule with twosingle-stranded
tails attached to RF DNA; about 10% of the tailed molecules had twotails.Figure 4 shows a a molecule spread at pH 11.1 in the presence of 50%
formamide,
condi-tions under which the double-strandedDNA ispartially denatured.That thefrequencyof such astructures didnotdecrease underthis condi-tion demonstrates thatthe ssDNA tails are in-deed
covalently
attachedtothedouble-strandedRF DNA
molecules,
and the absence of any675
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.500.303.395.74.297.2]6 76 ALLISON ET AL.
"Z-:r
'.&N V-6ft I, V' 0 .0tv
-,-t IV a. 1%,.I -W, .%O%*4
AM ire.0 0 'k
-K.Z b, .16qq,
q;I Al: J,.^+d. I& W.a'.,its& 4V A
Aj V
10 , 4*., .1 P 'L, . ..
-W Oes. P
aIvv; la.
t,-Q,
"o.4% 7;,t %,e
v 4.
vp4A.c.r.Z" o6Aoi k W
-.4' % ,
dr- "a 4" a P.00
V %F- AI%
R
C4iDh- 19
y!"s 40 W'P
Ir U.
L",
is0. t.r
'B- lae
P f 'A VP
r r
A VAOr. S,
r-0.'aj
b' j4. -'.''. -*J-% qP1.*to
After
I"-161
-At'"".' #4 *..P.
.4 V c.
L Ox k ..v
IV e:. 4:
'.re
.3 'J t44S.,-. 01,
16n
P
..P & r
roll
cl tOA kb
4? 4149g 06
V?4. V
V j Ix
6,4
% .4%
4, -J
If4 44
% % N v It 'fa r I.S190.11 It.
A Alr It 1,
1.VA
`V %1. N X%
6.4 4V.z r S
ZV
O
U, 4k A., I
0 A
f, Af rek
t
4J.,4-' rot In ju .I, A
04
4W, If
_J4 'S
d jo P,
'J. 4.
IS4. C' i
A
V 4 41 L. _. '%.,.F 'k,
P. A 400
.0 J A x.,I:
ile, 1.4- V'
?..-k.. :,;, I -11 '..,
..XJt 4
4, a
J A W'
k
T -v T J Qj. %
-6A. TW A
5IA, 7_
`4 4.
Jk A -Jo, X*1.6 Le .6,
'k
41 S" 4 te
V ok&;`
1k
V A.A q
A.010 F
x
V.
A.g
26t ft iAl V', 4- 4
A.L U
_d A 'P .40
*il. K-W, 8 A%
_0IL
It %
y/
.Ow,-J- IL
.0 4- 4,
1.` 4.4? 44 exf'
.x Ak;.;.e. t
at
4;:
I--4 q,
4 1P
4t.
41
A y
A( rzf 4
A V
%
..4.4.4 v
40N Z L 14
`X
-Z V
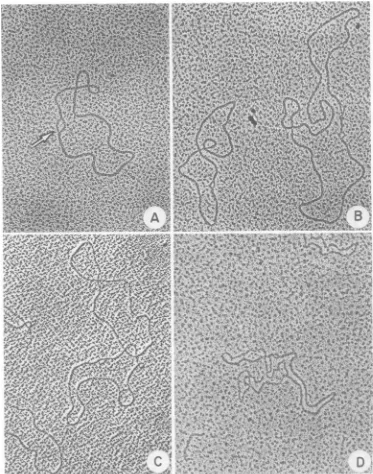
FIG. 2. Forms
of
intracellular M13DNA in theheavy
bandrepresented
in Fig. 1. Supercoiled RFImolecules
(A)
appearopenwhenspread
withformamide.
The small denaturedarea(arrow)isnearly alwayspresentwhen
supercoiled
moleculesarespread
under these conditions.Dimer-length, supercoiled
RF(B), concatenates(C),andafew Rstructures(D)arealsopresent. X57,560.denaturedareasinthetailsisaclearindication adouble-strandeda
intermediate,
andis,
in thisof their
single
strandedness.Nodouble-strandedrespect,
differentfrom that ofOX
174(28).
tailswere seenamongafew hundredastructures AfterE. coli infectionby
wild-type
M13,
RFSu
ejected
to denaturation. Thissuggests
thatreplication predominates
for the first 10 to 20RF
replication
of M13 DNA doesnotoccurviamin,
after which progeny ssDNA is themajor
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.500.75.448.77.551.2]j44
4 4
'A 4~~~~~~~~~~~~~~~4
~ ~ ~A
d.~~ ~ ~ ~ ~ ~ ~
4h-~ ~ ~ ~ ~
I4
S5
JP~ ~ ~
-a4
pS,L4~,
W, '60a)
4&&t3
W44
4~~~~~~~A
10~~ ~ ~
'S~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~.I
ir
~ ~ ~ ~ le
,6
al
^ssw.x.- - 4. M
FIG. 3. Formsof intracellular M13 DNAin thelight band represented in Fig. 1.(A) Relaxed RFII circles thatneverexhibit thesmalldenaturedareafound in the supercoiled circles. Unit-length, single-stranded
circles (B)are also present, as wellascircular RFmolecules withsingle-stranded linear tails (a). Most of
these molecules exhibit a single-stranded tail attached to a double-stranded circle (C). However, some
moleculesarefoundwith twosingle-strandedtailsissuing fromthe RF molecule(D). x57,560 formsynthesized (26). However, wesaw tailed
moleculesas early as8 min after wild-type in-fection(Fig. 3),and thefrequencyofthesetailed moleculesinthe total RF DNApopulation
ap-pearedtobeindependentof thetimeafter
infec-tion(Table 1).WethereforeagreewithForsheit etal.(7),who showedthatsomeprogenyssDNA
synthesisoccursveryearlyafterinfection. We found no tailed molecules in DNA ex-tracted from E. coli K38 10 to 15 min after
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.500.69.419.71.558.2]678 ALLISON ET AL.
FIG. 4. Partially denatured RF DNA with tail. DNA isolatedfromthe lightbandrepresentedinFig. I was adjusted topH11.1 for 10min
before spreading
for electron microscopy. Two denatured sites are evidentonthe circular RF molecule. The single-strandednessofthe tailcan be inferred because the tail doesnotcontain any denatured sites and appears less thick than the RF molecule. xl10,000.TABLE 1. PercentageofRF molecules with
single-stranded tailsearlyand latepostinfectiona
Expt Early Late
1 37/237(16)b 47/247(19)
2 4/38 (11) 5/41(12)
aLog-phase E. coli cultures(100ml)wereinfected withwild-type M13phage (multiplicityofinfection,
-100).Total intracellularDNA,after removal of the host DNA as described in the text, wasspread for electron microscopy after RNase treatment and
de-proteinization. "Early" and "late" refer to 8 and 45 minpostinfectionat
370C.
bNumber inparenthesesrepresents percent.
infectionwith M13 am5
phage
inthreeseparatepreparations, scoring approximately 500 RF
molecules. ThepresenceofM13 gene 5protein,
therefore,
appears to be essential for the exis-tenceof thea structure.Ultracentrifugal
studies of M13 ssDNAsynthesis.
Ultracentrifugal analysis
anddetec-tion of
longer-than-unit-length, pulse-labeled
DNA of virus-strand type led Ray (25) and Tseng and Marvin (33) to propose that M13 ssDNAsynthesisoccursbyarolling-circle
mech-anism.To
complement
ourelectronmicroscopic
evidencefor the involvementofa structuresin M13 ssDNA
synthesis
(to bepresented
later),
we extendedtheearlierultracentrifugal
studiesby
investigating
DNAfrom bothwild-type andam5
phages.
Band sedimentation analyses inneutralsucrose ofboth
pulse-labeled
M13 wildtypeandam5DNA
early
andlate postinfection showed that most of the label was present inRFI and
RFII,
withverylittle orno incorpora-tioninto maturessDNA, inagreementwithRay(25) (datanot
shown).
After infection withwild-type phage,a large fraction (70 to 80%) ofthis
label can,
however,
be transferred to ssDNA after a chase (4, 25). In contrast, inM13am5-infected
cells,
inthe absence of ssDNA synthesis, theeffect ofasimilarpulse
andchasewasonlytotransfer label from RFIItoRFI(S.Dasgupta
and S.
Mitra,
inpreparation).A more revealing setof experimentalresults wasobtained from extendedband sedimentation ofpulse-labeledDNAs inalkaline sucrose (Fig. 5) whereRFI DNAsediments into the cushion atthe bottom whileRFII DNA separates into
unit-length linear single strands, circularsingle
strands, and longer-than-unit-length
single
strands. It is evident by comparing with themarkerphage DNA that inboth
early
(A)
andlate(B) postinfectionwithwild-type phage,the
label is distributed
bimodally
intounit-length
linearDNA(16S) anda
faster-sedimenting
DNA with a sedimentationcoefficientof ca. 20S. Thefaster-sedimentingDNAdidnotcorrespond
ex-actly with, but contained material that moved
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.500.118.403.73.310.2]ORIGIN OF M13 SINGLE-STRANDED 679
1)20
301020
30 10 2030 40FRACTIONNO
FG5.Bandsedimentation in alkaline sucrose of pulse-labeled wild-type andam5M13 DNA. Twenty-milliliter log-phasecultures of E. coli K37 (A and B) and K38 (C) infected with wild type (A and B) and
am5phage (C) werepulse-labeled with 20 ,uCi of
[3Hlthymidineper ml as described in the text. (A) 30-spulse-label at37°C,8minpostinfection; (B) 30-spulse-label at37°C,45 minpostinfection; (C)1-mmn pulse-label at25°C, 3mi after shift-down to25°C from37°C,13 min postinfection. After termination of labeling, the infected cells were washed and lysed, and the bacterial DNA was removed by centrifuga-tion as described in the text. Portions ofthe DNA,
alongwith '4C-labeled M3phageDNA,were
centri-fuged in 5 to 20% alkaline sucrose gradients in a Beckman SW41 swinging-bucket rotor at 5°C at 39,000rpm for 11 h (A, B) or38,000)rpm for 12 h (C).
Symbols:*, 3H; C. The positions of circular and
unit-length, linearM13DNAareindicatedby C and L,respectively.RFI DNAsedimentinginto the
cush-ionis indicated by a dashed line.
fasterthan, the circular phage DNA.Incontrast, DNA extractedfrom M13 am5-infected culture
(C) early postinfection sedimented mainly as
unit-length linear DNA in alkaline sucrose, with
little labeled material sedimenting faster than circular DNA. Asignificant amount of label is
also in the RFI DNA. Figure 6shows that
fol-lowing the chase of pulse-labeled DNA after
wild-type M13 infection, the label from both
unit-length linear DNA and the faster-moving DNA(20Speak) isin the DNA that sediments
exactlywith circular DNAmarker.
These results can easily be explained by the
situation depicted in our electron micrographs, where longer-than-unit-length strands (i.e.,
cir-cles with tails) are clearly evident. If these are
indeed intermediates insSDNA synthesis, any
labelthey contain should be chased into mature
circular DNA during continued synthesis with unlabeled thymidine. This proposition was
testedintwoways:First, thepulse-labeled
frac-tions containing longer-than-unit-length linear
DNA (Fig. 6A) and the corresponding chased
fractions (Fig. 6B) wereseparately pooled. The
DNAs werethen tested for susceptibilitytoE.
coli exonuclease I, which attacks only ssDNA
from the 3' end. Circular phage DNA is
com-pletely resistant to this exonuclease (20). The majority of the label from pulse-labeled DNA issusceptible to the exonuclease (Table 2), in-dicating that most ofthe DNA sedimenting in theposition of circular DNA is, in fact, longer-than-unit-length linear DNA. The majority of the DNA isolated afterchasing is, asexpected,
resistanttotheexonuclease and, therefore,
cir-u
Il
.
18
-A
16 C
8-CL
L12
6-J~~~~~~~~~~~~
04 1
FRACTIO NO.
E)
Cli
Q
a.
FIG. 6. Bandsedimentation in alkaline sucrose of wild-type M13 DNA afterpulse and chase. E. coli K38infected withphagewaslabeled with
f3HjdThd
for30 s 45minpostinfection at370C.One half of the culture(A)wasstoppedwithethanol-phenolmix (22), while the other(B)wastreated with500,agof unla-beled dThd per mlfor10minbefore additionof the stopper. Furtherprocessing and centrifugation are described in thelegendtoFig. 5C, except M13virion [image:7.500.50.235.53.215.2]/32P]DNAwasusedasthe internal marker.
TABLE 2. Susceptibilityof alkali-denatured,
pulse-labeledM13DNAtoexonuclease Ia
DNAmade acidsoluble by exonuclease I(%) Type of DNA
[3H]DNA Control [32p]_
DNA After pulse-labeling (Fig.
7A) 74.4 91.2
After chase(Fig. 7B) 35.6 89.8
aThe fractions 8 to19and 10 to 17inFig.6Aand B,respectively,werepooled, quickly neutralized,and alcoholprecipitatedinthe presence of0.3Msodium acetate. Portions (-4 x 103 cpm) of the dissolved precipitate were mixed with 75 pmol ofpancreatic
DNase-treated and denatured 32P-labeled M13 RF DNA (4.7x 103cpm) (G.LavelleandS.Mitra,inP. Tattersall andD. Ward, ed., Parvoviruses, inpress)
and thendigestedwith0.6U of E.coliexonucleaseI in 0.3 ml at37°Cfor30min(20).
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.500.255.442.205.358.2]
12-- 8-I
I
4-0
0 20 40 20 40 20 40
FRACTION NO,
'4
6
k8
FIG. 7. Equilibrium centrifugation in alkaline CsCl. Pooled fractions of unit-length, linear and longer-than-unit-length M13 DNApooledfrom the alkalinesucrose gradientof Fig. 5were banded in
alkaline CsCl asdescribed in the text. (A) Longer-than-unit-length ssDNA from fractions 13 to 16of Fig.5B; (B) unit-length,linear ssDNAfromfractions 17to20ofFig. 5B;and(C)unit-length, linear ssDNA from fractions 18to22ofFig. 5C. Symbols:*, 3H; 0, `4C marker phage DNA. (v) and (c) denote the position ofviralandcomplementary strands,
respec-tively.
cular. Second, equilibrium centrifugation in
al-kaline CsCl (Fig. 7) was used to determine the
strandednessof(B) unit-lengthand (A)
longer-than-unit-length linear DNA samples from pulse-labeled DNA late after wild-type phage infection (pooled from fractions in Fig. 5B) and of (C) pulse-labeled, unit-length linear DNA after M13 am5 infection (Fig. 50). The pulse-labeled linear DNAfromM13am5-infected cells contained bothviralandcomplementary strands inanequimolaramountaftercorrecting for dif-ferences in thymine content (26), whereas the label in the DNA fromwild-type M13-infected cells late postinfection contains predominantly viral strands, which is in agreement with the results ofSuggs and Ray (31).
Origin of ssDNA synthesis on RF DNA
template.Alltheexperimentsdescribedsofar
indicate that progeny M13 ssDNA synthesis,
like that of 4X174 DNA, occurs bya a
mecha-nism where the 3' end of the growing virus strand displaces the 5' end of the same strand on the complementary strand template of RF
DNA. If the synthesis starts at a fixed site on
theRFtemplate,itshouldbepossibletolocate the sitebyvisually"folding"thesingle-stranded
tail backonthecircularpart oftheastructure
shownin electronmicrographs. The basic prob-lemofsuchamethodlies inestablishingapoint
of reference in the circular RF DNA, but we
circumvented thisdifficultybytakingadvantage of the site of cleavage by HindII restriction endonuclease on M13 RF (34). This enzyme
does not attack M13 ssDNA (2). Total phage DNA wasextracted from 100mlof E. coli K37
culture in supplemented M9 medium, 45 min
after infection withwild-type M13. After
treat-ment ofthe DNA solution with Hind ertdonu-clease (4), the DNA was spread for electron microscopyinthepresenceof formamideatpH
8.7 asdescribed in Materials and Methods. We were thus able to observe linear RF DNA (2
,Am long) with or without single-stranded tails
of various lengths attached to the double-stranded DNA. Thelengthof the tailwas
mea-sured in both directions from the three-point junction, giving two possible locations for the origin ofssDNAsynthesis.Assuming there isa
unique origin of synthesis, only oneof the two
sites from each molecule, inanarrayofdifferent molecules, would becommontoall.Becausethe "right" and "left" ends of the double-stranded RF DNA could not be distinguished, we could
only establish the distance of the origin from the Hind cleavage site. Figure 8 represents a
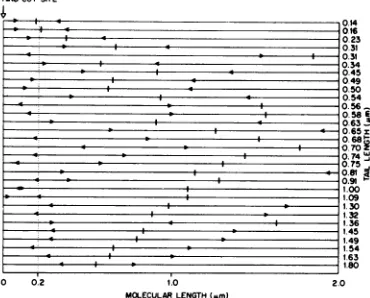
collection of molecules with the two possible origins,oneindicated foreachmoleculeoneither
side of thethree-point junction. It isquite clear thatonlyoneregion, correspondingtoabout 0.2 ,um from the Hindcleavage site, iscommonto mostof themolecules. The direction of replica-tion can be established in a relative sense in
H1DCUT SITE
0.2
b 140.14
:1 0.16
*|z~ ~ ~ ~ ~ ~ ~ ~ ~~~~~-30.2
.@ z 0~~~~~~~~~~~~~~~~.34
I$&Ie 0.45
* I6*:~0.49
**.- m4058E
a- -z 0is.63
a- '0.65
I.00
1.09
130 1.32
1.36
1.45 1.49
1.54
163 1.80
2.0
1.0
[image:8.500.65.254.52.175.2]MOLECULARLENGTH(I.m)
FIG. 8. Mapping ofsingle-stranded tailsonlinear
RF DNA. The total viral DNA waspurified from
K37cultures,maintainedat370C,45minafter infec-tion withwild-typeM13asdescribed inthetext.After digestion ofthe DNAwithHindendonuclease,itwas
spreadforelectron microscopy asdescribed in the text.The"Y"-shapedmoleculesarising from a
struc-tureswerephotographed. The double-strandedarms were measured and normalized to 2 wun, and the length ofthe single-stranded tailwas interpolated
onto the double-stranded arms. The two possible origins ofssDNA synthesis thusobtainedare
indi-cated by arrows on the lines representing the RF DNAmolecules. The moleculesareorientedsothat,
ofthe twopossible origins, the one closest to the Hindcutislocatedontheleft. Thenumbersatthe endofthelinesindicate taillength.
A B
8-2 4'
:I2
2cI
ICI ~
~~~~~.
--I0
_ * a0.31
_ b W&Os
b~~~~~~~~~~~~~~II-OS
I- 0.54
_ * 0. 56
_. _ 0.6 "
4 I V0.7X)Z
** I0.74'
*4 * 0.75
P * 0.81
SI II .0.91
1
4 0
4 0
0 4
4 b
-6 b
4, b
4 0
4 p
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.500.270.455.348.497.2]681
that the DNA chain grows either toward or away from the center of the double-stranded template. However,oneproblem thatmay com-plicate such a determination is branch migration
occurring in vitro (17) after replicating DNA
has been freed ofgene 5 protein, which in vivo covers up the peeled-off 5' end of the strand (24) and preventsbranch migration. Branch
mi-grationin DNAwould,ineffect,apparently re-verse the direction of synthesis. In fact, we can see both directions ofchain growth in Fig. 8. However, we may assume that the possibility of branch migration is significantly reduced in
moleculeswithlonger single-strandedtails, and thesemolecules (showninthelower half of the array) clearlyindicate that the direction ofchain growthisaway from the center of themolecules. To establish the absolute direction ofchain
growth and relate it tothe genetic map ofthe
phage,weutilized thepartial denaturation map-pingtechnique ofInman and Schnos (13). The
partial denaturation map (Dasgupta et al., J.
Biol.Chem., inpress) oflinear RFDNA
gener-ated by Hind endonuclease cleavage was then
related to the HaeII restriction endonuclease
map (35) that had already been related to the
genetic map of thephage (18). We utilized the
fact that glyoxal predominantly fixes a region in supercoiled M13 RF DNA that corresponds
to the major denaturable region and digested glyoxal-treated M13 RFI DNA with HaeII, whichcutsthe DNA into threepieces of3,500,
2,600, and320base
pairs,
denotedasA,B,
andC, respectively
(35).Acomparison
of theHaeIIrestriction map and the
partial
denaturation map of Hind endonuclease-generated linear DNA(Fig. 10) shows that theglyoxal-fixed
bub-blecorresponding
tothemajor
A-T-richregion
wouldbe located in the B
fragment
if theori-entation ofthedenaturation mapwerethesame
asthat of the conventional restrictionmap
(i.e.,
with theintragenic region locatedneartheHind site inaclockwise direction
[35]
or ontheright
side of the Hind site in a
similarly
oriented linearrepresentation). Alternatively,
thebubble wouldbe in the Afragment
ifthe molecules in thepartially
denatured map were oriented inthe
opposite
way. Out of 43 DNAfragments
scored, 12 contained
bubbles,
and 11 of these (>90%) fell in the size rangecorresponding
to2,300 to2,500basepairs.The
bubble-containing
fragments, therefore, clearly correspond to
HaeIIB
fragments.
This establishes the orienta-tionof thepartial denaturation maprelativetotheHaeIIrestriction map
and, by extension,
tothe
genetic
map.Toestablishtheoriginand direction of ssDNA
synthesis, we
partially
denatured circular RFIImolecules
(Fig.
4)by
raising
thepH
to11.1 for10minbefore spreading forelectron microscopy.
Molecules with single-stranded tailswere
mea-sured so that both the position and length of the denatured areas and the single-stranded tails
could be relatedto a convenient, but arbitrary,
starting point on the circular molecules. The data were fed into the computer, where the molecules were normalized to 2
pm
and alignedfor maximum overlap of denatured regions as described in Materials and Methods. We then
asked the computer to translate these data to
correspond to the partial denaturation profile of RFImoleculesthathadbeencleaved by the
site-specific HindII endonuclease before
dena-turation. TheHindII-cut moleculesrepresented
in Fig. 9A are plotted so that the site of the
predominant denatured area is in the left half
of themoleules; thearrayof partially denatured RFII molecules with single-stranded tails
ar-ranged by thecomputer arerepresented inFig. 9B. When the single-stranded tails ofvarious
lengths are superimposed onto these partially denatured RF molecules, they indicate a com-mon origin of replication. If the best-fit data plotted by thecomputer areused, outofthe39
moleculesshown, 31 have a common origin lo-cated 0.16,m (standard deviation = 0.12
tum
out of a total length of 2.0 ,um) or about 8%from the left endofthemap withthedirection
of chaingrowthaway fromthecenter.Weshould point out that eight molecules (indicated by dotted circles around the two possible origins
andthe tail inFig. 9B) do notfitourresultsas
plotted. However, sinceweasked thecomputer to align the molecules by the best fit of the denatured areas without
considering
where theorigin of
replication
would belocated and since we weredealing withasmall molecule with fewdenatured sites, we feel that these results are
quite good. If the 8 molecules that do not fit
wererotated 180° aboutthe major denaturation
area located in theleft half of the
molecules,
5 more molecules(asterisk,
Fig. 9B) would havean origin
coinciding
with that of the other 31molecules.
Acomparisonwith thegeneticmap
(Fig.
10)shows that this
origin
of ssDNAsynthesis
isbetween gene IV andgene IIofthe
phage
andthat
replication proceeds
ina counterclockwisedirection. Because both DNA and RNA chains grow in the 5'-+ 3' directionand because both
M13mRNAandprogeny ssDNA
syntheses
oc-cur on the complementary
strand,
one would expect the direction of DNAsynthesis
to becounterclockwise, asis RNA
synthesis
(21).DISCUSSION
The precise mechanism of
synthesis
of prog-eny M13 RF and ssDNA isnotyetcompletely
on November 10, 2019 by guest
http://jvi.asm.org/
682
ALLISON ET AL.)ERIVED HIND I SITE B
MOLECULARLENGTH (elm)
TAILLENGTH(Im) 0431 0 36
*--- 043
.053 05 0 56 0530
0601 0666 065
-.-075
-0
0-786
66 081 09 91--94
9
096
.02'
108
- i-41141111
116
26 1.27
-1-35- 1.7
171
81-l881.
2 00 2
inprogeny RF DNA
synthesis (26),
and the lack ofrequirement for many of the dnafunctions inprogenyssDNAsynthesis
(4, 20,26)
suggest that differentreplication
complexes
are respon-sible for the three different stages ofintracellular M13 DNAreplication.
.4X174,
which alsocon-tainscircular ssDNA asthe genome,
produces
intermediatesinDNAreplication thatare
anal-ogous to thoseofM13 (26). Dressler(6) showed that progeny ssDNA in
4)X174
issynthesized
via the a intermediate
by
displacement
of the 5' end of the viral strand in RF with anewly
synthesized 3' end of the same strand. Since then,
Schroder
and Kaerner (28) have shown that progeny RF DNAsynthesis
occursby
rep-lication of the
displaced single
strand in the aintermediates.It isobvious thatprogeny ssDNA
is synthesized most economically by a strand displacementmechanism,
i.e.,
oneinvolving
aastructure. On the other
hand,
RF DNA could besynthesizedinthesameway orviaacircular replicating intermediate, such as the structureobservedinothersystems(3, 14).Although this manuscript deals with theorigin of ssDNA syn-thesis, our
negative
findingsbring
out somein-teresting
possibilities
regarding RF synthesis, which we will discuss first. Evidence based onstudies with wild-type phage has been used to
IHINDICLEAVAGE SITE
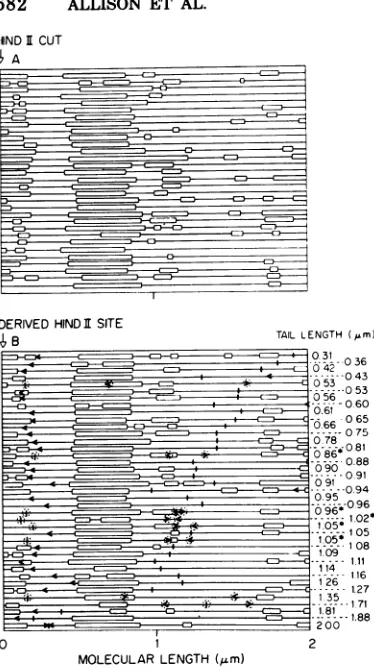
FIG. 9. Denaturation maps of M13 RF molecules and astructures. (A) M13 RFI DNA isolatedfrom
theheavy band described inFig.1wascutwith Hind endonuclease andpartially denaturedforelectron microscopy asdescribed in thetext. Themolecules, indicatedbyhorizontal lines(thedenaturedregions are shown by open blocks),are aligned so that the major asymmetrically located, denatured region is in the left half ofthe molecules. (B) Circular RF molecules with tails(astructures),presentinthe total phage DNA isolatedfromE. coli K37,45minafter infectionwithwild-typeM13 andpartiallydenatured asdescribed in the. text, were aligned by computer for the bestfitofthedenatured areas, and transposed by computertofit theprofile of the Hind-cut mole-cules. The location and directionof the arrows indi-catetheoriginanddirectionof replication, whereas the small vertical lines indicate theposition of the tail. Molecules that have bothorigins and tails cir-cled donothave anorigin that fitsourconclusion. Theasterisk indicates moleculeswhoseorigins would fitif the moleculeswererotated1800aboutthemajor denaturedarea.
understood. The lack ofrequirement for host
dna functions other than that of dnaE (DNA polymeraseIII) inparentalRFDNAsynthesis (27), therequirementforseveral dna functions
HAE n CLEAVAGE MAP
6 9 | . 3
A B C A
GENETIC MAP
IR. z
v3' ' i . a .... ..
1 I s I I ."
EARLYMELTINGREGIONS
ORIGIN OF ss DNA SYNTHESIS v3' a
[image:10.500.64.251.60.395.2]c5'
FIG. 10. Origin ofssDNAsynthesisonM13phage
genetic map. The HaeII restriction endonuclease cleavagemap ofRF DNAwasusedtocomparethe
genetic map and thepartial denaturation map as
described in the text. The origin and direction of ssDNAsynthesisindicatedbythearrow wasobtained
from Fig.9. (v)and(c) correspondtothe virus and thecomplementary strand,respectively. Thegenetic map and HaeII restriction map are redrawn from reference35.
t L
> -=_
K _:__
* C= _-1 I ah
h -L_
__n
.oC
2S.=
_ * _ _.
_ g
*__
An==
_=D {
£ -_ L_
=E he
C
5,.
3
:5
3s
l-
J. VIROL.1.
v
c .j
on November 10, 2019 by guest
http://jvi.asm.org/
indicatethat RF replication in M13 also occurs
by a a intermediate (29, 33), but our data do not support this model. We followed progeny RF DNA synthesis in the absence of progeny
ssDNAsynthesisbyinfectingsuppressor-freeE.
coliK38withM13am5,in whichRFreplication continues for at least 50 min at
340C
in thecomplete absence ofprogeny ssDNA synthesis
(S. Dasgupta andS. Mitra, unpublished data).
Under these circumstances,we found no a struc-turesof RF among several hundred DNA
mol-ecules scored by electron microscopy and no
pulse-labeled, longer-than-unit-length viral ssDNA afterbandsedimentationinalkaline su-crose(Fig.5C). Thesefindingssuggest that M13
RF isnotreplicatedin the same way asprogeny
ssDNA. Furthermore, the presence of DNA from both strands of RF DNA in both
unit-length linear and smaller-than-unit-unit-length frag-mentsin nascentRFDNA afterinfection with am5 phage (Dasgupta and Mitra, unpublished data) supportsamodel of discontinuous
synthe-sisof both strandsin RFsynthesis. In fact, we
did see a few Cairns-type 0 structures, which may be replicating intermediates of RF DNA.
Their paucity in the DNA population could result from the factthat the replication time for RF is expected to be in the order of seconds,iftherateofDNAchain growthinRF
is comparable to thatin the host. Hence, it is
quite possible thatmost of the molecules
com-pletearound ofreplicationevenwithin thetime
thepoison (usedtostopthepulse label) inhibits DNA
synthesis.
Alternatively, the replicating RFmoleculesmaybeselectively lost either by bindingverytightly
tothemembranouscomplex (29) orby existingin a structurethatcannotbespread
properly
forelectronmicroscopic visual-ization.Finally,
the lack of a structures with double-stranded tails after infectionwithwild-typephagemakesit
unlikely
that the a structure isinvolvedin M13RFreplicationinthemannerpostulated for 4X174 (28).
On the other
hand,
ourultracentrifugal
anal-yses of
pulse-labeled
M13 DNA confirm the amodeofprogenyssDNA
synthesis
suggested by Ray (25). Hisexperiments
and those ofTseng and Marvin(33)
indicated the presence oflonger-than-unit-length, pulse-labeled
strandsin thevirusstrand ofRF DNA after infectionwith wild-type phage.We showedbyultracentrifugal andenzymatic techniques
that bothlonger-than-unit-length and unit-length DNA derived
from pulse-labeled RFII DNA are of the viral-strand type andareindeedlinearbythe criterion of theirsusceptibilitytoexonuclease I. Further-more, afterachase,the labelcanbetransferred
intocircularDNA.These
results,
inagreement with therolling-circle
model of M13ssDNA synthesis, are further supported by elec-tronmicroscopic studies.Many astructureswith
single-stranded
tails covalently attachedto RFmoleculeswerereadily observed in the electron microscopeand constitutedasignificant
propor-tion ofthe total intracellularRF pool. The
oc-casional presence of RF molecules with two
single-stranded tails was puzzling at first, but
couldeasilybeexplained by incompletebranch
migration in vitro (17). That the two tails are
always attached to the RF in close proximity and that there isalwaysasingle-stranded region
on the RF molecules between these points of attachment support such apossibility. It is ob-vious that branch migration is prevented in vivo,
wherethedisplaced singlestrand of a structures
iscovered bygene 5protein (24).
Wehave also observed severalinteresting as-pectsof the control ofssDNAsynthesis. The a
modelpostulatesacontinuouselongationof the
tail witha5'end viadisplacement bythe grow-ing3'end of thevirusstrandonthe
complemen-tary strand template (10). After one round of replication, the single-stranded tail is cleaved
and circularized into mature progeny ssDNA. We have firstof all shown here that the tailin the a structures is neverlongerthan unitlength, which indicates an efficient cleavage reaction
independent
of the DNA chain growth.Sec-ondly,thefraction of RFDNAparticipatingin
ssDNA
synthesis
asrolling
circles does notchange
significantly
from early posinfection, when a very small amount ofprogeny ssDNAis
detected,
tolatepostinfection,
whenprogenyssDNAconstitutes the
bulk
of thephageDNAsynthesized.
The amountof gene5protein
alsoincreases
significantly
latepostinfection
(26); therefore, ourdata suggest that gene 5protein
stimulates the
synthesis
of viral DNA via astructuresandthuscontrolssuch
synthesis
inapositive manner
originally
postulated
byStau-denbauer and Hofschneider (30). Mazur and
Model(19) showedthat gene5
protein
controls M13 ssDNAsynthesis
in anegative
fashionby
preventing the ssDNA,
complexed
with gene 5protein, from
acting
asatemplate
for RFsyn-thesis.
We have established that the
unique origin
of M13 ssDNA
synthesis
is located about 8%from theHind
cleavage
site,
whichcorresponds
to a location inHpaII
fragment
F (34) andHaeIIIfragment G
(12),
and that the direction of itsreplicationiscounterclockwiseonthecon-ventional
genetic
map. Tabak et al.(32)
havealready established that the
origin
ofparental
RF DNAsynthesis
in vitro is also located inHpaIIfragment
F;
thisregion
isthe location of theintragenic
space between genes IV and II (12).on November 10, 2019 by guest
http://jvi.asm.org/
During the preparation of this manuscript, the establishment ofthe originof ssDNA
syn-thesis in M13phagewasreportedbytwo labo-ratories thatrelied uponthegradientofpulsed label in different regions of RF during
ssDNA synthesis (12, 31). Our results agree
re-markably well with thesereports,although our investigationwasentirelydifferent inapproach,
being based on physicalmapping of the origin andrelying on thea structure. The agreement
therefore provides positive evidence for the
in-volvement of a structures as intermediates in
ssDNA synthesis.
ACKNOWLEDGMENTS
This researchwassupported by theEnergyResearch and DevelopmentAdministration undercontractwiththe Union CarbideCorporation.A. T.Ganesanwassupportedby Public Health Service grant GM108andResearch Career Develop-mentAward 6M 50199 fromtheNationalInstituteofGeneral Medical Sciences.
Wethank Letha Oggs for her competent technical assist-anceandR.diLaurofor thegiftofHaeII restriction endonu-clease.
LITERATURE CITED
1. Bauer, W.,andJ.Vinograd.1971.Theuseof interca-lativedyesinthestudy of closed circular DNA. Prog. Subcell. Mol. Biol.2:181-215.
2. Blakesley, R. W., and R. D. Wells. 1975. Single-stranded DNA from OX174 and M13 is cleaved by certain restriction endonucleases. Nature (London) 257:421-422.
3. Cairns,J. 1963. The chromosome ofEscherichia coli. ColdSpring Harbor Symp.Quant. Biol. 28:43-46. 4. Dasgupta,S., and S. Mitra. 1976. The role of
Esche-richia coli dnaG function incoliphageM13DNA syn-thesis.Eur. J. Biochem.67:47-51.
5. Davis,R.W.,and N. Davidson. 1968.Electron micro-scopevisualization ofdeletion mutations.Proc. Natl. Acad. Sci. U.S.A.60:243-250.
6.Dressler,D. 1970. Therollingcircle forOX174replication.
II.Synthesisofsingle-stranded circles.Proc.Natl. Acad.
Sci.U.S.A.67:1934-1942.
7. Forsheit,A.P., D. S.Ray,and L.Lica.1971. Replica-tionofbacteriophageM13. V.Single-strand synthesis duringM13 infection. J.Mol. Biol.57:117-127. 8. Fujimura, R. K., and E. Volkin. 1968. Biochemical
analysis of the naturally repaired sections of bacterio-phage.T5 deoxyribonucleicacid.I.Bromodeoxyuridine incorporationintoparentaldeoxyribonucleicacid in the absence ofdeoxyribonucleicacidreplication. Biochem-istry7:3488-3498.
9. Gefter, M. L. 1975. DNAreplication. Annu. Rev. Bio-chem.44:45-78.
10. Gilbert, W.,and D.Dresser. 1968.DNA replications: the rolling circle model. Cold Spring Harbor Symp. Quant. Biol. 33:473-484.
11. Godson,G. N. 1973. Asimplemethod of preparing large
amountsofOX RFIsupercoiled DNA. Biochim. Bio-phys.Acta299:516-520.
12. Horiuchi, K.,and N. D.Zinder.1976.Origin and direc-tionofsynthesis ofbacteriophageF1 DNA. Proc. Natl. Acad.Sci.U.S.A.73:2341-2345.
13. Inman,R.B.,and M.Schnos.1970.Partialdenaturation ofthymine and 5-bromouracil-containing A DNA in alkali. J.Mol.Biol.49:93-98.
14. Inselburg,J., and M. Fuke. 1971.Isolationof catenated and replicating DNA molecules ofcolicin factor El from minicells. Proc. Natl. Acad. Sci. U.S.A. 68:2839-2842.
15. Jacobson,M.K.,and K.G. Lark. 1973.DNAreplication
inEscherichiacoli:evidencefor twoclassesof small deoxyribonucleotidechains. J. Mol. Biol. 73:371-396. 16.Kleinschmidt, A. K., D. Lang, D. Jacherts, andR. K.Zahn. 1962.Preparationandlengthmeasurements ofthetotaldeoxyribonucleicacid content ofT2 bacte-riophage. Biochim. Biophys.Acta61:857-864. 17. Lee,C. S., R. W.Davis, and N. Davidson. 1970. A
physical study by electron microscopy oftheterminally
repetitious, circularly permuted DNA from the
coli-phage particles ofEscherichia coli 15. J. Mol. Biol. 48:1-22.
18. Lyons, L. B.,and N. D. Zinder. 1972. Thegeneticmap of thefilamentousbacteriophage Fl. Virology 49:45-60. 19. Mazur, B.J., and P. Model. 1976. Regulationof coli-phage F1 single-stranded DNAsynthesisbya DNA-binding protein. J. Mol. Biol. 78:285-300.
20. Mitra, S.,andD.R.Stallions. 1976. Therole of Esche-richiacolidnaAgeneanditsintegrativesuppression in M13 coliphage DNA synthesis. Eur. J. Biochem. 67:37-45.
21. Model, P.,and N. D. Zinder. 1974. In vitrosynthesisof bacteriophageF1 proteins. J. Mol. Biol.83:231-251. 22. Okazaki, R. 1974. Short chain intermediates in DNA
replication,p. 1-32. In R. B. Wickner(ed.), Methods inmolecularbiology,vol. 7.Marcel Dekker,Inc.,New York.
23. Pratt, D., andW. S. Erdahl. 1968. Geneticcontrolof bacteriophage M13 DNA synthesis. J. Mol. Biol. 37:181-200.
24. Pratt, D.,P.Laws,and J.Griffith. 1974.Complexof bacteriophage M13 single-stranded DNA and gene 5 protein. J.Mol. Biol. 82:425-439.
25. Ray,D. S. 1969. Replicationofbacteriophage M13. II. Therole ofreplicativeforms in single-strand synthesis. J.Mol. Biol.43:631-643.
26. Ray, D. S. 1977. Replication offilamentous bacterio-phages,p. 105-178. In H.Fraenkel-Conrat and R.R. Wagner (ed.), Comprehensive virology,vol. 7.Plenum Publishing Corp.,NewYork.
27. Schekman, R., A. Weiner, and A.Kornberg. 1974. Multienzyme systems of DNA replication. Science 186:987-993.
28. Schroder,C.H.,and H.C.Kaerner.1972.Replication ofbacteriophage 4X174 replicativeformDNAin vivo. J. Mol. Biol.71:351-362.
29. Staudenbauer,W. L., and P. H.Hofschneider.1971. Membrane attachment of replicating parental DNA molecules of bacteriophage M13. Biochem. Biophys. Res.Commun. 42:1035-1041.
30. Staudenbauer,W.L., and P. H. Hofschneider. 1973. Replicationofbacteriophage M13: positive role of gene 5protein in single-stranded DNA synthesis. Eur. J. Biochem.34:569-576.
31. Suggs,S.V., and D. S. Ray. 1977.Replication of bac-teriophage M13.XI.Localization of the origin for M13 single-strand synthesis. J. Mol. Biol. 110:147-163. 32. Tabak,H.F.,J.Griffith,K.Geider,H.Schaller,and
A.Kornberg.1974.Initiationofdeoxyribonucleic acid synthesis. VII. Auniquelocation ofthe gapsinM13 replicative duplex synthesized in vitro. J. Biol.Chem. 249:3049-3054.
33. Tseng, B. Y., and D. A.Marvin. 1972. Filamentous bacterialviruses. V.Asymmetric replicationoffdduplex deoxyribonucleicacid. J. Virol. 10:371-383.
34. vandenHondel, C. A., andJ.G.G.Schoenmakers. 1975.Studiesonbacteriophage M13 DNA.I. Acleavage map of the M13genome.Eur. J. Biochem.53:547-558. 35. vanden Hondel, C. A., and J. G. Schoenmakers. 1976.Cleavage mapsofthefilamentous bacteriophages M13, fd,fl andZJ/2.J. Virol.18:1024-1039. 36. Young,I.T.,D.Levinstone,M.Eden, B.-K. Tye,and
D. Botstein. 1974.Alignmentofpartial denaturation mapsofcircularly permutedDNAby computer.J. Mol. Biol. 85:528-532.(Appendixin J. Mol. Biol.85:501-532, 1974.)

![FIG. 6.forK38/32P]DNAstopper.describedwild-typeculturewhilebeled Band sedimentation in alkaline sucrose of M13 DNA after pulse and chase](https://thumb-us.123doks.com/thumbv2/123dok_us/1538361.106405/7.500.50.235.53.215/dnastopper-describedwild-typeculturewhilebeled-band-sedimentation-alkaline-sucrose-pulse.webp)