JOURNAL OFVIROLOGY, June 1990,p. 2958-2966 Vol. 64, No.6 0022-538X/90/062958-09$02.00/0
Copyright © 1990, American Society for Microbiology
Intrachromosomal
Recombination
Mediated
by
Papovavirus
Large T
Antigens
LUC ST-ONGE, LOUISE BOUCHARD, SYLVETTE LAURENT,AND MARCEL BASTIN* Department ofMicrobiology, University ofSherbrooke, Sherbrooke, QuebecJJH5N4, Canada
Received 8December1989/Accepted 18 March 1990
Toinvestigate the mechanism by whichthelargeTantigen(T-Ag) ofpolyomavirusandsimian virus 40 can promote recombinationin mammaliancells,weanalyzed homologousrecombinationeventsoccurringbetween two defective copies of the polyomavirus middle T (pmt) oncogene lying in close proximity on the same chromosome in a rat cell line. Reconstitution of a functionalpmt geneby spontaneousrecombinationoccurred at a rateof about2 x 10-7per cellgeneration. Introduction of thepolyomavirus largeT (plt) oncogene into the cell line by DNAtransfection promoted recombination veryefficiently, with rates in therange of10-1 to 10-2percell generation.Recombination was independent of anyamplification of viral sequences andcould even be promoted by the large T-Ag from simian virus 40, which cannot activate polyomavirus DNA replication. To explain the role of large T-Ag, we proposea novel mechanismofnonconservative recombination involving slipped-strand mispairing between the twoviral repeatsfollowedby gaprepair synthesis.
Intrachromosomal recombination, that is, recombination between sister chromatids or between repeated sequences on a single chromatid, can play an important role in both gene expression and genome evolution in eucaryotes (30, 51). It is thought to involve both reciprocal and nonrecipro-cal modes ofexchange, although rigorous proof is lacking because of the inability to recover all products of recombi-nation (e.g., see Bollag and Liskay [6]). Gene conversion, one of the nonreciprocal modes of exchange, could be
responsible for maintaining sequencehomogeneity in multi-gene families and for transferring information between re-lated genes, at least when viewed on an evolutionary time scale (3, 18). It is also the process that operatesduring B-cell ontogeny to generate diversity within the chicken immuno-globulinlight-chain gene (24, 35, 49). In mammals,
recombi-nationinimmunoglobulin genes could involve another form ofexchange, such asunequal crossing over between sister chromatids (50, 52).
Despite substantial progress in understanding recombina-tion in bacteria, little is known about the process in eucary-otes, especially about cellular factors that promote
high-frequency intrachromosomal recombination. Recent studies onpapovavirusDNAreplication have suggested that one of the virally encoded proteins, the large T antigen (T-Ag), is associated with a DNA helicase activity and that the protein isresponsible for the duplex-unwinding activity that is likely to initiate replication (13, 42). Large T-Ag is known to promote phenomena such as amplification and excision of integrated viral sequences (7, 9, 27, 31), but the mechanism
leading to homologous recombination is still unclear.
Ac-cordingto the "onionskin" model (7), initiation of replica-tionat agiven proviral locus could result in multiple rounds of DNA synthesis so as to form a localized onionskin of
amplified sequences. This aberrant, polytenic structure could then represent afavorable substrate for homologous recombination, leading to excision or amplification.
Alterna-tively, large T-Ag could have a recombination-promoting
activity per se,independent of replication (8, 10, 12, 19, 33). To address this question, we have developed a system to
analyze homologous recombination events occurring
be-*Correspondingauthor.
tween two copies of the polyomavirus middle T (pmt) oncogenelying in close proximity on thesame chromosome in cultured ratcells. We reporthere that the polyomavirus large T-Ag can promote recombination between the viral sequences veryefficiently when the latter carry a functional origin of viral DNA replication. Furthermore, the simian virus 40(SV40) large T-Ag,which does not activate replica-tion at the polyomavirus origin, promotes recombinationas
efficiently as does the polyomavirus large T-Ag. We show that, contrary to the onionskin model, homologous recom-bination canoccurindependently of any amplification of the viralinsert, and we suggest that the role of large T-Ag isto melt and unwindthe DNA at the viralreplication origin, so as to create afavorable substrate forhomologous recombi-nation.
MATERIALS AND METHODS
Plasmids and mutants. pMT97.484 (see Fig. 1) encodes two defective copies of the pmt gene. The first copy was obtained by producinga 30-base-pair (bp)deletion between nucleotides (nt) 1366 and 1397 in the middle T-Ag-coding sequenceso as toinactivateitstransformationproperties (2). The second copy isaPstI-HindIIIfragment (nt 484 to1656) lacking thepolyomavirus origin of replication, the promoter, and the sequence coding for the first 104 amino acids of middle T-Ag. Bothcopies were separated by 3.5 kilobases
(kb)of pBR322 DNA(HindIII-PstIfragment,nt29 to3608). pneo-LT1 (seeFig. lb) carriesbothneoand the plt gene (8). Thisplasmidwasobtained by inserting the BamHI insert of pPyLT1, thelargeTplasmid constructed by Zhuet al.(54), intopSV2neo (41). pneo-LT97 carries bothneoandLT97,a pltmutantgenewitha30-bpdeletion (nt 1367to1396). This mutant is deficient in the initiation of viral DNA synthesis (2). pneo-SV2 carries both neoand the SV40 largeT gene. The sequences coding for large T-Ag were provided by mutantA2005, which does not produce small T-Ag. Details onthis constructhave beenpublished previously (1).
Cells and cultures. All cells were grown at 37°C in Dul-beccomodified Eagle medium supplemented with 10% fetal calfserum. ThepMT97.484 construct was transfected with
hygro (CG214 x SVHy; agift from C. Gdlinas) into mono-layers of FR3T3 cells, and colonies of hygromycin-resistant 2958
on November 10, 2019 by guest
http://jvi.asm.org/
HOMOLOGOUS RECOMBINATION BY LARGE T-Ag 2959
(a)
PstI
(b)
Pvull neo BamHI on Ssti
I
I
V
I
PI,
Sstl BamHI
I
I
pneo-LT1
FIG. 1. (a) Structure of the plasmid used in the construction of the Hy2 cell line. pMT97.484 contains two defective copies of thepmt oncogene(boxes) in the same orientation. The first copy (pmt-dll) has a 30-bp deletion (dll) around theSstIsite at nt 1373. The second copy
(pmt-d12)lacks the 5'portion of pmt up to thePstIsite at nt 484. Notethe presence of the intron inpmt-dl2. Polyomavirus sequences (thick linesandboxes) are separated by 3.5 kb of pBR322 DNA (pBR) on one side and by 3.3 kb of pAT153 DNA (pAT) on the other side. pBR and pAT sequences arein opposite orientations in the plasmid. Homologouspmtsequences are represented by black boxes. (b) Structure of theplt plasmid. pneo-LT1carriesboth theneogene and the genecoding for large T-Ag. The structure shown is inserted between theBamHI andPvuII sitesof pAT153 (not shown). The position of the deleted intron inpltis indicated by the triangle.
cells, designated Hyl, Hy2, Hy3, etc., were isolated. All of the cell lines isolated in this manner exhibited a normal
phenotypeindistinguishable from that of the parental FR3T3 cellline. After multiple passages in the absence of hygromy-cin, the cells retainedresistancetothe drug, indicating that the transgene wasstably integrated into the cell genome (37).
About half of the cell lines were able to reconstitute a
functionalpmt geneby homologous recombination following retransfection with plt. The rate of spontaneous transforma-tion was determined by the fluctuation test of Luria and Delbruck (23). Parallel clonal populations were grown to
confluence in either 6- or 15-mm Linbro microplates, i.e.,
sufficientlysmallpopulations so that no transformants would be observed in a significant proportion of cultures. The
mutation rateis given by the expression a =
(-lnPO
ln2)/(Nf-Nd)
per cellgeneration, whereP0
is the probability that nomutationwill occur and Nf andNi
arethefinal and initial numbers of cells, respectively.Tointroducelarge T into Hy2, we retransfected the cells withplasmids of the pneo series (e.g., LT1 and
pneo-SV2) and carried outneo selectionas describedpreviously
(8).
Analysis of middle and large T-Ags. The procedures for radiolabeling of cells with[35S]methionine,T-Agextraction, andimmunoprecipitation have beenpublishedin detail
pre-viously (38). The T-Ags were released from protein A-Sepharose by boiling for2min in 50
ALl
of dissociation buffer andwere purifiedfurtherbya secondimmunoprecipitation.Analysis ofintegrated sequences. The arrangement of the
plasmidsequenceswithin thecellularDNA wasanalyzed by
Southernblotting(40). The DNAwasdigested byrestriction endonucleases and fractionatedbyagarosegel
electrophore-sis. The fragments were transferred onto nitrocellulose pa-perandhybridized to 32P-labeled probes.
RESULTS
Strategyofdetecting homologousrecombination. The strat-egyweusedtoanalyze homologousrecombination involved theconstruction ofratcelllinescarryingtwomutatedcopies
of the pmt oncogene lying as direct repeats on the same chromosome. The recombination substrate, pMT97.484, is shown in Fig. la. It was designed such that homologous recombinations across some ofthe repeated sequences re-constitute a functional transforming gene and convert the cells from the normalto the transformed state. pMT97.484
was introduced into ratFR3T3 cells by cotransfection with
hygro, and colonies of hygromycin-resistant cells,
desig-nated Hyl, Hy2, Hy3, etc., were isolated and established into cell lines. All of the cell lines established in thismanner exhibitedanormal phenotypeindistinguishablefrom that of FR3T3. Three ofthem, Hy2, Hy3, and Hy5, were selected for furtheranalysis. Characterization of both Hy3 andHy5
will be presented elsewhere (L. Bouchard, unpublished data). In Hy2, the recipient cell line used in the present
study, thepmt-dll copy was interrupted between theAvaI site (nt 1016) and the AccI site (nt 1500), presumably as a result of the integration of pMT97.484 into the cellular genome. However, as shownbelow, Hy2was able toform an intactcoding region for the
polyomavirus
middle T-Ag,and the particular structure of its insert facilitated the detection ofhomologous recombination by Southern
blot-ting.
Effectofpolyomavirus large T-Ag. Toassess whetherthe
m
0
VOL. 64, 1990on November 10, 2019 by guest
http://jvi.asm.org/
2960 ST-ONGE ET AL.
TABLE 1. Activity ofrecombinant
Plasmid Coding
transfected capacity
pSV2neo neo
pneo-LT1 neo+plt
pneo-LT97 neo+plt(d197 mutant)
aColonies of G418-resistant cellswerepicked andt
Linbro microplates.The cultures wereobservedevery
changes. About half of the transformants appeare reached70%confluence (16 to 17 cellgenerations). T within the next 10days.
polyomavirus large T-Agcouldinduce rec viralinsert, we retransfected Hy2 with the neo and examined the resultingG418-resi morphological changes. The cells hadnorn the time of selection in G418. However, appeared during propagation of the col
About half of the lines establishedbytram (neoand plt) became transformed within confluence in 15-mm Linbro microplate
spontaneoustransformants appeared unde tions of transfection with pSV2neo (ne pneo-LT97, a plt mutant defective in the DNA synthesis (2). Furthermore, no
trn
observed when thelargeT construct wasit
originalFR3T3 cell line.
Toverify that the transformants expres wepicked six foci at random, subcloned th them by immunoprecipitation after lal
[35S]methionine.
The 56K middleT-Agal
allof thetransformants (Fig.2). The parer produced apolypeptide of about 53,000 d recognized by the polyomavirus anti T-remainedvisible in the Hy2transformants tide has not been detected before in F derived cell lines. It is, presumably, a t middle T-Ag produced by the resident pm
CY) LO OM C\M C\
>1 >11 >1 3
i-1 I
T-C\M C\M C\
'I- >-, >1
im
Kd
92
68
slilm.o...
Wlt-43
28
18
FIG. 2. Analysisof middle andlarge T-Ags
Cell lines of the Hy2LT series were isolate colonies by transfecting Hy2 with pneo-LT]
middle T-Ag (MT), large T-Ag (LT), and tl
indicated. Kd, Kilodaltons.
plasmids Although thesixcelllinesexpressed G418resistance, the
No. of transformed
100K
large T-Ag was barely detectable in only two of them. cultures/no. of A possible explanation is that large T could reconstitute a culturesa(%) functional pmt gene evenwhenexpressedatverylow levels0/75(0) orthat, in theabsence ofselection,the cellstended to lose 40/77 (51.9) the
ability
to express the gene. We verified that the trans-0/52(0) formants had stably integrated the transfectedplt
gene into the genomic DNA (see Fig. 3C). Furthermore, all of the transferred into15-mm representative transformants analyzed showed theproper-48 h formorphological
d before the cultures ties characteristic ofpolyomavirus transformation, such as 'he other halfappeared growth in soft agar and
production
oftumorsin animals(datanotshown).
Recombination in the viral insert. To
analyze possible
ombination in the changesin thestructureof theinsert,weisolated DNA from plt genelinkedto a number oftransformants that had presumably sustained istant colonies for recombination and examined itwith Southern blots
hybrid-nalmorphologyat ized to apmtprobe. The DNA from transformantsexhibited transformed cells major changes in restriction patterns (Fig. 3B and C). The [onies in culture. most obvious one was an amplification of the viral insert sfecting pneo-LT1 which occurred in 10 of 27 different cell lines analyzed. This 10 days following amplification was not surprising because it is well known s (Table 1). No that large T-Ag can initiate DNA synthesis at the viral origin
-rthe samecondi- evenwhen the DNA is
integrated
and when thecellsareonly
o withoutplt)
or semipermissivefor viral DNAreplication
(7, 31). Allof theinitiation of viral transformants analyzed exhibited a 2.2-kb
BamHI-HindIII
ansformation was fragment which was
produced
as a result ofhomologous
ntroduced into the recombination in the insert (see map in Fig. 4). Digestion by
;sed middleT-Ag, SstI
yielded
a742-bp fragment
which was not detected iniem, and analyzed
Hy2,
theparental
cell line(Fig.
3C).
Thisfragment
was beling cells with producedby
reconstitution of the SstI site in thepmt-dll
ppeared clearly in copy. Both 2.2-kb and742-bp fragments
wereamplified
inrital cell line, Hy2, some cell
lines,
suggesting
thatamplification
could occurltalt cellhline
H afterrecombination. Some transformants had sustainedre-serums andh
which combination without any evidence ofamplification
(data
notSuch a polypep-
shown).
However, one could argue that theamplified
se-'R3T3 or FR3T3- quences were lost following homologous recombination.
runcated form of
Effect
ofSV40
large T-Ag. To determine the role of larget-dll
copy. T-Ag in recombination, we attempted to obtain evidence ofrecombination
by using
alarge
Ttotally
defective in viral DNAreplication.
dl97, apolyomavirus large
T mutant unableto initiate viralDNAreplication,
was also unabletoJ _J promoterecombination in Hy2 (see
above).
However,sur->~
>~
prisingresultswereobtained whenHy2wastransfected with I I a plasmid encoding the large T-Ag fromSV40.
Here, selec-tion for recombinaselec-tion couldnotbeapplied
because the cellsL-T
acquired
themorphology
ofSV40transformants as soon asthey expressed T-Ag. Although the SV40 T-Ag does not activate thepolyomavirusoriginof
replication
(5),it recom-MT bined theHy2
insert soefficiently
that theproducts
of53K
_recombination could be observed in the cell
population
53K without any selection. Figure 4A shows the appearance of new restriction fragments in a transfectant (SV5) grown to ca. 108cells, i.e., ca. 27 cell
generations
after the introduc-tion oflargeT. On the basis of bandintensities,
we deter-mined that the cells that had sustainedrecombination repre-sented about 25% of the total cell population. This valuecorresponds to a recombinant rate of 1.5 x
10-2
per cellgeneration
(M.Bastin, unpublished
data). Ofeightdifferent SV40 transfectantsanalyzed,five exhibitedrecombination in the viral insert, while three did not. Similar results were intransfected cells. observed withplt
(see above), since only half of the trans-d as G418-resistant fectants yielded foci of transformed cells. The reason for this l. The positions of is unclear. It is unlikely due to a lack of T-Ag expressionhe 53K protein are because the SV40 transfectants
expressed
the transformedphenotype. We are currently
investigating
thepossibility
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.612.61.300.467.668.2]HOMOLOGOUS RECOMBINATION BY LARGE T-Ag 2961
A
H1.-`
0
B
!l!iC-~L: f{'.CLTI3r,! 4:
k
-= = _ _
,
o.
_ _ _ _
5 _ 1>;>
:
_
''3
= _ _
-__
E _ _
-rrx
! t.
___
PS
SH
PL..LI
1B PS
oriW
HSII
1-804- I-1229 t
-2430---1.9 1 3.6
1.11. -F-I.1 1
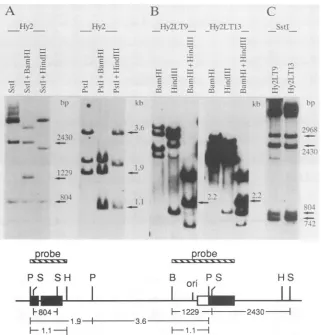
FIG. 3. Southernblotanalysis of Hy2 cells(top)andmapof theviral insert (bottom). (A)Parental Hy2 cell line. The 1,229-bp fragment
isproduced by cutting the BamHI site atthe pAT-polyomavirusjunction (nt4632) and the SstI site (nt 569) inpmt-dll. The804-bp SstI
fragmentoriginates from thepmt-d12copy.ThepresenceofaPstIfragment of 3.6 kb indicates that the pAT153sequenceis intactinHy2and
that thepBR322sequenceis interrupted. The 1.9-kb fragmentcontainspmt-d12andaportion of pAT. (B) Two of the transformants isolated
asG418-resistantcolonies by transfecting Hy2 withpneo-LT1. A similar analysis of Hy2 before transfection is shown in Fig. 4A. Note the
presenceofanamplified 2.2-kb BamHI-HindIII fragmentin both transformants. (C) Analysis of transformants bySstI.Reconstitution ofthe
SstI site(nt 1373) inpmt-dll by homologous recombination producesafragment of742bpinsteadof804bp becausepmt-dlllacks the intron
(seemapfordetails). The 2,968-bp fragmentcomesfrompneo-LT1 (nt 1373to4341)since ithybridizestoaspecificpltprobe (nt 1656to3918)
(notshown). Fragment sizesareindicated in basepairsorinkilobases. Abbreviations: B,BamHl;H, Hindlll;P,PstI, S,SstI.
thatonlyasubfraction of Hy2 cellscarrythe viral insert ina
configurationthatallows homologous recombination. To determine whether the viral insert could recombine spontaneously, we usedaprocedure baseduponthe
fluctu-ation test of Luria andDelbruck (23). Parallel clonal
popu-lations of Hy2 cells were grown in 15-mm Linbro
mi-croplatesandobserved formorphological transformation for
up to 4 weeks following confluence. Only 1 of96 cultures analyzed becametransformed, correspondingtoa
recombi-nationrate of about 2 x 10-7 percell generation.
To determine how T-Ag rearranged the viral insert, we
subcloned six differentSV40 transfectants after 30to35 cell generations and mapped the rearranged inserts in four dif-ferent subclonesfromtwocell lines aswellasinfourother
cell lines. In the parental cell line, BgIII, a noncutting enzyme,producedasinglefragmentof13 kb(Fig. 5A).After
recombination, the fragment was 19 kb. The DNA was
further analyzed with different restriction enzymes that cleaveonce(suchasBclI; Fig.5A)orseveraltimes(suchas
BamHI and HindIII; Fig. 4C) within the insert, and the structure depicted in Fig. 4 was obtained for all of the recombinantsanalyzed.Therearranged insertcontainedthe
entire viralsequencepresentinHy2withaduplicationof the
sequencebetween the tworepeats,resultinginthe addition of 5.6 kb of DNA. Astrikingfeatureof thisstructure isthat it can be obtained by unequal sister chromatid exchange. However, unequal recombinationbetweenchromatids(Fig. 6)shouldyieldtwodifferentproducts(panelsbandc), each segregating into different daughter cells. In the absence of selection fora particular product, onewould expect to find
aninsertwith adeletion foreveryinsert withaduplication.
The insert with a duplication was detected in Fig. 4A.
However, fragments characteristic ofarecombinant with a
deletionwere neverdetected inouranalyses (seethelegend
to Fig. 6 fordetails). We conclude, therefore, that reconsti-tution of thepmtgene in the Hy2cell line did not occurby a simple mechanism such as unequal sister chromatid
ex-change.
Propertiesofreplication-defective mutants. In view of the roleoflarge T-Aginviral DNAreplication,itwasofinterest to examine the effect of replication-defective mutants on
recombination. To thisend, Hy2cellswere transfected with fourSV40mutantsthat havesingle-amino-acidsubstitutions resulting in altered specific DNA binding, ATPase, and/or
C
* . ,
I
Im}4
probe
mn I I I i -~~~~~~~I
VOL. 64, 1990
I
.J.
*Moil
am
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.612.151.472.74.409.2]2962 ST-ONGE ET AL.
Ar
TI
n= 11 1
be 0
B
-- sitIl--I -7 to
S%R SV4 SV4
C
kb
8 1]I--- 81- 11 8r 1 --11
i3
I---]1 T3W}I I 3) 1 1311 1 i 131 1':
*b* %*56 eIhm.
~~ ~' %#h b
Hy2
HB H
B
HB
I1...
,'I.
I
.
I
} --- --- 4.9 - 7.4
W- 2.0 ---+--- 6.7
--1.6 --I 3.5
SVI
-SV7-SV3
HB
HI
ru8 I
4.9
F- 2.0 5.5
F-1.6 -1
ixw x !
B
Iori
L
I
H
B
HB
742l
orisL
5.6
--
7.4 - It
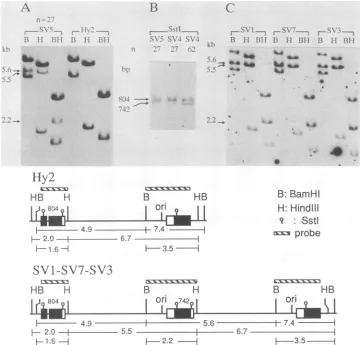
6t7 iFIG. 4. Southern blot analysis ofSV40 transfectants. Cell lines designated SV1, SV2, SV3,etc.,wereisolatedasG418-resistantcolonies
by transfecting Hy2 with pneo-SV2,aplasmid carrying bothneoand theSV40 large Tgene(1). (A)The DNA designatedSV5wasextracted fromapopulation ofca. 108 cells, i.e.,ca. 27cellgenerations after the introduction of large T into Hy2 (cell lineSV5). Note theappearance
offragments of 5.6, 5.5, and 2.2 kb whichwerenotdetected in Hy2. (B) Analysis bySstI. The 742-bp fragment is barelydetectable after 27 cell generations. However, after 62 cell generations, the 742- and 804-bp fragments are present in virtually equal amounts. (C) SV40 transfectantswereanalyzed after subcloning. Hy2,Structure of the insert inHy2before recombination.SV1-SV7-SV3, Structure of the insert afterrecombination. The duplication comprisesarepeataswellasthesequencebetween thetwo repeats.Inadditiontothefragments shown, thelocations of various sites, such asPstI, SstI,andAccI, wereconfirmedonthemaps(notshown).
unwinding activities of largeT-Ag (25, 26,43). The
proper-ties ofthe various mutants are listed in Table 2. Since the
mutants were transformation competent, about half of the G418-resistant colonies hada transformed morphology and
thus provided a source of Hy2 clones expressing mutant
T-Ags. DNA was extracted from a total of41 such clones
grown to 25 to 26 cell generations and was subjected to Southern blot analyses similar to those shown in Fig. 4. However, none of themutants wasableto promote
recom-bination in the viral insert (Table 2). This result indicated that, as with polyomavirus large T-Ag, recombination
re-quired an SV40 T-Ag functional in viralDNA replication.
Requirement for a functional origin of replication. To
determine whetherrecombination in the Hy2 insert required the polyomavirus origin of replication, we constructed a
plasmid similartopMT97.484 butwithanonfunctional origin
ofreplication. The replication defect in the construct was
introducedby the deletionof 23 bp in the origincoreand the
addition ofanXhoI linkerat nt 37 to 60 (4). Although this mutation inactivated the replication origin, it did not affect expression from the polyomavirusearly region. Six indepen-dent cell lines carrying one copy or several copies ofthe
construct were established by cotransfection with hygro. However, in no case did retransfection with polyomavirus
largeTandneoleadtothereconstitution ofafunctionalpmt
geneby homologousrecombination(datanotshown).These resultsindicate that the large T-Agcanpromote recombina-tion between the viral sequences only in thepresence ofa
functional originofreplication.
DISCUSSION
Homologous recombination promoted by large T-Ag. We havedevisedasystem to analyze intrachromosomal recom-bination between two copies of the pmtoncogene lying in
close proximity on the same chromosome in cultured rat cells. The advantageofrecombiningpmtsequences instead
ofneo(36, 48) or tk(22) is that oncogenically transformed
cells are readily detected in culture and can be isolated
without selectivekilling by cytotoxicdrugs. Thissystemhas enabledustoanalyzethestateoftheviral insert in the whole population oftransfected cells and to detect recombination events in the absence of any selection. Recombination
promoted by large T-Agoccursatveryhigh frequencies.The
0.0
Oka 6hw
'k:*
* I*
%.46 *lW
wz'o
-i:A 4'A
.4:
B: BamHI
H:
Hindill
:
Sstl
probe
II |
J. VIROL.
1
F
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.612.126.486.70.415.2]HOMOLOGOUS RECOMBINATION BY LARGE T-Ag 2963
(a)
~~~~I 1
A
i 1;
I11- isI
I3-11
K.%v0
(b)
JML
B
(c)
13(1-I Bcll 13cil Buill
L1~J
-1(-ilI
L-LII)iCltion
13c11 BclI
I ,
1.
I~
-FIG. 6. Diagram showing how unequal sister chromatid
ex-change (a)producesachromatid withadeletion (b) andachromatid
withaduplication(c).The small vertical barsrepresentHindlllsites
inthe cellular DNA.Chromatid b should yieldaHindlIl fragmentof
3.2kb.
Bcll Bglll
.0--- 4
FIG. 5. (A)Analysis of SV40 transfectants by BglII(anoncutting enzyme) and BclI (a single-cut enzyme). The cell lineswere
sub-clones after 30cell generations. (B) Arrangement of the viral insert inHy2 asdeduced from panel A. Top, Hy2 before recombination.
Bottom, Hy2 after recombination. Black boxes represent thepmt
repeats.Thesingle BcIl site is locatedat nt5051onthe polyomavi-rusmap.
appearance ofnewrestriction fragments in the experiment
shown inFig. 4A iscompatible witharecombinationrateof 1.5 x 10-2percell generation. Considering that the rate of spontaneousrecombination intheparental cell line is about 2 x
10',
recombination is promoted by large T-Ag byseveralorders ofmagnitude. Another study has shown that the transfer ofplt into ratfibroblasts induces anincreased
frequency of homologous recombination events and the
appearanceof abnormalkaryotypes(19). Thesame phenom-enonhas beenobserved withc-mycandfunctionally related oncogenes, and it has been suggested that immortalizing
oncogenes may contribute to the transforming process by
increasing genomic instability (10).
Theeffect ofSV40 onthepolyomavirus insertwas
unex-pected. Although the large T-Agsfrom both polyomavirus and SV40 recognize and bind to the same DNA sequence
motif in vitro(34, 39), theycannotfunctionallysubstitute for
oneanothertopromoteviralDNAreplication (5). Itislikely thatpolyomavirusDNAreplication requires species-specific permissive factors suchasDNApolymerase a-primasefrom
murine cells or proteins associated with this complex (28, 29). Interestingly, a recent studyhas shown that polyoma-virusDNAreplicationinmonkey cellsisnotunconditionally
limited. DNAs containing the polyomavirus origin replicate
efficiently in monkeycells which constitutively express the SV40large T-Ag (46).
Our results are incontradiction with several reports claim-ing that recombination within transfected plasmid DNAs does not depend on T-Ag expression (16) or that neither DNAreplication of the recombination substrate nor T-Ag is essential for recombination (44). The reasons for such dis-crepancies are not understood at present. They may arise from differences in the cell lines used or in the nature of the
experimental designs, such as recombination within trans-fected plasmids versus homologous sequences stably inte-grated into the host genome.
Theadvantage ofusing a heterologous system is that the SV40 large T-Ag does not amplify the sequences at the
polyomavirus origin of replication and the recombination products can be identified easily. All ofthe SV40 transfec-tants analyzed exhibited the same recombination product, i.e., a duplication of the repeat as well as the sequence between the tworepeats.Such aduplicationcanbeexpected
[image:6.612.79.280.80.419.2]from anevent ofunequal sister chromatid exchange. How-ever, unequal recombination between chromatids should
TABLE 2. Properties of SV40mutants
Mutanta Amino acid Origin
Recom-change binding binationb
pneo-SV2 None + + 5/8
pneo-C6-2 Asn-153- Thr - + 0/4
pneo-C8A Lys-224- Glu + + 0/11
pneo-CllA Pro-522 Ser + - 0/16
pneo-T22 His-203- Gln - + 0/10
aGenomicDNAwascleavedbyBamHIand inserted in the BamHIsiteof pSV2neo. pneo-SV2 carriesneoand thewild-typeSV40largeT gene.
bColoniesofG418-resistant cellswerepickedand grownto25to26cell generations.The DNAwasextractedandanalyzed bySouthernblottingas
described in thelegendtoFig.4.
VOL.64, 1990
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.612.353.523.83.307.2] [image:6.612.318.558.615.689.2]2964 ST-ONGE ET AL.
(a)
(d)
on_
(b)
L
ori
LargeT-Ag melts andunwinds the DNAatthereplication origin
(c) break 5
-7 7 break
(e)
Single-stranded loops are produced
by slipped-strand mispairing
[image:7.612.66.545.65.215.2]repair synthesis
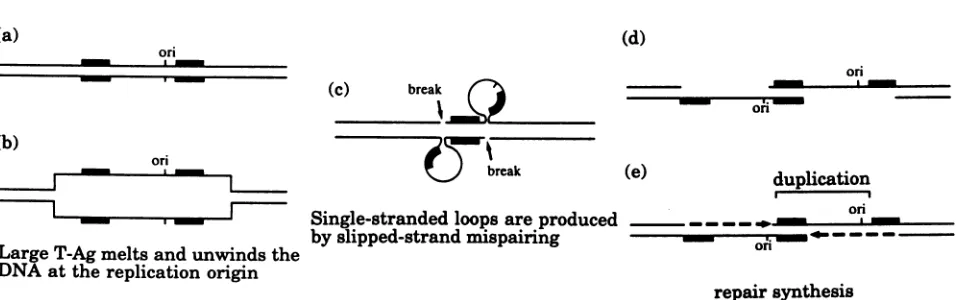
FIG. 7. Model explaining the role of large T-Ag in recombination. (a) AlthoughtheSV40large T-Agcannotinitiatepolyomavirus DNA replication, itrecognizessomeelements of thepolyomavirus replication origin. (b) Large T-Agmelts andunwinds thedouble-strandedDNA
attheorigin, allowing therepeats topair with each other byslipped-strand mispairing. (c)Twosingle-stranded loopsareproduced. (d)Breaks
occuronopposite strands, resulting in largegapsofsingle-stranded DNA. (e) Repairsynthesis producesthe structurewithaduplication. yield two different products, one with a duplication and
another with a deletion, each segregating into different
daughter cells. Since the productwith adeletion wasnever
detected in our analyses, we conclude that recombination
didnot occurby unequalsister chromatidexchange.
Recombination mechanism. Several studies havereported that intramolecular recombination during transfection in mammalian cells is anonconservativeprocess. When
mole-cules bearingdirect repeatsundergo intramolecular interac-tions intended to yield reciprocal exchange, the process
generates only one of the two expected products (11). Similar conclusions were reached by Lin et al. (21), who proposedamodel by which the removal of unpairedDNAat thejunction between the paired and unpaired regions
per-mitsagaprepairprocesstoreconstructan intactgene. Our
dataareinagreementwith such anonconservative mode of
homologous recombination. To account for the observed effect ofSV40 large T-Agonthe recombination of the viral
insert inHy2, wepropose themodel shown in Fig. 7. How the SV40 protein interacts with the polyomavirus origin of replication is not clear. In vitro studies have shown that SV40 T-Ag binds to multiple discrete regions on the
poly-omavirusorigin (34, 39). It doesnotunwindDNA containing thepolyomavirus originaswellasit unwindsitsownDNA.
However, it does exhibitpartial specificity for the sequence
atthepolyomavirus origin (E. WangandC. Prives, personal communication). Whethertheinteractionismore specificin vivo remains to be seen. We propose that the function of
T-Agistodestabilize thedouble-stranded DNA attheviral replication origin so as to create a favorable substrate for
recombination. Once the two strands are separated, the
repeats are free to pair with each other by slipped-strand mispairing. Such an event would produce two single-strandedloops, eachcontainingoneof therepeatsaswellas
thesequenceslying between thetwo repeats.Slipped-strand mispairing has already been invoked as a mechanism that can generate small deletions or duplications in the genome
(20). It can explain the generation of deletions in the lacI
geneofEscherichia coli(15) andinthe,B-globingene(14). A deletion isproducedwhen the single-stranded loop is
recog-nized by DNA repair enzymes which excise the loop and rejointheends of the brokenDNAstrand. However,breaks occurringontheopposite strands would produceastructure
with largegaps corresponding to the single-stranded loops. Filling of the gaps by repair synthesis would generate the structure observed in the cell lines after recombination.
Unequal sister chromatid exchange has been directly documented inyeast(32, 45)andDrosophilaspecies (47).In mammals, it has been implicatedin the evolution of
multi-genefamilies(3) and in the rearrangement of immunoglobu-lin genes (51, 52). However, cells carrying the putative reciprocal products cannot always be isolated (e.g., see
Tilley and Birshtein [50] and Wabl et al. [53]). In one
instance, it hasbeen shown that the switch rearrangement in the mouse heavy-chain locus occurs by a mechanism of looping out and deletion, thus excluding the model of
un-equal sisterchromatid exchange (17). Whether the mecha-nism proposed here for the large T-Ag applies to other recombinationaleventsinthecellulargenomeremainstobe investigated.
Spontaneousrecombination occursin theHy2 insertata
rate ofabout 2 x 10-7percellgeneration. Other
investiga-torshavedevised similarconstructsthatrecombineatarate
onthe order of 10-6percellgeneration (22, 36).It isunclear why some of these constructs, which include a functional
originofSV40DNAreplication, donotrecombineathigher frequencies when they are introduced into COS cells that
expresstheT-Agandwhythetypeofrecombinationproduct described here has neverbeenobserved in thesecell lines. Experimentsarepresentlyunderwaytodetermine whether the SV40 origin can substitute for that ofpolyomavirus in pMT97.484. However, itseemsthat thestructureor
config-uration of the insert is very critical in determining the mechanismbywhichhomologoussequenceswill recombine inagivencell line. Since theinceptionof thiswork,wehave constructed and characterized five different cell lines
carry-ingtandemrepeats ofpolyomavirus DNA. They all
recom-bine under theaction ofpolyomavirus large T-Ag, although
notnecessarilyatthesamerateandbythesamemechanism.
In oneofthem, the insertsustains deletions either sponta-neously or in response to large T-Ag (8). In another line, whichdiffersfromHy2 by the position of thepolyomavirus origin with respect to the two repeats, the rearrangements involve both reciprocal (inversion) and nonreciprocal (gene conversion)recombinationalevents(Bouchardetal., unpub-lished data). The most striking feature of this line is that recombination can be promoted by SV40
replication-defi-cientmutants.Itseems,therefore,that differentfunctions of large T-Agmaybeinvolved inrecombination indifferentcell lines.
RoleoflargeT-Ag.Themodelwepropose mayshedsome
light on the role of large T-Ag in phenomena such as
ori
on
duplication
I I
on
m w
o.
On
J. VIROL.
I
on November 10, 2019 by guest
http://jvi.asm.org/
HOMOLOGOUS RECOMBINATION BY LARGE T-Ag 2965 amplificationand excision of integrated viral sequences from
chromosomes. Cells transformed by polyomavirus andSV40 can undergo a high rate of amplification or excision of the integrated genome, and both phenomena require a functional large T-Ag as well as the presence of homology within integrated sequences such as complete or partial tandem insertions of viral DNA (7, 27). Several mechanisms leading to excision or amplification have been proposed. Currently, the prevailing model is that proposed by Botchan et al. (7). Upon initiation of replication at a given proviral locus, multiple rounds of DNA synthesis occur to form a localized onionskin of amplified sequences. This aberrant, polytenic structure could represent a favorable substrate for homolo-gous recombination, leading to excision or amplification. In agreement with these studies, we find that recombination in Hy2 requires a large T-Ag functional in the activation of viral DNA synthesis as well as a functional origin. However, in contrast to the onionskin model, recombination can occur in our cell lines without any detectable amplification of se-quences. The relationship between recombination in Hy2 and the replicative function of large T-Ag is of special interest. If the function of T-Ag is simply to melt and unwind the DNA at the replication origin, one would expect repli-cation-deficient mutants with helicase activity (such as C6-2, C8A, and T22) to promote recombination. Our data show that they do not. Large T could also be implicated, by its replicative function, in the repair synthesis leading to the duplication of the viral insert (Fig. 7e). If repair is achieved through the interaction between T-Ag and polymerasea,it is not clear why the SV40 T-Ag is inactive in extrachromo-somal polyomavirus DNA replication and why it fails to amplify the viral insert as does its polyomavirus counterpart. An interesting possibility is that a functional T-Ag is required for the repair of large gaps and that its role is to activate polymerasea or other enzymes involved in repair synthesis. To the best of our knowledge, this possibility has not yet been investigated.
ACKNOWLEDGMENTS
We thank J. Toutant and C. Bergeron for excellent technical assistance, Y. Gluzman for SV40 mutants, C. Gdlinasfor the hygro construct, J. Hassell for the polyomavirus replication origin-nega-tive mutant, B. Schaffhausen for the gift of polyomavirus anti-T serum, and P. Bourgaux, D. Gibson, and C. Prives for fruitful discussions.
This work was supported by the Medical Research Council of Canada and by the National CancerInstitute of Canada. L.S. and S.L. were supported by the Cancer Research Society and by the Fonds de la Recherche enSantdduQudbec, respectively.
LITERATURE CITED
1. Asselin, C., and M. Bastin. 1985. Sequences from polyomavirus and simian virus 40 large T genes capable of immortalizing primary rat embryo fibroblasts. J. Virol. 56:958-968.
2. Asselin, C., J. Vass-Marengo, and M. Bastin. 1986. Mutation in the polyomavirus genome that activates the properties of large T associated with neoplastic transformation. J. Virol. 57:165-172. 3. Baltimore, D. 1981. Gene conversion: some implications for
immunoglobulin genes. Cell 24:592-594.
4. Bautch, V. L., S. Toda, J. A. Hassell, and D. Hanahan. 1987.
Endothelial cell tumors develop in transgenic mice carrying polyoma virus middle T oncogene. Cell 51:529-538.
5. Bennett, E. R., M. Naujokas, and J. A. Hassell. 1989. Require-ments for species-specific papovavirus DNA replication. J. Virol. 63:5371-5385.
6. Bollag, R. J., and R. M. Liskay. 1988. Conservative intrachro-mosomal recombination between inverted repeats in mouse cells: association between reciprocal exchange and gene
con-version. Genetics119:161-169.
7. Botchan, M., W.Topp,and J.Sambrook.1979.StudiesonSV40 excision from cellular chromosomes. Cold Spring Harbor Symp. Quant. Biol.43:709-719.
8. Bouchard, L., F. Mathieu,andM.Bastin.1987.Polyomalarge T canactivate middle T expression by ahit-and-runmechanism. Oncogene 2:379-386.
9. Bourgaux, P., B. S.Sylla,and P.Chartrand. 1982. Excision of polyoma virus DNA from that of atransformed mouse cell: identification of a hybrid molecule with direct and inverted repeat sequences attheviral-cellular joints.Virology 122:84-97. 10. Cerni, C., E. Mougneau, M.Zerlin,M.Julius,K. B.Marcu,and F. Cuzin. 1986. c-myc and functionally related oncogenes in-duce bothhigh rates of sisterchromatidexchangeand abnormal karyotypes in rat fibroblasts. Curr. Top. Microbiol. Immunol. 132:193-201.
11. Chakrabarti, S., and M. M. Seidman. 1986. Intramolecular recombinationbetweentransfected repeated sequencesin mam-malian cellsis nonconservative. Mol. Cell. Biol. 6:2520-2526. 12. Colantuoni,V., L.Dailey, G.DellaValle,and C. Basilico. 1982.
Requirements forexcision andamplification ofintegrated viral DNA molecules in polyomavirus-transformed cells. J. Virol. 43:617-628.
13. Dean, F. B., P. Bullock, Y. Murakami, C. R. Wobbe, L.
Weissbach, and J. Hurwitz. 1987. Simian virus40(SV40) DNA replication: SV40 large T antigen unwinds DNA containingthe SV40 origin of replication. Proc. Natl. Acad. Sci. USA 84: 16-20.
14. Efstratiadis, A., J. W. Posakony, T.Maniatis, R. M. Lawn, C. O'Connell, R. A. Spritz, J. K. DeRiel, B. G. Forget, S. M. Weissman, J. L. Slightom, A. E. Blechl, 0. Smithies, F. E.
Baralle, C. C. Shoulders, and N. J. Proudfoot. 1980. The structureandevolution of thehuman
P-globin
genefamily.Cell 21:653-668.15. Farabaugh, P. J., U. Schmeissner, M. Hofer, andJ. H. Miller. 1978. Genetic studies of the lac repressor. VII. On the molec-ularnature ofspontaneous hotspots in the laclgene of Esche-richia coli. J. Mol. Biol. 126:847-863.
16. Finn, G. K.,B.W.Kurz, R. Z.Cheng,and R.J. Shmookler Reis. 1986.Homologous plasmidrecombination is elevated in immor-tallytransformed cells. Mol. Cell. Biol. 9:4009-4017.
17. Jack, H.-M., M. McDowell, C. M. Steinberg, and M. Wabl. 1988.Looping out and deletion mechanism for the immunoglob-ulin heavy-chain class switch. Proc. Nati. Acad. Sci. USA 85:1581-1585.
18. Klein, H. L., and T. D. Petes. 1981. Intrachromosomal gene conversion in yeast. Nature(London) 289:144-148.
19. Leopold, P., E. Mougneau,J. Vailly, C.Cerni,M. Rassoulzade-gan, and F. Cuzin. 1986. Genetic instabilities at the chromo-somal and the molecular levels induced bytheplt oncogeneof polyomavirus. Ann. Clin. Res. 18:304-306.
20. Levinson, G., and G. A. Gutman. 1987. Slipped-strand mispair-ing: a major mechanism for DNA sequence evolution. Mol. Biol. Evol. 4:203-221.
21. Lin, F.-L., K. Sperle, and N. Sternberg. 1984. Model for homologous recombination during transfer ofDNA intomouse
Lcells: role for DNA ends in therecombination process. Mol. Cell. Biol. 4:1020-1034.
22. Liskay, R. M., and J. L. Stachelek. 1983. Evidence for intra-chromosomal gene conversion in cultured mouse cells. Cell 35:157-165.
23. Luria,S. E.,and M. Delbruck. 1943. Mutations ofbacteriafrom virus sensitivity to virusresistance. Genetics 28:491-511. 24. Maizels, N. 1987. Diversity achieved by diverse mechanisms:
gene conversion in developing B cells of the chicken. Cell 48:359-360.
25. Manos, M. M., and Y. Gluzman. 1984. Simian virus 40 large
T-antigen point mutants that are defective in viral DNA
repli-cation but competent in oncogenic transformation. Mol. Cell. Biol. 4:1125-1133.
26. Manos,M. M., and Y. Gluzman. 1985. Genetic and biochemical analysis of transformation-competent, replication-defective simianvirus 40 large Tantigen mutants. J. Virol. 53:120-127.
VOL. 64, 1990
on November 10, 2019 by guest
http://jvi.asm.org/
2966 ST-ONGE ET AL.
27. Miller, J., P. Bullock, and M. Botchan. 1984. Simian virus40 T antigen isrequiredforviral excision fromchromosomes. Proc. Natl. Acad. Sci. USA 81:7534-7538.
28. Murakami, Y., T. Eki, M.-A. Yamada, C. Prives, and J. Hurwitz. 1986. Species-specific in vitrosynthesis ofDNA con-taining the polyoma virus origin of replication. Proc. Natl. Acad. Sci. USA83:6347-6351.
29. Murakami, Y., C. R. Wobbe, L. Weissbach, F. B. Dean, and J. Hurwitz. 1986. Role of DNA polymeraseaxand DNAprimasein simian virus40 DNAreplicationin vitro. Proc.Natl. Acad. Sci. USA 83:2869-2873.
30. Nasmyth, K. A. 1982.Moleculargenetics ofyeastmatingtype. Annu. Rev. Genet. 16:439-500.
31. Pellegrini, S., L. Dailey, and C. Basilico. 1984.Amplificationand excision ofintegratedpolyomaDNAsequencesrequirea func-tional originofreplication.Cell36:943-949.
32. Petes, T. D. 1980. Unequal meiotic recombination within tan-dem arraysofyeast ribosomal DNA genes. Cell 19:765-774. 33. Piche, A., and P. Bourgaux. 1987. Resolution of a
polyomavirus-mousehybrid replicon: viral function required for recombina-tion. J. Virol. 61:845-850.
34. Pomerantz, B. J., and J. A. Hassell. 1984. Polyomavirus and simian virus 40 large T antigens bind to common DNA se-quences. J. Virol. 49:925-937.
35. Reynaud, C.-A., V. Anquez, H. Grimal, and J.-C. Weill. 1987. A hyperconversion mechanism generates the chicken lightchain preimmunerepertoire. Cell48:379-388.
36. Rubnitz, J., and S. Subramani. 1986. Extrachromosomal and chromosomal geneconversion inmammalian cells. Mol. Cell. Biol. 6:1608-1614.
37. Scangos,G. A., K. M.Huttner, D. K. Juricek, and F. H. Ruddle. 1981.Deoxyribonucleic acid-mediatedgene transfer in mamma-lian cells:molecular analysis of unstable transformants and their progression to stability. Mol. Cell. Biol. 1:111-120.
38. Schafibausen, B., and T. L. Benjamin. 1981. Comparison of phosphorylationof two polyomavirus middle T antigens in vivo and invitro. J. Virol.40:184-196.
39. Scheller,A., and C. Prives. 1985. Simianvirus 40 and polyoma-virus large tumor antigens have different requirements for high-affinity sequence-specific DNAbinding. J. Virol. 54:532-545.
40. Southern, E. M. 1975. Detectionofspecific sequences among DNAfragments separatedbygelelectrophoresis. J.Mol. Biol. 98:503-517.
41. Southern,P.J.,and P.Berg. 1982.Transformationof
mamma-lian cells to antibiotic resistance with a bacterial gene under control ofthe SV40early regionpromoter.J. Mol.Appi.Genet. 1:327-341.
42. Stahl, H., P. Droge, and R. Knippers. 1986. DNA helicase activityofSV40largetumorantigen. EMBO J.5:1939-1944. 43. Stillman, B., R. D. Gerard, R. A. Guggenheimer, and Y.
Gluzman. 1985. Tantigenandtemplate requirementsforSV40 DNAreplicationin vitro.EMBO J. 4:2933-2939.
44. Subramani, S.,andP.Berg. 1983.Homologousand nonhomol-ogous recombination in monkey cells. Mol. Cell. Biol. 3: 1040-1052.
45. Szostak, J. W.,andR. Wu.1980.Unequal crossingoverin the ribosomalDNA ofSaccharomycescerevisiae.Nature(London) 284:426-430.
46. Tang,W.-J.,and W. R.Folk. 1989. Constitutiveexpressionof simian virus 40largeTantigeninmonkey cells activates their capacity to support polyomavirus replication. J. Virol. 63: 5478-5482.
47. Tartof,K.D. 1974. Unequalmitotic sister chromatidexchange asthe mechanism of ribosomal RNA gene magnification. Proc. Natl. Acad. Sci. USA 71:1272-1276.
48. Thomas,K.R., K.R. Folger,andM. R.Capecchi. 1986. High frequency targettingof genestospecificsites in the mammalian genome.Cell44:419-428.
49. Thomson,C.B.,and P. E. Neiman.1987.Somaticdiversification ofthe chickenimmunoglobulin lightchain gene is limitedtothe rearrangedvariable gene segment. Cell48:369-378.
50. Tilley, S. A., and B. K. Birshtein. 1985. Unequal sister chroma-tidexchange.A mechanismaffecting Iggene arrangementand expression.J. Exp.Med. 162:675-694.
51. Tonegawa, S. 1983. Somatic generation ofantibody diversity. Nature(London) 302:575-581.
52. Van Ness, B. G., C. Coleclough, R. P. Perry, and M. Weigert. 1982. DNA between variable and joining gene segments of immunoglobulin K light chain isfrequently retained in cells that rearrangethe K locus. Proc. Natl. Acad. Sci. USA 79:262-266. 53. Wabl, M., J. Meyer, G. Beck-Engeser, M. Tenkhoff, and P. D. Burrows. 1985. Critical test of a sister chromatid exchange model for theimmunoglobulin heavy-chain class switch. Nature (London) 313:687-689.
54. Zhu, Z., G. M. Veldman, A. Cowie, A. Carr, B.Schaffhausen, andR. Kamen. 1984. Construction and functional characteriza-tion ofpolyomavirusgenomes that separately encode the three earlyproteins. J. Virol. 51:170-180.
J. VIROL.