JOURNAL OF VIROLOGY, Dec. 1967,p.1117-1121 Copyright ( 1967 American Society for Microbiology
Replication of the Reticuloendotheliosis
Virus
(Strain T)
in
Chicken
Embryo Cell Culture
HENRY R. BOSE, JR., AND ALVIN S. LEVINE
DepartmentofMicrobiology, Indiana University MedicalSchool,Indianapolis,Indiana 46207
Received forpublication21August1967
Investigations wereconducted on the in vitro replication of the
reticuloendo-theliosis (RE) virus (strain T) in
specific-pathogen-free
chicken embryo fibroblast(CEF) cultures. Active virus productionwasdetectedin the tissue culture fluid24 hr
after infection. Wheninjected intochickens, samples taken 42 hr after infection of
the cell cultures killed approximately 50% of the birdsata1:100dilution. TheRE
virus titerremained at this levelfor5days before declining. Cell-free virus
prepara-tions from tissue culturesrarely resultedin 100%mortality oftheassaybirds. The
level of cell-associated virus was very low. Evidence that the
reticuloendotheliosis
wasnotinducedbyamycoplasmawasindicatedby failuretoisolateanorganismon
PPLOAgar (Difco) and failure ofkanamycin oramphotericin B toinhibit
multi-plication of RE virus in vitro. RE virus appearedtobe unrelatedtomembers ofthe
avian leukosis andsarcoma complex. It did notinduceresistance in CEF cultures
tosarcomaviruses of the A or Bsubgroup ofthiscomplex. Similarly, preinfection
of cell cultureswith leukosisvirusesofthe AorBsubgroupdidnotinhibitorreduce
thereplication ofRE virus.
The reticuloendotheiosis (RE) virus (strain
T) was isolated by Twiehaus and Robinson in
1958 from an adult turkey with leukosis-like
lesions(unpublished).The viruswassubsequently
adapted to young chickens (7) and later to
Japanese quail (9).Thisvirus differs significantly
fromother avian leukosisviruses in its decreased
length of incubation period, high mortality
pattern, and ability to infect several different
geneticlines ofchickens.
Although the virus replicates in chicken
embryofibroblast (CEF) cultures,noevidence of
a cytopathic alteration was observed (8). The
virus grown in quail embryo fibroblastsdid not
interfere with the Bryan strain (9) of Rous
sar-comavirus(RSV). Complementfixationtestsfor
avianleukosis viruses were negativewhen tissue
culturecellsservedasthesourceofantigen (10).
The results were variable when diseased tissue
was employed. Serum neutralization studies
indicatedthat RE viruswasnotrelated to several
commonleukosisviruses (10). However,antisera
prepared against the Bryan strain ofRSV
neu-tralizedRE virus. Electronmicroscopic
observa-tionsrevealedthat the virus sharedmorphological
characteristics with both avian leukosis and
murineleukemia viruses(12).
This reportdescribesthe in vitroreplicationof
RE virus in CEF cultures. The relationship of
the RE virus to members of the avian tumor
virus group was investigated by using
interfer-ence tests against viruses of the A and B sub-groupsofthiscomplex.
MATERIALS AND METODS
Viruses. The original preparation of RE virus (strain T) was obtained from E. K. Russell of the
National Cancer Institute through the courtesy of G. Theilen of theUniversity of California at Davis. This preparation consisted ofapool of chicken liver andspleen tissue fromaninfected bird.The material
was subsequently passed in specific-pathogen-free
strain 813, white Leghorn chickens (Kimber Farms, Niles,Calif.) and the plasma wascollected when the
birds became moribund. The RAV-1, RAV-2, RSV (RAV-1), and RSV(RAV-2) preparations were
kindly supplied by Peter Vogt, University of Colorado Medical Center, Denver.
Bioassay of infectivity. For assay, 1- to 3-day-old chickens were inoculated with 10-fold dilutions of tissueculture fluid. Each bird received 0.25 ml intra-abdominally. The birds used for assay were white Leghorn cockerelsstrain60F, 60X, and55Xobtained from the Indiana Farn BureauCooperative, Indian-apolis. These strainshadbeen foundequally sensitive to cell-free preparations of RE virus. Uninoculated birds were caged with the inoculated birds for the duration of theexperiment. Mortality occurring 7 days after inoculation was considered specific, based on
postmortem examination.
Infectious cycle tissue culture studies. Chicken embryo fibroblast cultureswerepreparedfrom
11-day-oldembryonated, strain 813, whiteLeghornchicken
1117
Vol. 1, No. 6 Printed in U.S.A.
on November 11, 2019 by guest
http://jvi.asm.org/
BOSE AND LEVINE TABLE 1. Replication ofreticuloenidotheliosis virus
[image:2.471.51.239.92.456.2] [image:2.471.255.444.385.611.2](strain T) inchickeni embryofibroblasts Extracellular virus Time after infection Inoculumb| 18 hr 24 hr 30hr 36 hr 42hr 5 days 6days 7 days Controlc
Virus iN dead dilution - o ed
log 0 -1 -2 0 0 -1 -2 0 -1 -2 0 -1 -2 0 -1 -2 -3 -1 -2 -3 -4
-1
-2 -3 -4 -1 -2 -3 -4 total 6/10 2/10 0/10 0/10 4/10 0/10 0/10 5/10 1/10 0/10 2/10 2/10 1/10 6/10 4/10 4/10 0/10 6/10 5/10 0/10 0/10 4/10 1/10 0/10 0/101/10
0/10 0/10 0/10 0/20 Mlortalityii ,C
60 20 0 0 40 0 0 50 10 0 20 20 10 60 '40 40 0 60 50 0 0 40 10 0 0 10 0 0 0 0
O
Average time to death days 18 25 42 34 48 25 27 43 18 25 40 23 47 27 32 35aVirus was assayed by injection of 0.25 ml of tissue culture supernatant fluidintra-abdominally
into 60FwhiteLeghorncockerels. Theexperiment was terminated 4 weeks after the last specific
death.
bFirst passage infectious tissue culture fluid
(0.5 ml/plate).
cUninoculated birds were caged with the test birds as contact controls for the duration of the
experiment.
eggs. Thecellsweregrown inDifco199medium with 8% heat-inactivated newborn calf serum (Hyland) and 11% Tryptose Phosphate Broth. The cultures wereincubatedat38CinaCO2 incubator(2% C02).
Secondaryculturescontaining 2.6 X 106 cells were
plated in 60-mm plastic petri dishes. The virus was added to thecells insuspension. The virus inoculum (0.5mlperplate) was undiluted second-passage tissue culture fluid or plasma collected from a moribund bird. The cellswereincubatedovernight andsamples
werecollected for3 days at 6-hrintervals, beginning
at 18 hr after infection. Samples werethencollected daily for 7 days. The culture fluidwas rendered cell-free bycentrifugation at2,000rev/minfor 20 minat 4 C. The cells were harvested with trypsin, were counted, andsuspended togiveafinal concentration
of 2.0 X 106 cells persample.Thematerialwasstored at -70 Cpriortotitrationinchickens.Todetermine
the level of cell-associated virus, the cells were rup-tured by three freeze-thawcycles,and the supernatant fluidwastitrated.
Interferencestudies. Proceduresfor theinterference
testwereessentially those ofRubin (6) and Vogt and
Ishizaki (10). Interference tests were conducted with viruses of the A andB subgroups of the avian tumor virus group. After four celltransfers,REvirus infected andcontrol cultureswerechallengedwith RSV(RAV-1) or RSV(RAV-2). The relativeplatingefficiencyon control and RE virus infected cells was determined. RSV assaytechniqueswereessentiallythosedescribed
byRubin(5).
Inthereciprocalexperiment,cultures of CEF were infected with RAV-1 or RAV-2. These cultures, to-gether with uninoculated control cells, were trans-ferredonthethird andsixth day after infection. After the final transfer, the cultures were challenged with RSV(RAV-1), RSV(RAV-2), or RE virus. The
rela-tiveplatingefficiency of theindicatorviruses on cells
preinfected with the different leukosis viruses was
determined. TheculturessuperinfectedwithREvirus
wereallowedto incubate3 days, and samples of the culturefluidwerecollected and titrated.
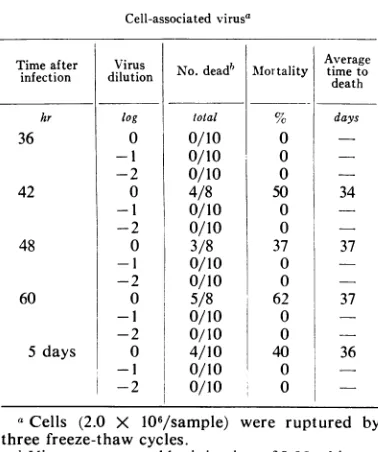
TABLE2. Replication of reticuloendotlheliosis virus
(strain T) in chicken embryo fibroblasts Cell-associated virusa
Time after Virus infection dilution hr log 36 0 -1 42 48 60 5 days -2 0 -1 -2 0 -1 -2 0 -1 -2 0 -I -2
No.deadb Mlortality
total
0/10
0/10
0/10 4/80/10
0/10 3/8 0/10 0/10 5/80/10
0/10 4/10 0/100/10
0 0 050
50
0 37 0 0 62 0 0 40 0 0 Average timeto death days 34 37 37 36aCells (2.0 X
106/sample)
were ruptured bythree freeze-thawcycles.
bViruswasassayed byinjection of 0.25 ml intra-abdominally into strain 55X white Leghorn cockerels. Theexperiment was terminated 4 weeks afterthelastspecific death.
J. VIROL.
1118
on November 11, 2019 by guest
http://jvi.asm.org/
REPLICATION OF RETICULOENDOTHELIOSIS VIRUS
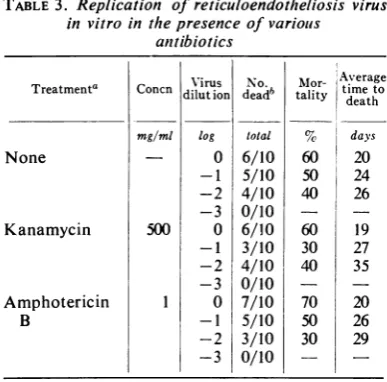
TABLE3. Replication of reticuloendotheliosis virus
invitro in thepresence ofvariouis anttibiotics
Treatmenta
None
Kanamycin
Amphotericin B
Concn
mg/ml
500
Virus dilut ion
log
0
-1 -2 -3
0
-1 -2 -3
0
-1 -2 -3
No.
deadb
total
6/10 5/10 4/10 0/10 6/10 3/10 4/10 0/10 7/10 5/10 3/10 0/10
Mor-tality
60 50 40 60 30 40 70 50 30
Average timeto
death
days
20
24 26 19 27 35 20 26 29
aAntibioticswere presentin the culture media
throughout the entire course of the experiment. Samplesoftheculture fluidwereharvested 3 days after infection.
bViruswasassayed byinjection of0.25 ml intra-abdominallyintoline60FwhiteLeghorncockerels.
Theexperiment wasterminated4 weeks afterthe lastspecificdeath. Uninoculatedbirds cagedwith the test birds for the duration ofthe experiment
werenegative.
RESULTS
Replication ofRE virus in vitro. Active virus
production in CEF cultures was detected in the
culture fluid on the first day after infection. As
noted in Table 1, samplescollected at24hrafter
infection produced a 40% mortality with an
induction period of 42 days. Virus production
and release into the culture fluid continued at a
slow but measurableratefor the first 12hr after
the onset of synthesis. Between 36 and 42 hr
after infection, there was a rapid rise in virus
synthesis and release into the culture fluid. The
percentage of
mortality
wassignificantly
in-creased at all dilutions, with a corresponding
decrease in the incubation periodofthe disease.
Samples of culture fluid collected at 42 hr were
infectious forapproximately
40%C
of thebirds ata 1:100 dilution.The maximalvirus titerinCEF
cultures infected with RE virus was reached at
this time. The titer remainedessentially constant
throughthe fifthday.
Tissue culture fluid from infected cultures
rarely produced
100%c
mortality. A variableproportionof chickenswasresistanttothe
high-estdoseofREvirus. Duringthe periodof
maxi-mal virus synthesis, the average time of death was 20 days with undiluted culture fluid, 23
days with a 1:10 dilution, and approximately 1
month with a 1:100 dilution. The range in the
incubation period
of
the disease was 5 days forsamples collected during the period of
maximal
virus
synthesis. Uninoculated birds, caged withthetest birds for the duration of the experiment,
remained
negative. Wheninfectious
plasma of the same titer was used as a source of virus inoculum, asimilar
growth pattern in vitro was observed.The amount of cell-associated virus was found
to be extremely low. As noted in Table 2,
cell-associated
virus was detected only in samplestakenduring theperiod of
maximal
virussynthe-sis. The
freeze-thaw
process was not responsiblefor
thelow virus in theintracellular
state.Effect of antibiotics on replication of RE virus. The
possibility
ofthis
agentbeing
a member ofthe order Mycoplasmatales was considered.
Amphotericin
B or kanamycin was added to theculture medium
atlevels known
tointerfere
withmycoplasma replication in
vitro (4).
Theseanti-biotics
were present throughout theentire courseof
theexperiment (Table 3).
Thereplication
of REvirus
in vitro
was notinhibited
or reducedby
theseantibiotics.
Similarly,
theadministration
of oxytetracycline
ortetracycline, during
thecourse
of
infection
with REvirus
inchickens,
didnot alter
either
thepercentageof
mortality
orthelatent
period of
thedisease.
Anattempt toisolate
anagent on PPLO
Agar (2) from diseased tissue
or
infectious
plasma
wasalsonegative.
Interference
studies.Experiments
werecon-ducted
toestablish
whether cellsinfected
with
RE
virus become resistant
tosuperinfection
with
A or B
subgroup of
the sarcomaviruses.
Theindicator viruses
wereadded
to thecultures
12days
after infection with
REvirus.
Asnoted in
Table 4,
preinfection
of
cellswith
REvirus
did
not
reduce their
susceptibility
toinfection
with
viruses
of
either
subgroup.
RSV(RAV-1),
at a1:100
dilution, produced
125foci
on thecontrol
plate
and 100foci
on theplate
containing
cells
infected
with
REvirus.
Theindicator virus
of
the B
subgroup
[RSV(RAV-2)]
produced
115foci on the
control
plate
and 150foci
onthe REvirus
infected
cells.
As noted in Table
5,
culturesinfected with
avian
leukosis viruses of both
subgroups
wereequally
sensitive
toinfection with
REvirus.
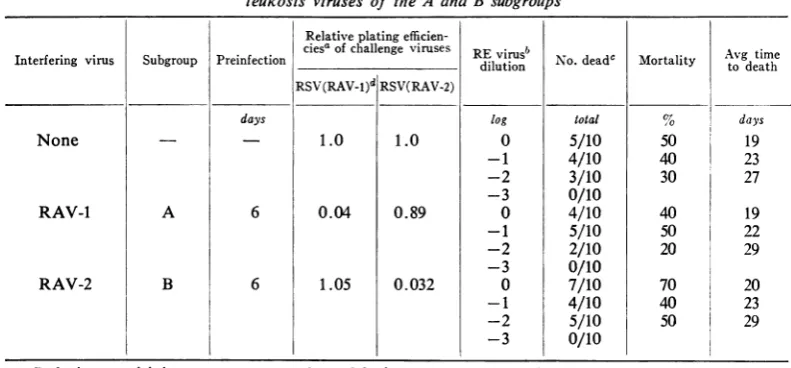
Culture
fluid collected with uninoculated cells
and cells infected withRAV-1 or RAV-2 6
days
prior
tochallenge
with RE virusproduced
the samemortality
and incubationperiod
in chickens.Specific
interferenceagainst
therespective
sub-groups had been established in the
preinfected
cultures
prior
to theadditonofRE virus.DISCUSSION
The
biological
properties
of
thereplication
of
RE virus inCEF cultures have many
character-VOL. 1, 1967
1119
on November 11, 2019 by guest
http://jvi.asm.org/
[image:3.471.35.229.76.267.2]TABLE 4.Susceptibility ofreticuloendotheliosis virusinfectedcellsto infection with sarcoma virusesoftheA andBsubgroups
Fociplate
Virusa Subgroup Dilution Relativesensitivityb
Control cellsControlcells RE virus~cellsinfected tog
RSV(RAV)c-1 A -2 TNTCd TNTC
-3 125 100 0.80
-4 10 15 1.50
-5 0 0
RSV(RAV)-2 B -2 TNTC TNTC
-3 115 150 1.30
-4 10 15 1.50
-5 0 0
aFourth-passage chicken embryo fibroblast cultures
containing
2.5 X 106 cells were infected with0.2ml ofRSV(RAV-1) or
RSV(RAV-2);
(5.5 X 105foci-forming units/ml) at the above dilutions.bRelativesensitivity = averagenumber offoci on testplatedivided by averagenumber offoci on
thecontrol plate.
cRSV = Rous sarcomavirus.
dToo numerousto count.
TABLE 5.Replication ofreticuloendotheliosis (RE) virusin cells
infected
with avian leukosis viruses of theA andBsubgroupsRelative plating
efficien-ciesaofchallenge viruses REvirusb N d tt Avg time Interfering virus Subgroup Preinfection _ _ __ _ dilution No.dead' Mortality todeath
RSV(RAV-l)d RSV(RAV-2)
days tog total % days
None 1.0 1.0 0 5/10 50 19
-1 4/10 40 23
-2
3/10
30 27-3
0/10
RAV-1 A 6 0.04 0.89 0 4/10 40 19
-1
5/10
50 22-2
2/10
20 29-3
0/10
RAV-2 B 6 1.05 0.032 0 7/10 70 20
-1 4/10 40 23
-2
5/10
50 29-3
0/10
aRelativesensitivity = average number offocionthe test platedividedby average number of foci
onthecontrol
plate.
Culture fluid was harvested3 days after infection withRE virus.
Viruswasassayedbyinjectionof 0.25 ml oftissue culture supernatant fluid intra-abdominally into
strain60X white Leghorn cockerels. Theexperimentwasterminated 4 weeks after the last specific death.
Uninoculated birdscaged withthetestbirdsfor the duration of the experiment remained negative.
dRSV = Rous sarcoma virus.
istics
in commonwith
the avian tumorvirus
group. The
comparatively
longincubation
period,
the time atwhich
themaximal
rate ofvirus
synthesis
is reached, and the low level ofcell-associated virusaresimilar
for
virusesof theavian leukosis and sarcoma complex. Virus pro-duction in infected cell cultures is detected 24 hr
after
infection
with
REvirus.
Thesubsequent
rise in
virus
synthesis
maybe due to anincrease
in thenumber of
cells
synthesizing virus or to anincrease
in the number ofcells
synthesizing virusor toan
increase
in the rate of synthesis by the individual cells, or to both. The time at which themaximal
rateofvirus synthesis is reached inthe RE virus
infected
culture is approximately42hr. At
this
time,
anequilibrium
between virussynthesis and thermal
inactivation
appears to beestablished.
Thedecline
invirus
titer detected on1120
BOSE AND LEVINE J. VIROL,on November 11, 2019 by guest
http://jvi.asm.org/
REPLICATION OF RETICULOENDOTHELIOSIS VIRUS
the sixth day after infection is
probably due
toexhaustion of nutrients in
the
culture medium.
The major difference in the
multiplication cycle
of RE virus and other avian tumor
viruses
is
quantitative. Perhaps
the reasonfor the lower
titer is that
only
aspecific
celltype iscapable
of
replicating
REvirus.Tissue
culture fluid collected from
REvirus
infected cultures
rarely produced 100%
mor-tality. Theilen
et al.(10) also did
notobserve
100% mortality in
cell-free preparations from
plasma
ortissue culture
fluid in
spite
of
50-fold
concentration. A variable
proportion
ofchickens
is
resistant
tothe
highest dose of
REvirus.
Whether
this is characteristic ofthe strain of
birds
used in the assay remains unknown. Serumneutralization studies, conducted with normal
sera collected from
1-day-old
cockerels of eachof
the strains used in thesestudies,
werenega-tive.
Thevariable response doesnotappeartobe
due
to maternal antibodies present in aportion
of
the birds used in theassay. Thevariability
insusceptibility
to RE virus may be due to animmune
reaction
developing in the hatched
birds
after inoculation. Thissuggestion,
how-ever, isnot consistent withTheilen's
(10)
obser-vation
that RE virus infectedembryonated
chicken
eggs also show thisvariability.
Geneti-cally
determined resistance of chickens to REvirus
hasnot beeninvestigated.
Efforts
to relate RE virus tomembers
ofthe
avian
leukosis andsarcomacomplex
werenega-tive. Members of the avian
tumorvirus
groupcontain
agroup-specific
antigen
which
canbe
detected
in cells infected with virusesbelonging
to
this
group(3).
Anattempt
todetect the
group-specific
antigen
of the avian tumorvirus
groupin RE
virus
infected cells hasbeen
negative
(10).
Failure
todetect
thegroup-specific
antigen,
however,
doesnotconstitute conclusiveevidence
for
its absence. Since RE virus attains a muchlower titer
than other avian tumorviruses,
the
antigen
could be present at levels belowdetec-tion.
Another
important
characteristic
of the avian
leukosis
viruses is theability
toinduceresistance
to
RSV
in vitro.Vogt
and Ishizaki(11)
havedemonstrated
that a distinct interference patternexists between
members of the avianleukosis
andsarcoma virus
complex.
Leukosis and sarcomaviruses
of
thesamesubgroup
show stronginter-ference,
whereas no interference isobserved
between viruses of different
subgroups.
Conse-quently,
interference tests must beconducted
separately
to detect viruses of bothsubgroups
(1).
Interference
studiesconducted
withviruses
of
the Aand Bsubgroups
indicate that REvirusis
antigenically
unrelated to this group. Ourpresent knowledge of RE virus does not permit
a
decision
as to which existing viral group thisagentmay be related to.
AcKNowLEDGmxNT
Thisinvestigation was supported by Public Health Service research grant CA-04692,07,08 from the
NationalCancerInstitute.
LrrERATuRE CrrED
1.
BURMESTER,
B. R. 1966. Report onavian leukosisconference. Polutry Sci. 45:1412-1415.
2. HAYFLICK,L.1965.Tissuecultures and
mycoplas-mas. Texas Rep. Biol. Med. Suppl. 1 23:285-303.
3. HUEBNER, R. J., D. ARMsrRONG, M. OKUYAN,
P. S.SARMA,A H. C.TURNER. 1964.Specific complement-fixing viral antigens in hamster
andguinea pigtumorsinducedby the
Schmidt-Ruppin strain of avian sarcoma. Proc. Natl.
Acad.Sci.U.S. 51:742-749.
4. PONTEN, J.,AND I. MACPHERSON. 1966. Interfer-encewith Rous sarcomavirusfocus formation
byamycoplasma-like factorpresentin human
cell cultures. Ann. Med. Exptl. Biol. Fenniae 44:260-264.
5. RUBIN,H. 1960. Ananalysis ofthe assayofRous
sarcoma cells in vitro by the
infective
centertechnique. Virology10:29-49.
6. RUBIN, H. 1960. Avirusinchickembryos which
induces resistancein vitro toinfection with Rous
sarcomavirus. Proc. Natl. Acad. Sci. U.S. 46: 1105-1119.
7. SEvoLuN, M., R. N.LAROSE,AND D. M. CHAMBER-LAIN. 1964. Avian lymphomatosis. VI.Avirus
of unusual potency and pathogenicity. Avian
Diseases 8:336-347.
8. SEVOIAN,M., R. N.LARoSE,ANDD.M.
CHAMBER-LAIN. 1964. Avian lymphomatosis. VIII.
Pathologicalresponseofthecickenembryo to
Tvirus. Natl. Cancer Inst. Monograph
17:99-119.
9. THEILEN, G. H., R. F. ZEIGEL, AND M. J.
TWEEHAUS. 1965.Biologicalstudieswithaviral
induced reticuloendotheliosis in avian species.
Proc.Tech.WorkshopConf., Athens,Ga.
10. THEILEN, G. H., R. F. ZEIGEL, AND M. J.
TwIEHAus. 1966.
Biological
studies with REvirus(strain T)that induces
reticuloendothelio-sis inturkeys,chickens, andJapanesequail. J. Natl. Cancer Inst. 37:731-743.
11. VOGT, P. K.,AND R. ISHIZAKI. 1966. Patternsof viral interference in the avian leukosis and sarcomacomplex. Virology30:368-374. 12. ZEIGEL, R. F., G. H. THEILEN, AND M. J.
TWIEHAUS. 1966.Electronmicroscopic
observa-tions on RE virus
(strain
T) that inducesreticuloendotheliosis in turkeys, chickens and
Japanese quail. J. Natl. Cancer Inst.
37:709-729.
VOL.