JOURNAL OF VIROLOGY, Dec. 1972, p. 1184-1198 Copyright ©1972 AmericanSocietyforMicrobiology
Vol.10,No. 6 Printed in U.S.A.
Mechanism of Interferon
Action: Inhibition of
Viral
Messenger
Ribonucleic
Acid Translation
in
L-Cell
Extracts
R. M. FRIEDMAN, D. H. METZ, R. M. ESTEBAN, D. R. TOVELL, L. A. BALL, AND I. M. KERR
National Institute for Medical Research, MillHill,LondonNW7IAA, Entglanid
Received for publication 21 August 1972
Encephalomyocarditis (EMC) virus ribonucleic acid (RNA) stimulated the
in-corporation of "4C-amino acids into polypeptides in cell-free systems using prein-cubatedS10extractsfrom Lcells. Incorporationwaslinear forover2hr. Analysis
ofthe tryptic peptides derived from thepolypeptide products formed in response
toEMC RNA showed themto bevirusspecific. Themajorproduct, apolypeptide
of140,000in molecularweight, migratedonsodiumdodecylsulfate-polyacrylamide
gels with one ofthe virus-specific polypeptides present in EMC-infected cells. A
minor component of molecular weight about 230,000 may correspond to the
product of complete translation of the EMC virus genome. Little or noeffect of
interferon or vaccinia virus infectionwas observed in the preincubated, cell-free system. The EMC RNA-stimulated incorporation of "4C-amino acids into poly-peptides wasnot inhibited in extracts derived from L cellsearlyinvirusinfection,
from interferon-treated cells, orfrom cellssubjectedtoboth treatments. Interferon treatmentdid appeartohave a slight inhibitoryeffect onchain elongation in this system. However, treatment of cells with highly purified interferon before virus
infection caused a decrease ofabout80% in thecapacity of non-preincubatedcell extracts totranslate added EMC RNA. This effect didnotextendto thetranslation ofpolyuridylicacidand could bereversed by preincubationof the extracts at37 C for 20 min.The inhibition oftranslation wasmanifestatinterferon concentrations
aslowas5IU/ml,and in this respectclosely paralleledtheinhibitionof virusgrowth.
Inactivationof the antiviral activity oftheinterferonbyheating or digestion with
trypsin also abolished the effect on cell-free protein synthesis. The EMC-specific
polypeptidesformed in reducedamounts inextracts ofinterferon-treated vaccinia-infected cells were smaller than those formed in extracts ofuntreated, vaccinia-infected cells. Thus, inhibition ofinitiationorelongation of polypeptides, orboth, canbedemonstrated incell-freesystemsemploying non-preincubatedextractsfrom interferon-treated, virus-infected cells. These resultsindicate that antiviral activity
ofinterferon is directed against the translation of viral messenger RNA.
With the exception of some recent evidence
implicatingtranscription of viralribonucleic acid
(RNA)asthe basicdefect in the virus replication
mechanism in interferon-treated cells (2, 18, 23),
most studies have suggested that translation of viral RNA is theprimary point at which inter-feronactionisexpressed(25). Joklik and Merigan
(9) demonstrated that early vaccinia virus
mes-senger RNA (mRNA) is produced in increased
amounts in interferon-treated L cells, but that this mRNA failsto enterintopolyribosomesand isnottranslated (9). In anRNAvirusinfection, the translation of input viral RNA is inhibited
(7). However, attempts to reproduce this inhibi-tion in cell-free systems haveeither proved non-reproducible (15, 19) or have uncovered effects which have not been sufficiently clear-cut for it
to be established beyond doubt that they were
duetotheantiviral activity of interferon (3, 12).
We present here evidence that both preincu-bated and non-preincubated cell-free systems from L cells can translate virus mRNA with
fidelitytoyield virus-specific polypeptides.
Inter-feron pretreatment of the L cells had little effect
on the ability of conventional, preincubated
extracts to translate encephalomyocarditis virus
1184
on November 10, 2019 by guest
http://jvi.asm.org/
INHIBITION OF EMC VIRUS mRNA TRANSLATION
(EMC) RNA in the cell-free system, but an
inhibitory effect of interferon treatment on the
subsequent ability of cell extracts to translate added EMC RNA has been demonstrated. This
required that the extracts be taken not simply
frominterferon-treated
cells,
but frominterferon-treated, virus-infected cells. Moreover, the effect
was seen in the systems from interferon-treated,
vaccinia virus-infected cells only if the usual
preincubation step was omitted from the prepa-ration of the cell extracts.
MATERIALS AND METHODS
TheKrebs 2 mouseascitestumorcells(20) and the preparation of cytoplasmic extracts fromthem (21), the preparation of EMC virus (14) and EMC RNA (14), thelabelingofpolypeptide products inthe cell-free system (4), theperformate oxidation and tryptic digestion of these products, and the analysis ofthe tryptic digests by electrophoresisandchromatography onthin-layer silica gel plates (4) have already been described, as has the qualitative and quantitative
analysisofthepolypeptideproductsbyelectrophoresis on 5 or7.5%' polyacrylamide gels (4) aftertreatment with sodiumdodecylsulfate (SDS). The values forthe molecular weights of polypeptides synthesized inthe cell-free system were obtained by comparison with marker polypeptides from purified reovirus run in parallelgelsorinsplitgels.
Preparationof L-cell extracts:preincubatedextracts. Lcellswereoriginally obtained fromN.Finter (Well-come Research Laboratories) and were grown in EagleSpinner medium with10%( calf serum to a den-sityof80 to 100 X 104cells/ml.Cellextracts were pre-pared basically by the methods of Mathews and Korner (21) originally described for Krebs mouse ascites tumor cells. Cells were washedthreetimes by centrifugation in cold buffer (35 mM tris[hydroxy-methyllaminomethane [Tris]-hydrochloride [pH 7.51,
and140 mmNaCl). Theywerepacked by sedimenta-tion at400 X g for 10 min and resuspended in 1.5 packedcell volumes of buffer (10mm
Tris-hydrochlo-ride, pH 7.5, 7 mm 2-mercaptoethanol, 10 mm KCl, and 1.5 mmmagnesium acetate). After5mintheywere homogenized with 20strokes ofa glass Dounce ho-mogenizer, the homogenate was brought to 30 mM Tris-hydrochloride (pH7.5), 90 mmKCl,3.5mM mag-nesium acetate, and 7 mm 2-mercaptoethanol, and centrifuged at10,000 X gfor 10 min.Thepelletwas discarded. Theextract was incubated at 37Cfor 40 min after the addition of adenosine triphosphate (ATP; 1 mM), guanosine triphosphate (GTP; 0.1
mM), cytidine triphosphate (CTP; 0.6 mM), creatine phosphate (10mM), creatinekinase(0.16mg/ml),and 40
yM
eachof amino acids.The extract wasthenpassedthrough a fine stainless-steel mesh prior to passing through a Sephadex G-25 column which had been equilibrated with buffer containing30 mm Tris-hydro-chloride (pH 7.5), 120 mm KCl, 5 mM magnesium acetate, and 7 mm 2-mercaptoethanol. Samples of about 0.2 ml, containing the maximal concentration
of protein andRNA, were frozenat -70 C inglass
vials. Activitywasstable in the frozen material for at least2months. These extracts contained 5 to 9 mg of proteinperml as measured by the method of Lowry etal. (17) and had anabsorbancy at 260 nm (A60) of 20to 30 units ml. Preparations with A260 <20 units / ml had little or no activity. All operations, unless otherwisencted, werecarried out at 4 C.
Non-preincubated extracts. Lcellsat aconcentration of1.5 X 106cells/mlweretreatedfor4hrat 37 Cwith theindicated dose ofinterferon, diluted to 7.5 X 105 cells'ml,andincubatedovernight(about 17 hr).
Thecells were theninfected (1) with vaccinia virus at a multiplicity of infection of 500 virus particles (about 5plaque-formingunits [PFU]) added per cell, andafter75 minwere washed three times with 35 mNM
Tris-hydrochloride (pH 7.5) and 140 mm NaCI. The preparation thenproceeded as described for preincu-batedextractsuntilafterthe10,000X gsedimentation step. At this point, samples of 0.2 ml were frozen at -70C in glass vials. Thawed preparations were not refrozen.These extractscontained 14to 16 mgof pro-teinper mnlasmeasuredbythemethodofLowry etal. (17) and had anA2Wof 50 to 60units/ml.No signifi-cantdifferenceswerenoted intheproteinorRNA con-tent between extracts from interferon-treated or un-treatedcells.
In the case of infection with EMC, treatment was identical except thatinfection was at anadded multi-plicity of300 PFU percell inthe presenceof actinomy-cin D (1 jig ml). Virus wasallowed toabsorb overa 30-min period, and extracts were prepared after a further 2-hrperiodof incubationat37C.
Preparations wereassayedasdescribed below with anMg2' concentrationof4.5 mmexceptinthe caseof the polyuridylic acid experiments where the Mg28
concentrationwas 10 mmand theincubation timewas 1 hrat37C.
Assay for amino acidincorporation. Each 50-,liter
reaction mixturecontained the following constituents in thegiven concentration: 110mmKCI, 30mM Tris-hydrochloride (pH 7.5), 7 mm 2-mercaptoethanol,
magnesium acetate as indicated (usually 4.5 mM), 1 mMATP, 0.1 mmGTP,0.6 mmCTP, 10mM creatine phosphate, 0.16 mg of creatine kinase per ml, 5 MCi
of"4C-amino acid mixtureperml(The Radiochemical Centre, Amersham, Bucks, England), 40 Mm each of amino acidsnotpresent in the14C-amino acid mixture (methionine, histidine, glutamine,asparagine, cysteine, andtryptophan),and 10 to 15,ulitersof L-cellextract.
Inthe caseoflabeling with35S-methionine, 100
MuCi
of 35S-methionineper ml and 40 ,uM ofcold amino acids(minus methionine) were added per
50-Muliter
incu-bation mixture. Each L-cell preparation was tested todetermine whichvolume of the preparation yieldedoptimal incorporation. Incubation was usually for 120 minat 30C. The reaction was halted, and the mixtures wereanalyzedfor acid-precipitable radioac-tivityaspreviouslydescribed (4).
Interferonpreparation andassay. Mouseinterferon was prepared by the method of Paucker (24). The preparationsusedcontainedatleast1.5 X 10'IU of mouse interferon activity per mg of protein. Inter-feronwasassayedbyitsinhibitoryeffectonthegrowth 1185 VOL. IO, 1972
on November 10, 2019 by guest
http://jvi.asm.org/
FRIEDMAN ET AL.
ofEMCvirusinLcells. Virusyieldwasestimatedby hemagglutininproduction (20). In the case of vaccinia virus-infectedcells, the inhibitionof vaccinia virus pro-tein synthesis andits effecton thepolyribosome pat-ternwere alsoused to monitor interferon action (9). The mouseinterferon usedinthis studywasprepared by K. Paucker and purchased through special funds fromtheNationalCancerInstitute,Bethesda, Md.
Chick interferon was prepared by the method of Fantes (6). The preparation used was donated by K. H. Fantesandcontained13,000 international chick unitsper mg of protein.
RESULTS
Characteristics ofthe translation of EMC RNA by L-cell extracts. When we tested extracts of Krebs ascites cells prepared by previously de-scribedmethods,weconfirmed thatincorporation of 14C-amino acids was stimulated by the
addi-tion of EMC RNA (Table 1). We prepared, in a similar manner, extracts of L cells and tested these at 37 Cand 30 C. With L-cellpreparations at 37 C, we found eightfold stimulation, but a
60-fold stimulation was seen when these extracts
wereassayedat 30C(Table 1). In L-cell extracts, thestimulationby EMCRNA wasoptimalwhen the Mg2+ concentration was about 4.5 mm. The
optimal K+ concentrationwas 110 mm. An addi-tional 10 to 20'" incorporation of amino acid
was occasionally observed by including transfer
RNA in the L-cellincubation mixtures.
We used similarly prepared L-cell extracts to testwhethermouseglobinmRNA(27)orvaccinia mRNA (F. Fournier, D. Tovell, and D. Metz, manuscript in preparation) (11) produced by vaccinia virus cores would also stimulate amino
acid incorporation. Both did to some degree
(about five- to sixfold over endogenous
incorpo-ration) but toa much lesser extentthandid EMC
RNA. TheconcentrationsofMg2+ andK+which
rABLE 1. EMC RNA-directedproteini synithesis in
Krebsascites or L-cellextracts
Counts/min/50-,liter incubation mixture Originofpreparation Assa iuo r
-EMC +EMC
R-NA RNA
Krebs cellsa 37 1,400 18,200
Lcells 37 970 7,600
L cells 30 1,000 62,100
Krebs ascites cell or non-preincubated L-cell extracts were prepared and assayed as described in Materials and Methods. Each50-iAlitersofassay mixturecontained 10-,uliters of extract.
were optimal for stimulation of amino acid in-corporation by each of the mRNAs used were
somewhat different although each mRNA used
was more effective at 30 C than at 37 C in the L-cell systems.
The stimulation of amino acid incorporation by EMC RNA in L-cell extracts was linear for 120min. Thereafter until 300 min, therateof the reactiondecreased (Fig. 1). Part of the explanation for theprolonged ability of EMC RNAto
stimu-lateamino acidincorporation in L-cell extractsat
30 Cwasduetothe marked stability of EMC RNA under these conditions. After1hr at 30 C, 80' of thisRNAranwithmobility ofEMC RNA on elec-trophoresisin polyacrylamide gels.
Theeffect of theconcentration of viralmRNAs on stimulation of 14C-amino acid incorporation by L cells was also studied. Increasing
concen-trations of EMC RNA, up to 2 or 3 ,ug per
50-,uliter incubation
mixture,
stimulatedpropor-tional increases in incorporation of 14C-amino
acids by L-cell extracts. Higher concentrations
wereinhibitory.
Nature of the product. Thepolypeptides formed in response to EMC RNA in the cell-free systems
0I
0 I
x
zI
Z 'A
+EMC
RNA
-EMC RNA
*I -4_- -s_-* - *
--,~~~~
I I1
2
3
4
5
TIME -HRS.
FIG. 1. Stimulationi of amino acidintcorporationi by EMC RNA in L-cellextracts: time course. Two 500-,uliter inicubationi mixtures were prepared conitainin1g the contcentrationis ofconistituenits listed in Materials anzdMethods, 150 ,uliters of ani L-cellpreparationi, 50 ,uliters of i4C-ami,io acids, and 0 or 20 mg of EMC RNA. Ateach inidicatedtimepoinit, a25-,ulitersample wasremovedan1dassayed fori4C-aminoacid incorpora-tionI. 0 -0,0.04 mgofEMC RNA permlpresen7t;
*0-,noEMC RNA added.
1186 J. VIROL.
o
on November 10, 2019 by guest
http://jvi.asm.org/
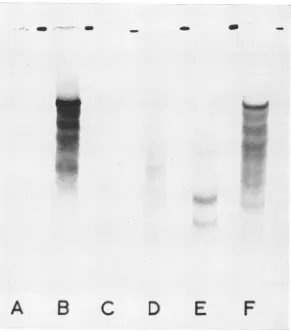
[image:3.499.260.450.343.538.2]INHIBITION OF EMC VIRUS mRNA TRANSLATION
were analyzed by polyacrylamide gel electro-phoresis and by two-dimensional peptide mapping on thin-layer plates. In both L-cell and Krebs ascites cell extracts, product size was found to increase with increasing time of incubation at 30C. Theidentification of virus-specific peptides was facilitated by the polydisperse nature ofthe product and the very low incorporation by the extracts in the absence of EMC RNA (Fig. 2). Thegeneral patterns seen onSDS-polyacrylamide gels weresimilar to those previously reported for Krebs ascites cells with the exception that, by 120 min, the largest prominent peptide found (molecular weight about 140,000) was more conscpicuous in L-cell than Krebs ascites cell preparations (13). The nature and origin of these polypeptides have been extensively discussed in previous communications from this laboratory (4, 13). Itis sufficient to note here that the forma-tion of very large polypeptide products was
stimulated by EMC RNA in L-cell extracts and that theseproducts closely resembled in size those previously identified as being characteristic of
polypeptides stimulated by EMC RNA in Krebs
ascites cellextracts.
35S-methionine-labeledEMCpolypeptidesfrom
L-cell extractsprogrammedwith EMCRNA were
also subjected to electrophoresis on
polyacryl-amide gels with the EMC-specific polypeptides
labeled by 3H-methionine in virus-infected
HeLa cells (Fig. 3).Theelectropherogram shows
*1' * .
that the polypeptide with a molecular weight of 140,000 which is formed in the in vitro L-cell system is the same size as one of the large poly-peptides formed in virus-infected cells. Similar results have recently been reported (5). The in vitro product may therefore be related to one of the large polypeptides formed in virus-infected cells.
We have analyzed the tryptic peptide maps
derived from EMC RNA-directed products
labeled with 35S-methionine (Fig. 4). The L-cell and the Krebs ascites extract products were com-pared. The numbers assignedtothedifferent spots seenin theautoradiograms of the thin-layer plates
designate peptides which have been previously
shownbydetailed analysis of peptide maps to be present in EMC virus-infected Krebs cells and, in the case of some of these, also in purified EMC virus (4). These tryptic peptides are definitely specified by the EMC virus genome. There is a
close correspondence in Fig. 4 between the pep-tides formed by the two systems, and it can be
concluded thatL-cell extracts arecapableof
pro-ducing a number of EMC-specific polypeptides.
Lack of effect of vacciniavirusinfectionor
inter-ferontreatment ontranslation of EMCvirus RNA inpreincubated extracts. In cell-free systems de-rived from L cells treated with 50 IU of mouse interferon per ml, which was sufficient to inhibit virusproduction bymorethan 99%, noeffect on
.~
~~~_a
I...
*F
*.:l:..
I.
C
D
E
F
G
H
I
FIG. 2. Electrophoretic anialysis of products ofL cell anid Krebs cell-free system onipolyacrylamide gels. A
500-jlditer intcubation mixtureconitainied35S-methionine, 100 j.ditersofL cellorKrebs cell extract, anld 0 or 20 ,ug ofEMCRNA.Afterintcubbationzat30 C, samples were removed anidprepared Jor polyacrylamide gel elec-trophoresis, and samples conitaininlg50,000countspermiiiwereappliedtoeachgel. The 5%1- gelsweresubjected toelectrophoresisoverniight, fixed, stainied, sliced, dried, and autoradiographed aspreviously described(4). The auttoradiographsweredeveloped after1week. The molecularweights oftheproductswereestimatedfrom thoseof reovirusproteinis which weresubjected to electrophoresis with the samples. A, D, anid G; L-cell extracts with EMC RNA,30, 60,anid120miiiofinicubation,respectively. B, E, anid H;L-cell extracts; nio EMCRNAadded;
inlcabationts werefor 30, 60, alid120 miti. C,FandI; Krebs ascites cell extracts with EMC RNA; 30, 60 anid
120nmii ofinicubationi.
A
B
1187 VOL. 10, 1972
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.499.107.404.408.543.2]FRIEDMAN ET AL.
14
(V)r¶
IS
(x-z~~~~~
z ~ ~ z
z
10
2030
405067s
SLICE
NUMBER
FIG. 3. Co-electrophoresis ofEMCRNA-directed productsfrom L cell-free system anidEMCvirus-inifected HeLa cells. The 35S-methionine-labeled cell-freeproduct (a) was the EMC-directed 120 mill L-cell specimeni from Fig. 2G. 3H-methionine-labeled EMC-directedproductfrom HeLa cells (0) was produtcedby inifectinig
actinomycilt D (2
lig/ml)-treated
HeLacellmoniolayers withEMCvirus atamuiltiplicity of100.After3 hrwhen,uniider these conditions, theprotein syiithesisin thesecells is almost enitirely virus-directed (J. J. Skehel,personal communiication), the monolakyers were wvashed five times with balaniced salt solution anid inzcuibatedfor 15 mill withmethioniine-free mediiunm to wvhich25jiCi of 3H-methionin2e had beeniadded. Thecells were washed fivetimes, anidantextractwasprepared fbr gelelectrophoresis, mixedwith the35S-labeledin vitroproduct, the mixtlure was
siubjectedtoelectrophoresisalndthegels wereslicedanzd counltedaspreviouslydescribed (16).
translation of EMC RNA was observed (Table
2A).
Amongtheexplanationsforthisfailurewasthe
possibility that the antiviral effect of interferon
might only be expressed in virus-infected cells (25). To test this, we first had to determine
whether extracts of cells infected by a
heter-ologous virus would still translate EMC RNA. Wetherefore examined whetherextractsfrom
vac-ciniavirus-infected cells early in thecourse of in-fection (1hr afterinfection) could translate EMC RNA. The results(Table 2B) indicate that they do
so at least asefficiently as extracts from control
cells. By 1hrafterinfection, vaccinia virus
mark-edly inhibited cell-directed protein synthesis and
vaccinia virus proteins dominated thepattern of protein being produced; however, no cytopathic
effect of the viruswasobservedatthis time(22). Itwasthuspossibleto testwhether interferon-treated,vaccinia virus-infectedcellswould trans-late EMC RNA. Indeed, cell-free systems from these cells were just as capable of translating
EMCRNAasthose from vaccinia virus-infected
cells which had not been treated with interferon (Table 2C).
Changes in severalelementsinthe assay system also failed to bring out any effect of interferon
treatment. We varied theconcentration of EMC RNA, the time of incubation, and the
concentra-tions of magnesium or potassium ion without
demonstrating any effect of interferon
pretreat-ment on the ability of cell extracts to translate
EMCRNA.
Onepossibly significanteffectwasfound,
how-ever.Inextractsfrom both vaccinia virus-infected and uninfected cells, the products formed were
analyzedonpolyacrylamidegels. Theproportion
ofcounts inthe highest-molecular-weight
EMC-specificpolypeptide obtained (about140,000) was
larger in extracts from controls than from inter-feron-treatedcells (Fig. 5). Thiswassuggested in the results seen after 120min of incubation but
wasevenmoreevident in the 240-min specimens. Thiscanbeseenat a quantitative levelin the
re-sults of the experiment shown in Fig. 6 which illustrates an additional point of interest. With
1188 J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.499.111.408.54.323.2]INHIBITION OF EMC VIRUS mRNA TRANSLATION
28
20)
(-29
2.
w
.3S*:
A
X
.41
32
40
4° -42
51
34
48
-50
7"
.... 2
19-
20
30
41
40
-W
11
12
1I
23
25'
32
51
,V.
34
x
B
48-50
FIG. 4. Radioautogranis oftwo-dime,isiontal tryptic peptide mapsfrom EMC-directed productsformedin L-cell and Krebs L-cell extracts. Tlhe 35S-methionine-labeled products were the 120-mill L-cellandKrebs cell speci-meiis inicubated with EMCRNA in Fig. 2 (G anid1).
Perfornmate
oxidatioll,trypsini
treatment, electrophoresis,anidchromatographly onithiin-layerplates, alnd autoradiography werecarriedout aspreviouslydescribed(4). The
numbers are those previouslyassigntedin thislaboratory to EMC-specificpeptides (4). Productsformedin (A)
L-cellextractsanid (B) Krebscellextracts.
long exposures of the autoradiograms, small amounts of a
polypeptide
of at least 230,000 in molecularweighthave beenconsistentlyobserved among theproductsformedbyextractsfromcon-trol cells(Fig. 6, arrow). Thispolypeptideis
suffi-ciently largefor itto correspondto thecomplete
translationproductof the EMC RNA genome. It
is not seen in extracts from interferon-treated
cells(Fig. 6).
Inhibition of EMC RNA-directed polypeptide
synthesis in non-preincubated extractsfrom
inter-feron-treatedLcells.L-cell extractswhich hadnot
VOL. 10, 1972 1189
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.499.56.446.78.518.2]FRIEDMAN ET AL.
beenpreincubated for40minat
preparation are capable of transl (Table 3A). As with theconver
bated extractsdescribedinTable treatment of the cellswith suffici interferon to inhibit EMC virusI culturesby more than
99%c
hadTABLE2.Lack of e-ffect of
interje
vaccinia virus infection on EM( proteinsyntlhesisin L-ce
1'reatment of cells'
A. None... Interferon....
B. None... Vaccinia virus infection ....
C. Vacciniavirus infection .... Interferon +
vacciniavirus infection ...
Preincuba-tion of extractb
+
+
+
+
-1-a L cells were treated with 5(
interferon per ml for 14 hr or i
cinia virus, or both, and extraci assayed as described in Materi; Each
50-Aliter
mixture contain EMCRNA and wasincubatedfcb37 C,40'min.
37Cduring their hibitory effect on chain elongation during the
latingEMC RNA translation of added EMC RNA in this system.
ntionally preincu- In addition (Table 3B), non-preincubated,
cell-e2,however,pre- free systems, which werederived from L cells
in-ient (50 units/ml) fected with vacciniavirus,translated EMCRNA.
growth inparallel Accordingly, as neither interferon treatment nor
I only a small in- virus infection appeared individually to have a
significanteffectonthesesystems, itwaspossible 7rontreatment anld to test their combined effect. When this
experi-CRNA-directed ment was performed, a marked inhibitionin the
11 extracts translation ofEMCRNA wasobservedwith
non-preincub4ted
extracts frominterferon-treated,
Counts/min/50-mAiter
vacciniavirus-infected cells(Table
4).
incubationmixture___ ________ That anantiviral state had been established by
-EMC +EMC the interferontreatment
employed
wascheckedby
RNA RNA (i) testing the ability of samples of cells (not in - this caseinfected with vaccinia virus) to support 400 V 400 EMC virus growth, (ii) checking the pattern of 800 .~.2,,700 polyribosomes in the cytoplasm of the
interferon-treated,
vaccinia virus-infected cellsagainst
that 4007,z ofuntreated,
vaccinia virus-infected cells, and440 12,000 (iii) analyzing on polyacrylamide gels the poly-peptides produced in interferon-treated and
tin-treated, vaccinia virus-infected cells. The results 640 6,200 of these checkson the presenceofaviral inhibi-tory state showed the following. (i) EMC virus growth wasinhibited by more than99c%, in inter-670
7,200)
feron-treated cells. (ii) The polysome pattern ininterferon-treated,
vaccinia virus-infected cellsnfected
with vac- was, aspreviously
described(9),
disaggregated
ts were made and
with
a marked increasein the number ofmono-als and Methods. somes as compared to untreated, virus-infected led 0 or 2 ,ug of cells.
(iii)
Almost no protein (virus or cell) was )r120 min at 30 C. produced in interferon-treated, vaccinia virus infected cells, whereas a pattern characteristic oI . *. a
A
B
C
D
E
F
G
H
FIG. 5. Analysis ofproducts ofcell-free systems byelectrophoresisonpolyacrylminidegels:effect ofinterferon treatment ancd vacciiiia viruis infection. Extractsfrom vaccinia virus-infected cells were treated fbr 14hr with 50 uiiitsofpurifiedmouseinterferoniper mlprior toiiifectioii,andvirus-intfectedcontrols wmerepreparedanldanalyzed
asdescribedin thelegend to Fig. 2. In thisexperimelnt, however, sampleswere takeni after 30, 60, 120, aiid 240 min ofincubation with EMC RNA. A, B, C, andD:extracts from controlcells at 30, 60, 120, and 240 miii,
respectively. E,F, G,antdH:extractsfrominiterferoii-treated cells at 30, 60, 120, aiid 240 miii.
1190 J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.499.57.251.170.362.2] [image:7.499.112.399.443.590.2]INHIBITION OF EMC VIRUS mRNA TRANSLATION
A
B
FIG. 6. Analysis of products of cell-freesystemsby electrophoresis onipolyacrylamide gels: possible effect
of interferoni treatmenit anid vaccinia virus infectiont. Sampleswerepreparedinamanniieriden7ticaltothosein Fig.5.However,films wereexposedto thedehydrated gels for14daysinisteadoj5. A,Extractfrom vacciniia
virus-inifectedcells; B, extractfrom initerferoni-treated, vacciniia virus-infectedcells.
vacciniavirusinfectionwasalreadyestablished in
untreatedcellsby1hrafterinfection(22).
One additional pointshould be made
concern-ingthe results in Table 4. At 1 hrafterinfection,
almost all of the protein being producedin cells infected with vaccinia virus is vaccinia virus di-rected (22). Therefore,theendogenous
incorpora-tion observedinthe absenceof added EMC RNA
[image:8.499.56.244.80.434.2]largely represents vaccinia virus-directed protein synthesis. It might be expected that interferon treatment would have some effect on translation ofendogenous vaccinia virus mRNAand indeed
TABLE 3. TranislationzofEMCRNA by L-cell extracts
Counts/min/50-pliter
incubatio nmixture Treatmentof cells
Treatment
of-EMC +EMC
RNA RNA
A. None None 3,830 8,700
Interferon" None 3,970 8,370
B. Vaccinia in- Presentb 580 7,300 fection
Vaccinia in- None 1,330 8,310
fection
Cells were treated with 50units of interferon per ml, and non-preincubated or preincubated extracts were prepared and assayed as described in Materials and Methods. Each 50-jiliter incuba-tion mixture contained 15
Aliters
of the L-cellpreparationand0or2ugof EMC RNA.
I After 1 hr of infection with vacciniavirus,
ex-tracts were preincubated for 40 min at 37 C and then filteredthrough Sephadex G25.
TABLE 4. Inlhibitioni of EMC RNA tranislatio,i in
non-preinicubatedextractsof
initerferoni-treated,
vaccintia viruts-inifectedL cells
Treatmentofcellsa Volume ofL-
intin5mitcr
cellextractper inuainmixture50-jAliter Interferon
Interfits/
Vaccinia incubationvirus mixture -EMC +EMfC
ml) infection (uics N N
_ + 10 1,750 6,930
15 1,560 8,320
20 1,230 5,840
+ + 10 291 738
15 323 597
20 337 631
aLcellswere treated with interferon (50units/
ml) and infected withvaccinia virus as described. Each
50-juliter
incubation mixture contained the indicated volume ofthe non-preincubated L-cellpreparations employed.
a three- to fourfold inhibition of 'IC amino acid
incorporation wasobserved (Table 4).
Taken together, these results suggest that the antiviral effect of interferon is directedagainstthe translation ofviral mRNA and thatoperationof this inhibition is only fully manifest in extracts
from virus-infected cells. As shown below, this effect of interferonisreversible. Previousattempts
todemonstrate this effect in conventionally pre-pared extracts (S-10 preparations) failed,
prob-ably because somestep inthepreparative
proce-dureinactivatedafactor(s)responsible.
1191 VOL. 10, 1972
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.499.259.452.82.220.2] [image:8.499.258.453.344.477.2]FRIEDMAN ET AL.
Characteristics of theinterferon effect in extracts ofinterferon-treated,vaccinia virus-infectedLcells. At all concentrations of EMC RNA tested, ex-tracts from interferon-treated, vaccinia virus-infected cells showed less stimulation of amino acid incorporation thandid those from untreated, vacciniavirus-infected cells (Fig. 7). Theoptimal concentration ofEMC RNA with both extracts was 40
,g/ml.
With extracts from both interferon-treated, virus-infected and untreated, virus-in-fected cells,incorporation was linear for about 60 min (Fig. 8). At all times studied, preparations frominterferon-treated,virus-infected cells incor-porated amino acids less well than did prepara-tions from untreated, virus-infected cells. The difference notedwas notdueto morerapid break-down of EMC RNA by extracts from theinter-K
48 _
O 4C 0
x
Z 32
I-w 24 z O 16
8
_~~~~~~~~~~
2 CONTROL
--fr
---'
IEE TREATED-
INTERFERON-TREATED;
I
0 1 2
TIME -HRS.
2
0
x
16
z
I-Z 8
0
U
CONTROL
EMC
-RNA
ADDEI
FIG. 7. InhibitionI of EMC RNA tra,isla) tracts of interferon-treated, vaccilnia viri
cells: effect of EMC RNA conIcentErationt. treated with 50 unlits of interferontperinl ali With vaccinia virus. Controls were olnly! viru
Extracts wereprepared fromthesecells. Eac incubationi mixtureconitainted the indicated EMCRNA and 15,uliters of non-preincubat6 Incubation was at 30 C for 2 hr. 0-- 0
from untreated, vaccinia viruis-ilifected cells -- 0, extracts from interferoni-treated virus-infected cells (intferferon -treated).
FIG. 8. Inihibitioni of EMC RNA tranislationi ill extracts of intterferon2-treated, vacciniia virus-infected cells: effect of inicutbationz time. Two250-i.litermixtures
were prepared conttainting constituents in the
conicent-trationi inidicated in Materials anid Methods antd 75 ul1iters of nion-preinicubated extract from initerferoni-treated, vacciniia viruis-inifected cells (initerferoni-treated; *---0) or uiiitreated, viruts-inifected cells (conttrol; Q--O). At the inidicated times, 20-Mlliter -0 samiipleswereremovedan1dassayedfor inicorporationi of
'4C-aminio acids.
feron-treatedcells sincethepatternof thecounts, when analyzed on polyacrylamide gels, was the same for radioactive EMC RNA incubated with
either cellextract.
P In addition, extracts from both
interferon-treated, virus-infected cells and from untreated, virus-infected cells incorporated amino acids
optimallyat4.5 mm Mg2+; however, the extracts from interferon-treated, virus-infected cells had much lower levels ofactivity atall Mg2+andK+ concentrations where significant stimulation of
4 amino acidincorporation was seen in untreated,
(g)
virus-infected controls.The polypeptide products synthesized in re-tioi inex- sponse to EMC RNA in the different cell-free
is-inlfected systems usedwereanalyzed byelectrophoresis on
Cells were SDS-polyacrylamide gels (Fig. 9B, D, and F).
,idinifected Parallel analysesof theproductsofsystemsin the
s inifected, absenceof EMC RNAare shown in Fig. 9A,C,
/h
S0-ilite- and E. With material from untreated L cells,amouniit Of whether the cell-free systems were preincubated d
Extracts
(Fig. 9B)
or not(not
shown),
a characteristic(conttrol);
(Fig. 2)
series ofpolypeptides
wassynthesized
in r, vaccilnia response to the added virus RNA, thepredomi-nant product after 2 hr of incubation at 30 C
3 4
1192 J. VIROL.
)I
q
i.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:9.499.262.450.71.280.2] [image:9.499.55.250.265.533.2]INHIBITION OF EMC VIRUS mRNA TRANSLATION
A
B
C
D
E
F
FIG.9. Inhibition of EMC RNA translation in extracts ofinterferon-treated, vaccinia virus-infected cells: electrophoretic analysis of polypeptidesformed in the cell-free system. "S-labeled products wereprepared for analysison 5% SDS-polyacrylamide gelsaspreviously described. About 50,000 countspermin were loadedon eachgel. Thegelswerepreparedfor radioautography, and thefilmwasexposedfor7days.Preincubatedextracts from uninfectedLcellsin the absence (A) and presence (B) of EMC RNA. Non-preincubated extractsfrom
in-terferon-treated, vaccinia virus-infected cells in the absence (C) and presence (D) ofEMCRNA.
Non-preincu-batedextractfrom untreated, vacciniavirus-infected cellsinthe absence (E)and presence(F) ofEMCRNA.
being
a large polypeptide of approximately140,000in molecular weight (Fig. 9B). Vaccinia virusinfectiondid not alterthenatureof the
poly-peptides synthesizedin response to EMC RNA in
these systems. This is shown for a
non-preincu-bated system in Fig. 9F. Interferon treatment
alonehad, at most, a
marginal
effect on therela-tive amounts of the higher-molecular-weight
products synthesized (Fig. 5 and 6). In contrast, with non-preincubated systems from cells
sub-jectedtobothinterferon treatment andinfection,
only smallamountsoflow-molecular-weight
poly-peptideswereproduced in response to the added
EMC RNA(Fig. 9D). These resultsindicatethat
notonly is there lessproductmade in responseto
the added RNA, but also the product size is altered. This would argueagainst aneffect
exclu-sively on initiation of translation of the viral
RNA.
Clearly, the translation of added viral RNA
(EMCRNA) is inhibited in cell-free systems from
interferon-treated, vaccinia virus-infected cells.
Theresults of theanalysisof these systemsinthe absenceof EMC RNAareinaccord witha
corre-sponding inhibition of the translation of
endoge-nous vaccinia virus mRNA in the
interferon-treated, virus-infected cell system. Although the
polypeptides detectable in the non-preincubated
systemfrom vaccinia virus-infected cells(Fig.9E) have not beenfullycharacterized, theyarealmost
certainlyvacciniavirus-specific(22),andtheyare
absent in systems from cells
subjected
to inter-feron treatmentpriortoinfection(Fig.9C). These resultsindicate thatadded EMC RNA andendog-VOL. 10, 1972 1193
on November 10, 2019 by guest
http://jvi.asm.org/
[image:10.499.106.397.72.404.2]FRIEDMAN ET AL.
enous vaccinia virus mRNA are not efficiently translated in extracts from
interferon-treated,
virus-infected cells.Requirement for virus infection for expressionof
antiviral activityincell-free systemsis notspecific forvaccinia virus. Theantiviralactivityinduced by interferon is not apparent unless the cells arealso virus-infected (Table 3).Intheseexperiments,
vac-ciniavirus was usedastheinfecting agent. Some
objection has been raisedtotheuseofthe vaccinia
virus-L cell system in studies of interferon action
on the basis of theprofoundcytopathic eftFectseen
in cellstreated inthis manner (10). Although for preparation of extracts we used cells 1 hr after infection, in which no cytopathic effect and no
effect of virusinfection on amino acid uptakeor
pool sizes wasnoted (22), wealsoperformed
ex-periments on cells infected with EMC instead of vaccinia virus(Table 5, A).
Inthis study, interferon-treatedor untreatedL cells were infected with EMC virus at a multi-plicity of 300 PFU per cell in the presence of actinomycinD(1
Ag/ml).
Extractswereprepared2hr afterinfection asdescribed in Materials and Methods. The results of assayson these extracts are shown in Table 5, A.Littleor no stimulation by EMC RNA was seen in theextractsfrom
in-terferon-treated, virus-infected cells, whereas
ex-tracts from untreated, virus-infected cells had a
response toadded EMC RNA.Wealsoobserved
amarked inhibition in theendogenous
incorpora-tion of extracts from interferon-treated as
com-paredtountreated cells. Thiswasprobablydueto
the factthat, by 2 hr afterinfection, most of the
polyribosomesin cellsnottreatedwith interferon
were engaged inmaking EMC proteins, whereas
in interferon-treated cells neither cell nor viral products were beingproduced, since in the
pres-enceofactinomycinD,arapid inhibitionofL-cell
protein synthesis takes place on infection with EMCvirus (4).
Additional studies have shown that
multiplici-ties of vaccinia or EMC virus of 1 or 25 PFU,
respectively, are sufficient to establish antiviral
activity in cell-free systems from
interferon-treated cells. In the case of vaccinia virus, the
antiviral state iswellestablished by 15 minafter infection ininterferon-pretreatedLcells.
Inhibition of translation is interferon mediated. Krebs ascites cells were treated with interferon
(50units/ml),infected withEMCvirusat a
multi-plicity of 300PFUper cell,and non-preincubated extracts werepreparedasdescribed forL cellsin Materials andMethods. Interferon treatmentwas
ineffective ininducinganinhibition of translation in extracts of Krebs ascites cells which are
re-sistant to induction of antiviral activity by inter-feron (TableSB).
Treatmenitofcellsa
A. Lcells EMCinfection.
Interferon, thenEMC in-fection...
B. Krebs cells EMCinfection. Interferon, then EMC infection.... ELICvirus growth he-magglutinin (units/ml) 1,280 <20 2,560 2,560 Counts/min, '50-pliter incubationmixture -EMC +EMC RNA RNA
4,300 9,900
1,530 5, 620 9,180 1,840 24,200 28,300
(l Krebs ascites or L cells were treated with 50 units ofmouse interferon per ml.They were then infected with EMC virus in thepresenceof actino-mycin D (1 ,yg/ml). After 2 hr,5-ml samples were
removed, and virus was allowedtoreplicate for 9 hr to assay virus growth and the effectiveness of interferon treatment. The major portionls of the cultureswereusedat2hrafter infection for mak-ing non-preincubated extractswhich wereassayed as described in Materials andMethods.
Interferon itself and the antiviral activity
in-ducedbyinterferon have some verytypical
prop-erties (25). The antiviral activity and the
inhibi-tion of EMC RNAtranslation in cell-freesystems, which was inducedby theinterferon preparation we used, were both stable to pretreatment ofthe interferon atpH 2. Also, neither activitywas re-versedby washingthe cellswhichhadbeen treated with the interferon preparation. The antiviral activityinducedby interferon and the invitro in-hibition ofEMC RNA translation were stableat
37 C for 30min butwere destroyed by80 Cor
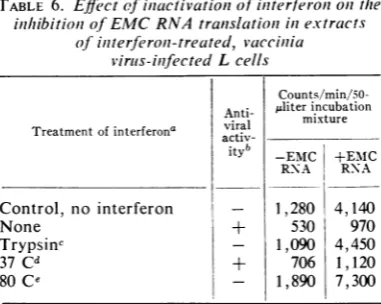
trypsin treatment for 30 min (Table 6). Partially
purified chickinterferon, in titer sufficiently high (80 units/ml) to inhibit Semliki Forest virus growth by morethan99%- inchick cells, had no effect on L cells, and an interferon preparation
(kindly donated byR. Z. Lockart),purified bya differentmethod(26) thanPaucker's (24),wasas effective as the standard preparation which we used inmostofour studies.
In addition, Table 7shows that both the anti-viral activity induced and theinhibition of EMC RNA translation in cell-free systems varied
[image:11.499.264.456.78.268.2]di-rectlywith theconcentration ofinterferon which hadbeen usedtotreat Lcells. Maximumactivity ofbothwasreachedbetween5and 15units/ml.It
TABLE 5. EMC RNA traislationt in lioli-preincuhatedextractso
interferon-treated EMC virlus-inifected L cells or Krehs aiscites cells
1194 J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
INHIBITION OF EMC VIRUS mRNA TRANSLATION
TABLE 6. Effectofilnactivationz ofiniterferonioiithe inthibitioni of EMCRNAtranslationi inextracts
ofinterferon-treated, vaccinia virus-infected Lcells
Counts/min/50-Anti-
ntira
julitermi.xtureincubation Treatment ofinterferonaactiv--EMC +EMC RNA R'NA Control, no interferon - 1,280 4, 140
None + 530 970
Trypsinc - 1,090 4,450
37 Cd + 706 1,120
80 Ce 1,890 7,300
a Inall cases 50 units ofinterferon per ml or its
equivalent prior to inactivation was employed to treat cells.
IAntiviral activity was assayed by inhibition of
EMC virus hemagglutinin production, inhibition of vaccinia virus-directed protein synthesis, and effect on polyribosome pattern in vaccinia virus-infected cells.
cTrypsin (100 ,ig/ml) was added to a concen-trated interferon preparation (1,500 units/ml) for 30 minat 37C. At theendof this time, soya bean trypsin inhibitor (200 qg/ml) was added and the preparation was diluted appropriately.
dTreated exactly as footnote c except that trypsin was omitted.
eInterferon (1,500 units/ml) was heated to 80 C for 30 min, and diluted.
is also interesting to note (Table 7) that, in cell-free systems, the level of endogenous incorpora-tion, which is almost certainly directed by vaccinia virusmRNA,also varied with the interferon dose.
Thisagreedquitewellwiththedegreeof cell
poly-somedisaggregation (9) noted in cells treated with different interferon doses. In cells treated with 5 units ofinterferon per ml, some viral polysomes could be found, but with higher concentrations a clear-cutdisaggregation of polysomes occurred.
We also tested the ability of these extracts to direct the incorporation of '4C-phenylalanine in the presence ofpolyuridylic acid [poly(U)]. At the lowestMg2+ concentrationwhereeffective phenyl-alanineincorporation was observed (10 mM) and
athigher Mg2+ concentrations, nodifference was
seen in the incorporation of
"4C-phenylalanine
between the extracts from interferon-treated, virus-infected cells and untreated, virus-infected
cells (Fig. 10).This result indicatesthatthe cyto-plasmic elements, such as ribosomes andvarious factors necessary for the functioning of the
poly(U) system, are present inequal amounts in
preparations from both interferon-treated,
virus-infected cells and untreated, virus-infected controls.
Reversal of theantiviral activity incell-free sys-temsinduced byinterferon treatment of cells. It has been observed that there is no difference in the ability to translate EMC RNA by extracts from
TABLE7.Effectofthe interferonconcenttrationused in thetreatmentofcellsontheinhibition of EMC
RNA translationt in extracts of
interferon2-treated, vaccinia virus- iiifectedLcells
Counts/min/50-uliter
EMCvirus incubation mixture Interferonconcn growth
(he-(units/ml) magglutinin
unitsproduced)a -EMC +EMC
RNA RNA
Ob 1,280 2,080 18,450
5 30 1,180 6,940
15 <20 540 2,820
50 <20 630 2,630
aBeforevacciniavirusinfection,L-cellsamples
wereremoved, washed,andinfected with EMCfor 8 hr, and yields of EMC hemagglutinin were
as-sayedtoestimate the effectiveness of the interferon treatment.
bCells were treated with the indicated concen-tration of interferon and then infected with
vac-cinia virusasdescribedinMaterials and Methods.
481
c 40 1 0
32
I-"n'
24z
0 16
8
INTERFERON
-_ // TREATED
I I I I
0 4 8 12 16 20
POLY U ADDED(pg)
FIG. 10. Lack ofeffect on poly(U) translation in extracts of
interferoln-treated,
vaccinia virus-infectedcells. ExtractswerepreparedasdescribedinMaterials
andMethods. Concentrationsoftheconstituenms ofthe reaction mixture were as describedexcept that Mg2+ was 10 m.r andthe incubation wasfor I hr at 37C. The amount ofpoly(U) indicatedisper
SO-aiter
reac-tion mixture. 0- O, Non-preincubated extracts
from untreated, vaccinia virus-infectedcells (control); *---,
noni-preinicubated
extracts frominterferon-treated, virus-infectedcells (interferon-treated).
1 195 VOL. 10, 1972
on November 10, 2019 by guest
http://jvi.asm.org/
[image:12.499.254.449.133.267.2] [image:12.499.257.451.357.552.2]FRIEDMAN ET AL.
interferon-treated, virus-infected cells or
un-treated, virus-infected cells whentheyare preincu-batedfor40 min at 37 Cduringtheir preparation (Table 2). The conventional preincubation pro-cedure used also involves filtration of extracts
through a Sephadex G25 column, but thisalone
did not reverse the inhibition of the translation of EMC RNAobservedwith extractsfrom
inter-feron-treated, vaccinia virus-infected cells. When
all ofthestepsinthis
procedure
wereemployed,however, the antiviral activityoftheextract was
destroyed (Table 8). When only incubation at
37 Cfor 40min was carried out, the inhibition of EMC RNA translation was also lost. Indeed,
preincubation of extractsfrom such cells at 37 C
for as little as20min in the presence or absence of
aminoacids, ATP, and an ATPgeneratingsystem
was sufficient to reverse the inhibitory effect of
interferon treatment on EMC RNA translation.
This was true despite the fact that, underthese latterconditions, amino acidincorporationduring
the preincubation was inhibited by more than
85%. Onthispreliminary basis, therefore, itseems
unlikelythat protein synthesis isrequired during
the preincubation for the reversal of the
inter-feron-mediated inhibitionto occur.
DISCUSSION
Inthis study wehave discussed two basic
sys-tems. The first involved preincubated extracts
frominterferon-treatedbutuninfectedcellswhich,
with the possible exception of an inhibition of
[image:13.499.61.254.416.576.2]chainelongation (Fig. 5 and 6), translatedEMC
TABLE 8. Effect of preincubation of extracts of interferon-treatedandvirus-infectedL cellson
theirability to iranslate EMC RNA
Counts/min/50-,uliter incubation
Treatment
~~~~~~mixture
Treatment Treatmentof extract
-EMC +EMC
RNA RNA
None None 2,740 5,470
Interferon None 944 1,450
None 37C,Sephadex G25a 635 6,480 Interferon 37 C, Sephadex G25 528 4,510
None 37 Conlyb 764 5,020
Interferon 37 Conly 786 4,360
a Extracts were treated at 37 C for 40 min and
werethenpassed throughaSephadex G25 column
(5).
bExtracts weretreated as in footnote a except that amino acids wereomitted from the preincu-bation mixture and no gel filtration was carried out.
RNA as well as controls fromcellswhich hadnot
been treated with interferon (Table 2). InfectioP.
with vacciniavirusdid not impair the ability of extractsfrominterferon-treatedoruntreatedcells totranslate EMC RNA (Table 3). The second cell-free system consisted of non-preincubated ex-tractsfrom interferon-treated,virus-infected cells. The results from this system clearly show marked inhibition of translation of EMC RNA. Both in-terferon treatment and virus infection were re-quired for the demonstration of this inhibitory
state.
Taken together, these results and previously
published work suggest the following mechanism ofaction for interferon. In interferon-treated cells, apotentialantiviral state is induced. This process seems to require cellular RNA and protein syn-thesis and may manifest itselfby a slight inhibition of cell growth (24) and of translation of viral RNA (3, 8). Such a potential state may account for the inability to demonstrate any profound effect ofinterferon treatment on the processes of
uninfected cells (25). When interferon-treated
cells areinfected with virus, the antiviral state is fullyrealized, and the translation of viral RNA in cell-free systems derivedfrom such cells is mark-edly inhibited. The factor(s) responsible for this
inhibition in cell-free systems from
interferon-treated,vacciniavirus-infectedcells isheat labile.
Current studies on suchextracts indicate that the factor(s) is present in both inicrosomal and cell
sap fractions. Interestingly, the addition of cell
sap from vaccinia virus-infected,
interferon-treated L cells to ribosomesfrom
interferon-insen-sitiveKrebs cellsalso resultsin aninhibition of
thetranslationof EMC RNA.
Anintriguingaspectofthesesystems isthat
cur-rent results obtained on infection of interferon-treated cellswith EMCdo differ in some details
from those obtained with theinterferon-treated,
vacciniavirus-infected cellsystems. With systems
from theinterferon-treated, EMC-infected cells,
theinhibitoryeffect is confinedto thecellsap
frac-tion (D. Tovell et al., manuscript in prepara-tion). Moreover, the results of two experiments would suggest that the translation ofboth mouse globin mRNA and EMC RNA is reduced in cell-free systems from vaccinia virus-infected, inter-feron-treated cells. This reflects the complete
inhibition of host and viral protein synthesis in
these cells (9).
Althoughit ispossible that the results obtained
were due to a nonspecific effect of interferon
1196
J. VIROL.on November 10, 2019 by guest
http://jvi.asm.org/
INHIBITION OF EMC VIRUS mRNA TRANSLATION
treatment and virus infection, we feel this is
un-likely for several reasons. The mouse interferon
preparations employed were highly purified and, aside from inhibitingvirus growth, had no marked effect on the cells. The activity in the interferon preparation which induced the antiviral state in both cells and cell-free systems had the chemical andphysical characteristics of an interferon. Two different viruses could be used to activate the anti-viralstate and, finally, in extracts from interferon-treated, vacciniavirus-infected cells, the inhibition of viral RNA translation was reversed by preincu-bation at 37 C for 40 min.
Viral RNA was not degraded more quickly in extracts from interferon-treated, virus-infected cells than in extracts from untreated, virus-in-fected cells (L. A. Ball, unpublished observation). Our results suggest that the interferon-induced in-hibition we have observed may be due to an effect on theelongation and possibly also on the
initia-tionstep ofvirus polypeptide synthesis.Total
in-corporationof aminoacidsintoviral products was
markedly inhibited, andthose polypeptides which wereproduced were of a small size compared to the EMC RNA-specific polypeptides normally produced in cell-free systems.
Two recent studies have suggested that inter-feron pretreatment inhibits the synthesis of viral mRNAs mediated in the infected cell by the
virion polymerases of parental vesicular
stoma-titis (18) and vaccinia (2) viruses. The question
arises whetherthiseffectortheinhibitionof
trans-lation reported here is the basis of the antiviral
activity of interferon. The crucial points in this
respect are (i) how general is the effect on tran-scription, (ii) can the apparent inhibition of the virion polymerases in the intact cell beseenwith low doses of highly purified interferon
prepara-tions, and (iii) can the effect be shown to be
directlyonthe viral polymerasesby studiesinthe
cell-freesystem? However, theinhibition of trans-lation reported in this study parallels a similar effect in the intact cell (22, 25). Translation
in-hibition is seen with low doses ofthe two most
highly purified interferon preparations available
and isquantitatively sufficient to accountfor the inhibitory effect of interferon treatment onvirus
replication.
ACKNOWLEDGMENTIS
WeareindebtedtoD. Risbyfortechnicalassistance. R.M.F., National Cancer Institute staff member at The National InstituLtesofHealth, isavisitingscientist. D.R.T. is therecipient ofa Canadian M.R.C. Fellowship, and R.M.E. of an EMBO Fellowship. Globinmessenger RNAwasdonatedbyR.
William-soin,TheBeatson Institute for CancerResearch, Glasgow, Scot-land.
LITERATURECITED
1. Becker, Y., and W. K.Joklik. 1964. Messenger RNA in cells infected with vacciniavirus.Proc. Nat. Acad. Sci. U.S.A. 51:577-585.
2. Bialy, H. S., and C. Colby. 1972. Inhibition of early vaccinia virus ribonucleic acid synthesis in interferon-treated chicken embryo fibroblasts. J. Virol. 9:286-289.
3. Carter, W. A., and H. B. Levy. 1968. The recognition ofviral RNA by mammalianribosomes. An effect ofinterferon. Biochim. Biophys. Acta 155:437-443.
4. Dobos, P.,I. M. Kerr, and E. M. Martin. 1971. Synthesis of capsid and noncapsid viral proteins in response to en-cephalomyocarditisvirusribonucleic acid in animal cell-free systems. J. Virol.8:491-499.
5. Eggen, K. L., andShatkin,A.J.1972. In vitro translationof cardiovirus ribonucleic acid by mammalian cell-free ex-tracts.J. Virol.9:636-645.
6. Fantes, K. H. 1967. Purification of interferon from chick embryoallantoicfluidsandfibroblast tissue infected with inifluenza virus. J. Gen. Virol. 1:257-267.
7. Friedman, R. M. 1968. Interferon inhibition ofarbovirus proteinsynthesis.J.Virol. 2:1081-1085.
8. Friedman,R.M., R. M. Esteban,D. H. Metz, D. R.Tovell, and I. M. Kerr. 1972.Translation ofRNA by L cell ex-tracts:effectofinterferon. FEBSLett.24:273-277. 9. Joklik, W. K., and T. C. Merigan. 1966. Concerning the
mechanism of action of interferon.Proc. Nat. Acad.Sci. U.S A.56:558-565.
10. Jungwirth, C., I. Horak, G. Bodo, J. Linder, and R. Schultze. 1972. Synthesis of poxvirus-specific RNA in interferon-treatedcells.Virology48:59-70.
11. Kates,J., and J. Beeson. 1970.Ribonucleic acidsynthesis in vaccinia virus. 1. Themechanismof synthesis and release of RNAinvacciniacores.J.Mol.Biol.50:1-18. 12. Kerr,I.M. 1971. Proteinsynthesisincell-free systems; an
effectof interferon. J.Virol.7:448-459.
13. Kerr,I. M., R. E. Brown, and D. R. Tovell. 1972. Char-acterization of the polypeptides formed in response to encephalomyocarditis virus ribonucleic acid ina cell-free systemfrommouseascitestumorcells.J.Virol. 10:73-81. 14. Kerr,1.M., and E. M.Martin. 1972.Simple method forthe
isolation of encephalomyocarditis virus ribonucleic acid. J.Virol.9:559-561
15. Kerr,I. M., J.A.Sonnabend,and E.M.Martin. 1970. Pro teinsynthetic activityofribosomesfrominterferon-treated cells. J.Virol. 5:132-144.
16. Levin, J.G., and R. M.Friedman. 1971. Analysis of arbo-virus ribonucleic acid forms by polyacrylamide gel elec-trophoresis.J.Virol. 7:504-514.
17. Lowry, 0.H.,N.J.Rosebrough,A. L.Farr,and R. J. Ran-dall. 1951. Protein measurement with the Folin phenol reagent. J.Biol.Chem.193:265-275.
18. Marcus, P. I., D. L.Engelhardt, J. M.Hunit,and M. J. Se-kellick. 1972. Interferon action: inhibition of vesicular stomatitis virus RNA synthesis induced by virion-bound polymerase. Science 174:593-597.
19. Marcus, P. I.,and J. M. Salb. 1966. Molecularbasisof in-terferon action. Inhibition of viral RNA translation. Vi-rology30:502-516.
20. Martin, E. M., J.Malec, S. Sved,and T. S. Work. 1961. Studies onproteinandnucleic acidmetabolism of virus-infected mammalian cells. I. Encephalomyocarditis virus in Krebs 2 mouse ascites tumour cells. Biochens. J. 80: 585-597.
21. Mathews, M. B., and A. Korner. 1970. Mammalian cell-free protein synthesisdirectedbyviral RNA. Eur. J. Bio-chem. 17:328-338.
VOL. IO, 1972 1197
on November 10, 2019 by guest
http://jvi.asm.org/
FRIEDMAN ET AL.
22. Metz,D. H., andM. Esteban. 1972. Interferoninhibitsviral protein synthesis in L cells infected with vatccinia virus. Nature(London) 238:385-388.
23.Oxman,M. N., and M. J. Levin. 1971.Interferon aind tran-scription of early virusspecific RNA incellsinfected with simian virus 40. Proc. Nat. Acad. Sci. U.S.A. 68:299-302. 24.Paucker, K.,B. J. Berman, R.R. Golgher,anidD.Stanc~ek.
1970. Purification, characterization, and attempts at iso-topic labeling ofmouseinterferon.J.Virol.5:145-152.
25. Sonnabend, J. A., and R. M. Friedman. 1966. Mcchanisms ofinteiferon action. In N. B. Finter (ed.), Interferons.
North-Holland Publishing Co., Amsterdam.
26. Stewart, W. E.,It,L.B.Gosser, andR. Z.Lockart, Jr. 1971.
Priming: a nonantivirall functioni of interferon. J. Virol. 7:792-801.
27. Williamison, R., M.Morrison,G. Lanyon,R.Eason,andJ. Paul. 1971. Properties of mouse globin messenger ribo-nucleic acid and its preparation in milligram quantities. Biochemistry 10:3014-3021.