Copyright ©1972 AmericanSocietyforMicrobiology
Isolation and Preliminary Characterization of
Bacteriophages for Bdellovibrio bacteriovorus
MARY ALTHAUSER, WILLIAM A. SAMSONOFF, CAROL ANDERSON, AND S. F. CONTI Thomas Hunt Morgan School of BiologicalSciences, University of Kenttucky, Lexinigtoni,Kenitucky40506
Received forpublication 15 June 1972
Tenbacteriophages that attack and lyse saprophytic strains of Bdellovibrio bac-leriovoruswereisolated.Morphological, serological, and host-range studies revealed that there werefour different bdellovibrio phagespresentamongthe isolates. One of thephages lysedastrain ofB.bacteriovorusthatrequires thepresenceofasuitable bacterial host forgrowth.Thephageattachedtothe bdellovibrio cells in the absence of the bacterial host cells; lysisoccurred onlyin the presenceof host cells. The 19
saprophytic bdellovibrio strains employed in the phage host-range studies were
groupedonthe basis of theirsusceptibilitytophage lysis.
Bdellovibrio bacteriovorus was initially de-scribed as an ectoparasitic and bacteriolytic
or-ganism that attacks gram-negative bacteria (18). Subsequently, saprophytic strains were isolated (11, 13, 15, 17); some of these strains were capableofgrowth onartificial media andinitially
retained their parasitic abilities. A facultative strain designated UKi2, which is capable of growing in host-free media or endoparasitically onEscherichiacoli B/r, wasreported (5). Studies
of these parasitic, saprophytic, and facultative strains have considerably expanded our knowl-edge ofthe unique bacteria (14, 16). Neverthe-less, the classification ofB. bacteriovorus andthe interrelationships of the various strains have not
been clearly established.
Sincebacteriophages for bdellovibrio could be usedtoclassify thevarious strains of B.
bacterio-vorusand asprobes for elucidating someaspects
ofthehost-endosymbiont relationship, the avail-ability of phages could prove to be useful. We have reported (6) theisolation ofabacteriophage which attacks and lyses B. bacteriovorus strain UKi2; this bdellovibrio can grow either in the presence or absence of host cells (5). We have
isolated additional bdellovibrio phage which not
only lysed saprophyticstrains of B.
bacteriovorus,
but alsowereinfective for obligately endosymbi-oticstrains of bdellovibrio inasystem consisting ofbdellovibrio, bacterial host, and phage (Mary Althauser and S. F. Conti, Bacteriol. Proc., p.
173, 1971; Abstr. Annu. Meeting Amer. Soc. Microbiol., p. 220, 1972). Varon and Levisohn
(19) have recently also reported the isolation of bacteriophageswhich will attackaparasiticstrain
ofB. bacteriovorus.
This paper describes the isolation and
pre-liminary characterization offour different addi-tional bacteriophages forB. bacteriovorus.
MATERIALS AND METHODS
Media. The liquid medium used throughout this investigation for growing the bacteria and diluting the phage suspensions was peptone-yeast extract (PYE) whichcontains iS0 (w/v) peptone (Difco) and 0.3% (w/v) yeast extract (Difco). The pHwas adjustedto
7.2with 1 M NaOH. PYE agar plates for enumera-tion ofphage and parasiticbdellovibriowereprepared by adding1% (w/v)agartoPYE broth for the bottom layer and 0.7%agarin theoverlay.
Bacterialstrains and cultural conditions. The sapro-phyticB.bacteriovorusstrains (100,109, 110, 114, 118, 120, B, D, E, OX9-2, OX9-3, A3.12, 2484-2, 2484-3,
Sa 109, SaD3Smr, Xty) werekindly supplied by R. J. Seidlerand M. P.Starr. AfacultativestrainUKi2(5) and an additional saprophytic strain (UKil) were isolated fromraw sewage.Erwiniaamylovora, Proteits mirabilis, Serratia marcescenis, Spirillum serpenis, Sal-monlella typhimurium, and E. coli (ATCC 15144), furnishedby D. AbramorM.Starr,wereemployed in thehost-range determinations.
The saprophytic bdellovibrio strains were
trans-ferred every 12 hr using a 10% (v/v) inoculum. The members of the othergenera weregrowninasimilar manneremployinga 1% (v/v) inoculum for transfer. B.bacteriovorus 114 (ATCC 15362),aparasitic strain, was grown onE. coli (ATCC 15144) in PYE broth. Forstartercultures of theparasitic bdellovibrio, 9 ml
of a 12-hr PYE broth culture of E. coli (4 X 109)
cells/ml) ina16by120mmtesttubewasinfected with
1 ml ofan 18- to 24-hr B. bacteriovorus 114 culture
(108 cells/ml).
All liquid cultures were incubated at 30 C in a
gyratoryshaker (New Brunswick Scientific Co., New
Brunswick, N.J.).
Enumeration of bacteria. The saprophytic cells of
516
Prinlted in U.S.A.
on November 10, 2019 by guest
http://jvi.asm.org/
B. bacteriovorus and the bacterial host cells were enumerated by colony counts on PYE agar and the parasiticcells byplaque assayaspreviously described (5).
Isolation of bacteriophage. Two procedures were used to isolate bacteriophage. In the first isolation procedure 100ml of raw sewagefrom the Lexington, Kentucky, sewage treatment plant were clarified by lowspeed centrifugation, and the supernatant fluid
waspassed throughdisposable filters of 0.45- and
0.2-/Am
poresize(NalgeCo., Rochester, N.Y.). The filtratewascentrifuged at40,000 X g for 1 hrinaBeckman modelL-2ultracentrifuge. Thepelletwasresuspended in 10.0ml ofPYEbroth, and 1 mlof thesuspension
was plated with 0.05 ml ofa 12- to 14-hr culture of each of thefollowing strains ofB. bacteriovorus: 100, 109, 118, D, E, UKil, UKi2, 2484-2, and Xty, using thedouble-layer platingproceduredescribedbelow.
The secondmethod usedwasavariation of the
con-ventional enrichmenttechniquedescribed forisolating bacteriophage. A 2-liter flaskcontaining 1 liter ofraw
sewage was inoculated with 20 ml of an overnight culture of each of the nine strains and incubated at
25 Cfor72hr.The flaskswereoccasionallyshakenby handduringthisperiodof time. Afterincubation, the suspensions were clarified and filtered as described above. A 10-ml amount ofa 12- to 14-hr culture of each saprophytic bdellovibrio was added to a 1-liter flask containing 500 ml of filtrate from thefirst
en-richment.A5-mlamountofa20%- (w/v) yeastextract
(Difco) solution was then added to the flask. After incubation at 25 C for 72 hr,the suspensions were
clarified, filteredthroughadisposablefilter of0.45-jim pore size, and plated as described in thefirst enrich-ment procedure.
Plaques which appeared to be ofdifferent size or
morphology, or both, were picked separately, and if subsequent platings showed these differences were
consistent they were treated as different isolates. Plage werepurified by atleastthree successivesingle plaque isolations. High-titered stocks of each of the phagewereprepared by the plate lysis technique (1). Enumeration ofphage. Forthe phage isolation
ex-periments, petri dishes containing approximately 20 ml ofPYEagarwereoverlaid withamixture of2ml ofPYEoverlay agar, either 0.05 ml or0.3 mlofan
exponential-phase culture oftheorganism,and 1.0 ml
of the material being tested for phage. The plating procedurewas modified in the experiments to deter-minethe hostrange ofthephageandphagetitersby mixing0.1 mlofanappropriatedilution ofthephage suspension with the overlay agar. All plates were
incubatedat30Cfor24to48hr. Theorganismsused
inthephage host-range studies included allthestrains
ofbdellovibrio and the members ofthe othergenera
listed underbacterialstrains.
Effect ofphageMAC-3 inathree-memberedsystem.
The effect ofone ofthe phages (MAC-3) on the B.
bacteriovoruis 114-E. colisystemwastested by
adding
4 X 108
plaque-forming
units(PFU)/ml
to a 12-hrculture ofE. coli (4 X 109/ml)
immediately
after theaddition of the bdellovibrio (108
cells/ml). Samples
were taken from the tube with the three-membered system at zerotimeandafter 12 and 24 hr of
incuba-tion at 30 C and assayed for numbers ofbdellovibrio, E.coli, and phage. Control tubes, lacking one or more components of the system, were treated similarly; ex-periments were repeated a number of times with equiv-alent and reproducible results.
To determine the adsorption kinetics of MAC-3 to to B.bacteriovorus 114, 3 X 109 PFU of bdellovibrio cells per ml were infected with MAC-3 at a multi-plicity of infection (MOI) of 0.2. Samples (0.1 ml)
were removed at various time intervals and diluted with 9.9 ml of cold PYE broth containing a few drops ofCHCl3. The mixtures were shaken on a Vortex shaker for 20 sec and titrated after 1 hr.
Totestdirectly the effect of MAC-3 on the motility of thebdellovibrio cells, 2 X 109 B. bacteriovorus 114 cells/ml were mixed with MAC-3 at ratios of phage to bdellovibrio of 30, 3, and 0.2 and incubated at 30 C. Control tubes contained bdellovibrio cells without phage. Samples were removed after 15, 30, and 60 min of incubation, and the motility of the cells was immediately checked by observation with a Zeiss Universal phase microscope.
Todetermine the effect of the phage on the ability of thebdellovibrio to attach to and penetrate the host cells, samples from the two-membered system des-cribed above and samples from the control tubes were added to 4 X 109 E. coli cells. The attachment and penetration of the parasites within the host cell were observed by phase-contrast microscopy over a 2- to 3-hrperiod.
Serology. Antigen preparations were obtained by propagating each phage in PYE broth cultures of an appropriate saprophytic strain of B.
bacteriovorius.
The phage from the broth lysates were then purified by differential and sucrose density gradient cen-trifugation, followed by dialysis against phosphate-buffered salineasdescribed by Bradley (2). The purity of each phage preparation was examined by direct electron microscopy, the amount of protein was determined by the procedure of Lowry et al. (7), and plaques were assayed. Six intravenous injections, each containing30 to 50
jig
ofviral protein, were given to rabbits at 3- and 4-day intervals. Rabbits were bled fromtheheart 10 days after the last injection.Each antiserum was tested first against the homol-ogousphageand thenagainsttheremaining phagesto
detect cross-neutralization reactions. In both
neu-tralization tests, heat-inactivated (56 C, 30min) anti-serum wasdilutedandmixed withanequal volume ofa
phage preparation containing 107 PFU/ml. At
10-min intervals, 0.1-ml samples of the phage-serum mixture were withdrawn and added to 9.9 ml of diluent to stop the neutralizing action. From these tubes, further dilutions were madeand samples were
plated bythedouble layer procedure. Neutralization
velocityconstants (k min-') foreach antiserum were determined as described by Adams (1). The per-centage of phage neutralized was determined, and a
velocity constant,k,wascalculatedat
99'%
neutraliza-tion by using the relationship k = 2.3 D/t X log Po/P ; where D is the dilution ofantiserumused in the determination; t, the time required to obtain 99% neutralization; pa, the plaque count before the
addi-tion of antiserum; and p, the plaque count after neutralization.
on November 10, 2019 by guest
http://jvi.asm.org/
ALTHAUSER AL. Electron microscopy. Phagespecimens for negative
staining were obtained by placing a drop of the purified phage preparation on a carbon-coated grid or by lightly touching the grid to an isolated phage plaque; specimens were stained with 0.2% uranyl acetate.
Thin sections were prepared by using previously described procedures (4); all specimens were
ex-amined in a Philips EM 200 electron microscope operatingat 60kv.
RESULTS
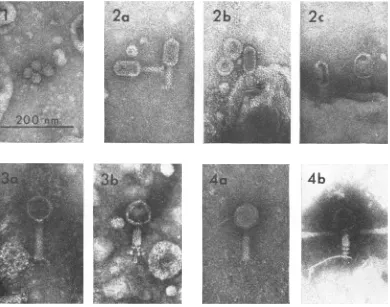
Phage isolation and morphology. Ten bacterio-phages that attacked and lysed saprophytic bdellovibrios were isolated from raw sewage (Table 1). Two different
morphological
plaque types were observed on the plates ofthe
phages initially designated as MAC-1 and MAC-4; each plaque wassubsequently purified,
andthe
second typewas designated with a prime notation.Microscopic
examination of all ten phage iso-lates revealed four distinctmorphological
types. Seven of thephage
isolates(MAC-1,
1', 2, 4, 4', 5, and7)
were ofidentical structure and corre-spond to Bradley's group E (3). All are tailless, have a regularhexagonal outline,
and areap-proximately
25nmindiameter (Fig.
1).The
remaining
isolates weretypical
of Brad-ley's type Abacteriophage, possessing
hexagonalheads,
longtails,
andcontractilesheaths.
MAC-3 (Fig. 2a) has an elongatedhexagonal
head 75 nmby
50 nm indiameter,
and an overall length of150 nm. Subunits ofthe
tailareclearly visible
(Fig. 2a), and its double
base
plate and four tail pins are best seen in Fig. 2b.The apparentswell-ing
orcollapsing
ofthe
headfrequently
seen when this phage attaches toits host isshown
inTABLE 1. Neutralizilg velocitiesaofB.
bacteriovoruis
by phageanitiserutm
Phage AIAC-36 -MAC-6 TIDC-2 HDC-1 AIAC-1
MAC-3 ... 698 -C
MAC-6 ... - 115
_-HDC-2 -- 41
HDC-1'1.
70MAC-1 2,705
MAC-1'
.
- 1,468MAC-2 2,188
MAC-4
._
- 2,482MAC-4' -- - -- 1,889
MAC-5 .. 2,610
MAC-7 2,595
a Expressed as k values (min-'); k values
deter-mined as described in Materials and Methods. h Phage antisera tested.
ck values ofless than I arerecorded as -dThis phage was previously isolated and described (6).
Fig. 2c. MAC-6 hasan overalllength of 180 nm
and a head diameter of 65 nm (Fig.
3a).
The complex tail assembly, consisting ofasingle base plate and three or four tail pins with spherical structures on their distal end, is shown in Fig. 3b. HDC-2 hasahead of 70to 75nmand is 150to 160 nm
long.
A complex tail assembly asseen on MAC-6 was not seen on the intact or
contracted HDC-2particles (Fig.4a,
b).
Table 1 summarizes the results of cross-neu-tralization tests conducted to detect serological relationships among the various phage isolates. Antisera to phages MAC-3, MAC-6, HDC-2, andHDC-1 had neutralizing activity onlyagainst the homologous phage. The MAC-1
antiserum,
however, neutralized the homologous phage, MAC-1, and also neutralized phages MAC-l', MAC-2, MAC-4, MAC-4', MAC-5, and MAC-7. Similar first-order inactivation kinetics were observed when each of the seven phages was exposedto MAC-1 antiserum, indicating that the seven spherical phage are closely related, if not identical.Host range of the phage isolates. The host ranges of the phages among the saprophytic bdellovibrio strains are summarized in Table 2. The spherical phage MAC-1, like all ofthe other spherical phage isolates had a rather broadhost range andlysed 12 ofthe 19 bdellovibrio strains tested. MAC-3, one of the tailed phages, had a more narrow host rangelysing7 of the 19strains and was the only isolate to lyse strains 110 and 120. MAC-6, HDC-2, and HDC-1 had very specific host ranges. None ofthe phage isolates lysedstrainsUKil, A3.12, oranyofthebacterial hosts used topropagatethe bdellovibrio.
Effect of MAC-3 on a host-dependent B. bac-teriovorus. The effect of MAC-3 on a parasitic strain ofB. bacteriovorus 114 grown on E. coli is shown in Table3. Inthetestsystemcontaining bdellovibrio and host cells, there was a 500-fold increase in bdellovibrio cells after 24 hr of incu-bation. In the presence of MAC-3 phage, the number of bdellovibrio cells decreased 100-fold in 24 hr, with an
accompanying
1,000-fold increase in the phage titer. -he MAC-3 phage did not multiply on either the parasitic bdello-vibrio cells in the absence of host or on E. coli. Inaccordance withtheseobservations,
therewas an increase in the number ofviable E. colicells remaining after incubation with the bdellovibrio in thepresenceofphage.Since thefrequency ofmutation ofparasiticto
saprophytic cells is relatively high (11), during thecourse ofthe experimentation weassayed for saprophytic cells in the B. bacteriovorus strain 114cultureemployed; none weredetected.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
2a
2b
:
2c
[image:4.493.49.439.73.380.2]4b
FIG. 1. Negativestainpreparationofthetailless, hexagonallyshapedphageisolate MAC-i. Scalemarker rep-resents200irmandappliestoFig. Ithrough4.
FIG. 2. Negativestainpreparationsofphage MAC-3. Theelongatedhead and subunits ofthetail assemblyare
shownt(a);the double baseplate (arrows)and small tail pinsareevident(b).The headsof MAC-3areusually spher-icalorcollapsed after attachmenit toits host(c).
FIG. 3. Negativestainpreparationsofphage MAC-6. The subunitstructureof the uncontracted tail (a) and the complexstructureofthetailpinsotn the contractedphage particles(arrows, b)areillustrated.
FIG. 4. NegativestainpreparationsofphageHDC-2. Thephagehasahexagonalhead anddistinct tailsubunits
(a). The contractedphage particle (b) doesnotshowanycomplex tailstructures.
TABLE 2. Host range oJfive B. bacteriovorusphages
LysisofsaprophyticB.bacieriovoruisteststrai.'sb
Phage__
100 109 114 SaD3Sm' 118 D |x9-2 (x9-3 2484-2 2484-3 Xty Sa109 110 120 B E UKi2
MAC-I +r + + + + ++ + + + + +
MAC-3 + + + + ±-- - - +
--MAC-6 - -- - --- - -
-+
+-HDC-2 - T- 't - _ - .._ +
HDC-1 - --
-
-.+
aThehomologous host (saprophytic bdellovibrio strains used for initial phage isolation) ofphage
MAC-1, MAC-3,andMAC-6areB.bacteriovorus strainsXty, 118,and E,
respectively.
Thefacultatively parasitic bdellovibriostrain UKi2was usedfor initial isolation ofphagesHDC-1 andHDC-2.bB.bacteriovorusstrains UKil and A3.12 were notlysed byanyofthe phages.
c+, Lysis ofcells; -,no lysis of cells.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.493.44.440.506.601.2]TABLE 3. Effect ofphage MAC-3 o01 the host-depenzdent Bdellovibrio bacteriovorus straini 114a
Testsystemb
1. Escherichia coli
B. bacteriovorus 114 MAC-3
2. E. coli
B. bacteriovoruts 114
3. B. bacteriovorus 114
MAC-3
4. E. coli MAC-3
5. MAC-3
6. B. bacteriovorus 114
7. E. coli
to
4 X 109
1x 107
4 X 107
4 X 109
1 X 107
I X 107 4 X 107
4 X 109 4 X 107
4 X 107
1 X 107
4 X 109
aThe test systems which contained bdellovibrio cells were assayed for the presence of saprophytic
bdellovibrio cells atzero time and after 12 and 24 hr ofincubation; none weredetected. E. coli viable
cellcounts per milliliterwere enumerated byplating onPYEagar, and parasiticcells by plaque assay
asdescribed inMaterialsandMethods;phagecountspermilliliterweredeterminedby thedouble-layer technique (1).
bPYE brothwasaddedtothe testsystems which containedone ortwomemberstomaintainavolume of 10ml. All cultureswereincubated at 30 Con agyratoryshaker.
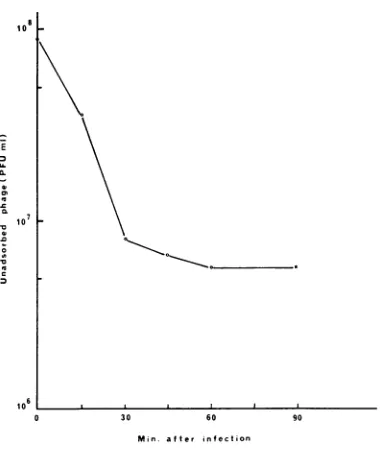
Whenthe bdellovibrio cellsandthephagewere mixedinPYE brothataratio of 50 to 1,
99%/
of MAC-3 adsorbed to B. bacteriovorus 114 after 1.5 hr of incubation at 30 C (Fig. 5). Electron micrographs of negatively stained preparations from thethree-membered system ofphage, bdel-lovibrio, and E. coli (Fig. 6) revealed theattach-ment ofa bdellovibrio cell to host after 1 hr of
incubation of the bdellovibrio and MAC-3 in a
ratio of1 to 30. A section ofa sample from the three-membered system of phage, bdellovibrio, and host which was incubated at 30 C for 4 hr
shows MAC-3 particles insidea bdellovibrio cell which is withinanE. colicell(Fig. 7).
MAC-3 didnotaffecteither themotility of the bdellovibriocells in the two-membered systemor the attachment and penetration ofthe parasites into the hostcells,evenwhenratiosashighas 30
phageto 1 bdellovibrio cellwere employed.
These data and observations support the
con-clusion that MAC-3 adsorbs to and multiplies
on B.bacteriovorus strain 114growingon E.coli.
DISCUSSION
Although ten bacteriophages wereinitially iso-lated for various strains of B. bacteriovorus our
results indicate that only four different phages
were present among the initial isolates. he
morphological and serological data and the host
30 60 90
FIG. 5. Adsorption of'MAC-3 to B. bacteriovoruis 114.Bde/lovibrio cells (3 X 109PFU/ml inP YEbrotlh)
wereilifected wvithMAC-3ataiiMOIof0O.2.Samnplesof
0.1 m/l were renmoved at various time intervals and
diluted in9.9 nil of coldPYEbroth conitaininlg a
fiew
dropsofCHC/3. Themixtures wereslhakeliina Vortex
mixerfor20secanidassayed/clrphagealterIhr.
12hr
3 X 109
7 X 107 6 X 108
5 X 109
7 X 108
1 X 107
8 X 107
4 X 109
1 X 108
1 X 10,
1 X 107
5 X 109
24 hr 3 X 109 1 X 105
3 X 101°
5 X 103
5 X 109
3 X 101 5 X 107
4 X 109 1 X 10t
1 X 108
5 X 106
4 X 109
E
CL
0 a, n
CL.C
-2
c
n
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.493.58.455.82.279.2] [image:5.493.266.456.355.581.2]BACTERIOVORUS
P
.A
El,
/B
Bd
0.5
pm
[image:6.493.46.438.64.606.2]hWA.W.
FIG. 6. Negativestainlpreparationlofathree-memberedsystem. C0ontractedMAC-3phlageparticlescanlbeseen
adherinigto thehdellovibriocell, which in turntisattachedtoani E.colicell. Uniattached, iiliconitractedphageare
presenit
(triplearrow).FIG. 7.
Thlinl-sectionz preparationz
illustratinlgMAC-3phage particles (P) withinabdellovibriocell(Bd),w1hic1his. locatedintheperiplasmicspace ofaniE.coli cell(E).52-1
1972
.i,,..A"
N.,
II
on November 10, 2019 by guest
http://jvi.asm.org/
ranges of the phage support the conclusion that phages MAC-1, 1', 2, 4, 4', 5, and 7 are very
similar, ifnotidentical; MAC-1 has been selected astherepresentative of thisgroup.The data also
support the conclusion that MAC-3, MAC-6, and HDC-2areseparateand distinctbdellovibrio phage types. It thus appears that seven dif-ferent phages now havebeen isolated which are
infective forsomestrains ofbdellovibrio: MAC-1,
MAC-3, MAC-6, HDC-2, the previously de-scribed HDC-1 (6), and the VL-1 and VL-2 phages recently isolated by Varon and Levisohn (19). The apparent ease with which phages for B. bacteriovorushave been isolated indicates that utilization ofconventional procedures, or minor modifications thereof, should result in the isola-tion ofadditional phages from natural habitats containing B. bacteriovorus.
Themost extensive studieson thesaprophytic strains of bdellovibrio (9, 11, 12) indicatea very close similarity between all of the saprophytic strains tested with the exceptionofstrains A3.12 and the facultative B. bacteriovorus UKi2. Fur-thermore, there are no completely satisfactory methods available for differentiating between the 20 or so saprophytic strains used in various laboratories, or for determining the number of truly distinct saprophytic strains. Our data (Table 2) on the susceptibility of the various saprophytic strains to the phage isolates illus-trates that some of the strains can be differen-tiated from others. On the basis of phage
sus-ceptibility,
thesaprophyticstrainscanbegrouped as follows: Group I, strains 100, 109, 114,SaD3Smr, 118; Group II, strains D,
Ox9-2,
2484-2, 2484-3, Xty, Sa 109; Group III, strains 110 and 120; Group IV,strains B andE; Group V, strains UKi2; and Group VI, strains A3.12 andUKil, whichare strains notinfected byany
of the phage isolates. The availability of addi-tional phages and appropriate antiserum,
com-bined with otherapproaches, should allow differ-entiation between strains within a particular
group, and perhaps establish more definitively
whetheraseparatestraindesignationiswarranted
for allofthesaprophytic bdellovibrio isolates. TherecentreportbyVaron and Levisohn(19) andthe datapresentedin thispapersupport our
previous contention (Mary Althauser and S. F. Conti, Bacteriol. Proc., p. 173, 1971) that some phage which attack, lyse,andreplicateon sapro-phytic strains of bdellovibrio are also infective
for parasitic strains, provided that a suitable
host bacterium is present inthetestsystem. The requirementfor thepresenceofahostbacterium ispresumably duetotheinability of the parasitic bdellovibrio togrowand divideintheabsence of
hostcells, although they are metabolically active
(14).
The
three-membered
system (host,bdello-vibrio, and phage)
described
by Varonand
Levi-sohn
(19)
issimilar
to our system,but there
are somesignificantdifferences.
Intheir
system, and undertheir conditions, host-free cultures of bdel-lovibrio which are unable to supportphage
growth can
adsorb the
phage particles and aresubsequently
killed.
As a consequence, even adelay of
afew
minutes in the addition
ofthe host
cells
tothe
bdellovibrio-phage
system resultsin
adiminished
phage yield.
In our systemthe
incu-bation of the
phage-bdellovibrio
mixture for as long as 6hr before addition of host
cells did nothave
any noticeable effect onthe
phage yield. Varonand
Levisohn
alsoobserved that the
motility
ofthe
bdellovibrio cells
ceased 10 to 20 minafter
addition of
the phage; this
did not occur in our system.The observations that
phage
which can infectsaprophytic strains
can also attackbdellovibrio
which
require suitable bacterial
host cells forgrowth
is notunexpected in
view of the
observa-tion of
Reiner and
Shilo (8) that
socalled
host-dependent
orwild-type strains
of B. bacteriovorus canelongate in
the absence of host cells when
incubated in cell-free microbial
extracts.Subse-quent work
has resulted in the isolation
of afactor
which, when added
toaparasitic strain
of B.bacteriovorus,enables
cells
togrowand
divide,
i.e.,
cells
gothrough
atleast
onecycle of
elonga-tion and
fragmentation (Shilo,
personal
com-munication).
Itwould
therefore
appearthat the
major difference
between
saprophytic and
para-sitic
bdellovibrios
is
their
ability
to growand
divide in the absence of
a"factor"; parasitic
bdellovibrio
canobtain the "factor" from
suit-able host bacteria whereas
saprophytic
strains
can
either
synthesize
this
factor
ordo
notrequire
it.
Presumably, all so-called host-dependent
bdellovibrio
canbe "transformed" into
host-independent
cells
by addition
ofthe "factor"
tothe medium under
appropriate
conditions. We
are
presently
testing
the
infectivity of
ourfive
phage isolates
against
anumber
ofparasitic
bdellovibrio
withthe expectation
thatphage
infective
forsaprophytic
strains will infect theclosely related
parasitic strains.
ACKNOWLEDGMENT
This researchwassupportedbyNational Science Foundation grantGB 7972.
LITERATURE CITED
1. Adams, Mark. 1959. Bacteriophages, Wiley (Interscience), NewYork.
on November 10, 2019 by guest
http://jvi.asm.org/
2. Bradley, D. E. 1966. Thefluorescent staining cf bacteriophage nucleic acids. J. Gen. Microbiol. 44:383-391.
3. Bradley, D. E. 1967. Ultrastructure of bacteriophages and bacteriocins. Bacteriol. Rev. 31:230-314.
4. Burnham, J. C., T. Hashimoto, and S. F. Conti. 1968.Electron microscopic observationsonthe penetration of Bdellovibrio bacteriovorus into gram-negative bacterial hosts. J. Bac-teriol. 96:1366-1381.
5.Diedrich, D.L.,C. F.Denny,T.Hashimoto,andS. F. Conti. 1970. Facultativelyparasitic strainofBdellovibrio
bacterio-vorus.J. Bacteriol. 101:989-996.
6. Hashimoto, T.,D. L.Diedrich, and S. F. Conti.1970. Isola-tion of bacteriophage for Bdellovibrio bacteriovorus. J. Virol.5:97-98.
7. Lowry, 0. H., N. J. Rosebrough, A. L. Farr, and
R. J. Randall. 1951. Proteinmeasurementwith the Folin phenolreagent.J. Biol. Chem. 193:265-275.
8. Reiner, A. M., and M.Shilo. 1969. Host-independent growth ofBdellovibrio bacteriovorus in microbial extracts.J. Gen. Microbiol. 59:401-410.
9. Seidler, R. J., M. Mandel,and J. N. Baptist. 1972. Molecular heterogenlity of the bdellovibrios: evidence of two new
species. J. Bacteriol. 109:209-217.
10. Seidler, R. J., and M. Starr.1969. Factors affecting the intra-cellular parasitic growth of Bdellovibrio bacterioi'orus de-veloping within Escherichia coli. J. Bacteriol. 97:911-923.
11. Seidler,R.J., and M. P. Starr. 1969. Isolation and
characteri-zation of host-independent bdellovibrios. J. Bacteriol. 100:769-785.
12. Seidler, R. J., M. Starr, and M. Mandel. 1969. Deoxyribo-nucleic acid characterization of bdellovibrio. J. Bacteriol. 100:786-790.
13. Shilo, M. 1966. Predatory bacteria. Sci. J. (Londorn) 2:33-77. 14. Shile, M. 1970. Morphological and physiologicalaspectsof the interaction of Bdellovibrio with host bacteria. Curr. Top. Microbiol.50:174-204.
15. Shilo, M., and B. Bruff. 1965. Lysis of gram-negative bacteria by host-independent ectoparasitic Bdellovibrio bacteriovorus isolates. J. Gen. Microbiol.40:317-328.
16. Starr, M. P., and R. J. Seidler. 1971. The bdellovibrios. Annu. Rev. Microbiol. 25:649-678.
17. Stolp, H. 1968. Bdellovibrio bacteriovorus-ein rauberisher Bakterienparasit.Naturwissenschaften 55:57-63.
18.Stolp, H., and M. P. Starr. 1963. Bdellovibrio bacteriovorus
gen.etsp. n., apredatory, ectoparasitic and bacteriolytic microorganism. Antonie van Leeuwenhoek J. Microbiol. Serol.29:217-248.
19. Varon, M., and R. Levisohn. 1972. Three-membered parasitic system: a bacteriophage, Bdellovibrio bacteriovorus, and Escherichia coli. J.Virol. 9:519-525.