Topoisomerase II

for Latent DNA Replication of the Terminal
Repeats
Pravinkumar Purushothaman, Maria E. McDowell, James McGuinness, Ruth Salas, Sharif M. Rumjahn, and Subhash C. Verma
Department of Microbiology & Immunology, University of Nevada, Reno, School of Medicine, Center for Molecular Medicine, Reno, Nevada, USA
The latency-associated nuclear antigen (LANA) encoded by Kaposi’s sarcoma-associated herpesvirus (KSHV) plays a major role in maintaining latency and is critical for the perpetual segregation of viral episomes to the progeny nuclei of newly divided cells. LANA binds to KSHV terminal repeat (TR) DNA and tethers the viral episomes to host chromosomes through the association of chromatin-bound cellular proteins. TR elements serve as potential origin sites of KSHV replication and have been shown to play important roles in latent DNA replication and transcription of adjacent genes. Affinity chromatography and proteomics analysis using KSHV TR DNA and the LANA binding site as the affinity column identified topoisomerase II(TopoII) as a LANA-inter-acting protein. Here, we show that TopoIIforms complexes with LANA that colocalize as punctuate bodies in the nucleus of KSHV-infected cells. The specific TopoIIbinding region of LANA has been identified to its N terminus and the first 32 amino acid residues containing the nucleosome-binding region crucial for binding. Moreover, this region could also act as a dominant negative to disrupt association of TopoIIwith LANA. TopoIIplays an important role in LANA-dependent latent DNA repli-cation, as addition of ellipticine, a selective inhibitor of TopoII, negatively regulated replication mediated by the TR. DNA break labeling and chromatin immunoprecipitation assay using biotin-16-dUTP and terminal deoxynucleotide transferase showed that TopoIImediates a transient DNA break on viral DNA. These studies confirm that LANA recruits TopoIIat the origins of latent replication to unwind the DNA for replication.
K
aposi’s sarcoma-associated herpesvirus (KSHV), also calledhuman herpesvirus 8 (HHV-8), is linked to Kaposi’s sarcoma, primary effusion lymphomas (PELs), and multicentric
Castle-man’s disease (MCD) (40,41,64). KSHV predominantly causes
tumors in individuals that are immunocompromised either by HIV infection or by immunosuppressive drug therapies and is
among the leading cause of AIDS-related deaths (12). Like other
herpesviruses, KSHV exhibits latent as well as lytic modes of in-fection and persists predominantly in the latent form, wherein only a subset of proteins are expressed, including the
latency-associated nuclear antigen (LANA) (16,24,63,69). LANA is
con-sistently expressed in all forms of KSHV-positive tissues and cell
lines (14,38,45,64). However, a small fraction (1 to 5%) of
in-fected cells spontaneously undergo lytic replication (reactivation), which is likely to be essential for maintaining the population of
newly infected cells and the development of viral pathogenesis (10,
20,46,66). LANA, encoded by open reading frame 73 (ORF73), is
a large nuclear protein (222 to 234 kDa) that regulates transcrip-tion, cellular signaling, viral DNA replicatranscrip-tion, and genome
main-tenance (44,63). In its lifelong latent state, KSHV genomic DNA
exists as a closed circular episome tethered to host chromosomal
DNA and is packaged onto nucleosomes with cellular histones (2,
6,14,63). This maintenance function is mediated by direct and
indirect binding of LANA to the viral DNA and host
chromo-somes (3,6,8,33,54).
LANA is a multifunctional protein that plays a central role in maintenance of latency, segregation of episomes, and oncogenesis
(26,63). LANA has been shown to modulate cellular transcription
by altering various cellular and viral promoters and transcription
factors (1,4,8,51,62,65). LANA has also been shown to regulate
various proto-oncogene and tumor suppressors at the
posttran-scriptional level (9,13,17,43,49,52,63). Several of these
interac-tions have crucial effects on proliferation and survival of the in-fected cells. LANA has been shown to induce chromosome instability and Survivin (a cellular inhibitor of apoptosis)
expres-sion to enhance proliferation of KSHV-infected cells (35, 52).
LANA interacts with K-bZIP and suppresses lytic origin (ori-Lyt
)-dependent DNA synthesis (48). LANA also interacts with Bub-1
and CENP-F to promote long-term persistence of KSHV episome
in the infected cell (68). Further, LANA can deregulate host-cell
interactions with the immune system and attenuate the antiviral
response (29) and inhibits interleukin-4 (IL-4)-mediated STAT6
phosphorylation to regulate apoptosis and maintain latency (7).
In addition, LANA maintains KSHV latency by repressing the
transcriptional activity of viral immediate-early gene, rta
(ORF50), which activates the switch from latency to lytic
replica-tion (28,32).
In addition to modulating the transcription of viral and cellu-lar genes, LANA recruits a number of molecules to regulate repli-cation of the viral episome and the segregation of the newly syn-thesized genome copies to daughter nuclei by tethering to the host
chromosomes (18,30,31,50,51,59). LANA has three distinct
domains: a proline-rich N-terminal region, important for binding with host chromosomes; a long glutamic acid-rich internal repeat
domain; and a carboxy-terminal domain (63). LANA mediates
tethering of the KSHV genome by binding to the terminal repeats
Received3 April 2012Accepted28 June 2012
Published ahead of print3 July 2012
Address correspondence to Subhash C. Verma, scverma@medicine.nevada.edu.
Copyright © 2012, American Society for Microbiology. All Rights Reserved.
doi:10.1128/JVI.00839-12
on November 7, 2019 by guest
http://jvi.asm.org/
through its carboxy terminus and associating with components of the human chromatin at its amino terminus, which includes
his-tones and MeCP2 (3,6,14,18,37). The LANA C-terminal domain
binds directly to two LANA-binding sites (LBS) in the KSHV ter-minal repeats (TR) adjacent to the replication element (RE),
which confers DNA replication origin of the TR (3,18,22,23,55).
The long-term persistence of KSHV depends on its effective interaction with the host cellular machinery. Genome replication and viral gene transcription are consistently dependent on the involvement of a number of cellular processes and appear to be
synchronized with the host cell cycle (4,53,63). KSHV genomes
replicate once per cell cycle during latency and are partitioned perpetually into daughter cells along with host chromosomes
dur-ing mitosis (3,4,63). KSHV-infected PEL cells maintain between
50 and 100 copies of episomes per cell, and the copy number appears to be retained at the same number over time after multiple
rounds of cell division (2,11,42,57). Since LANA has no
detect-able polymerase or helicase activity required for DNA replication, this strongly suggests that replication of the KSHV genome is de-pendent on enzymes that contain these activities and core
com-ponents of the cellular replication machinery (42). Association of
topoisomerase II (TopoII) with the KSHV TR region was
iden-tified by DNA affinity chromatography and proteomics analysis
using KSHV TR DNA orori-LytDNA as an affinity ligand and was
furthermore demonstrated to be essential for KSHV lytic DNA
replication (19,53,67).
TopoIIis an enzyme that controls and alters the topologic
state of DNA during transcription and replication. TopoIIhas
been shown to induce double-stranded (ds) breaks required for
regulated transcription/replication (15,25,56). Our results show
that LANA interacts with TopoIIand colocalizes with TopoII
as punctuate bodies in the nuclei of KSHV-infected BCBL-1 and
JSC-1 cells. The binding domain of LANA to TopoIImapped to
its amino-terminal chromosome-binding region. TopoII was
shown to play an essential role in LANA-dependent latent DNA replication of TR-containing plasmids, since cells treated with ellipticine, a selective inhibitor of TopoII, negatively regulated replication mediated by the TR. Additionally, we show that
TopoIImediates transient DNA breaks on KSHV DNA in order
to initiate replication. These studies confirm that LANA recruits
TopoIIat the origins of latent replication to unwind the DNA for
replication.
MATERIALS AND METHODS
Plasmids, antibodies, and cell lines.pA3F-LANA, pA3F-LANA deletion constructs carrying the Flag-tagged ORF73 amino-terminal domain (amino acids [aa] 1 to 340) and carboxy-terminal domain (aa 940 to 1162), and KSHV TR-containing plasmids were described earlier (58,59, 61). The GFP-LANA deletion constructs and their mutants carrying Myc-tagged ORF73 aa 1 to 340 and aa 1 to 32 were constructed by PCR ampli-fication from LANA constructs and inserted into pEGFP-myc vector. Lentiviral construct pLVX-LANA-YFP-Flag was constructed similarly by PCR amplification from LANA constructs and inserted into pLVX-AcYFP-C1-Flag vector. Alanine substitutions in green fluorescent protein (GFP) LANA aa 1 to 32 were introduced by PCR mutagenesis with the oligonucleotides described earlier (6). KSHVori-Lytplasmid was ob-tained from the Greg Pari laboratory (University of Nevada, Reno). GFP-TopoIIplasmid expressing full-length TopoIIisoform fused to GFP in the pEGFP-C3 vector was a generous gift from William T. Beck, Univer-sity of Illinois at Chicago (39). Myc-tagged proteins were detected using mouse hybridoma 9E10. The following commercially available antibodies
were used: rabbit anti-TopoII-H286 (Santa Cruz Biotechnology Inc., CA), rat LANA (Advanced Biotechnologies, Inc.), mouse anti-GAPDH (US Biological), and mouse anti-Flag (Sigma-Aldrich).
The KSHV-negative cell line BJAB and the KSHV-positive cell lines BCBL-1 and JSC-1 were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mML-glutamine, and
penicillin-strepto-mycin (5 U/ml and 5g/ml, respectively). Human embryonic kidney 293 (HEK 293) cells, mouse embryonic fibroblast (MEF) wild-type cells, and TopoIIknockdown (TopoII⫺/⫺) MEF cells (36) (gift from Yi Lisa Lyu,
UMDNJ-Robert Wood Johnson Medical School) were cultured in Dul-becco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 2 mML-glutamine, and penicillin-streptomycin (5 U/ml and 5g/ml, respectively). All cell lines were grown at 37°C in a humidi-fied environment supplemented with 5% CO2.
Indirect immunofluorescence microscopy.BCBL-1 and JSC-1 cells were washed with phosphate-buffered saline (PBS) and spread evenly on coverslips before air drying. Vero cells containing KSHV bacterial artifi-cial chromosome (BAC) (BAC36) were grown on coverslips for 24 h in order to attach and spread. The cells were fixed for 10 min at room tem-perature with 4% paraformaldehyde followed by permeabilization with 0.2% Triton X-100 in PBS for 10 min at room temperature. For blocking, cells were incubated with PBS containing 0.4% fish skin gelatin and 0.05% Triton X-100. Fixed cells were then incubated with primary antibodies for 1 h at room temperature, washed with PBS, incubated with Alexa Fluor secondary antibodies (Molecular Probes) for 45 min at room tempera-ture, and washed with PBS. Nuclear stain TO-PRO 3 (Molecular Probes) was used to counterstain the nucleus. Images were obtained using a laser scanning confocal microscope (Carl Zeiss, Inc.).
Immunoprecipitation.For immunoprecipitation, cells were washed with PBS and lysed in NP-40 buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, and 1% NP-40 supplemented with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 10g of pepstatin/ml, 10g of leupeptin/ml, and 10g of aprotinin/ml). The lysates were cen-trifuged at high speed to remove the cell debris. The lysates were incubated with protein A beads for 1 h at 4°C to preclear before incubation with specific antibodies. The precleared lysates were then incubated with anti-Flag or anti-LANA antibody overnight at 4°C with rotation followed by capture of the immune complex with protein A and G Sepharose beads at 4°C for 1 h. The resulting immunoprecipitates were collected by centrif-ugation at 2,000⫻gfor 3 min at 4°C. The beads were washed four times with 1 ml of ice-cold NP-40 buffer to remove loosely bound proteins. The immunoprecipitated pellets were resuspended in 30l of sodium dodecyl sulfate (SDS) protein sample buffer followed by resolving of the protein and Western transfer using standard protocols (Bio-Rad Laboratories). Proteins of interest were detected using specific antibodies followed by incubation with appropriate infrared-dye-tagged (IR680 and IR800) sec-ondary antibodies and scanning with an Odyssey infrared scanner (LI-COR Biosciences, Lincoln, NE).
In vitrobinding assay.Escherichia coliBL21 expressing glutathione S-transferase (GST) fusion proteins was harvested and stored at⫺80°C until use. Cell pellets were resuspended in binding buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1 mM MgCl2, 1 mM EDTA [pH 8.0], 1% Non-idet P-40) and lysed with sonication. After removal of cell debris, the supernatants were incubated with glutathione-Sepharose 4B beads (GE Healthcare Life Sciences, Inc.) at 4°C for 1 h. The resin was washed three times with binding buffer, and lysates containing overexpressed protein from 293T cells prepared using the same binding buffer were added after preclearing with protein A Sepharose 4B beads (GE Healthcare Life Sci-ences, Inc.). The resin was washed four times with binding buffer after 3 h of incubation at 4°C, and the bound proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting. Transient replication assay.Transient replication assay was done as described earlier (60). Briefly, 293L cells in 100-mm dishes were cotrans-fected with 20g of KSHV TR-containing plasmid with either 20g of KSHV LANA expression plasmid or with empty vector pA3F/pA3M as
on November 7, 2019 by guest
http://jvi.asm.org/
filler DNA. At⬃96 h posttransfection, 293L cells were collected by cen-trifugation (⬃5⫻106cells per sample) for DNA extraction using modi-fied Hirt’s method, described earlier (61). Similarly, BCBL-1 cells were transfected with 30g of KSHV TR-containing plasmid by electropora-tion using a Bio-Rad gene pulser at 975-F capacitance and 210 V. Fol-lowing electroporation, cells were induced with 20g/ml tetradecanoyl phorbol acetate (TPA) and 1 mM sodium butyrate for⬃96 h to induce lytic replication. Cells were collected by centrifugation (⬃107cells per sample) and washed twice with phosphate-buffered saline followed by extraction of DNA using modified Hirt’s lysis method (21). Extracted DNA was dissolved in 50l of distilled water containing RNase. Ten percent of the extracted DNA from⬃5⫻106293L cells and⬃107BCBL-1 cells was digested with EcoRI and the remainder with DpnI and EcoRI to remove the nonreplicated DNA. Digested DNA was separated on 0.8% agarose gel followed by Southern transfer on Hybond N⫹membrane (GE Healthcare) and hybridized with32P-labeled TR probes. Probes specific for the KSHV TR region were synthesized with a New England Biolabs (NEB) random prime kit and signals kit, and signals were detected using a PhosphorImager according to the manufacturer’s instructions (Molecu-lar Dynamics, Inc.). Signals were quantified using ImageQuant software (Molecular Dynamics, Inc.). Replicated DNA was determined by analyz-ing the relative densities of the DpnI-resistant band and normalizanalyz-ing with the respective EcoRI bands in the input DNA.
DNA break labeling and chIP.In order to detect TopoII-mediated transient-DNA break formation on KSHV TR DNA, we applied a DNA break detection method using biotin-16-dUTP (Roche Applied Science) and terminal deoxynucleotidyl transferase (TdT; Promega, Inc.) in the nucleus (25). Briefly, wild-type (TopoII⫹/⫹) and TopoII null
(TopoII⫺/⫺)MEF NIH 3T3 and HEK293L cells transfected with KSHV TR plasmid were fixed with Streck tissue fixative (STF) (Streck Laborato-ries) in the presence of 10 mM EDTA for 20 min at room temperature. STF does not cause any DNA damage during the processing (25). The cells were washed with cold Tris-buffered saline (TBS) twice and subsequently resuspended in buffer A (0.25% Triton X-100, 10 mM EDTA, 10 mM HEPES [pH 6.5]) followed by suspension in buffer B (200 mM NaCl, 1 mM EDTA, 10 mM HEPES [pH 6.5]). The nuclei were permeabilized with buffer C (100 mM Tris-HCl [pH 7.4], 50 mM EDTA, 1% Triton X-100) for 30 min at 4°C. The nuclei were sequentially washed with cold PBS, deionized water, and 1⫻TdT reaction buffer. The DNA breaks were la-beled with biotin-16-dUTP using TdT for 30 min at 37°C. After washing the residual biotin-16-dUTP with buffer D (100 mM Tris-HCl [pH 7.4], 150 mM NaCl), nuclei were fixed again with 1% formaldehyde. The nu-clear pellet was then resuspended in 100l digestion buffer (50 mM Tris-HCl [pH 7.5], 15 mM NaCl, 5 mM KCl, 3 mM MgCl2, 1 mM CaCl2, 10 mM NaHSO4, 0.25 M sucrose, 0.15 mM spermine, 0.5 mM spermidine, and 0.15 mM -mercaptoethanol). Chromatin immunoprecipitation (ChIP) was performed without sonication on MEFs and with sonication on HEK 293L cells using streptavidin-conjugated magnetic Sepharose beads (GE Healthcare Life Sciences, Inc.).
Flow cytometry.HEK 293 cells were transfected with KSHV TR plas-mid along with either pA3F LANA or the empty vector pA3F. Cells were pretreated with ellipticine for 1 h before transfection, and the treatments continued posttransfection. After 24 h, cells were harvested and fixed in cold 70% ethanol for 30 to 60 min. The fixed cells were washed twice by 1⫻PBS and stained with propidium iodide (PI) as described previously (34,47). Data were acquired on FACSCalibur equipped with CellQuest Pro software and analyzed using FlowJo software.
Real-time PCR.Quantitative real-time PCR was performed in a total volume of 20l, including 10l of SYBR green PCR 2⫻Master mix (Applied Biosystems) and 0.5M each KSHV TR primer (forward, 5=-G GGGGACCCCGGGCAGCGAG-3, and reverse, 5-GGCTCCCCCAAAC AGGCTCA-3) flanking TR nucleotides 677 to 766. The ampicillin gene region was amplified with forward (5=-GTAGATAACTACGATACGGG AGGG-3=) and reverse (5=-GCGAACTACTTACTCTAGCTTCCC-3=) primers. Purified DNA samples of the ChIP fraction and the input DNA
samples were amplified on an ABI StepOne plus real-time PCR machine (Applied Biosystems). Relative copies of immunoprecipitated TR were calculated by the⌬CTmethod.
RESULTS
KSHV LANA associates with TopoIIin KSHV-positive cells.
KSHV establishes a lifelong latent infection after primary infec-tion in the target cells. LANA, a nuclear protein, is expressed con-sistently in all the infected cells in relatively large amounts and is solely responsible for maintaining the viral genome into the divid-ing tumor cells. Besides tetherdivid-ing, LANA recruits the host cellular replication complex to replicate the terminal repeat-containing
plasmids (55,59,63). DNA affinity column as well as LANA
pull-down assays identified topoisomerase IIas one of the
LANA-interacting proteins (26,58). To identify whether LANA interacts
with TopoIIin KSHV-positive cells, a coimmunoprecipitation
(co-IP) assay was performed using the KSHV-positive cell lines BCBL-1 and JSC-1. Immunoprecipitation with LANA
anti-body and subsequent detection with anti-TopoII antibody
showed that LANA precipitated TopoIIfrom the KSHV-positive
BCBL-1 and JSC-1 cells (Fig. 1AandC, respectively). To further
analyze the specificity of this interaction, a reverse co-IP assay was
performed using anti-TopoIIantibody on the KSHV-positive
cell lines BCBL-1 and JSC-1. Immunoprecipitation and
subse-quent detection with anti-TopoIIand LANA antibodies showed
that LANA precipitated with TopoIIfrom the KSHV-positive
cells BCBL-1 and JSC-1 (Fig. 1BandD), respectively. Similarly,
co-IP analysis with anti-Flag antibody from BJAB cells expressing either yellow fluorescent protein (YFP)-Flag or YFP-LANA-Flag
showed coimmunoprecipitation of TopoIIfrom the BJAB cells
expressing YFP-LANA-Flag (Fig. 1E, lane 4) but not from
YFP-Flag (Fig. 1E, lane 3). Expressions and immunoprecipitations of
LANA and YFP were detected with anti-Flag antibody.
YFP-FIG 1KSHV LANA associates with TopoIIin KSHV-positive cells. (A to D) Coimmunoprecipitation assays were performed using 25 million KSHV-pos-itive cells (BCBL-1) with anti-LANA antibody (A); using 25 million BCBL-1 cells with anti-TopoIIantibody and subsequent detection with anti-TopoII and LANA antibodies (lane 4) (B); using 25 million KSHV-positive cells (JSC-1) with LANA antibody (C); using 25 million JSC-1 cells with anti-TopoIIantibody and subsequent detection with anti-TopoIIand LANA antibodies (lane 4) (D). (E) Co-IP analysis with anti-FLAG antibody from 25 million BJAB cells expressing either YFP-Flag (Y-Flag) or LANA-YFP-Flag (Y-LFlag) and subsequent detection with anti-TopoIIantibody. TopoIIwas found to coimmunoprecipitate with exogenously supplied LANA (lane 4).
on November 7, 2019 by guest
http://jvi.asm.org/
[image:3.585.302.542.66.238.2]LANA-Flag also showed a band corresponding to the YFP-Flag
due to cleavage of YFP from the fusion protein (Fig. 1E, lanes 2
and 4). These results show that TopoIIforms a complex with
LANA in KSHV-infected cells as well as in cells expressing exoge-nous LANA.
LANA colocalizes with TopoIIin KSHV-positive cells.To
further confirm the association of LANA and TopoII, we
per-formed an immunofluorescence assay (IFA) on KSHV-positive BCBL-1 and JSC-1 cells. These cells were stained with rat
anti-LANA and rabbit anti-TopoIIantibodies followed by detection
of LANA with goat anti-rat Alexa Fluor 488 (green) and chicken anti-rabbit Alexa Fluor 594 (red). LANA showed a distinct
punc-tate pattern in both of the PEL cells as detected earlier (14).
TopoIIproteins, shown in red, localized primarily in the nuclei
of the infected cells and were in the same nuclear compartment as
LANA, thus suggesting colocalization (Fig. 2A, detected as a
yel-low signal in the merge panels). Nuclei detected by TO-PRO 3 staining showed that the colocalization signals were in the nuclei. A differential interference contrast (DIC) image showed that the cells were healthy and had distinct nuclei. Additionally, IFA for the
localization of LANA and TopoIIon Vero cells harboring KSHV
BAC36 showed punctate LANA staining and colocalization with
TopoIIin the nucleus (Fig. 2B). Since Vero BAC36 cells have
GFP, we were unable to use TO-PRO 3 to localize the nucleus, but
a DIC image showed that colocalization of LANA and TopoII
was in the nucleus (Fig. 2B). These localization assays confirm that
LANA and TopoIIare in the same nuclear compartment of the
infected cells and may have a role in latent replication of the viral genome.
The amino terminus of LANA interacts with TopoII.To
identify the distinct domain of LANA responsible for TopoII
interaction, we transiently expressed either full-length LANA (LANA-FL) or truncation mutants, one expressing the amino ter-minus and the other the carboxy terter-minus tagged with Flag
epitope, along with TopoIIin HEK 293T cells.
Immunoprecipi-tation analysis with anti-Flag antibody and subsequent detection
with anti-TopoIIantibody showed that TopoIIwas
coimmu-noprecipitated with full-length LANA as well as the amino
termi-nus of LANA but not with the carboxy termitermi-nus (Fig. 3A, lanes 6
and 7, and B, lane 5). Relative binding of LANA-FL and LANA-N
terminus with TopoIIshowed that LANA-FL had stronger
bind-ing affinity, which could possibly be due to the involvement of
additional factors recruited with the full-length LANA (Fig. 3A,
compare lanes 6 and 7). Empty flag vector with TopoIIdid not
show any precipitation of TopoII, confirming the specificity of
the interaction (Fig. 3A, lanes 1 and 5, and B, lanes 1 and 4).
Expression of LANA and its truncation mutants in the lysates (input) and immunoprecipitated lanes are marked with red ar-rows. These interactions were consistently observed with
endog-enous TopoII. HEK 293T cells were transfected with either
full-length LANA or truncation mutants expressing amino terminus, LANA amino acid residues 1 to 32, and the carboxy terminus tagged with Flag epitope. Immunoprecipitation analysis with
anti-Flag antibody and subsequent detection with anti-TopoII
and LANA antibodies showed that endogenous TopoII
coim-munoprecipitated with full-length LANA as well as the amino terminus and aa 1 to 32 of LANA but not with the carboxy
termi-nus (Fig. 3C, lanes 7, 8, and 9). Further to ensure the specificity of
FIG 2LANA colocalizes with TopoIIin KSHV-positive cells. KSHV-posi-tive cells BCBL-1 and JSC-1 (A) and Bac36 Vero cells (B) were stained with rat anti-LANA and rabbit anti-TopoIIantibodies. LANA is shown in green and TopoIIin red. Nuclear stain TO-PRO 3 is shown in blue. LANA and TopoII colocalize in the nucleus as punctate bodies. DIC images were used to show the cells’ morphology.
FIG 3The amino terminus of LANA interacts with TopoII. (A) Twenty million HEK 293T cells were transfected with flag epitope-tagged pA3F empty vector, pA3F LANA, pA3F LANA-N, and pA3F LANA-C along with GFP-TopoII. At 36 h posttransfection, cells were harvested and immunoprecipi-tated with anti-Flag antibody and subsequently detected with anti-TopoII antibody (lanes 6 and 7). (B) Similarly, HEK 293T cells were transfected with pA3F empty, pA3F LANA-N, and pA3F LANA-C along with GFP-TopoII. At 36 h posttransfection, cells were harvested and immunoprecipitated with anti-Flag antibody and subsequently detected with anti-TopoII antibody. TopoIIspecifically interacts with full-length LANA and the N-terminal re-gion of LANA (panel A, lane 7, and panel B, lane 5). (C) HEK 293T cells were transfected with pA3F empty, pA3F LANA, pA3F LANA-N, LANA 1 to 32, and pA3F LANA-C. At 36 h posttransfection, cells were harvested and immuno-precipitated with Flag antibody and subsequently detected with anti-TopoIIand LANA antibodies. Endogenous TopoIIspecifically interacts with full-length LANA and the N-terminal region of LANA (lanes 7, 8, and 9). (D) HEK 293T cells were transfected with pA3F empty, pA3F LANA, and pA3F LANA-N along with GFP-TopoII. At 36 h posttransfection, cells were har-vested and the lysate was incubated with 2,000 U micrococcal nuclease (NEB) for 30 min at 37°C and immunoprecipitated with anti-Flag antibody and sub-sequently detected with anti-TopoIIantibody. TopoIIinteracts specifically with full-length LANA and its amino terminus irrespective of nuclease treat-ment (lanes 5 and 6). (E) Micrococcal nuclease-digested DNA on ethidium bromide (EtBr)-stained agarose gel.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:4.585.300.542.65.273.2] [image:4.585.43.286.66.195.2]this interaction, cell lysates were pretreated with micrococcal nu-clease prior to immunoprecipitation analysis with Flag
anti-body. Results showed that TopoIIinteracts specifically with
full-length LANA and its amino terminus irrespective of nuclease
treatment (Fig. 3D, lanes 5 and 6). These results confirmed that
LANA directly interacts with TopoIIand that the
internucleo-somal DNA does not mediate their association.
The residues localized between aa 1 and 32 of the amino ter-minus of LANA mediate association with TopoII.To
deter-mine the domains of LANA responsible for TopoIIinteraction,
we made further truncations of the amino terminus of LANA into expression constructs containing aa 1 to 32, 1 to 150, 1 to 250, 33
to 275, and 33 to 340, tagged with Myc epitope (Fig. 4A). Transient
expression of these constructs along with TopoIIin HEK 293T
cells followed by immunoprecipitation analysis with anti-Myc
an-tibody and subsequent detection with anti-TopoII antibody
showed that both LANA truncation constructs aa 1 to 150 and aa
1 to 250 were able to bind with TopoII(Fig. 4B, lanes 6 and 7).
LANA mutants lacking aa 1 to 32 (constructs aa 33 to 275 and aa
33 to 340) were unable to precipitate TopoII(Fig. 4B, lanes 5 and
8) suggesting that LANA binding domain to TopoIIlies between
amino acid residues 1 and 32. In order to determine the binding of
TopoIIwith LANA aa 1 to 32, we cloned aa 1 to 32 in frame with
GFP and Myc epitope tag and generated a stably expressing cell line in BJAB. An immunoprecipitation assay with Myc anti-body to precipitate LANA aa 1 to 32 showed coprecipitation of
TopoII(Fig. 4C, lane 5). Vector control with GFP-Myc did not
show precipitation of TopoII, suggesting specific association of
LANA aa 1 to 32 with TopoII(Fig. 4C, lane 4). In an attempt to
identify the specific residues of LANA aa 1 to 32 associating with
TopoII, we used LANA alanine substitution mutants between
residues 5 and 15, which were shown to associate with host
chro-matin and be important for replication (5,6). BJAB, stably
ex-pressing GFP-Myc LANA aa 1 to 32 with substitution mutation at
aa 5 to 15 (M5), did not show precipitation of TopoII, suggesting
that residues 5 to 15 are critical for TopoIIrecruitment (Fig. 4C,
lane 6).
We further confirmed the association of TopoIIwith LANA
through residues 5 to 15 in the context of the entire amino terminus region (aa 1 to 340) and its alanine substitution mutant (aa 1 to 340 with mutations at aa 5 to 15) along with the aa 1 to 32 region of LANA. Immunoprecipitation with anti-Myc antibody to precipitate LANA
and its mutants showed that TopoIIcoimmunoprecipitated with
LANA aa 1 to 340 and aa 1 to 32 but not with alanine substitution
mutants of both of these truncations (Fig. 4D, compare lanes 7 and 9
with 8 and 10). These experiments indicated that the residues 5 to 15
of LANA are required for recruiting TopoII.
The interaction of LANA residues 1 to 32 with TopoIIwas
further confirmed by an in vitrobinding assay. Bacterially
ex-pressed GST-tagged LANA aa 1 to 32 and its alanine substitution mutant M5 (where residues 5 to 15 were replaced by alanine) were used for the binding assay with transiently overexpressed
TopoIIprotein from HEK 293T cells. GST protein prepared
from empty vector served as control. As shown inFig. 4E,
bac-terially expressed GST-LANA aa 1 to 32 efficiently precipitated
TopoII, whereas LANA mutant M5 did not show any binding
to TopoII and showed results similar to those of the GST
control lane (Fig. 4E).
LANA aa 1 to 32 have been previously shown to be responsible for the association of LANA with host nucleosome through
inter-action with cellular histones (6). Therefore, we wanted to map the
residues of aa 5 to 15 specifically involved in the TopoII
interac-tion with LANA. To this end, we made alanine substituinterac-tion mu-tations of LANA in sets of three amino acids in both aa 1 to 32 and
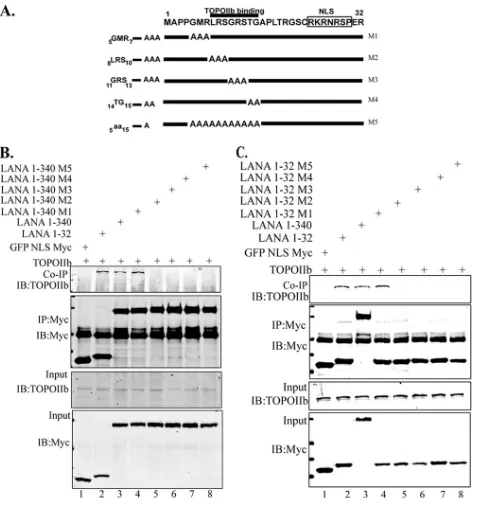
aa 1 to 340 LANA truncation mutants (Fig. 5A), as follows:5GMR7
changed to AAA (M1),8LRS10 to AAA (M2),11GRS13to AAA
(M3),14TG15to AA (M4), and all residues between 5 and 15
changed to alanine (M5). Transient expression of these LANA
mutants along with TopoIIin HEK 293 cells followed by
im-munoprecipitation analysis with anti-Myc to immunoprecipi-tate LANA mutants identified residues 8 to 15 as being essential
for binding with TopoII, since mutants M2 through M4
(8LRS10to AAA,11GRS13to AAA, and14TG15to AA) failed to
coimmunoprecipitate TopoII(Fig. 5BandC). These results
were consistent with both LANA truncation (aa 1 to 32 and aa 1 to 340) mutants.
TopoIIis required for KSHV latent DNA replication.To
determine whether TopoIIis important for the latent replication
of KSHV DNA, a transient replication assay with the KSHV TR was conducted in the presence of ellipticine, a selective inhibitor of
FIG 4The aa 1 to 32 region at the amino terminus of LANA is responsible for the association with TopoII. (A) Schematic showing the truncations of LANA N-terminal region. (B) Twenty million HEK 293T cells were trans-fected with pEGP-Myc LANA aa 33 to 275, pEGFP-Myc LANA aa 1 to 250, pEGFP-Myc LANA aa 1 to 150, and pEGFP-Myc LANA aa 33 to 340 with GFP-TopoII. At 36 h posttransfection, cells were harvested and immuno-precipitated with anti-Myc antibody (9E10) and subsequently detected with anti-TopoIIantibody (lanes 6 and 7). (C) Twenty-five million BJAB cells stably expressing pEGP-Myc empty, pEGP-Myc LANA aa 1 to 32, and pEGP-Myc LANA aa 1 to 32 with substitutions at aa 5 to 15 (5aa15) were
harvested and immunoprecipitated with anti-Myc antibody (9E10) and subsequently detected with anti-TopoIIantibody (lane 5). (D) HEK 293 T cells were transfected with pEGFP-Myc empty, pEGFP-Myc LANA aa 1 to 32, pEGFP-Myc LANA aa 1 to 325aa15, pEGFP-Myc LANA aa 1 to 340, and
pEGFP-Myc LANA aa 1 to 3405aa15with GFP-TopoII. At 36 h
posttrans-fection, cells were harvested and immunoprecipitated with Myc anti-body (9E10) and subsequently detected with anti-TopoIIantibody (lanes 7 and 9). (E)In vitroGST binding: GST, LANA aa 1 to 32, and GST-LANA aa 1 to 325aa15fusion proteins were expressed inE. coli, purified
with glutathione-Sepharose beads, and incubated with TopoIIcell lysate prepared from HEK 293T cells transfected with GFP-TopoII. The aa 1 to 32 region of LANA interacted with TopoII(lane 2).
on November 7, 2019 by guest
http://jvi.asm.org/
[image:5.585.43.285.64.272.2]TopoII. HEK 293L cells were transfected with TR plasmid along with Flag epitope-tagged KSHV-LANA, pA3F-LANA, or empty vector pA3F. Ninety-six hours posttransfection, cells were har-vested and subjected for DNA extraction by modified Hirt’s pro-cedure. Extracted DNA was subjected to restriction digestion with either EcoRI alone (to linearize) or with EcoRI plus DpnI (to remove nonreplicated plasmid DNA) followed by the detection of
replicated DNA in a Southern blot assay using32P-labeled TR
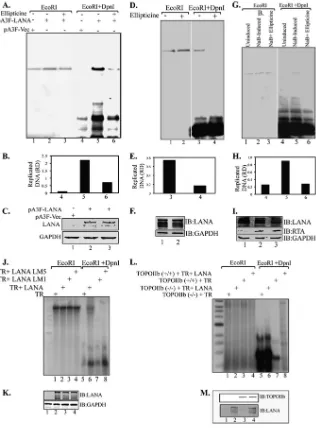
probe. Hybridization signals were detected by PhosphorImager and analyzed using ImageQuant software (Molecular Dynamics, Inc.). Results showed that KSHV latent DNA replication is
TopoIIdependent, since treatment of cells with ellipticine
effec-tively reduced latent DNA replication (Fig. 6A). LANA-expressing
cells treated with ellipticine showed a faint DpnI-resistant band, whereas the untreated cells showed significantly larger amounts of
replicated DNA (Fig. 6A, compare lanes 5 and 6). Relative
amounts of the replicated DNA determined based on the densities of the DpnI-resistant band normalized with input lanes suggest
effective inhibition of replication by ellipticine (Fig. 6B). Cells
without LANA expression did not show any DpnI-resistant band,
as expected. These results suggest that TopoIIis essential for
latent DNA replication. The levels of LANA were determined by anti-Flag Western blotting in ellipticine-treated as well as non-treated cells to ensure that ellipticine did not have any adverse
effect on LANA expression (Fig. 6C).
Similar results were observed in transient replication assays conducted on KSHV-positive BCBL-1 cells in the presence of
ellipticine (Fig. 6D). BCBL-1 cells were transfected with KSHV TR
plasmids, and replication assays were performed in the presence and absence of ellipticine to determine whether ellipticine was able to block replication of TR plasmids in KSHV-infected cells. Ninety-six hours posttransfection, cells were harvested, followed by the extraction of DNA using modified Hirt’s procedure. Ex-tracted DNA was digested with either EcoRI (to linearize) or with DpnI and EcoRI to identify the replicated plasmids after Southern transfer as mentioned above. Cells treated with ellipticine did not show any DpnI-resistant band, whereas the untreated cells showed a prominent DpnI-resistant band, confirming that
ellip-ticine can effectively block TR DNA replication (Fig. 6DandE,
compare lanes 1 and 2). Expression of LANA was not affected by
ellipticine treatment (Fig. 6F, IB: LANA). As a control, a similar
transient replication assay was conducted on induced BCBL-1
cells transfected withori-LytDNA. Induced BCBL-1 cells,
con-tainingori-LytDNA, were treated with ellipticine following the
extraction of DNA using modified Hirt’s procedure and detection
of replicated DNA copies in a Southern blot. Anori-Lyt-specific
32P-labeled probe was used for the detection of replicated plasmid.
Consistent with the previous report,ori-Lytplasmid did not
rep-licate in uninduced cells (Fig. 6G, lane 1). Induction of these cells
with sodium butyrate showed replication of ori-Lyt plasmids,
which was blocked by ellipticine treatment as reported earlier (Fig.
6GandH, compare lanes 2 and 3). This suggests that TopoIIis
required for both latent and lytic KSHV DNA replication. The levels of LANA and RTA were determined in a Western blot assay to show that the treatment with sodium butyrate induced RTA expression, which is required for the lytic DNA replication (Fig. 6I).
Additionally, transient replication assays with the KSHV TR
along with full-length LANA and its mutants, LANA5GMR7-AAA
and LANA with residues 5 to 15 replaced by alanine, showed that the residues 5 to 15 are crucial for KSHV latent DNA replication,
which may be due to the loss of TopoIIbinding to LANA. This
was done by transfecting 293L cells with equal amounts of TR plasmids with either full-length Myc-tagged LANA
(pA3M-LANA) or its mutants, LANA5GMR7-AAA and LANA with
resi-dues 5 to 15 changed to alanine. Ninety-six hours posttransfec-tion, cells were harvested and the DNA extracted by Hirt’s procedure was digested with either EcoRI or DpnI and EcoRI to detect the replicated copies as mentioned above. Extracted DNA from cells expressing pA3M LANA showed a prominent
DpnI-resistant band indicating replicated DNA (Fig. 6J, lane
6), whereas the mutants LANA5GMR7-AAA and LANA with
residues 5 to 15 changed to alanine were not able to support replication and hence failed to show any DpnI-resistant band (Fig. 6J, lanes 7 and 8). Expression of LANA detected in the Western blot showed comparable levels of protein expression (Fig. 6K).
To further substantiate the requirement of TopoIIfor KSHV
latent DNA replication, we conducted a transient replication assay
with the KSHV TR on TopoIIknockout mouse embryonic
fibro-FIG 5The aa 1 to 32 region of the amino terminus of LANA is responsible for TopoIIassociation. (A) Schematic showing the alanine substitution muta-tions of aa 1 to 32 of the LANA N-terminal region. (B) Twenty million HEK 293T cells were transfected with pEGFP-Myc empty, pEGFP-Myc LANA aa 1 to 32, pEGFP-Myc LANA aa 1 to 340, pEGFP-Myc LANA aa 1 to 340 M1, pEGFP-Myc LANA aa 1 to 340 M2, pEGFP-Myc LANA aa 1 to 340 M3, pEGFP-Myc LANA aa 1 to 340 M4, and pEGFP-Myc LANA aa 1 to 340 M5 with GFP-TopoII. At 36 h posttransfection, cells were harvested and immu-noprecipitated with anti-Myc antibody (9E10) and subsequently detected with anti-TopoIIantibody. (C) Similarly, HEK 293T cells were transfected with pEGFP-Myc empty, pEGFP-Myc LANA aa 1 to 32, pEGFP-Myc LANA aa 1 to 340, pEGFP-Myc LANA aa 1 to 32 M1, pEGFP-Myc LANA aa 1 to 32 M2, Myc LANA aa 1 to 32 M3, Myc LANA aa 1 to 32 M4, pEGFP-Myc LANA aa 1 to 32 M5 with GFP-TopoII. At 36 h posttransfection, cells were harvested and immunoprecipitated with anti-Myc antibody (9E10) and subsequently detected with anti-TopoIIantibody. The residues 8 to 15 of LANA are crucial for binding TopoII(B and C).
on November 7, 2019 by guest
http://jvi.asm.org/
[image:6.585.43.286.65.320.2]blast cells (TopoII⫺/⫺) MEF NIH 3T3. TopoIIknockout MEF NIH 3T3 and wild-type cells were transfected with KSHV TR plas-mid along with LANA expression plasplas-mid. Ninety-six hours post-transfection, cells were harvested, and the DNA extracted by Hirt’s procedure was digested with either EcoRI or DpnI and EcoRI to
detect replicated DNA copies using32P-labeled TR probe as
men-tioned above. TopoIIknockout MEF NIH 3T3 cells did not show
any detectable DpnI-resistant band in the presence of LANA (Fig.
6L, lane 6). However, MEF NIH 3T3 wild-type cells expressing
LANA showed a DpnI-resistant band, indicating replication of
FIG 6TopoIIis required for KSHV latent DNA replication. (A) HEK 293L cells were transfected with TR plasmid along with pA3F-LANA or empty vector pA3F. Cells were treated with ellipticine 24 h posttransfection. Ninety-six hours posttransfection, cells were harvested, and the DNA extracted by Hirt’s procedure was subjected to Southern blotting with a TR probe after digestion. Cells expressing LANA without ellipticine treatment show a prominent DpnI-resistant replicated DNA (lane 5), whereas cells treated with ellipticine showed a faint band (lane 6). (B) Quantitation of the replicated DNA based on the relative density (RD). (C) Expression of LANA and GAPDH. (D) BCBL-1 cells transfected with TR plasmid were treated with ellipticine 24 h posttransfection. Ninety-six hours posttransfection, cells were harvested, and the DNA extracted by Hirt’s procedure was subjected to Southern blotting with TR probe after digestion. Ellipticine effectively blocked KSHV latent DNA replication in KSHV-positive cells (lane 4). (E) Quantitation of the replicated DNA. (F) Expression levels of LANA and GAPDH. (G) BCBL-1 cells transfected withori-Lytplasmid were first induced with 3 mM sodium butyrate and 20g/ml TPA followed by treatment with ellipticine 24 h posttransfection. Ninety-six hours posttransfection, cells were harvested, and the DNA extracted by Hirt’s procedure was subjected to Southern blotting withori-Lytprobe after digestion. Ellipticine blocked KSHV lytic DNA replication (lane 6). (H) Quantitation of the replicated DNA. (I) Western blots showing the expression of LANA, RTA, and GAPDH. (J) LANA mutants M1 and M5 do not support KSHV latent DNA replication (lanes 7 and 8): HEK 293L cells were transfected with TR plasmid along with pA3M-LANA, pA3M-LANA-M1, or pA3M-LANA-M5. Ninety-six hours posttransfection, cells were har-vested, and the DNA extracted by Hirt’s procedure was subjected to Southern blotting with TR probe after digestion. (K) Expression of LANA and GAPDH. (L) TopoIIis required for KSHV latent DNA replication: wild-type (TopoII⫹/⫹) and TopoIIknockout (TopoII⫺/⫺) MEF NIH 3T3 cells were transfected with
TR plasmid along with empty vector pA3F or pA3F-LANA. Ninety-six hours posttransfection, cells were harvested, and the DNA extracted by Hirt’s procedure was subjected to Southern blotting with TR probe after digestion. (M) Expression of LANA and TopoIIin the cells used for replication assays.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:7.585.131.457.66.495.2]transfected plasmid (Fig. 6L, lane 8). These data suggest that
TopoIIis essential for a LANA-dependent KSHV latent DNA
replication. Expression of LANA detected in Western blotting
showed comparable levels of protein expression (Fig. 6M).
The minimal binding region of LANA can act as a dominant negative and disrupts LANA-TopoIIinteraction. To further
understand the biological significance of TopoIIrecruitment by
LANA, we used the minimal TopoIIbinding region of LANA
(construct 1 to 32) as a dominant negative in both immunopre-cipitation and transient replication assays. To study the effect of LANA 1 to 32 as a dominant negative in coimmunoprecipitation
assay, HEK 293T cells were transfected with GFP-tagged TopoII
and the amino terminus of LANA aa 1 to 340 tagged with Flag
epitope along with two different amounts (20g and 40g) of
LANA aa 1 to 32 (Myc tagged) as dominant negative. Cells were
transfected with TopoIIalong with empty vector, pA3F,
pA3F-LANA C terminus, pA3F-LANA aa 1 to 32, the M5 mutant (alanine substitutions at aa 5 to 15), full-length LANA (pA3F-LANA), and pA3F-LANA N terminus (aa 1 to 340) as negative and positive controls. Immunoprecipitation analysis with anti-Flag antibody
showed coimmunoprecipitation of TopoII with LANA
full-length and amino-terminal domain as seen earlier (Fig. 3A, lanes 2
and 4). Interestingly, overexpression of LANA aa 1 to 32 was able
to adversely affect the interaction of LANA-N with TopoIIand
thus acted as dominant negative (Fig. 7A, lanes 5 and 6). Cells
expressing increased amounts of LANA aa 1 to 32 showed a
dose-dependent response in abolishing the association of TopoIIwith
LANA-N (Fig. 7A, compare lanes 4, 5, and 6). However,
overex-pression of LANA aa 1 to 32 with alanine substitutions at aa 5 to 15
had no effect on the association of TopoIIwith LANA-N and was
similar to LANA-FL (Fig. 7A, lanes 2 and 7). Overexpression of
LANA aa 1 to 32 may have slightly reduced the expression of
LANA-N (Fig. 7A, compare lanes 4 and 5); however, the
immu-noprecipitation of LANA-N was not affected (Fig. 7A, IB: FLAG,
LANA-N). Subsequent increase of LANA aa 1 to 32 expression did
not affect LANA-N expression but drastically reduced TopoII
association with LANA-N.
Similarly, transient replication assays using LANA aa 1 to 32 also revealed that the expression of LANA aa 1 to 32 as a dominant negative could effectively reduce KSHV latent DNA replication (Fig. 7B). For the transient replication assay, HEK 293L cells were transfected with equal amounts of KSHV TR plasmid with either empty vector, LANA aa 1 to 32, full-length LANA, or full-length
LANA with two different amounts (20g and 40g) of LANA aa
1 to 32 as dominant negative. As a control, 40g of LANA aa 1 to
32 mutant M5 (alanine substitutions) was transfected with TR and full-length LANA. Ninety-six hours posttransfection, cells were harvested, and the DNA extracted by Hirt’s procedure was sub-jected to either EcoRI or DpnI and EcoRI to detect replicated TR
plasmid copies in a Southern blot assay using a32P-labeled TR
probe. Cells transfected with TR with full-length LANA showed a
prominent DpnI-resistant band as expected (Fig. 7B, lane 9).
LANA aa 1 to 32 did not show any detectable replication,
suggest-ing that additional factors besides TopoIIare required for
repli-cation (Fig. 7B, lane 8). Interestingly, cells transfected with TR and
full-length LANA along with LANA aa 1 to 32 showed decreased
replication of TR plasmids (Fig. 7B, compare lanes 9 and 10).
Additionally, increasing amounts of LANA aa 1 to 32 progres-sively reduced LANA-dependent KSHV latent DNA replication (Fig. 7B, compare lanes 10 and 11). Interestingly, cells
trans-fected with M5 of LANA aa 1 to 32 with TR and full-length LANA did not show any reduction in the replication of TR
plasmid (Fig. 7B, lane 12), confirming that residues 5 to 15 are
critical for recruiting TopoIIat the site of replication
initia-tion. A quantitation of the replicated DNA, based on the rela-tive density normalized to the respecrela-tive EcoRI band, showed a dose-dependent response of LANA aa 1 to 32 in blocking DNA
replication (Fig. 7C). These data clearly show that LANA aa 1 to
32 could act as a dominant negative, specifically disrupting the
association of LANA with TopoII. Moreover, LANA aa 1 to 32
is crucial for the association of LANA with TopoIIand
LANA-dependent KSHV latent DNA replication. Expression of LANA detected in a Western blot showed comparable levels of protein
expression (Fig. 7D).
TopoII makes dsDNA breaks on the KSHV genome.
TopoIIplays a central role in altering the degree of supercoiling
of double-stranded DNA molecules (15). TopoIIcleaves both
strands of the DNA helix simultaneously and is essential in the separation of intertwined and supercoiled DNA strands during
replication and transcription (25). It has been shown that
TopoII-mediated dsDNA breaks are also required for
transcrip-tion (15,25). We performed a DNA break labeling and ChIP assay,
shown in a schematic diagram (Fig. 7E), to detect whether
TopoII could mediate nicks on KSHV TR DNA. Wild-type
(TopoII⫹/⫹) and TopoIIknockdown (TopoII⫺/⫺) MEF NIH
3T3 cells were transfected with TR-containing plasmid either with pA3F-LANA or with empty vector pA3F. The nuclei were subse-quently labeled with biotin-16-dUTP using terminal deoxynu-cleotidyl transferase (TdT) to label the ends of nicked DNA and subjected to chromatin immunoprecipitation using streptavidin. Quantitative real-time PCR was done on the input and on ChIP DNA samples using KSHV TR-specific primers to calculate the ratios of immunoprecipitated TR copies in the presence and
ab-sence of LANA expression in both TopoII⫺/⫺and TopoII⫹/⫹
cells. Fold change analysis, based on the⌬CTvalues, showed
sig-nificant increase in copies of ChIP DNA in TopoIIsufficient
(TopoII⫹/⫹) cells in the presence of LANA, suggesting LANA
was able to increase dsDNA breaks in TopoII⫹/⫹cells (Fig. 7F).
TopoII⫺/⫺cells showed minimal TR copies with biotin ChIP,
which did not show any change with LANA expression, suggesting
background levels of biotin incorporation in those cells (Fig. 7F).
To further evaluate the role of TopoIIin KSHV latent DNA
replication, DNA break labeling and ChIP assays were performed on HEK 293L cells transfected with KSHV TR-containing plasmid either with pA3F-LANA or with empty vector pA3F. One set of the
transfected cells was treated with a 5M concentration of
ellipti-cine. After 24 h, cells were harvested and a portion (1 million) of the cells were used for cell cycle analysis after propidium iodide (PI) staining and flow cytometry. The remaining cells were fixed with Streck tissue fixative and used subsequently to label with biotin-16-dUTP followed by ChIP and quantitative PCR as de-scribed earlier. The flow cytometry data showed that the transient overexpression of LANA slightly affected the cell cycle and pushed the cells into S phase as expected, whereas the treatment with
ellipticine appeared to arrest the cells at S phase (Fig. 7H).
Fur-thermore, the quantitative PCR data from biotin-16-dUTP ChIP showed that transient expression of LANA indeed increased the incorporation of biotin-16-dUTP, suggesting increased incidence
of double-stranded breaks in LANA-expressing cells (Fig. 7G). To
determine whether the dsDNA break was limited to the TR region,
on November 7, 2019 by guest
http://jvi.asm.org/
we compared the copies of TR and the vector backbone (ampicil-lin gene) in sonicated and biotin ChIP DNA, which showed
in-creased copies of the TR but not the vector backbone (Fig. 7G,
compare black and gray bars in⫺LANA and⫹LANA).
Interest-ingly, a treatment with ellipticine effectively reduced the
incorpo-ration of biotin-16-dUTP, strongly suggesting a role for TopoII
in mediating double-stranded breaks in the KSHV TR DNA.
These data suggest that TopoII mediates nicks on KSHV TR
FIG 7The minimal binding region of LANA acts as a dominant negative to disrupt LANA-TopoIIinteraction and replication. (A) Twenty million HEK 293T cells were transfected with GFP-TopoIIalong with pA3F, pA3F-LANA, pA3F-LANA C terminus, pA3F-LANA N, and pA3F-LANA N with two different amounts (20g and 40g) of GFP-Myc-LANA aa 1 to 32 and 40g GFP-Myc-LANA aa 1 to 32 mutant M5 (with alanine substitutions at aa 5 to 15). At 36 h posttransfection, cells were harvested and immunoprecipitated with anti-flag antibody to precipitate LANA and its mutants, followed by detection of TopoII as the coimmunoprecipitated proteins. LANA aa 1 to 32 was found to interfere with LANA TopoIIinteraction (lanes 5 and 6), whereas transfection of LANA aa 1 to 32 mutant M5 did not interfere with LANA and TopoIIinteraction (lane 7). Expression of increased amounts of LANA aa 1 to 32 further decreased TopoIIbinding (lane 6). Expressions of LANA aa 1 to 32 were detected by anti-Myc immunoblot (asterisk). (B) HEK 293L cells were transfected with TR plasmid along with GFP-Myc-LANA 1 to 32, pA3F-LANA, or pA3F-LANA with two different amounts (20 and 40g) of GFP-Myc-LANA 1 to 32 and pA3F-LANA along with 40g of GFP-Myc-LANA 1 to 32 M5. Ninety-six hours posttransfection, cells were harvested, and the DNA extracted by Hirt’s procedure was subjected to Southern blotting with TR probe after digestion. LANA aa 1 to 32 interfere with KSHV latent DNA replication as detected by a reduced level of DpnI-resistant band (compare lanes 9 and 10). Increasing expression of LANA aa 1 to 32 further reduced the replication of TR plasmid (compare lanes 11 and 10), however; mutant 5 (M5) of LANA aa 1 to 32 was unable to suppress replication (compare lanes 11 and 12). (C) Quantitation of the replicated DNA. (D) Expression levels of LANA and GFP-Myc-LANA aa 1 to 32 and GFP-Myc-LANA aa 1 to 32 M5. (E) Schematic showing DNA break labeling and ChIP assay procedure. Wild-type (TopoII⫹/⫹) and TopoIIknockdown (TopoII⫺/⫺) MEF NIH 3T3 cells were transfected with KSHV TR plasmid either with
pA3F-LANA or with empty vector pA3F. The nuclei were subsequently labeled with biotin-16-dUTP using terminal deoxynucleotidyl transferase (TdT) and subjected to chromatin immunoprecipitation (ChIP). (F) Quantitative real-time PCR was done on the input and ChIP DNA samples using KSHV TR-specific primers. Relative copies of the dUTP-labeled ChIP DNA were determined as the ratio of cells without LANA to cells with LANA in TopoII⫺/⫺and TopoII⫹/⫹cells to
determine the effect of LANA on dsDNA breaks. TopoII⫹/⫹cells showed significant increase in the presence of LANA. (G) DNA break labeling and ChIP assay
was performed on HEK 293L cells transfected with KSHV TR-containing plasmids with either pA3F-LANA or the empty vector pA3F. One set of cells with LANA with TR was treated with 5M ellipticine. dUTP-labeled DNAs were determined in a real-time PCR using primer for TR (black bar) and vector backbone (gray bar). An increase in dUTP-labeled ChIP DNA in the TR region but not in the ampicillin region (vector) in LANA-expressing cells suggests the existence of a dsDNA break in the TR region. Ellipticine treatment blocked dUTP incorporation, suggesting a TopoII-mediated dsDNA break. (H) At 24 h posttransfection, 293L cells were harvested for flow cytometry and cell cycle analysis.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:9.585.131.456.62.448.2]DNA to unwind the supercoiled DNA and thus assists in latent DNA replication.
DISCUSSION
Kaposi’s sarcoma-associated herpesvirus is tightly associated with multiple human malignancies including Kaposi’s sarcoma (KS), primary effusion lymphomas (PELs) and multicentric
Castle-man’s disease (MCD) (63). KSHV establishes a lifelong latent
in-fection following primary inin-fection and propagates into dividing tumor cells following duplication of the episomes along with the
human chromosomes (68). During latency, protein expression is
tightly regulated by transcriptional repression and is limited to a
few crucial oncogenic proteins (63). LANA, encoded by ORF73, is
consistently expressed in KS lesions and is crucial for the
mainte-nance of viral episomes in proliferating cells (4,63,64,69). LANA
not only modulates the transcription of viral and cellular genes but also recruits a number of molecules to regulate the replication of the viral episome and the segregation of the newly synthesized genome copies to daughter progeny nuclei by tethering to the host
chromosomes (3,4,63).
Others and we have shown that LANA is critical for the repli-cation of TR-containing plasmids, but the molecular mechanism
of replication initiation at the TR is poorly understood (4,8,42).
DNA affinity and coimmunoprecipitation analysis using KSHV,
TR DNA, orori-LytDNA as the affinity ligand identified
topo-isomerase II (TopoII) as an interacting protein (53,67). KSHV
and the other herpesviruses studied so far do not encode topo-isomerases. Thus, it could be assumed that these viruses use
cel-lular topoisomerases for the viral DNA replication (19,67).
In-deed, the importance of TopoIIin KSHV lytic DNA replication
was recently demonstrated (67). It has been shown that gene
si-lencing of TopoI and TopoII with specific short hairpin RNA (shRNA) or use of ellipticine, a specific inhibitor of TopoII, could
abolish KSHV lytic DNA replication (19). Additionally, it has
been shown that a wide variety of potential topoisomerase inhib-itors effectively block KSHV lytic DNA replication and could be
further screened as therapeutic drugs (19).
Since TopoIIwas identified as a LANA-interacting protein in
a DNA affinity column and proteomics analysis, it was suggested
that TopoIImay have a role in DNA replication at the latent
origins of the TR (53). In this study, we show that LANA recruits
the cellular protein topoisomerase IIfor KSHV latent DNA
rep-lication. TopoIIproteins form complexes with LANA and
colo-calize as punctuate bodies in the nuclei of KSHV-infected BCBL-1
and JSC-1 cells. TopoIIis an enzyme that controls and alters the
topological state of DNA during transcription and replication and has been shown to induce double-stranded breaks required for
regulated transcription/replication (15,25).
The amino terminus of LANA is identified as the TopoII
binding domain; more specifically, the first 32 amino acids, con-taining the nucleosome-binding region, seem to be crucial for
TopoIIbinding. Additionally, this region of aa 1 to 32 acted as a
dominant negative and disrupted association of TopoIIwith
LANA when expressed in excess, suggesting that TopoII
inter-acts only through aa 1 to 32 (Fig. 7AandB). Since the region
encompassing aa 1 to 32 of LANA has also been shown to be the region of nucleosomal attachment, one could argue that the
DNA-linking nucleosomes may have a role in associating TopoIIwith
LANA (6,14). Nuclease treatment of the lysates prior to
immu-noprecipitation excludes the possibility of DNA facilitating their
interaction. Additionally, the binding analysis with bacterially ex-pressed protein confirmed that the association of LANA aa 1 to 32
with TopoIIis more likely a direct interaction. Alanine
substitu-tion mutasubstitu-tions within LANA aa 1 to 32 and subsequent co-IP
assays showed that the TopoIIbinding region lies very close to
the chromatin-binding region identified previously (6).
Amino-terminal regions of LANA carrying mutations 8LRS10 to AAA,
11GRS13to AAA, and14TG15to AA and mutant M5, where
resi-dues between aa 5 and 15 were mutated to alanine, failed to bind to
TopoII(Fig. 5BandC). These studies suggest that amino acid
residues 8 to 15 are essential for TopoIIbinding (Fig. 5BandC).
Interestingly, aa 5 to 15 of LANA are also shown to bind with histones for chromosome tethering and are required for episome
replication (5,6,27). This suggests that these residues may serve as
binding sites for other cellular proteins along with the histones. The C-terminal domain of LANA has been shown to bind directly to the LANA-binding sites (LBS) in the KSHV TR, and the binding
is required for origin firing in the RE region of the TR (3,4,59,63).
Also, the amino and carboxy termini of LANA have been shown to
associate with each other (26). Thus, it could be hypothesized that
LANA brings TopoIIin close proximity to the LANA binding
site through its amino terminus to initiate replication in the RE region of TR.
The requirement of TopoIIin latent DNA replication of TR
was confirmed in a transient replication assay on TopoII
knock-out MEF NIH 3T3 cells (TopoII⫺/⫺). Only wild-type
(TopoII⫹/⫹) MEF NIH 3T3 cells expressing LANA supported
KSHV latent DNA replication, which strongly suggests that
TopoIIis required for latent replication. The role of TopoIIin
replication was also confirmed by treating the cells with ellipticine, a selective inhibitor of TopoII, which abolished latent DNA repli-cation mediated by TR in BCBL-1 cells. Ellipticine, which has previously been shown to block DNA replication mediated by the
lytic origin (ori-Lyt), was used as a positive control in our
replica-tion assays to demonstrate that ellipticine treatment and the
rep-lication assay were working in our hands (67). These data suggest
that TopoIIplays an important role in KSHV latent DNA
repli-cation.
The role of specific residues of LANA important for binding
with TopoIIwas further confirmed in replication assays to
de-termine whether the alanine substitution mutants, which were
unable to bind to TopoII, can support DNA replication. LANA
mutants with mutations at aa 5 to 7 (GMR-AAA) and aa 5 to 15 were unable to support replication of TR plasmid, suggesting that
TopoIImay be essential for DNA replication. Alternatively,
teth-ering of the TR plasmids to the host chromosome may also be critical for replication, which may bring the cellular replication machinery at the TR origin. Dominant negative aa 1 to 32 may
have disrupted the association of TopoIIalong with other
cellu-lar factors including histones in order to block DNA replication. This is evidenced by the fact that the LANA mutant with
substitu-tions at aa 5 to 7 (GMR) bound with TopoIIbut was unable to
support replication, therefore suggesting the involvement of ad-ditional factors for replication initiation at the TR. Previous stud-ies have shown that LANA mutants lacking chromosome binding
(5GMR7,8LRS10, and11GRS13) were unable to support replication,
but the mechanisms were not determined (5). Here, we show that
TopoIImay be the one factor required for LANA-mediated
rep-lication, as the mutants lacking TopoIIbinding were unable to
support replication. Also, our dominant negative data whereby
on November 7, 2019 by guest
http://jvi.asm.org/
the association of TopoIIwith LANA was inhibited by express-ing an excess amount of LANA aa 1 to 32, which reduced
replica-tion, confirmed that the recruitment of TopoIIby LANA is
re-quired for replication. In other words, close proximity of TopoII
to the site of replication initiation is important for replication. Since the replication element (RE) is adjacent to the LANA
bind-ing site (23), recruitment of TopoIIby LANA may bring the
TopoIIprotein at the RE site to initiate replication (Fig. 8).
TopoIIcontrols and alters the topologic state of DNA during
transcription and replication by changing the degree of
supercoil-ing of double-stranded DNA molecules (15, 25). TopoIIhas
been shown to induce double-stranded breaks required for
regu-lated transcription/replication (25). Our DNA break labeling and
ChIP assay to detect nicks in the KSHV TR DNA suggests that
TopoII mediates transient DNA breaks on KSHV DNA in a
LANA-dependent manner (Fig. 7E). Moreover, ellipticine was
also found to effectively interfere with the incorporation of biotin-16-dUTP in the double-stranded nick assay, strongly suggesting a
role for TopoIIin mediating double-stranded breaks on KSHV
TR DNA (Fig. 7G). Therefore, we propose that LANA recruits
TopoIIat the latent replication origins of TR to create a nick in
order to unwind the complex supercoiled DNA for the facilitation
of DNA replication (Fig. 8).
ACKNOWLEDGMENTS
We thank Erle S. Robertson, University of Pennsylvania, for providing the cell lines and LANA expression plasmids.
This work was supported by K99/R00, CA126182 to S.C.V.
REFERENCES
1.An FQ, et al.2005. The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus modulates cellular gene expression and protects lymphoid cells from p16 INK4A-induced cell cycle arrest. J. Biol. Chem.280:3862–3874.
2.Ballestas ME, Chatis PA, Kaye KM.1999. Efficient persistence of extra-chromosomal KSHV DNA mediated by latency-associated nuclear anti-gen. Science284:641– 644.
3.Ballestas ME, Kaye KM.2001. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol.75:3250 –3258.
4.Ballestas ME, Kaye KM.2011. The latency-associated nuclear antigen, a multifunctional protein central to Kaposi’s sarcoma-associated herpesvi-rus latency. Future Microbiol.6:1399 –1413.
5. Barbera AJ, Ballestas ME, Kaye KM. 2004. The Kaposi’s
sarcoma-associated herpesvirus latency-sarcoma-associated nuclear antigen 1 N terminus is essential for chromosome association, DNA replication, and episome per-sistence. J. Virol.78:294 –301.
6.Barbera AJ, et al.2006. The nucleosomal surface as a docking station for Kaposi’s sarcoma herpesvirus LANA. Science311:856 – 861.
7.Cai Q, Verma SC, Choi JY, Ma M, Robertson ES. 2010. Kaposi’s sarcoma-associated herpesvirus inhibits interleukin-4-mediated STAT6 phosphorylation to regulate apoptosis and maintain latency. J. Virol.84: 11134 –11144.
8.Cai Q, Verma SC, Lu J, Robertson ES. 2010. Molecular biology of Kaposi’s sarcoma-associated herpesvirus and related oncogenesis. Adv. Virus Res.78:87–142.
9.Cai Q, et al.2012. Kaposi’s sarcoma herpesvirus upregulates aurora A expression to promote p53 phosphorylation and ubiquitylation. PLoS Pathog.8:e1002566. doi:10.1371/journal.ppat.1002566.
10. Cannon JS, et al.2000. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi’s sarcoma herpesvirus-containing supernatant. J. Virol.74:10187–10193.
11. Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM.1995. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med.332:1186 –1191. 12. Chang Y, et al.1994. Identification of herpesvirus-like DNA sequences in
AIDS-associated Kaposi’s sarcoma. Science266:1865–1869.
13. Chen W, Hilton IB, Staudt MR, Burd CE, Dittmer DP.2010. Distinct p53, p53:LANA, and LANA complexes in Kaposi’s s-associated herpesvi-rus lymphomas. J. Virol.84:3898 –3908.
14. Cotter MA, II, Robertson ES.1999. The latency-associated nuclear anti-gen tethers the Kaposi’s sarcoma-associated herpesvirus anti-genome to host chromosomes in body cavity-based lymphoma cells. Virology264:254 – 264.
15. Cowell IG, Papageorgiou N, Padget K, Watters GP, Austin CA.2011. Histone deacetylase inhibition redistributes topoisomerase IIbeta from heterochromatin to euchromatin. Nucleus2:61–71.
16. Fakhari FD, Dittmer DP.2002. Charting latency transcripts in Kaposi’s sarcoma-associated herpesvirus by whole-genome real-time quantitative PCR. J. Virol.76:6213– 6223.
17. Friborg J, Jr, Kong W, Hottiger MO, Nabel GJ.1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature402:889 – 894.
18. Garber AC, Shu MA, Hu J, Renne R.2001. DNA binding and modula-tion of gene expression by the latency-associated nuclear antigen of Kapo-si’s sarcoma-associated herpesvirus. J. Virol.75:7882–7892.
19. Gonzalez-Molleda L, Wang Y, Yuan Y.2012. Potent antiviral activity of topoisomerase I and II inhibitors against Kaposi’s sarcoma-associated herpesvirus. Antimicrob. Agents Chemother.56:893–902.
20. Grundhoff A, Ganem D.2004. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sar-coma pathogenesis. J. Clin. Invest.113:124 –136.
21. Hirt B.1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol.26:365–369.
22. Hu J, Garber AC, Renne R.2002. The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus supports latent DNA replica-tion in dividing cells. J. Virol.76:11677–11687.
23. Hu J, Renne R.2005. Characterization of the minimal replicator of Ka-posi’s sarcoma-associated herpesvirus latent origin. J. Virol.79:2637– 2642.
24. Jenner RG, Alba MM, Boshoff C, Kellam P.2001. Kaposi’s sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J. Virol.75:891–902.
25. Ju BG, et al.2006. A topoisomerase IIbeta-mediated dsDNA break re-quired for regulated transcription. Science312:1798 –1802.
26. Kaul R, Verma SC, Robertson ES.2007. Protein complexes associated with the Kaposi’s sarcoma-associated herpesvirus-encoded LANA. Virol-ogy364:317–329.
27. Komatsu T, Ballestas ME, Barbera AJ, Kelley-Clarke B, Kaye KM.2004. KSHV LANA1 binds DNA as an oligomer and residues N-terminal to the oligomerization domain are essential for DNA binding, replication, and episome persistence. Virology319:225–236.
28. Lan K, et al.2005. Induction of Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen by the lytic transactivator RTA: a novel mechanism for establishment of latency. J. Virol.79:7453–7465. 29. Lee HR, Lee S, Chaudhary PM, Gill P, Jung JU.2010. Immune evasion FIG 8Schematic model showing the association of LANA with TopoII.
LANA recruits TopoIIto the sites of latent origin of replication. TopoIIin turn mediates double-stranded breaks required for viral DNA replication, fur-ther facilitated by the cellular replication machinery.