The HIV-1 Tat Protein Enhances Splicing at the Major Splice
Donor Site
Nancy Mueller,
aAlexander O. Pasternak,
aBep Klaver,
aMarion Cornelissen,
aBen Berkhout,
aAtze T. Das
aaLaboratory of Experimental Virology, Department of Medical Microbiology, Academic Medical Center,
University of Amsterdam, Amsterdam, The Netherlands
ABSTRACT
Transcription of the HIV-1 proviral DNA and subsequent processing of
the primary transcript results in the production of a large set of unspliced and
differ-entially spliced viral RNAs. The major splice donor site (5
=
ss) that is located in the
untranslated leader of the HIV-1 transcript is used for the production of all spliced
RNAs, and splicing at this site has to be tightly regulated to allow the balanced
pro-duction of all viral RNAs and proteins. We demonstrate that the viral Tat protein,
which is known to activate viral transcription, also stimulates splicing at the major
5
=
ss. As for the transcription effect, Tat requires the viral long terminal repeat
pro-moter and the
trans
-acting responsive RNA hairpin for splicing regulation. These
re-sults indicate that HIV-1 transcription and splicing are tightly coupled processes
through the coordinated action of the essential Tat protein.
IMPORTANCE
The HIV-1 proviral DNA encodes a single RNA transcript that is used
as RNA genome and packaged into newly assembled virus particles. This full-length
RNA is also used as mRNA for the production of structural and enzymatic proteins.
Production of other essential viral proteins depends on alternative splicing of the
primary transcript, which yields a large set of differentially spliced mRNAs. Optimal
virus replication requires a balanced production of all viral RNAs, which means that
the splicing process has to be strictly regulated. We show that the HIV-1 Tat protein,
a factor that is well known for its transcription activating function, also stimulates
splicing. Thus, Tat controls not only the level of the viral RNA but also the balance
between spliced and unspliced RNAs.
KEYWORDS
RNA splicing, Tat protein, VP16, human immunodeficiency virus,
transcription
T
he retrovirus HIV-1 has an
⬃
9.7-kb RNA genome that upon infection of cells is
reverse transcribed into double-stranded DNA. This DNA integrates into the cellular
genome and can subsequently be transcribed by RNA polymerase II (RNAP II), which
results in the production of new viral RNA transcripts. The full-length 5
=
-capped and
3
=
-polyadenylated transcript is used as mRNA for translation of the Gag and Pol
proteins and as genomic RNA that is packaged into new virus particles. The primary
transcript contains several 5
=
and 3
=
splice sites (5
=
ss and 3
=
ss), and differential usage
of these sites during the cotranscriptional splicing process results in a large variety of
spliced mRNAs that are translated into the other viral proteins, including the Tat protein
that stimulates HIV-1 transcription (1).
The RNAP II transcription complex that initiates at the 5
=
long terminal repeat (LTR)
promoter in the absence of Tat is paused at the stable
trans
-acting responsive (TAR)
RNA structure formed at the 5
=
end of the nascent transcript, which causes premature
termination of transcription (2, 3). As a consequence, predominantly short transcripts
are produced (4, 5). Binding of Tat to TAR results in the recruitment and activation of
the positive transcription elongation factor B (P-TEFb). P-TEFb preassembles at the U3
Received24 October 2017Accepted25 April 2018
Accepted manuscript posted online9 May 2018
CitationMueller N, Pasternak AO, Klaver B, Cornelissen M, Berkhout B, Das AT. 2018. The HIV-1 Tat protein enhances splicing at the major splice donor site. J Virol 92:e01855-17.
https://doi.org/10.1128/JVI.01855-17.
EditorWesley I. Sundquist, University of Utah
Copyright© 2018 American Society for Microbiology.All Rights Reserved.
Address correspondence to Atze T. Das, a.t.das@amc.uva.nl.
OF VIRAL GENE EXPRESSION
crossm
on November 6, 2019 by guest
http://jvi.asm.org/
region of the LTR promoter in a catalytically inactive state bound to the inhibitory 7SK
small nuclear ribonucleoprotein (snRNP) (6–8). Tat mediates transfer of P-TEFb to TAR
RNA, resulting in displacement of 7SK snRNA. The cyclin-dependent kinase 9
compo-nent of P-TEFb can subsequently activate RNAP II through phosphorylation of the
C-terminal domain (9–11). P-TEFb also phosphorylates the negative elongation factors,
which causes their release from the transcription complex, and P-TEFb directs the
recruitment of TATA box binding protein to the LTR promoter, which stimulates the
assembly of new transcription complexes (12). Tat may also recruit several
chromatin-modifying proteins to remodel the promoter region (reviewed in references 10, 13, and
14). In addition, Tat has been reported to stimulate phosphorylation of several
tran-scription factors that bind the LTR promoter, like Sp1 and NF-
B (reviewed in reference
13). These combined Tat effects result in the formation of a more processive
transcrip-tion complex that is not paused at the TAR RNA structure. Thus, Tat activates the
production of the complete set of unspliced and spliced viral RNAs needed for virus
replication.
HIV-1 splicing has to be tightly regulated for the balanced production of all viral
RNAs and proteins. The major 5
=
ss in the 5
=
untranslated leader is used for the
generation of all spliced mRNAs. The level of splicing at this 5
=
ss is controlled by several
factors. First, splicing regulatory elements that bind splicing enhancing or silencing
factors are located in close proximity to the 5
=
ss (15, 16). Second, incomplete sequence
complementarity between the 5
=
ss nucleotides and the U1 snRNA results in suboptimal
spliceosome assembly during the first step of the splicing process (17). Third, the
sequence surrounding the major 5
=
ss can fold a repressive hairpin structure (18). We
demonstrated that destabilization of this splice donor (SD) hairpin increased splicing,
whereas stabilization reduced splicing, which indicates that splicing is restricted by the
RNA structure, most likely by limiting the access of splicing factors, including U1 snRNA
and regulatory factors (19, 20).
Transcription and splicing are tightly coupled processes (reviewed in references 21
to 23). Several studies suggested a role for transcription factors in HIV-1 splicing and a
role for splicing factors in HIV-1 transcription. The transcription elongation regulator 1
(TCERG1; also known as CA150) that associates with the RNAP II complex and is
involved in Tat-activated HIV-1 transcription (24) also binds to the splicing factor SF1
(25). SF1 was originally identified as a constitutive splicing factor (26, 27), but other
studies indicated that it represses transcription (28, 29). The Tat stimulatory factor
Tat-SF1 was first discovered as a cellular protein required for activation of HIV-1
transcription (30–34). Several studies indicated that Tat-SF1 associates with RNAP II and
other factors, including Tat, P-TEFb, and TCERG1 (CA150) (30, 35–39). More recent
studies, however, demonstrated that Tat-SF1 is not required for Tat activation of
transcription but rather influences the ratio of unspliced to fully spliced HIV-1 RNAs (40,
41). Tat-SF1 interacts with several components of the spliceosome, including U1 snRNP
proteins and all five spliceosomal U snRNAs (42). The human splicing factor SKIP
(splicing-associated c-Ski-interacting protein) also influences both HIV-1 transcription
and splicing. SKIP associates with P-TEFb and subsequently stimulates transcription
elongation. SKIP also associates with U5 snRNP proteins and tri-snRNP110K, but not
with other splicing factors (including Tat-SF1 and TCERG1/CA150), and enhances
utilization of an alternative 3
=
ss for a Tat-encoding RNA (43). Recently, the splicing
factor SRSF1 (SF2/ASF) and Tat were shown to recognize overlapping sequences within
TAR and 7SK RNA (44). A model was proposed in which the ubiquitously expressed
SRSF1 can activate basal HIV-1 transcription in the absence of Tat by recruiting P-TEFb
to TAR from the 7SK snRNP. When present, Tat can substitute for SRSF1, which results
in more efficient transcription elongation.
The Tat protein has a major role in the regulation of HIV-1 transcription, but other
functions have been suggested to support HIV-1 replication. For example, Tat may
influence reverse transcription, capping, and translation of the viral RNAs (45–50) and
stimulate production of an HIV-1 TAR-encoded miRNA-like small RNA (5). Tat also
influences 3
=
ss selection and promotes usage of splice sites in Rev- and Env/Nef-specific
on November 6, 2019 by guest
http://jvi.asm.org/
mRNAs (45). We investigate here the role of Tat in splicing at the major splice donor site
that is used for the production of all spliced RNAs.
RESULTS
Tat activates splicing at the major 5
=
ss.
To analyze the effect of Tat on splicing at
the major 5
=
ss site, we used a simple reporter construct in which the 5
=
LTR promoter
and leader RNA sequence of the HIV-1
LAIstrain, including the major 5
=
ss (SD
wt;
CUG
Œ
GUGAGUAC, with “
Œ
” indicating the splice site), was fused to the firefly luciferase
gene that encodes a cryptic 3
=
ss (
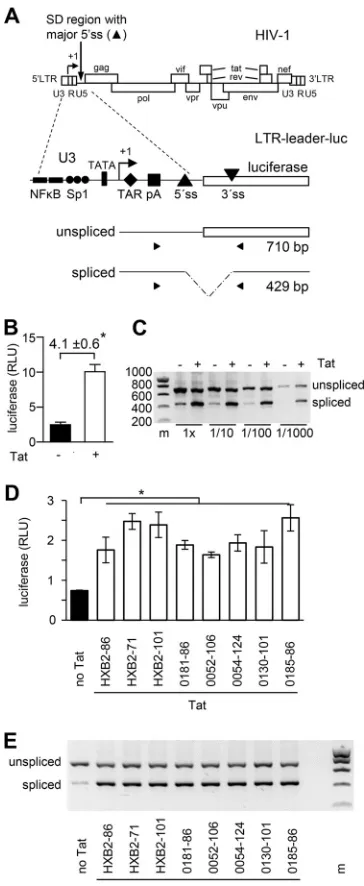
in Fig. 1A) (19). Upon transfection of this
LTR-leader-luc construct in cells, transcription and subsequent RNA processing will result in
the production of unspliced RNAs that encode luciferase and spliced RNAs that do not.
These RNA products can be detected by reverse transcription followed by PCR
ampli-fication (reverse transcription-PCR [RT-PCR]), which results in 710- and 429-bp products
for the unspliced and spliced RNAs, respectively (Fig. 1A). Moreover, the intracellular
luciferase level can be measured to quantify splicing. We previously used this reporter
to determine the effect of local RNA structure and binding of splice regulatory SR
proteins in the SD region on splicing frequency (15, 20).
The LTR-leader-luc construct (pLTR-leader-luc) was cotransfected into 293T cells
with either the pTat plasmid expressing the HIV-1
LAITat protein or the empty pcDNA3
vector. Transcription from the LTR promoter is activated by Tat and we indeed
measured an increased luciferase level with pTat (15, 20) (Fig. 1B). Both in the absence
and in the presence of Tat, unspliced and spliced RNAs were detected by RT-PCR, but
the ratio between these products varied. The unspliced RNA product was more
abundant in the absence of Tat, whereas the spliced RNA product was more abundant
in the presence of Tat (Fig. 1C). A similar pattern was observed when the cells were
transfected with smaller amounts of the reporter construct (data not shown) or when
the PCR analysis was repeated with different dilutions of the cDNA input (Fig. 1C). These
results indicate that the observed splicing effects are not due to quantitative
differ-ences in RNA transcripts resulting from Tat-induced activation of transcription but likely
reflect Tat activation of splicing at the major 5
=
ss site.
The HIV-1 Tat protein is encoded by two exons and varies in size in different HIV-1
isolates. Whereas most subtype B isolates produce a 101-amino-acid (101-aa) protein,
some subtype B viruses that are frequently used in experimental settings (e.g., the
HIV-1
LAIand closely related HIV-1
HXB2strains), but also most subtype D and H isolates,
produce an 86-aa protein (51). We therefore cotransfected the pLTR-leader-luc
con-struct with different Tat plasmids, expressing either the complete (86-aa), a
first-exon-only (71-aa), or an extended (101-aa) Tat protein of the HIV-1
HXB2strain, or the Tat
protein of other HIV-1 subtype B primary isolates that varied in size (0181-86aa,
0052-106aa, 0054-124aa, 0130-101aa, and 0185-86aa) (51). The Tat variants similarly
enhanced LTR-driven luciferase production (Fig. 1D). When analyzing the luciferase
transcripts by RT-PCR, all Tat proteins were found to increase the spliced/unspliced RNA
ratio (Fig. 1E). These results indicate that the splicing stimulatory effect is a general Tat
characteristic and that the 71-aa fragment encoded by the first exon is sufficient for this
activity.
The Tat splicing effect does not depend on local RNA structure or SR protein
binding.
The sequence surrounding the major 5
=
ss can fold an RNA stem-loop
struc-ture, the splice donor (SD) hairpin, that exposes the splice site in the loop (Fig. 2A). To
analyze the role of this hairpin structure in Tat activation of splicing, we analyzed the
RNAs produced by LTR-leader-luc constructs with different nucleotide substitutions
that destabilized the RNA structure but did not affect the 5
=
ss sequence (Fig. 2A). These
mutations, except for the A
Lmutation, also removed some of the putative SR protein
binding sites, in particular putative sites for the SRSF2 (SC35) protein (Fig. 2B). We
previously demonstrated that most of these mutations slightly increase the splicing
efficiency— both in the absence and in the presence of Tat—and resulted in a reduced
luciferase level compared to the wild-type (wt) construct (15, 20). RT-PCR analysis of the
RNAs produced by the different variants revealed that the unspliced RNA product was
on November 6, 2019 by guest
http://jvi.asm.org/
FIG 1Tat activates splicing at the HIV-1 major 5=ss. (A) The HIV-1 provirus (DNA) is shown with its ORFs and LTR regions. The 5=and 3=LTRs consist of U3, R, and U5 regions. Transcription starts at the U3-R border (⫹1) of the 5=LTR, and the transcripts are polyadenylated at the R-U5 border of the 3=LTR. The major 5=ss is used for the production of all spliced RNAs. In the pLTR-leader-luc construct, the 5=LTR, and leader region (including the major 5=ss) of the HIV-1LAImolecular clone are coupled to the firefly
luciferase gene that encodes a cryptic 3=ss. The primers used for RT-PCR analysis of the RNA products are indicated. This RT-PCR will result in a product of 710 bp for the unspliced RNA and 429 bp for the spliced RNA. (B) 293T cells were transfected with the pLTR-leader-luc construct with or without the plasmid expressing the HIV-1LAITat protein (pTat). The intracellular luciferase production was measured after 48
h. The average values of four independent experiments are shown, with the error bars representing the standard deviations. The fold induction in luciferase expression observed upon Tat administration is indicated. (C) The unspliced and spliced RNAs were analyzed by RT-PCR. Different dilutions of the reverse transcription cDNA product were used as input in the PCR (1⫻, undiluted). (D and E) 293T cells were transfected with the pLTR-leader-luc construct and different Tat plasmids, expressing either the complete (86-aa), the first-exon-only (71-aa), or the extended (101-aa) Tat protein of the HIV-1HXB2strain, or the Tat
protein of different HIV-1 subtype B primary isolates that varied in size (0181-86aa, 0052-106aa, 0054-124aa, 0130-101aa, and 0185-86aa). The intracellular luciferase production was measured after 48 h (D; average values of three experiments, with the error bars representing the standard deviations, are shown), and the unspliced and spliced RNAs were analyzed by RT-PCR (E). A two-sided unpaired sample
t test was used to identify statistically significant differences (*, P ⬍ 0.05). RT-PCR analyses were performed two times, and representative agarose gels are shown (m, DNA size marker in bp).
on November 6, 2019 by guest
http://jvi.asm.org/
[image:4.585.114.296.70.513.2]nevertheless always more abundant in the absence of Tat (Fig. 2C, upper panel), and
the spliced RNA product was more abundant in the presence of Tat (lower panel). In
fact, the Tat-induced increase in splicing efficiency was more pronounced than the
mutation-induced increase. These results demonstrate that destabilization of the SD
FIG 2Role of SD hairpin stability and putative SR protein binding sites in Tat activation of splicing. (A) The sequence of the SD region with the putative SR protein binding sites (63) and the SD stem-loop structure with the introduced mutations are shown (Œ, cleavage site; gray circles indicate the 5=ss nucleotides to which the U1 snRNA can bind). (B) The presence (⫹) or absence (⫺) of putative SR protein binding sites, the thermodynamic stability of the SD hairpin (ΔGin kcal/mol), and the splicing efficiency (as previously determined [17, 20]) of the wt and mutant SD sequences are shown. (C) 293T cells were transfected with the wt or mutant pLTR-leader-luc constructs without (upper panel) or with (lower panel) pTat, and the RNA products were analyzed by RT-PCR. This RT-PCR analysis was performed three times, and a representative agarose gel is shown.on November 6, 2019 by guest
http://jvi.asm.org/
[image:5.585.79.328.66.580.2]hairpin or removal of the putative SR protein binding sites did not impede Tat
activation of splicing. In other words, the Tat splicing effect does not depend on local
5
=
ss characteristics such as hairpin structure and SR protein binding.
Disentanglement of the Tat transcription and splicing effects.
These results
suggest that Tat not only activates transcription from the LTR promoter, but also
stimulates splicing at the major 5
=
ss. These Tat effects have opposing effects on the
read out in LTR-leader-luc transfection experiments. Whereas an increase in
transcrip-tion will boost the luciferase level, an increase in splicing will reduce this level. The
observed increase in luciferase production observed upon Tat administration (Fig. 1B)
thus reflects the sum of these opposing effects. To disentangle these transcription and
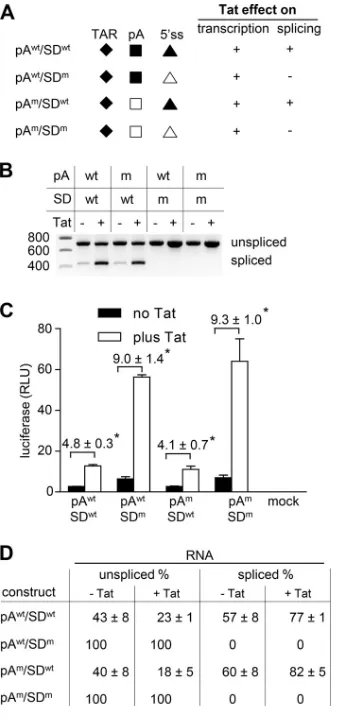
splicing effects, we designed an LTR-leader-luc construct with an inactivated major 5
=
ss
sequence (SD
m; CUG
Œ
GUGCGCAC, with mutations underlined). The introduced SD
mmutation reduces the sequence complementarity between the 5
=
ss and U1 snRNA and,
as previously demonstrated, completely blocks splicing at the major 5
=
ss (20). As a
consequence, an increase in luciferase production measured upon Tat administration
will solely reflect Tat-mediated activation of transcription. It has previously been
described that binding of U1 snRNA to the 5
=
ss prevents polyadenylation at the
polyadenylation signal present in the 5
=
LTR (52, 53). We anticipated that the SD
mmutation could thus cause premature polyadenylation of the transcripts, which would
result in very short RNAs [
⬃
97 nucleotides plus poly(A) tail] and complicate the analysis.
We therefore included SD
wtand SD
mconstructs with a mutation in the 5
=
polyadenyl-ation signal (pA
wt, AAUAAA; pA
m, AACAAA) that inactivates this site (Fig. 3A).
293T cells were transfected with the LTR-leader-luc constructs with pTat or the
empty pCDNA3 vector, and the RNA products and luciferase level were analyzed after
48 h. Analysis of the RNAs produced by the wt construct (SD
wt/pA
wt) demonstrated the
typical shift toward spliced RNA products upon Tat administration (Fig. 3B). For the
SD
m/pA
wtconstruct, only the unspliced RNA was detected both in the absence and in
the presence of Tat, which confirms that the 5
=
ss mutation did completely block
splicing. Accordingly, the SD
mconstruct consistently produced significant higher
lucif-erase levels than the wt construct (Fig. 3C). Moreover, Tat activated luciflucif-erase
expres-sion 4.8-fold for the wt construct, whereas expresexpres-sion from the SD
mconstruct was
induced 9.0-fold. This difference in fold induction can be attributed to the Tat-splicing
effect, which tempers Tat-induced luciferase production by the SD
wtconstruct.
Muta-tion of the polyadenylaMuta-tion signal did not influence the RNA patterns or the luciferase
levels (compare SD
wt/pA
wtwith SD
wt/pA
mand compare SD
m/pA
wtwith SD
m/pA
min
Fig. 3B and C), which indicates that this site was not used for polyadenylation in the
SD
wtand SD
mtranscripts. As observed for the pA
wtconstructs, Tat administration
induced luciferase expression from the splicing-competent pA
mconstruct (SD
wt/pA
m)
to a lesser extent (4.1-fold) than from the splicing-defective variant (SD
m/pA
m) (9.3-fold;
Fig. 3C), which confirms the Tat-splicing effect.
We calculated the splicing frequency of the different constructs in the absence or
presence of Tat. For every SD
wt/SD
mset, the luciferase level observed for the SD
mvariant reflects the total RNA production (100% unspliced RNA). The luciferase level
observed in mock-transfected cells (which is close to zero) reflects the absence of
unspliced RNA (0% unspliced RNA). These values were used to interpolate the unspliced
RNA level of the SD
wtvariants (luciferase level SD
wtconstruct/luciferase level SD
mconstruct
⫻
100%; Fig. 3D). In the absence of Tat, both the SD
wt/pA
wtand SD
wt/pA
mconstructs produced
⬃
40% unspliced RNA (
⬃
60% spliced RNA). In the presence of Tat,
both constructs produced only
⬃
20% unspliced RNA (
⬃
80% spliced RNA) (Fig. 3D),
corroborating the stimulatory effect of Tat on splicing at the 5
=
ss site.
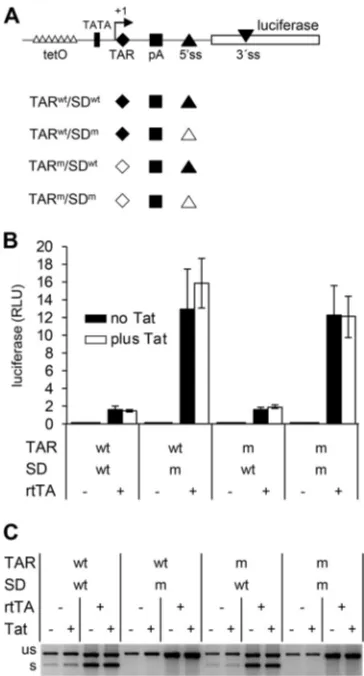
Tat requires TAR for the splicing effect.
Tat binds the TAR hairpin that is present
at the 5
=
ends of nascent viral transcripts to activate transcription at the 5
=
LTR. To test
whether splicing activation also depends on the Tat-TAR interaction, we tested an
LTR-leader-luc construct with several nucleotide substitutions in TAR that prevent Tat
binding (TAR
m; Fig. 4A). These mutations block the Tat-TAR transcription activation
on November 6, 2019 by guest
http://jvi.asm.org/
mechanism (54, 55). This construct contains two binding sites (tet operators; tetO) for
the doxycycline (dox)-inducible transcriptional activator protein rtTA in the U3
pro-moter region, which allows the exogenous activation of transcription by the
adminis-tration of dox and rtTA (56).
Whereas the wt construct (TAR
wt) revealed the Tat-induced increase in spliced RNA
in transfected cells (Fig. 4B), the TAR-mutated construct (TAR
m) demonstrated an
identical RNA pattern in the absence or presence of Tat, which indicates that Tat did not
activate splicing. These results demonstrate that Tat requires a wt TAR element for its
splicing effect. The TAR
mconstruct produced a slightly increased level of luciferase in
the presence of Tat (Fig. 4C), which likely reflects TAR-independent activation of
transcription that requires the presence of the Sp1 sites in the U3 promoter region (57).
The U3 promoter region is required for the Tat splicing effect.
For its
transcrip-tion activating functranscrip-tion, Tat preassembles with P-TEFb at the U3 region of the LTR
FIG 3Tat activates transcription and splicing. (A) LTR-leader-luc variants were constructed with a wt (SDwt, CUGŒGUGAGUAC) or inactivated major 5=ss (SDm, CUGŒGUGCGCAC) and a wt (pAwt, AAUAAA) orinactivated 5=polyadenylation site (pAm, AACAAA; wt and mutated elements are indicated by filled and
open symbols, respectively). The anticipated effect of Tat on transcription and splicing is indicated (⫹, effect;⫺, no effect). (B) 293T cells were transfected with the LTR-leader-luc constructs without or with pTat. The RNA products were analyzed by RT-PCR. This RT-PCR analysis was performed two times, and a representative agarose gel is shown. (C) Analysis of the luciferase production. The average values and standard deviations of four independent experiments are shown. The fold induction in luciferase expression observed upon Tat administration is indicated. A two-sided unpaired samplettest was used to identify statistically significant differences (*, P⬍ 0.05). (D) The percentage of spliced RNA was calculated for every mutant. The luciferase level of the SDmmutant (100% unspliced) was used to
interpolate the unspliced RNA level for the SDwtconstructs (luciferase level SDwtconstruct/luciferase level
SDmconstruct⫻100%).
on November 6, 2019 by guest
http://jvi.asm.org/
[image:7.585.120.290.70.430.2]promoter and is subsequently transferred to the TAR hairpin at the 5
=
end of nascent
viral transcripts (6). To test whether or not the splicing-stimulating activity of Tat also
requires the viral U3 promoter region, we designed pTet-leader-luciferase reporter
constructs in which the U3 sequences are replaced by the dox-inducible pTet promoter,
which consists of 7 tetO sites coupled to a TATA box containing minimal promoter
sequence (Fig. 5A). This promoter activates transcription exclusively in the presence of
dox and rtTA (56). Cells were transfected with pTet constructs with wt or mutant TAR
and SD sequences, either with or without rtTA- and Tat-expressing plasmids. After
culturing the transfected cells with dox for 48 h, the luciferase level was measured (Fig.
5B), and the RNA transcripts were analyzed by RT-PCR (Fig. 5C). For both the SD
wtand
SD
mvariants, the luciferase level was low in the absence of rtTA and increased
FIG 4Tat requires TAR to activate splicing. (A) LTR-leader-luc constructs with a wt (TARwt) or mutant(TARm) TAR hairpin. The TARmconstruct contains several nucleotide substitutions in the TAR loop and
bulge domains to prevent Tat binding. This construct contains two binding sites (tet operators; tetO) for the dox-inducible transcriptional activator protein rtTA in the U3 promoter region, which allows the exogenous activation of transcription by the administration of dox and rtTA. (B and C) 293T cells were transfected with the TARm construct, an rtTA-expressing plasmid, and either pTat or pcDNA3 and
cultured with dox. Cells were transfected with the TARwtconstruct and either pTat or pcDNA3 in parallel.
The RNA products (B) and luciferase production (C) were analyzed at 48 h after transfection as described in Fig. 3. The RNA (RT-PCR) analysis was performed two times, and a representative agarose gel is shown. The luciferase analysis was performed four times, and average values and standard deviations are shown. The fold induction in luciferase expression observed upon Tat administration is indicated. A two-sided unpaired samplettest was used to identify statistically significant differences (*,P⬍0.05).
on November 6, 2019 by guest
http://jvi.asm.org/
[image:8.585.113.294.66.468.2]significantly in the presence of rtTA (Fig. 5B), which is in agreement with rtTA control
of transcription. As anticipated, the SD
wtconstructs produced both spliced and
un-spliced RNAs, whereas the SD
mvariants produced only unspliced RNA (Fig. 5C), which
resulted in higher luciferase levels (Fig. 5B). Tat did not influence the luciferase and RNA
transcript levels or the ratio between the spliced and unspliced SD
wtRNAs, even when
the construct carried a wt TAR element. These results indicate that Tat requires the U3
promoter region to stimulate splicing.
Notably, rtTA did increase the ratio between the spliced and unspliced SD
wtRNAs
in a similar way as Tat increased this ratio for the wt LTR-leader luciferase constructs
(compare the increased relative abundance of the spliced SD
wtRNAs in the presence of
rtTA in Fig. 5C and of the spliced TAR
wtRNAs in the presence of Tat in Fig. 4B). This
result suggests that rtTA can also enhance splicing at the major 5
=
ss. Such an effect on
the spliced/unspliced RNA ratio was less apparent upon rtTA activation of the TAR
mconstruct in Fig. 4B, which could relate to the presence of only two tetO binding sites
in the U3 region of this construct.
Tat activates HIV-1 splicing.
Analysis of the Tat effect on splicing efficiency in the
complete HIV-1 genome context is complicated by pleiotropic effects that Tat
muta-tions may have on viral gene expression. Tat may not only affect transcription and
splicing at the major 5
=
ss but also other processes, such as 3
=
ss selection (45). The fact
FIG 5The U3 promoter region is required for the Tat effect on splicing. (A) In the pTet-leader-luc construct, transcription is driven by the dox-inducible pTet promoter, which consists of seven tetO sites coupled to a TATA box containing minimal promoter sequence. Variants were constructed with a wt (TARwt) or mutant (TARm) TAR element and a wt (SDwt) or mutant (SDm) SD sequence. (B and C) 293T cellswere transfected with the pTet-leader-luc constructs, an rtTA-expressing plasmid, and/or pTat and cultured with dox. The luciferase expression and RNA products were analyzed as described in Fig. 3. The luciferase analysis was performed three times, and average values and standard deviations are shown. The RNA (RT-PCR) analysis was performed two times, and a representative agarose gel is shown.
on November 6, 2019 by guest
http://jvi.asm.org/
[image:9.585.114.296.70.408.2]that the regulatory Tat and Rev proteins are produced from fully spliced transcripts
further complicates the analysis. Rev stimulates nuclear export of the unspliced and
singly spliced viral RNAs. Moreover, Rev has been reported to influence other viral
replication processes, such as translation and RNA encapsidation (58). Thus, changes in
the splicing efficiency of the complete HIV-1 genome will have complicated direct and
indirect effects on RNA production and processing.
We nevertheless set out to confirm the stimulatory effect of Tat on splicing at the
major 5
=
ss in the complete HIV-1 genome context. To prevent the Tat and Rev
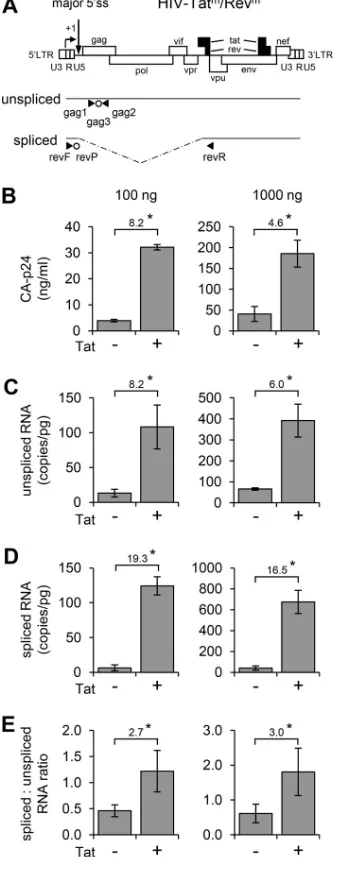
regulatory loops during viral gene expression, we constructed a Tat- and Rev-deficient
HIV-1 variant (HIV-Tat
m/Rev
min Fig. 6A). In this virus, the production of Tat was blocked
FIG 6Tat enhances HIV-1 splicing. (A) Schematic of the HIV-Tatm/Revmgenome with the primers (‹andŠ) and probes (Œ) used in the qRT-PCR to detect unspliced and spliced viral RNAs. (B to E) 293T cells were transfected with 100 or 1,000 ng of HIV-Tatm/Revm(right and left panel, respectively), 25 ng of Rev
plasmid, and 0 or 50 ng of Tat plasmid. The CA-p24 protein level was measured in the culture supernatant after 48 h (B). The intracellular RNA was isolated after 48 h and used for the qRT-PCR analysis to determine the level of the unspliced and spliced viral RNAs (C and D) and their ratio (E). The average values and standard deviations of three independent experiments are shown. The fold induction observed upon Tat administration is indicated. The effect of Tat was statistically significant in all panels, as determined by unpaired samplettests (*,P⬍0.05).
on November 6, 2019 by guest
http://jvi.asm.org/
[image:10.585.119.289.74.511.2]by substitution of the Gly
15and Glu
17codons in the Tat open reading frame (ORF) with
translation stop codons, which will cause premature termination of translation (57). To
prevent Rev production, the translation start and Gly
6codons in the Rev ORF were
replaced by translation stop codons. These mutations did not affect other ORFs. Upon
transfection into 293T cells, this construct did not produce detectable viral Gag-derived
CA-p24 protein (data not shown), whereas viral gene expression could be rescued by
cotransfection of Tat- and Rev-expressing plasmids. Cotransfection of the Rev plasmid
resulted in a detectable level of CA-p24, which was further increased by Tat (Fig. 6B).
We used quantitative RT-PCR (qRT-PCR) to quantify both the unspliced and the spliced
RNAs. The unspliced RNAs were detected with primers and a probe recognizing the gag
region (59), whereas the spliced RNAs were analyzed with primers/probe designed to
detect RNAs resulting from splicing from the major 5
=
ss to a 3
=
ss upstream of the rev
gene (Fig. 6A) (60). Low levels of the unspliced and spliced RNAs were measured in the
absence of Tat, and both RNA levels were significantly increased in the presence of Tat
(Fig. 6C and D). Notably, the ratio between the spliced and unspliced RNAs was also
increased by Tat (
⬃
3-fold; Fig. 6E), confirming the stimulatory effect of Tat on splicing
at the major 5
=
ss. Similar results were obtained upon transfection of the HIV-Tat
m/Rev
m,
Rev, and Tat plasmids into SupT1 cells, a T cell line that expresses the CD4 and CXCR4
receptors for HIV infection and that fully supports virus replication (Fig. 7). Also in these
physiologically more relevant cells, Tat stimulated splicing and significantly increased
the ratio between the spliced and unspliced HIV RNAs (9.7-fold increase; Fig. 7C).
FIG 7Tat enhances HIV-1 splicing in T cells. SupT1 T cells were transfected with 1,000 ng of HIV-Tatm/Revm, 25 ng of Rev plasmid, and 0 or 50 ng of Tat plasmid. The intracellular RNA wasisolated after 48 h and used for qRT-PCR analysis to determine the level of the unspliced and spliced viral RNAs (A and B) and their ratio (C). The average values and standard deviations of three independent experiments are shown. The fold induction observed upon Tat administration is indicated. Due to the relatively high variation in transfection efficiency, which we frequently observe in SupT1 electroporation experiments, the Tat effect was not statistically significant in the unspliced and spliced RNA panels, but this effect was statistically significant for the spliced/unspliced RNA ratio, as determined by unpaired samplettests (*,P⬍0.05).
on November 6, 2019 by guest
http://jvi.asm.org/
[image:11.585.151.262.73.362.2]DISCUSSION
Transcription and splicing are tightly coupled processes (reviewed in references 21
to 23). Several studies suggested a role for cellular transcription factors (e.g., TCERG1/
CA150 and Tat-SF1) in HIV-1 splicing and a role for cellular splicing factors (e.g., SKI and
SRSF1) in HIV-1 transcription (25, 40, 42, 43). We here demonstrate that the viral Tat
protein, a strong activator of viral transcription, also stimulates splicing at the major
5
=
ss that is located in the untranslated leader RNA and that is used for the generation
of all spliced RNAs. As for the transcription mechanism, Tat requires the viral LTR
promoter and the TAR hairpin for the splicing effect, which indicates that both activities
are closely intertwined.
The C-terminal domain (CTD) of RNAP II may have a central role in both Tat effects
on transcription and splicing, since differential phosphorylation of this domain affects
the association of both transcription and RNA processing factors (21–23). At the HIV-1
LTR promoter, this phosphorylation is mediated by the P-TEFb complex that is recruited
in an inactive, 7SK snRNP-bound state. Binding of Tat to the nascent TAR RNA and
P-TEFb is thought to displace 7SK snRNP and activate P-TEFb, which results in
phos-phorylation of the CTD (6–8). This Tat-induced RNAP II phosphos-phorylation may not only
stimulate recruitment of transcription factors and enhance transcription (9–11) but also
stimulate recruitment of splicing factors to enhance splicing. In agreement with this
explanation, we observed that both HIV-1 LTR DNA elements and TAR RNA are required
for the Tat splicing effect. Furthermore, we observed that the transcriptional activator
protein rtTA can similarly activate both transcription and splicing. This fusion protein
consists of the
Escherichia coli
-derived Tet repressor protein, which mediates specific
binding to tet operator sequences, and three copies of the herpes simplex virus
VP16-derived minimal transcription activation domain (AD). Like Tat, the VP16 AD can
recruit P-TEFb to the transcription complex (61, 62), which leads to phosphorylation of
the RNAP II CTD.
Jablonski et al. (45) previously demonstrated that Tat activates splicing to the 3
=
ss
sites of the Rev- and Env/Nef-specific mRNAs. Surprisingly, this Tat effect required the
TAR hairpin but not the HIV-1 LTR promoter and was also observed with nonviral
promoters that were not activated by Tat. The latter result suggests that this Tat
splicing effect can be uncoupled from the transcription effect. Further analyses
indi-cated the involvement of Tat-SF1 and TCERG1 (CA150) that are recruited to the RNAP
II complex and stimulate assembly of the SRSF1 (SF2) splice factor onto the GAR splicing
enhancer downstream of the Rev- and Env/Nef-specific 3
=
ss. This interaction promotes
the upstream 3
=
ss sites and recruitment of U1 snRNP to the downstream 5
=
ss, which, by
an unknown mechanism, leads to increased expression of the Env-encoding mRNA (45).
Although the Tat splicing effect that we describe at the major 5
=
ss requires the HIV-1
LTR promoter, a similar mechanism that involves recruitment of Tat-SF1, TCERG1
(CA150), and splicing factors may be involved as 5
=
ss splicing is influenced by several
nearby positioned splice regulatory elements (16, 63).
MATERIALS AND METHODS
DNA constructs.The LTR-leader-luc plasmid (pLTR-gag-flag-lucfirefly) and derivatives with a mutated
polyadenylation site (pAm) or a mutated SD site (SDm; a.k.a. J1) were described previously (15, 19, 20). To
combine the pAm and SDm mutations, the HindIII-NcoI fragment from pLTR-gag-flag-luc-SDJ1 that
contained the SDmmutation was ligated into the corresponding sites of pLTR-gag-flag-luc-pAm. To
construct an LTR-2ΔtetO-leader-luc variant with an inactivated TAR element (TARm) and tetO elements
in the U3 promoter region, we inserted the HindIII-NcoI leader fragment from pLTR-gag-flag-luc into the corresponding sites of plasmid pLTR-2ΔtetO-luc (56) that contains the HIV-1 LTR promoter with two tetO elements between the NF-B and Sp1 sites, an inactivated TAR element, and a truncated leader region. In the plasmid HIV-LTR-7dtetO-n-CMV2-dNF-luc (to be published elsewhere), seven tetO sequence elements and the 30-bp minimal TATA box containing promoter region derived from the cytomegalo-virus immediate-early (CMV IE) promoter replaced the HIV-1 U3 promoter region in pLTR-2ΔtetO-luc. To introduce the wild-type (wt) and SD-mutated (SDm) HIV-1 RNA leader regions in this construct, the leader
region in the pLTR-gag-flag-luc and pLTR-gag-flag-luc-SDJ1plasmids was PCR amplified with the primers
CMV-TATA-mut2 (GAAAGTCGACTATATAAGCAGAGCTCGTTTAGTGAACCGGTCTCTCTGGTTAGACCAGA; the AgeI site is underlined; the TAR sequence is italicized) and the reverse luciferase primer TA016 (15). The resulting PCR fragments were digested with HindIII and NcoI and ligated into the corresponding sites of
on November 6, 2019 by guest
http://jvi.asm.org/
HIV-LTR-7dtetO-n-CMV2-dNF-luc, which resulted in the pTet-leader-TARm/SDwt-luc and
pTet-leader-TARm/SDm-luc plasmids. To construct variants with a wt TAR sequence, the PCR fragments were digested
with AgeI and NcoI and ligated into the corresponding sites of HIV-LTR-7dtetO-n-CMV2-dNF-luc, which resulted in pTet-leader-TARwt/SDwt-luc and pTet-leader-TARwt/SDm-luc constructs.
The Tat and Rev ORFs in the HIV-1 pLAI molecular clone were inactivated by mutagenesis PCR, using the overlap extension method (64) and HIV-Tatstop(5) as the template. PCR fragment 1 was produced
with the primers Vpr-primer1-Not1 (ACCGCGGCCGCAAGGGCCACAGAGGGAG) and Tat-splice1 (57). PCR fragment 2 was produced with primers Rev-Augstop1 (AGGCATCTCCTTAGGCAGGAAGAAGCTGAGACAG CGA) and WS3 (57). The fragments 1 and 2 were combined and used as the template in a third PCR with the outer primers Vpr-primer1-Not1 and WS3. The resulting PCR fragment was digested with SalI and KpnI and ligated into the corresponding sites of pUHD6100-8911, a shuttle vector containing the NcoI-BamHI Tat-Vpu-Env fragment from pLAI (57). After digestion with NcoI and BamHI, the mutated Tat-Vpu-Env fragment was ligated into the corresponding sites of pLAI, which resulted in HIV-Tatm-Revm.
The pRL-CMV plasmid contains theRenillaluciferase reporter gene under the control of the CMV-IE promoter (Promega). The plasmid pTat-exon expresses the HIV-1 LAI Tat protein under the control of the CMV-IE promoter (57). Similar Tat plasmids expressing Tat HXB2-71, Tat HXB2-86, Tat HXB2-101, Tat 0181-86, Tat 0185-86, Tat 0130-101, Tat 0052-106, or Tat 0054-124 are recently described by van der Kuyl et al. (51).
Cell culture and transfection.293T cells were cultured at 37°C and 5% CO2in Dulbecco modified
Eagle medium containing 10% fetal bovine serum, nonessential amino acids, 40 U/ml penicillin, and 40
g/ml streptomycin. Unless stated otherwise, cells were transfected by calcium phosphate precipitation as previously described (56).
Luciferase activity assays.293T cells (⬃1.5⫻105cells per 2 cm2, 60% confluence) were transfected
with 20 ng of the LTR-leader-luc or pTet-leader-luc construct, 0.5 ng of pRL-CMV (as an internal control), 5 ng of Tat plasmid or the empty pcDNA3 vector, and 0.4 ng of pCMV-rtTA-V1 (56) when indicated. The DNA was completed to 500 ng with pBluescript as carrier DNA. When indicated, 1,000 ng/ml doxycycline was added to the culture medium to activate rtTA. The cells were cultured for 48 h and subsequently lysed in passive lysis buffer (Promega). Firefly andRenillaluciferase activities were determined with the dual-luciferase assay (Promega), and the firefly luciferase activity was normalized to theRenillaluciferase activity. Data were corrected for between-session variation with the factor correction software (65).
RT-PCR analysis.293T cells (⬃1.5⫻105cells per 2 cm2, 60% confluence) transfected with 1g of
the LTR-leader-luc or pTet-leader-luc construct, 50 ng of Tat plasmid or pcDNA3, and 0.4 ng of pCMV-rtTA-V1 (56) (when indicated) were cultured for 48 h (with 1,000 ng/ml dox when indicated). Total cellular RNA was isolated with the RNeasy kit (Qiagen), and contaminating DNA was removed with RNase-free DNase during isolation (Qiagen). The RNA was used as the template for cDNA synthesis with oligo(dT) and random hexamers as the primer (ThermoScript RT-PCR system; Invitrogen). The cDNA product obtained with the LTR-leader-luc constructs was PCR amplified with primers TA033 (66) and TA113 (19), as described previously (19). The cDNA product for the pTet-leader-luc constructs was PCR amplified with primers LTRalu1 (CTGGCTAACTAGGGAACCCACTG) and TA113 (19). The PCR products were analyzed by ethidium bromide/agarose gel electrophoresis.
Quantitative RT-PCR.293T and SupT1 cells were transfected with 100 or 1,000 ng of HIV-Tatm/Revm,
25 ng of pRSV-Rev, 0 or 50 ng of pTat-exon, and 500 or 450 ng of pcDNA3 (total amount of pTat-exon and pcDNA3 kept constant at 500 ng) using Lipofectamine 2000 (Invitrogen) and electroporation (56), respectively, and cultured for 48 h. The culture supernatant was harvested and virus production was measured by CA-p24 enzyme-linked immunosorbent assay (67). The intracellular RNA was isolated using the Boom isolation method (68). The unspliced and spliced RNAs were analyzed by qRT-PCR as described previously (59, 60).
ACKNOWLEDGMENTS
This research was sponsored by the Netherlands Organization for Scientific Research
(Chemical Sciences Division; NWO-CW; TOP grant) and the Aidsfonds (The Netherlands;
grant 2012025).
We thank Alex Harwig for critical readings of the manuscript.
REFERENCES
1. Purcell DF, Martin MA. 1993. Alternative splicing of human immunode-ficiency virus type 1 mRNA modulates viral protein expression, replica-tion, and infectivity. J Virol 67:6365– 6378.
2. Berkhout B, Silverman RH, Jeang KT. 1989. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell 59:273–282.
https://doi.org/10.1016/0092-8674(89)90289-4.
3. Palangat M, Meier TI, Keene RG, Landick R. 1998. Transcriptional pausing at⫹62 of the HIV-1 nascent RNA modulates formation of the TAR RNA structure. Mol Cell 1:1033–1042.https://doi.org/10.1016/ S1097-2765(00)80103-3.
4. Kao SY, Calman AF, Luciw PA, Peterlin BM. 1987. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature 330:489 – 493.https://doi.org/10.1038/330489a0.
5. Harwig A, Jongejan A, van Kampen AH, Berkhout B, Das AT. 2016. Tat-dependent production of an HIV-1 TAR-encoded miRNA-like small RNA. Nucleic Acids Res 44:4340 – 4353.https://doi.org/10.1093/nar/gkw167. 6. D’Orso I, Jang GM, Pastuszak AW, Faust TB, Quezada E, Booth DS, Frankel
AD. 2012. Transition step during assembly of HIV Tat:P-TEFb transcrip-tion complexes and transfer to TAR RNA. Mol Cell Biol 32:4780 – 4793.
https://doi.org/10.1128/MCB.00206-12.
7. Barboric M, Lenasi T. 2010. Kick-sTARting HIV-1 transcription elongation by 7SK snRNP deporTATion. Nat Struct Mol Biol 17:928 –930.https://doi .org/10.1038/nsmb0810-928.
8. D’Orso I, Frankel AD. 2010. RNA-mediated displacement of an inhibitory snRNP complex activates transcription elongation. Nat Struct Mol Biol 17:815– 821.https://doi.org/10.1038/nsmb.1827.
on November 6, 2019 by guest
http://jvi.asm.org/
9. Parada CA, Roeder RG. 1996. Enhanced processivity of RNA polymerase II triggered by Tat-induced phosphorylation of its carboxy-terminal do-main. Nature 384:375–378.https://doi.org/10.1038/384375a0. 10. Gatignol A. 2007. Transcription of HIV: Tat and cellular chromatin. Adv
Pharmacol 55:137–159.https://doi.org/10.1016/S1054-3589(07)55004-0. 11. Bieniasz PD, Grdina TA, Bogerd HP, Cullen BR. 1999. Recruitment of cyclin T1/P-TEFb to an HIV type 1 long terminal repeat promoter prox-imal RNA target is both necessary and sufficient for full activation of transcription. Proc Natl Acad Sci U S A 96:7791–7796.https://doi.org/10 .1073/pnas.96.14.7791.
12. Raha T, Cheng SW, Green MR. 2005. HIV-1 Tat stimulates transcription complex assembly through recruitment of TBP in the absence of TAFs. PLoS Biol 3:e44.https://doi.org/10.1371/journal.pbio.0030044. 13. Romani B, Engelbrecht S, Glashoff RH. 2010. Functions of Tat: the
versatile protein of human immunodeficiency virus type 1. J Gen Virol 91:1–12.https://doi.org/10.1099/vir.0.016303-0.
14. Easley R, Van Duyne R, Coley W, Guendel I, Dadgar S, Kehn-Hall K, Kashanchi F. 2010. Chromatin dynamics associated with HIV-1 Tat-activated transcription. Biochim Biophys Acta 1799:275–285.https://doi .org/10.1016/j.bbagrm.2009.08.008.
15. Mueller N, Berkhout B, Das AT. 2015. HIV-1 splicing is controlled by local RNA structure and binding of splicing regulatory proteins at the major 5=
splice site. J Gen Virol 96:1906 –1917. https://doi.org/10.1099/vir.0.00 0122.
16. Erkelenz S, Theiss S, Otte M, Widera M, Peter JO, Schaal H. 2014. Genomic HEXploring allows landscaping of novel potential splicing regulatory elements. Nucleic Acids Res 42:10681–10697.https://doi.org/10.1093/ nar/gku736.
17. Mueller N, Klaver B, Berkhout B, Das AT. 2015. Human immunodeficiency virus type 1 splicing at the major splice donor site is controlled by highly conserved RNA sequence and structural elements. J Gen Virol 96: 3389 –3395.https://doi.org/10.1099/jgv.0.000288.
18. Mueller N, Das AT, Berkhout B. 2016. A phylogenetic survey on the structure of the HIV-1 leader RNA domain that encodes the splice donor signal. Viruses 8:200.https://doi.org/10.3390/v8070200.
19. Abbink TE, Berkhout B. 2008. RNA structure modulates splicing efficiency at the human immunodeficiency virus type 1 major splice donor. J Virol 82:3090 –3098.https://doi.org/10.1128/JVI.01479-07.
20. Mueller N, van Bel N, Berkhout B, Das AT. 2014. HIV-1 splicing at the major splice donor site is restricted by RNA structure. Virology 470: 609 – 620.https://doi.org/10.1016/j.virol.2014.09.018.
21. Saldi T, Cortazar MA, Sheridan RM, Bentley DL. 2016. Coupling of RNA polymerase II transcription elongation with pre-mRNA splicing. J Mol Biol 428:2623–2635.https://doi.org/10.1016/j.jmb.2016.04.017. 22. Naftelberg S, Schor IE, Ast G, Kornblihtt AR. 2015. Regulation of
alterna-tive splicing through coupling with transcription and chromatin struc-ture. Annu Rev Biochem 84:165–198. https://doi.org/10.1146/annurev -biochem-060614-034242.
23. Herzel L, Ottoz DSM, Alpert T, Neugebauer KM. 2017. Splicing and transcription touch base: cotranscriptional spliceosome assembly and function. Nat Rev Mol Cell Biol 18:637– 650.https://doi.org/10.1038/nrm .2017.63.
24. Sune C, Hayashi T, Liu Y, Lane WS, Young RA, Garcia-Blanco MA. 1997. CA150, a nuclear protein associated with the RNA polymerase II holoen-zyme, is involved in Tat-activated human immunodeficiency virus type 1 transcription. Mol Cell Biol 17:6029 – 6039.https://doi.org/10.1128/MCB .17.10.6029.
25. Goldstrohm AC, Albrecht TR, Sune C, Bedford MT, Garcia-Blanco MA. 2001. The transcription elongation factor CA150 interacts with RNA polymerase II and the pre-mRNA splicing factor SF1. Mol Cell Biol 21:7617–7628.https://doi.org/10.1128/MCB.21.22.7617-7628.2001. 26. Kramer A. 1992. Purification of splicing factor SF1, a heat-stable protein
that functions in the assembly of a presplicing complex. Mol Cell Biol 12:4545– 4552.https://doi.org/10.1128/MCB.12.10.4545.
27. Kramer A, Utans U. 1991. Three protein factors (SF1, SF3 and U2AF) function in pre-splicing complex formation in addition to snRNPs. EMBO J 10:1503–1509.
28. Zhang D, Childs G. 1998. Human ZFM1 protein is a transcriptional repressor that interacts with the transcription activation domain of stage-specific activator protein. J Biol Chem 273:6868 – 6877.https://doi .org/10.1074/jbc.273.12.6868.
29. Zhang D, Paley AJ, Childs G. 1998. The transcriptional repressor ZFM1 interacts with and modulates the ability of EWS to activate transcription. J Biol Chem 273:18086 –18091.https://doi.org/10.1074/jbc.273.29.18086.
30. Parada CA, Roeder RG. 1999. A novel RNA polymerase II-containing complex potentiates Tat-enhanced HIV-1 transcription. EMBO J 18: 3688 –3701.https://doi.org/10.1093/emboj/18.13.3688.
31. Zhou Q, Sharp PA. 1995. Novel mechanism and factor for regulation by HIV-1 Tat. EMBO J 14:321–328.
32. Zhou Q, Sharp PA. 1996. Tat-SF1: cofactor for stimulation of transcrip-tional elongation by HIV-1 Tat. Science 274:605– 610.https://doi.org/10 .1126/science.274.5287.605.
33. Wu-Baer F, Lane WS, Gaynor RB. 1998. Role of the human homolog of the yeast transcription factor SPT5 in HIV-1 Tat-activation. J Mol Biol 277:179 –197.https://doi.org/10.1006/jmbi.1997.1601.
34. Li XY, Green MR. 1998. The HIV-1 Tat cellular coactivator Tat-SF1 is a general transcription elongation factor. Genes Dev 12:2992–2996.
https://doi.org/10.1101/gad.12.19.2992.
35. Zhou Q, Chen D, Pierstorff E, Luo K. 1998. Transcription elongation factor P-TEFb mediates Tat activation of HIV-1 transcription at multiple stages. EMBO J 17:3681–3691.https://doi.org/10.1093/emboj/17.13.3681. 36. Kim JB, Yamaguchi Y, Wada T, Handa H, Sharp PA. 1999. Tat-SF1 protein
associates with RAP30 and human SPT5 proteins. Mol Cell Biol 19: 5960 –5968.https://doi.org/10.1128/MCB.19.9.5960.
37. Fong YW, Zhou Q. 2000. Relief of two built-in autoinhibitory mecha-nisms in P-TEFb is required for assembly of a multicomponent transcrip-tion elongatranscrip-tion complex at the human immunodeficiency virus type 1 promoter. Mol Cell Biol 20:5897–5907.https://doi.org/10.1128/MCB.20 .16.5897-5907.2000.
38. Sanchez-Alvarez M, Goldstrohm AC, Garcia-Blanco MA, Sune C. 2006. Human transcription elongation factor CA150 localizes to splicing factor-rich nuclear speckles and assembles transcription and splicing compo-nents into complexes through its amino and carboxyl regions. Mol Cell Biol 26:4998 –5014.https://doi.org/10.1128/MCB.01991-05.
39. Smith MJ, Kulkarni S, Pawson T. 2004. FF domains of CA150 bind transcription and splicing factors through multiple weak interactions. Mol Cell Biol 24:9274 –9285. https://doi.org/10.1128/MCB.24.21.9274 -9285.2004.
40. Miller HB, Robinson TJ, Gordan R, Hartemink AJ, Garcia-Blanco MA. 2011. Identification of Tat-SF1 cellular targets by exon array analysis reveals dual roles in transcription and splicing. RNA 17:665– 674.https://doi.org/ 10.1261/rna.2462011.
41. Miller HB, Saunders KO, Tomaras GD, Garcia-Blanco MA. 2009. Tat-SF1 is not required for Tat transactivation but does regulate the relative levels of unspliced and spliced HIV-1 RNAs. PLoS One 4:e5710.https://doi.org/ 10.1371/journal.pone.0005710.
42. Fong YW, Zhou Q. 2001. Stimulatory effect of splicing factors on tran-scriptional elongation. Nature 414:929 –933.https://doi.org/10.1038/414 929a.
43. Bres V, Gomes N, Pickle L, Jones KA. 2005. A human splicing factor, SKIP, associates with P-TEFb and enhances transcription elongation by HIV-1 Tat. Genes Dev 19:1211–1226.https://doi.org/10.1101/gad.1291705. 44. Paz S, Krainer AR, Caputi M. 2014. HIV-1 transcription is regulated by
splicing factor SRSF1. Nucleic Acids Res 42:13812–13823.https://doi.org/ 10.1093/nar/gku1170.
45. Jablonski JA, Amelio AL, Giacca M, Caputi M. 2010. The transcriptional transactivator Tat selectively regulates viral splicing. Nucleic Acids Res 38:1249 –1260.https://doi.org/10.1093/nar/gkp1105.
46. Zhou M, Deng L, Kashanchi F, Brady JN, Shatkin AJ, Kumar A. 2003. The Tat/TAR-dependent phosphorylation of RNA polymerase II C-terminal domain stimulates cotranscriptional capping of HIV-1 mRNA. Proc Natl Acad Sci U S A 100:12666 –12671.https://doi.org/10.1073/pnas.183572 6100.
47. Charnay N, Ivanyi-Nagy R, Soto-Rifo R, Ohlmann T, Lopez-Lastra M, Darlix JL. 2009. Mechanism of HIV-1 Tat RNA translation and its activation by the Tat protein. Retrovirology 6:74.https://doi.org/10 .1186/1742-4690-6-74.
48. SenGupta DN, Berkhout B, Gatignol A, Zhou AM, Silverman RH. 1990. Direct evidence for translational regulation by leader RNA and Tat protein of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A 87:7492–7496.https://doi.org/10.1073/pnas.87.19.7492. 49. Braddock M, Thorburn AM, Chambers A, Elliott GD, Anderson GJ,
Kings-man AJ, KingsKings-man SM. 1990. A nuclear translational block imposed by the HIV-1 U3 region is relieved by the Tat-TAR interaction. Cell 62: 1123–1133.https://doi.org/10.1016/0092-8674(90)90389-V.
50. Chiu YL, Ho CK, Saha N, Schwer B, Shuman S, Rana TM. 2002. Tat stimulates cotranscriptional capping of HIV mRNA. Mol Cell 10:585–597.
https://doi.org/10.1016/S1097-2765(02)00630-5.
on November 6, 2019 by guest
http://jvi.asm.org/
51. van der Kuyl AC, Vink M, Zorgdrager F, Bakker M, Wymant C, Hall M, Gall A, Blanquart F, Berkhout B, Fraser C, Cornelissen M. 2018. The evolution of subtype B HIV-1 tat in the Netherlands during 1985-2012. Virus Res 250:51– 64.https://doi.org/10.1016/j.virusres.2018.04.008.
52. Ashe MP, Griffin P, James W, Proudfoot NJ. 1995. Poly(A) site selection in the HIV-1 provirus: inhibition of promoter-proximal polyadenylation by the downstream major splice donor site. Genes Dev 9:3008 –3025.
https://doi.org/10.1101/gad.9.23.3008.
53. Ashe MP, Pearson LH, Proudfoot NJ. 1997. The HIV-1 5=LTR poly(A) site is inactivated by U1 snRNP interaction with the downstream major splice donor site. EMBO J 16:5752–5763.https://doi.org/10.1093/emboj/16.18 .5752.
54. Berkhout B, Jeang KT. 1989.trans-Activation of human immunodefi-ciency virus type 1 is sequence specific for both the single-stranded bulge and loop of thetrans-acting-responsive hairpin: a quantitative analysis. J Virol 63:5501–5504.
55. Feng S, Holland EC. 1988. HIV-1 Tattrans-activation requires the loop sequence within tar. Nature 334:165–167.https://doi.org/10.1038/3341 65a0.
56. Das AT, Zhou X, Vink M, Klaver B, Verhoef K, Marzio G, Berkhout B. 2004. Viral evolution as a tool to improve the tetracycline-regulated gene expression system. J Biol Chem 279:18776 –18782. https://doi.org/10 .1074/jbc.M313895200.
57. Das AT, Harwig A, Berkhout B. 2011. The HIV-1 Tat protein has a versatile role in activating viral transcription. J Virol 85:9506 –9516.https://doi .org/10.1128/JVI.00650-11.
58. Groom HC, Anderson EC, Dangerfield JA, Lever AM. 2009. Rev regulates translation of human immunodeficiency virus type 1 RNAs. J Gen Virol 90:1141–1147.https://doi.org/10.1099/vir.0.007963-0.
59. Pasternak AO, Adema KW, Bakker M, Jurriaans S, Berkhout B, Corne-lissen M, Lukashov VV. 2008. Highly sensitive methods based on seminested real-time reverse transcription-PCR for quantitation of human immunodeficiency virus type 1 unspliced and multiply spliced RNA and proviral DNA. J Clin Microbiol 46:2206 –2211.https://doi .org/10.1128/JCM.00055-08.
60. Boritz EA, Darko S, Swaszek L, Wolf G, Wells D, Wu X, Henry AR, Laboune
F, Hu J, Ambrozak D, Hughes MS, Hoh R, Casazza JP, Vostal A, Bunis D, Nganou-Makamdop K, Lee JS, Migueles SA, Koup RA, Connors M, Moir S, Schacker T, Maldarelli F, Hughes SH, Deeks SG, Douek DC. 2016. Multiple origins of virus persistence during natural control of HIV infection. Cell 166:1004 –1015.https://doi.org/10.1016/j.cell.2016.06.039.
61. Kurosu T, Peterlin BM. 2004. VP16 and ubiquitin; binding of P-TEFb via its activation domain and ubiquitin facilitates elongation of transcription of target genes. Curr Biol 14:1112–1116.https://doi.org/10.1016/j.cub.2004 .06.020.
62. Guo L, Wu WJ, Liu LD, Wang LC, Zhang Y, Wu LQ, Guan Y, Li QH. 2012. Herpes simplex virus 1 ICP22 inhibits the transcription of viral gene promoters by binding to and blocking the recruitment of P-TEFb. PLoS One 7:e45749.https://doi.org/10.1371/journal.pone.0045749.
63. Asang C, Erkelenz S, Schaal H. 2012. The HIV-1 major splice donor D1 is activated by splicing enhancer elements within the leader region and the p17-inhibitory sequence. Virology 432:133–145.https://doi.org/10 .1016/j.virol.2012.06.004.
64. Mikaelian I, Sergeant A. 1992. A general and fast method to generate multiple site directed mutations. Nucleic Acids Res 20:376.https://doi .org/10.1093/nar/20.2.376.
65. Ruijter JM, Thygesen HH, Schoneveld OJ, Das AT, Berkhout B, Lamers WH. 2006. Factor correction as a tool to eliminate between-session variation in replicate experiments: application to molecular biology and retrovirology. Retrovirology 3:2.https://doi.org/10.1186/1742-4690-3-2. 66. Berkhout B, Arts K, Abbink TE. 2011. Ribosomal scanning on the 5=
untranslated region of the human immunodeficiency virus RNA ge-nome. Nucleic Acids Res 39:5232–5244. https://doi.org/10.1093/nar/ gkr113.
67. Jeeninga RE, Jan B, van den Berg H, Berkhout B. 2006. Construction of doxycycline-dependent mini-HIV-1 variants for the development of a virotherapy against leukemias. Retrovirology 3:64. https://doi.org/10 .1186/1742-4690-3-64.
68. Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. 1990. Rapid and simple method for purification of nucleic acids. J Clin Microbiol 28:495–503.
on November 6, 2019 by guest
http://jvi.asm.org/