A 45,000-M(r) glycoprotein in the Sendai virus envelope triggers virus-cell fusion.
Full text
Figure
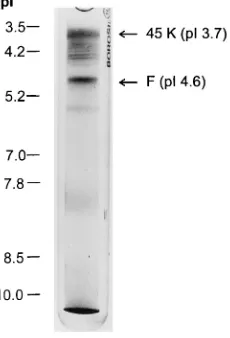
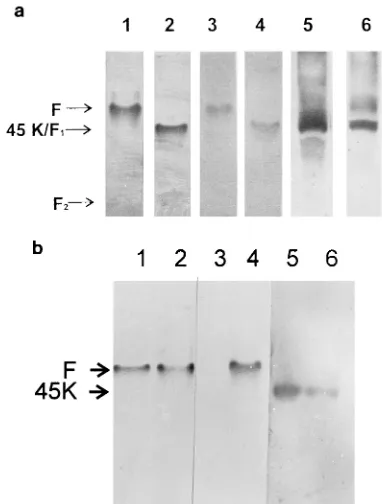


Related documents
We have now examined the recombinational history and evolution of all viruses belonging to the WEE antigenic complex, including the Buggy Creek, Fort Morgan, Highlands J,
A small fraction of nonpermeabilized cells exhibited weak fluo- rescence after staining with anti-gK antibodies; however, the pattern of fluorescence was similar to that
The relationship between the expression of ULli and resistance to HSV infection in Us11cl19 cells has not been defined, but the block to infection with wild-type HSV-1 was overcome
Two DNA sequence-specific protein complexes were detected with DNA probes spanning the region containing the cis-acting positive element and human cytomegalovirus- infected
Binding of trans-dominant mutant Rev protein of human immunodeficiency virus type 1 to the cis-acting Rev-responsive element does not effect the fate of viral mRNA.
The role of the origin-binding domain of simian virus 40 large tumor antigen (T antigen) in the initiation of virus DNA replication was investigated by analyzing the
Several C/EBP binding sites within the Rous sarcoma virus (RSV) long terminal repeat (LTR) and gag enhancers were mutated, and the effect of these mutations on viral gene expression
The primary sequence alterations in stem regions I (TM22) and II (TM23) greatly reduced protein binding activity (Fig. 5, lanes 11 and 12) and thereby demonstrate that the