Copyright © 2000, American Society for Microbiology. All Rights Reserved.
Establishment of Monoclonal Anti-Retroviral gp70 Autoantibodies
from MRL/
lpr
Lupus Mice and Induction of Glomerular gp70
Deposition and Pathology by Transfer into
Non-Autoimmune Mice
NOBUTADA TABATA,
1MASAAKI MIYAZAWA,
1,2* RYUICHI FUJISAWA,
2†
YUMIKO A. TAKEI,
2HIROYUKI ABE,
1ANDKEIJI HASHIMOTO
1,3Department of Immunology
1and Third Department of Internal Medicine,
3Kinki University
School of Medicine, Osaka-Sayama, Osaka 589-8511, and Department of Pathology,
Tohoku University School of Medicine, Sendai 980-8575,
2Japan
Received 6 October 1999/Accepted 1 February 2000
Several strains of mice, including MRL/MpJ mice homozygous for the Fas mutant
lpr
gene (MRL/
lpr
mice),
F
1hybrids of New Zealand Black and New Zealand White mice, and BXSB/MpJ mice carrying a Y-linked
autoimmune acceleration gene, spontaneously develop immune complex-mediated glomerulonephritis. The
involvement of the envelope glycoprotein gp70 of an endogenous xenotropic virus in the formation of
circu-lating immune complexes and their deposition in the glomerular lesions have been demonstrated, as has the
pathogenicity of various antinuclear, antiphospholipid, and rheumatoid factor autoantibodies. In recent
genetic linkage studies as well as in a study of cytokine-induced protection against nephritis development, the
strongest association of serum levels of gp70–anti-gp70 immune complexes, rather than the levels of
antinu-clear autoantibodies, with the development and severity of glomerulonephritis has been demonstrated,
sug-gesting a major pathogenic role of anti-gp70 autoantibodies in the lupus-prone mice. However, the
pathoge-nicity of anti-gp70 autoantibodies has not yet been directly tested. To examine if anti-gp70 autoantibodies
induce glomerular pathology, we established from unmanipulated MRL/
lpr
mice hybridoma clones that secrete
monoclonal antibodies reactive with endogenous xenotropic viral
env
gene products. Upon transplantation, a
high proportion of these anti-gp70 antibody-producing hybridoma clones induced in syngeneic
non-autoim-mune and severe combined immunodeficiency mice proliferative or wire loop-like glomerular lesions.
Further-more, deposition of gp70 in glomeruli and pathological changes were observed after intravenous injection of
representative clones of purified anti-gp70 immunoglobulin G, demonstrating pathogenicity of at least some
anti-gp70 autoantibodies.
Several strains of mice such as MRL/MpJ mice homozygous
for the Fas mutant
lpr
gene (MRL/
lpr
mice), F
1hybrids of New
Zealand Black (NZB) and New Zealand White (NZW) mice
[(NZB
⫻
NZW)F
1], and BXSB/MpJ mice carrying a yet
un-defined Y-chromosome-associated autoimmune acceleration
gene (
Yaa
) spontaneously develop an autoimmune syndrome
closely resembling human systemic lupus erythematosus (SLE)
(1, 4, 35, 41). Both human and murine SLE are serologically
characterized by elevated levels of multiple autoantibodies (1,
4, 20, 35). These include antibodies (Abs) reactive with DNA
and other nuclear components, Abs to extracellular matrices
and cytoplasmic proteins, and in mice Abs reacting to the
major envelope glycoprotein (gp70) of an endogenous
xeno-tropic retrovirus that is expressed as a normal constituent of
mouse serum (8). These autoantibodies and resultant
circulat-ing immune complexes (IC) have been implicated in the
de-velopment of fatal glomerulonephritis. However, not all
auto-antibodies are primary pathogens (20, 39); in some cases they
may instead be a secondary consequence of tissue damage.
Several different approaches have been used to delineate the
relationship between types of autoantibodies and the
develop-ment of renal pathology. Several different clones of anti-DNA
antibodies have been shown to induce glomerular lesions
as-sociated with immunoglobulin (Ig) deposition and/or
protein-uria when transferred into non-autoimmune mice (13, 20, 36,
38). On the other hand, recent genetic analyses using simple
sequence length polymorphisms as positional markers have
identified several chromosomal loci in linkage with the
devel-opment and severity of the renal disease. Interestingly, one of
the genetic linkage analyses performed by using (NZB
⫻
NZW)
F
1⫻
NZW backcross mice (40) demonstrated that the loci
linked with anti-gp70 Ab production, rather than those
asso-ciated with levels of antinuclear Ab, had the strongest
influ-ence on the development of glomerulonephritis. In a similar
study performed with C57BL/6
⫻
(NZW
⫻
C57BL/6.
Yaa
)F
1backcross mice (26), association of serum levels of gp70 IC
with severe glomerulonephritis was much stronger than that
between levels of IgG anti-DNA autoantibodies and the renal
disease. In addition, transgenic expression of interleukin-4
(IL-4) in the (NZW
⫻
C57BL/6.
Yaa
)F
1mouse model of SLE
resulted in almost complete protection against the
develop-ment of lupus-like nephritis in association with the lack of
IgG3 production and marked decrease in the amount of serum
gp70–gp70 IC, while the serum concentrations of
anti-DNA IgG were not markedly reduced (25). These data suggest
that autoantibodies reactive to endogenous retroviral gp70
comprise the major pathogenic Abs in the mouse models of
* Corresponding author. Mailing address: Department of
Immunol-ogy, Kinki University School of Medicine, 377-2 Ohno-Higashi,
Osaka-Sayama, Osaka 589-8511, Japan. Phone and fax: 81 723-67-7660.
E-mail: masaaki@med.kindai.ac.jp.
† Present address: Department of Microbiology, Kinki University
School of Medicine, Osaka-Sayama, Osaka 589-8511, Japan.
4116
on November 9, 2019 by guest
http://jvi.asm.org/
lupus nephritis. However, suggested pathogenicity of anti-gp70
autoantibodies has not yet been directly proven. Therefore, we
decided to develop a new screening system and establish from
MRL/
lpr
mice hybridoma clones that secrete monoclonal Abs
(MAbs) reactive with endogenous xenotropic virus
env
gene
products. MRL mice were chosen so that passive transfer into
syngeneic mice of hybridoma cells and MAbs were more easily
performed than in the cases of the F
1hybrid models with a
complex genetic background.
Tryptic peptide mapping analyses of gp70 molecules eluted
from IC revealed that the serum gp70 involved in the
produc-tion of circulating IC both in (NZB
⫻
NZW)F
1and MRL/
lpr
mice is structurally related to the envelope glycoprotein of an
infectious NZB xenotropic virus (5, 12). Subsequent studies
have shown that almost all strains of mice, healthy and SLE
prone, produce endogenous xenotropic viral gp70 in the liver
as an invariable serum constituent, and its expression is
con-trolled as an acute-phase reactant (8). A cDNA clone encoding
the serum gp70 was isolated from the liver of a
lipopolysac-charide (LPS)-injected NZB mouse, and Northern blot
analy-ses confirmed the expression of this message as an acute-phase
reactant (29). Therefore, we used this cDNA clone, along with
the
env
gene from an infectious molecular clone of NZB
xe-notropic virus (21), for in vitro expression of the endogenous
retroviral
env
gene products to screen anti-gp70 Ab-producing
hybridoma cells. Resultant hybridoma clones established from
unmanipulated MRL/
lpr
mice induced severe glomerular
le-sions upon transplantation into syngeneic (BALB/c
⫻
MRL)F
1and severe combined immunodeficiency (SCID) mice.
More-over, purified IgG molecules of representative anti-gp70
auto-antibodies induced glomerular deposition of gp70 and renal
pathology when injected intravenously (i.v.) into
non-autoim-mune mice.
MATERIALS AND METHODS
Mice.The original breeding pairs of MRL/MpJ-⫹/⫹(MRL/⫹) and MRL/lpr
mice were purchased from The Jackson Laboratory, Bar Harbor, Maine. These strains of mice were maintained by sister-brother mating in our animal facilities under specific-pathogen-free conditions. BALB/cCrSlc, NZW/NSlc, and C57BL/ 6CrSlc (B6) mice were purchased from Japan SLC, Inc., Hamamatsu, Japan, and (BALB/c⫻MRL/⫹)F1hybrid mice were bred in our animal facilities. C.B-17/
Icr-scid/scid(SCID) mice were produced from the breeding pairs originally donated by S. Ikehara, Kansai Medical University, Moriguchi, Japan, and were kindly provided by M. Nose, Tohoku University School of Medicine. All animal experiments described in this report were approved by the institutions and performed under the guidelines of our animal facilities.
NZB xenotropic virus-producing cells.NZB-AR cells that are chronically infected with a biological clone of NZB xenotropic virus were kindly provided by L. Evans, Laboratory of Persistent Viral Diseases, National Institute of Allergy and Infectious Diseases, Hamilton, Mont. Control uninfected Mv1Lu mink lung cells were purchased from the American Type Culture Collection, Manassas, Va. Expression of xenotropic murine leukemia viralenvgenes and their chimeras in recombinant vaccinia viruses.Vaccinia virus transfer vectors used for the expression of mouse retrovirusenvgenes and their chimeras were constructed as described previously (10, 17, 18). The structures of the expressedenvgenes and their chimeras are diagrammatically presented in Fig. 1. Plasmid clones pGP6-8, containing the gp70 cDNA isolated from a LPS-injected NZB mouse liver (29), and pNZB9-1, containing the whole permuted infectious molecular clone of an
[image:2.612.62.542.73.336.2]NZB xenotropic virus, IU-6 (21), were used as sources of endogenous xenotropic virusenvgene sequences. Amino acid sequence analyses have revealed only three substitutions near the C terminus of gp70 between these twoenvgene products, although the C terminus of the transmembrane portion (p15E) contains five additional substitutions (21, 29), four of which are located within the R peptide that is cleaved from the mature transmembrane protein (31). ASalI oligonucle-otide linker (New England Biolabs, Beverly, Mass.) was ligated onto both ends of the 2.2-kbAccI-HaeII fragment harboring the entireenvsequence and a part of the long terminal repeat (LTR) isolated from pGP6-8 (Fig. 1), and the modifiedenv-containing fragment was recloned into the uniqueSalI site of the
FIG. 1. Diagrammatic representation of the liver-derived gp70 cDNA and viralenvgenes and their chimeras expressed in recombinant vaccinia viruses. The nucleotide sequence of the gp70 cDNA isolated from an LPS-injected NZB mouse liver (29) is 99% homologous to that of the infectious NZB xenotropic virusenv
gene (21), except for several base changes clustered near the gp70/p15E cleavage site and in the 3⬘flanking region and LTR (□), which are reflected by the indicated differences in restriction sites. The SFFVenvgene (
,
) is a product of natural recombination between endogenous polytropic and exogenous Friend ecotropic viruses with a large in-frame deletion (ƒ) encompassing the 3⬘portion of gp70- and the 5⬘portion of p15E-encoding regions. Dashed lines represent vector-derived sequences. A,AccI; B,BamHI; E,EcoRI; Ha,HaeII; Hc,HincII; Hd,HindIII; K,KpnI; S,SmaI; V,EcoRV; X,BstXI; AAAAA, poly(A) tail.
on November 9, 2019 by guest
http://jvi.asm.org/
vaccinia virus expression vector pSC11-SS (10). This construct was used to generate a vaccinia virus-NZB liver cDNA recombinant. TheHincII-SmaI frag-ment harboring the entireenvgene and portions of thepoland LTR from pNZB9-1was reconstructed in pBluescript-KS(⫹) vector from purifiedHin cII-EcoRI andEcoRI-SmaI fragments (Fig. 1), and the uniqueAccI site was re-placed with aBamHI linker (New England Biolabs). ABamHI-digested frag-ment containing the entireenvgene and a part of the LTR was cloned into the uniqueBglII site of the previously described modified vaccinia virus expression vector pSC11-SB (17), resulting in the generation of a vaccinia virus-infectious NZB xenotropic virusenvgene recombinant. Theenvclones derived from the infectious NZB xenotropic virus and those derived from the NZB liver gp70 cDNA were easily distinguishable by the presence of a few different restriction sites (Fig. 1).
For the construction ofenvgene chimeras, a plasmid clone (BT4-1a3 [42]) that contains a permuted infectious molecular clone of the Friend spleen focus-forming virus (SFFV) was used as a source of nonxenotropicenvsequences (Fig. 1). A vaccinia virus recombinant expressing the whole SFFVenvgene has been described elsewhere (18). TheEcoRI-SmaI fragment from pNZB9-1was
sub-cloned into pBluescript-KS(⫹) and was ligated with the 1.3-kbHindIII-EcoRI fragment harboring the 3⬘portion of thepoland the 5⬘portion of the SFFVenv
genes from BT4-1a3. TheBamHI-digested fragment containing the entire chi-mericenvgene was then inserted to pSC11-SB at the uniqueBglII site. The resulting construct was used to generate a recombinant vaccinia virus that ex-pressed the chimeric SFFV-NZB xenotropic virusenvgene. For the construction of a reciprocal chimera, the uniqueKpnI site in the LTR of BT4-1a3 was replaced with theBamHI linker, and the 1.8-kbBamHI-digested fragment har-boring the entire SFFVenvgene was subcloned into pUC19. TheHincII-EcoRI fragment containing the 5⬘portion of the infectious NZB xenotropic virusenv
gene was ligated to theEcoRI-KpnI (BamHI) fragment of the subcloned SFFV
envgene, taking advantage of the uniqueHincII site in the vector, and theAccI site upstream of the initiation site of NZB xenotropic virusenvgene was replaced with a BamHI linker to insert theBamHI-digested fragment containing the chimericenvgene into pSB11-SB. The resultant plasmid was used to generate a recombinant vaccinia virus that expressed the chimeric NZB xenotropic virus-SFFVenvgene. Recombinant vaccinia viruses were produced by homologous recombination as described elsewhere (10, 17, 18). A recombinant vaccinia virus expressing the influenza virus hemagglutinin (HA) gene (30) was used as a negative control throughout the experiment.
Production and screening of hybridoma cells.Spleen and lymph node cells were prepared aseptically from unmanipulated MRL/lprmice. P3/NSI/1-Ag4-1 (NS-1) and P3X63Ag8.653 (8.653) myeloma cells were purchased from the American Type Culture Collection and used as fusion partner cells. Hybridoma cell fusion, hypoxanthine-aminopterin-thymidine selection, and cloning by col-ony formation in fibrin gels were performed as described previously (16, 24). For immunofluorescence detection of the reactivities of hybridoma Abs to expressed
envgene products, monkey CV-1 cells were grown in wells of 96-well tissue culture plates, infected with a recombinant vaccinia virus at 100 to 200 PFU/well for 20 to 36 h, and incubated at 4°C overnight with a hybridoma culture super-natant added at 100l/well. Culture supernatants were then aspirated, and the wells were washed twice with phosphate-buffered balanced salt solution (PBBS) (3) containing 2% fetal bovine serum (FCS), and once with PBBS not containing FCS. Cells in each well were fixed with methanol, blocked with 10% skim milk, and stained with a 1/150 dilution of fluorescein isothiocyanate (FITC)-conju-gated goat anti-mouse Ig Ab (Cappel, Organon Teknika Corporation, West Chester, Pa.) as described elsewhere (17). For observation, the plates were placed upside-down under a Zeiss Axioplan fluorescence microscope (Zeiss, Overkochen, Germany). Antinuclear Ab activity was detected by treating unin-fected CV-1 cells with methanol before incubating them with hybridoma-derived Ab. Methanol-fixed cells in wells of 96-well plates were washed with phosphate-buffered saline (PBS), blocked with 10% skim milk, and incubated with Ab as described above. To prepare representative immunofluorescence photographs, CV-1 cells were grown on glass coverslips and processed similarly.
Hybridoma cells producing reference MAbs that react with various mouse retrovirusenvgene products (2, 22, 23) were kindly provided by B. Chesebro, Laboratory of Persistent Viral Diseases, National Institute of Allergy and Infec-tious Diseases. Hybridoma cell line N-S.7, producing mouse IgG3 reacting with sheep red blood cells (SRBC), was purchased from the American Type Culture Collection. Another IgG3-producing hybridoma clone, 11, reactive with mumps virus nucleoprotein (37), was kindly provided by Y. Ito, Department of Micro-biology, Mie University School of Medicine, Tsu, Japan. Ig isotypes of MAbs were determined by an Ouchterlony immunodiffusion method using an isotype-specific Ab kit (The Binding Site, Birmingham, United Kingdom) as described previously (16, 24). The above-described reference MAbs and others of previ-ously defined isotypes were used as controls in the Ig isotype determination.
Western blotting.Western blotting analyses of polypeptide specificity of the Abs was performed as described previously (17, 19, 22, 23), using extracts from NZB-AR and control Mv1Lu cells. In brief, cells were washed four times with ice-cold PBS and incubated with 0.5% NP-40 in 50 mM Tris-buffered saline (pH 7.4) containing 10 mM EDTA, 5 mMn-ethylmaleimide, 1 mM phenylmethyl-sulfonyl fluoride, and 0.002% leupeptin at 4°C for 15 min. The supernatant was collected after centrifugation at 15,000⫻gfor 10 min. The extract was mixed with an equal volume of 4% sodium dodecyl sulfate (SDS) sample buffer (17, 19)
without a reducing agent and was subjected to SDS-polyacrylamide gel electro-phoresis. Proteins separated through 7.5% polyacrylamide gels were transferred onto polyvinylidene difluoride membranes (Immobilon; Millipore Corporation, Bedford, Mass.) as described previously (17, 19), and the blotted membrane was blocked with 10% skim milk. Incubation with MAb and detection of bound Ab by using biotinylated horse anti-mouse Ig secondary Ab and avidin-biotinylated peroxidase complex (Vector Laboratories, Burlingame, CA) has been described elsewhere (17, 19).
For the detection of serum gp70, sera from NZW, (BALB/c⫻MRL/⫹)F1, and
B6 mice were mixed at 1:20 with the SDS sample buffer containing no reducing agent, and serum proteins were separated through 7.5% polyacrylamide gels and blotted as described above. Serum gp70 molecules were detected with biotin-conjugated anti-gp70 MAb 24-6 by chemiluminescence reaction using horse-radish peroxidase-conjugated streptavidin (Vector Laboratories) and ECL⫹
reagent (Amersham Pharmacia Biotech, Uppsala, Sweden) according to the manufacturers’ instructions.
Transfer of hybridoma cells or purified Abs into mice and pathological anal-yses.Hybridoma cells were grown in Dulbecco’s modified Eagle medium sup-plemented with glucose (4.5 g/liter [final concentration]), gentamicin sulfate (50 mg/liter), and 10% FCS, washed twice with PBBS, and resuspended in PBBS at 107cells/ml. (BALB/c⫻MRL/⫹)F
1and SCID mice were transplanted
intra-peritoneally (i.p.) with 1⫻107to 2⫻107hybridoma cells after a pretreatment
with a 0.5-ml/mouse i.p. dose of 2,6,10,14-tetramethylpentadecane (pristane; Aldrich Chemical Co., Inc., Tokyo, Japan) given 1 to 3 weeks prior to hybridoma transplantation. Serum concentrations of IgG in transplanted SCID mice were measured by single radial immunodiffusion assays using isotype-specific antisera (anti-mouse IgG2a and anti-mouse IgG3; Zymed Laboratories, Inc., South San Francisco, Calif.) as described previously (34). For purification of a clonal anti-gp70 IgG, hybridoma cells were grown in a serum-free medium (Hybridoma SFM; Gibco BRL, Rockville, Md.) in 4-liter spinner flasks, and culture super-natants were concentrated by using a tangential flow ultrafiltration system (Mini-tan II; Millipore Corporation). IgG was purified by protein A-Sepharose (Am-ersham Pharmacia Biotech) affinity chromatography as described previously (19, 24). Special care was taken to perform the purification aseptically at room temperature. Purified MAbs dissolved in PBBS at 0.5 to 1.0 mg/ml were injected into the tail vein after removing possibly contaminating Ig aggregates by centrif-ugation at 10,000⫻gfor 15 min.
The methods of preparation and staining of formalin-fixed, paraffin-embedded tissue sections and specimens for electron microscopy have been described else-where (19, 34). A part of the kidneys from each mouse was snap frozen in a mixture of dry ice and acetone after being embedded in O.C.T. compound (Miles Scientific, Naperville, Ind.), and frozen sections were prepared as described previously (16, 19, 24). For immunofluorescence detection of mouse IgG and C3 in frozen sections, FITC-conjugated goat anti-mouse IgG and anti-mouse C3 Ab (Cappel, Organon Teknika Corporation) were used. To detect the deposition of retroviral gp70, MAbs specific for xenotropic viralenvgene products, 24-6 and 24-9 (23), were purified as described above and labeled with biotin (19, 24). Localization of the biotinylated anti-xenotropic viral envelope MAb was visual-ized by using the avidin-biotinylated peroxidase complex (Vector Laboratories) as described previously (16, 24).
Histopathologic severity of each glomerular lesion in periodic acid-Schiff (PAS)-stained sections was semiquantitatively determined according to previ-ously described criteria (34), and an average index of glomerular pathology (IGP) was calculated by examining⬎20 glomeruli per mouse. In brief, grade 1 was given when there was apparent increase in the number of mesangial cells (⬎3 nuclei in a single separate section of a mesangial area) but no inflammatory cell infiltration into capillaries, grade 2 was given when cellular components were increased in at least one capillary lumen, and grade 3 was given when obliteration of at least one capillary lumen with fibrin- or collagen-containing materials was observed. Grade 0 means that none of the above histologic changes were ob-served in a glomerulus in question. Mice in which⬎80% of examined glomeruli showed significant histologic changes (IGPⱖ1) or in which 30 to 80% of glomeruli showed severe histologic changes (IGPⱖ2) are designated nephritic in this study. Incidences and average IGP were statistically compared with those of control mice by Fisher’s exact probability test and by Student’sttest, respec-tively.
RESULTS
Expression of endogenous xenotropic viral
env
cDNA and
establishment of MAbs reactive with the
env
gene product
from MRL/
lpr
mice.
A DNA fragment containing the entire
env
gene sequence from the cDNA clone isolated from a
LPS-injected NZB mouse was inserted into a vaccinia virus
expres-sion vector, and a recombinant vaccinia virus that expressed
the liver-derived xenotropic viral envelope glycoprotein was
constructed (Fig. 1). The whole
env
gene from a molecular
clone of an infectious xenotropic virus isolated from an NZB
mouse, IU-6, was also expressed in another vaccinia virus
on November 9, 2019 by guest
http://jvi.asm.org/
combinant as a positive control. Reactivities of a panel of
antiretroviral MAbs previously established from
non-autoim-mune mice (2, 22, 23) to these two xenotropic viral
env
gene
products showed no difference (Fig. 2 and Table 1), reflecting
their almost identical amino acid sequences.
Fifteen separate hybridoma clones were selected from a
total of three fusions of spleen and lymph node cells, using
eight unmanipulated, female MRL/
lpr
mice, both for reactivity
of secreted Ab with CV-1 cells expressing the liver-derived
gp70 cDNA and for lack of reactivity to cells infected with the
control vaccinia virus-influenza virus HA recombinant, as
ex-emplified in Fig. 2. Of these, three were established from the
first fusion performed by using spleen and lymph node cells
from two 2.5-month-old female mice and 8.653 myeloma cells,
seven others were established from the second fusion in which
four 2.5 month-old female mice and NS-1 myeloma cells were
used, and the remaining five clones were derived from the third
fusion performed by using two 4.5-month-old female mice and
NS-1. An additional clone, 17D7.1, was similarly selected from
a fusion made with spleen and lymph node cells of two
4.5-month-old male MRL/
lpr
mice and 8.653 myeloma cells. A few
nonproducer clones were also established from these fusions,
as represented by clone 4E9.1 in Table 2, and were used as
negative controls in the following experiment along with the
fusion partner cells. During the initial screening procedure for
Ab-producing hybridoma cells, wells containing antinuclear Ab
were also observed at roughly the same frequency as those
containing anti-gp70 Ab; however, none of the MAbs selected
for reactivity to the xenotropic viral
env
gene product
cross-reacted with nuclear antigens in the immunofluorescence
as-say. Western blotting analysis confirmed the reactivity of these
MAbs with the whole
env
gene product gp85 (gp70 plus p15E),
which was detected from the lysate of NZB-AR cells
chroni-cally infected with an NZB xenotropic virus but not from the
lysate of uninfected Mv1Lu cells (Fig. 3). Although Abs
reac-tive with the surface components of CV-1 cells other than
expressed gp70 were eliminated through the screening
proce-dure, by selecting MAbs reactive with the plaques of
gp70-expressing cells but not with the surrounding uninfected CV-1
cells (Fig. 2), a few bands other than that of gp85 were readily
detectable with some of these MAbs in blots of both NZB-AR
and uninfected Mv1Lu cell lysates, suggesting possible
cross-reactivity with normal cellular components.
[image:4.612.117.491.72.244.2]The 16 MAbs reactive with the xenotropic viral
env
gene
products were further analyzed for reactivities to the products
of the SFFV
env
gene and the chimeras between NZB
xeno-tropic virus and SFFV
env
genes (Fig. 1 and Table 2) for rough
epitope mapping. The SFFV
env
gene is a naturally produced
recombinant with a large deletion encompassing the
C-termi-nal one-fourth of the gp70 and the N-termiC-termi-nal half of the
transmembrane p15E (42). Its gp70 sequence is unrelated to
that of Friend murine leukemia virus, but the N-terminal
one-FIG. 2. Antigenic characterization of expressedenvgene products using a panel of MAbs. Representative immunofluorescence micrographs of infected CV-1 cells are presented. 24-6, 24-8, 514, and 603 are anti-retroviral envelope MAbs of previously defined virus type and polypeptide specificities (2, 22, 23), whose reported reactivities to different types of mouse C-type retroviruses are given in parentheses; 12H5.1 is a representative MAb newly established from MRL/lprmice in this study. Note that only the foci of cells infected with a relevant recombinant vaccinia virus, not the uninfected cells surrounding the foci, are stained.TABLE 1. Reactivities of reference MAbs of known specificity to various murine leukemia virus
env
gene products
Hybridoma clone
Virus type, polypeptide specificitya
Reactivity with CV-1 cells expressingb:
NZB liver
gp70 cDNA NZB IU-6env SFFVenv NZBenv⫹SFFV SFFVenv⫹NZB Influenzavirus HA
24-6
Xeno
⫹
Poly, gp70
⫹
⫹
⫺
⫹
⫺
⫺
24-8
Eco
⫹
Poly, gp85
⫺
⫺
⫺
⫺
⫺
⫺
514
Poly, gp70
⫺
⫺
⫹
⫺
⫹
⫺
603
Xeno, gp70
⫹
⫹
⫹
⫹
⫹
⫺
11
Mumps, NP
⫺
⫺
⫺
⫺
⫺
⫺
aVirus type specificities of reference MAbs are summarized from the previous reports (2, 22, 23). Xeno, xenotropic viruses; Poly, polytropic viruses; Eco, ecotropic
viruses; NP, nucleoprotein.
bTested by indirect immunofluorescence assays using CV-1 cells infected with an indicated recombinant vaccinia virus as target cells as shown in Fig. 2. Assays were
repeated at least three times for each MAb, with consistent results.
on November 9, 2019 by guest
http://jvi.asm.org/
[image:4.612.52.552.605.694.2]third is most similar to endogenous polytropic viruses (31, 42),
a type distinct from xenotropic viruses. Eight of the MRL/
lpr
-derived MAbs (17D7.1 through 42D3.2 in Table 2) reacted
with the products of the both xenotropic viral
env
genes but
lost reactivity when the 5
⬘
one-third of the NZB xenotropic
viral
env
gene was replaced at the
Eco
RI site with the
corre-sponding portion of SFFV
env
. Based on their reactivity to the
reciprocal chimera (NZB xenotropic viral
env
-SFFV
env
), they
are most likely to react with epitopes located in the N-terminal
[image:5.612.55.551.91.307.2]one-third of xenotropic viral gp70. On the other hand, two
clones, 37C4.1 and 42D3.1, reacted with the products of the
whole NZB xenotropic viral
env
and the SFFV
env
-NZB
xe-notropic viral
env
chimera but not with the products of the
SFFV
env
and the NZB xenotropic viral
env
-SFFV
env
chi-mera. Therefore, they seem to recognize epitopes located in
the C-terminal portion of the xenotropic viral
env
gene
prod-ucts. Six other clones were reactive to the products of
xeno-tropic viral and SFFV
env
genes and both of the chimeras.
TABLE 2. Characteristics of anti-xenotropic viral MAbs established from MRL/
lpr
mice and incidence and severity of
glomerular lesions induced by transplantation of the hybridoma cells
aHybridoma clone Heavy-chainisotype
Reactivity with CV-1 cells expressing: Glomerular pathology NZB liver
gp70 cDNA NZB IU-6env SFFVenv NZBenv⫹SFFV SFFVenv⫹NZB Influenzavirus HA Incidence Avg IGPSEM ⫾
8.653
(⫺)
⫺
⫺
⫺
⫺
⫺
⫺
1/7
0.42
⫾
0.10
NS-1
(⫺)
⫺
⫺
⫺
⫺
⫺
⫺
0/7
0.12
⫾
0.03
4E9.1
b(nonproducer)
(⫺)
⫺
⫺
⫺
⫺
⫺
⫺
0/6
0.22
⫾
0.12
17D7.1
⫹
⫹
⫺
⫹
⫺
⫺
0/17
0.52
⫾
0.05
34B4.1
⫹
⫹
⫺
⫹
⫺
⫺
0/8
0.34
⫾
0.08
58C5.1
⫹
⫹
⫺
⫹
⫺
⫺
10/12
c1.07
⫾
0.08
e12H5.1
␥3
⫹
⫹
⫺
⫹
⫺
⫺
12/13
c2.28
⫾
0.18
e36D1.1
␥2a
⫹
⫹
⫺
⫹
⫺
⫺
0/11
0.23
⫾
0.05
37C6.1
␥2a
⫹
⫹
⫺
⫹
⫺
⫺
10/14
c0.96
⫾
0.06
e42B4.1
␥2a
⫹
⫹
⫺
⫹
⫺
⫺
0/8
0.49
⫾
0.05
e42D3.2
␥2a
⫹
⫹
⫺
⫹
⫺
⫺
1/7
0.54
⫾
0.11
f6F12.3
⫹
⫹
⫹
⫹
⫹
⫺
0/12
0.30
⫾
0.06
7C6.3
⫹
⫹
⫹
⫹
⫹
⫺
1/7
0.49
⫾
0.09
51C4.1
␥3
⫹
⫹
⫹
⫹
⫹
⫺
1/4
0.75
⫾
0.15
f51D1.1
␥3
⫹
⫹
⫹
⫹
⫹
⫺
14/19
c1.20
⫾
0.12
e59C4.1
␥3
⫹
⫹
⫹
⫹
⫹
⫺
4/8
0.88
⫾
0.16
f60A5.1
␥3
⫹
⫹
⫹
⫹
⫹
⫺
6/9
d1.01
⫾
0.09
e37C4.1
␥3
⫹
⫹
⫺
⫺
⫹
⫺
12/12
c2.10
⫾
0.19
e42D3.1
␥2a
⫹
⫹
⫺
⫺
⫹
⫺
0/10
0.49
⫾
0.06
aReactivities of MAbs were tested by indirect immunofluorescence assays as described for Table 1. Criteria for histopathologic diagnosis of glomerulonephritis and
the standards for determination of the IGP are described in Materials and Methods. The incidence of nephritis and IGP for each MAb were statistically compared with those for relevant fusion partner cells.
bThree other nonproducer or non-anti-gp70 hybridoma clones were similarly tested for potential pathogenicity, and none induced significant glomerular pathology
in transplanted (BALB/c⫻MRL/⫹)F1mice.
cP⬍0.003 by Fisher’s exact probability test. d0.01⬍P⬍0.02 by Fisher’s exact probability test. eP⬍0.001 by Student’sttest.
f0.001⬍P⬍0.01 by thettest.
FIG. 3. Representative results of Western blotting assays showing virus polypeptide specificities of the MAbs. Mr, molecular mass markers, with positions indicated in kilodaltons at the left; U, uninfected mink Mv1Lu cells; AR, NZB-AR cells chronically infected with a biological clone of NZB xenotropic virus. N-S.7 is a negative control IgG3 specific for SRBC; 603 is a positive control IgM specific for xenotropic viral gp70. The arrowhead indicates bands of the viralenvgene product, gp85 (gp70 plus p15E).
on November 9, 2019 by guest
http://jvi.asm.org/
[image:5.612.135.470.520.692.2]These MAbs, therefore, may recognize epitopes common to
different types of mouse retrovirus
env
gene products. It is
notable that these latter MAbs showed more prominent
cross-reactivity to normal cellular components in Western blotting as
exemplified by clones 51D1.1 and 603 in Fig. 3.
Pathogenicity of gp70-reactive autoantibodies produced
from transplanted hybridoma cells in non-autoimmune mice.
To test possible pathogenicity of these MAbs reactive with
retroviral gp70, each hybridoma clone was injected i.p. into
syngeneic (BALB/c
⫻
MRL/
⫹
)F
1mice that had been injected
with a single i.p. dose of pristane to facilitate hybridoma
trans-plantation. Injected mice were killed before dying of a tumor
burden, and the organs were examined histopathologically.
The average interval between pristane injection and organ
removal was 23.8 days, and that between hybridoma
transplan-tation and organ removal was 14.3 days. Six of the 16
hybrid-oma clones induced in syngeneic (BALB/c
⫻
MRL/
⫹
)F
1mice
significant glomerular lesions at a considerable frequency,
while the transplantation of fusion partner cells or a control
nonproducer clone did not induce significant pathology at this
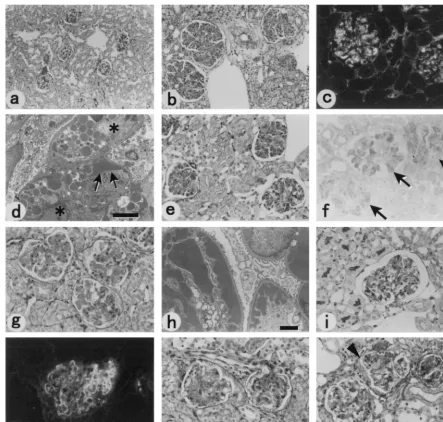
early stage after a single pristane treatment (Fig. 4 and Table
2). Another clone, 59C4.1, induced histologically evident
glo-merular lesions (Fig. 4l) at a low frequency (in four of eight
mice). Among the six clones that consistently induced
glomer-ular lesions, four IgG3-producing hybridoma clones, 12H5.1,
37C4.1, 51D1.1, and 60A5.1, caused diffuse and histologically
more severe glomerular pathology compared with other
anti-gp70 hybridomas when transplanted into (BALB/c
⫻
MRL/
⫹
)F
1mice. The glomerular lesions induced by transplantation
of hybridoma clone 12H5.1 were characterized by
intracapil-lary proliferation and/or infiltration of cells with granular
sub-endothelial and intracellular deposition of IgG and C3 (Fig. 4b
to d). Massive deposition of fibrin was also demonstrated in
affected glomeruli by phosphotungstenic acid-hematoxylin
stain-ing (not shown). On the other hand, hybridoma clone 37C4.1
induced diffuse lupus-like glomerular lesions characterized by
light microscopic wire loops and massive subendothelial IgG
deposition (Fig. 4g and h). Two other IgG3 clones, 51D1.1 and
60A5.1, induced proliferative glomerular lesions at a high
in-cidence with dilatation of capillary lumina and occasional
ac-cumulation of red cell fragments (Fig. 4k). One clone (58C5.1)
out of five IgM- and another (37C6.1) from five
IgG2a-pro-ducing hybridoma cells also induced proliferative glomerular
lesions at a high frequency (Table 2).
Induction of glomerular lesions in transplanted SCID mice
and deposition of gp70 in glomeruli.
To exclude the possibility
that the induction of glomerular lesions by transplantation of
the hybridoma cells was due to host immune responses against
hybridoma-derived Abs or cellular components, or a result of
autoantibody production in response to pristane injection, we
next injected three representative clones of the hybridoma cells
into SCID mice. Both hybridomas 12H5.1 and 37C4.1 induced
in the transplanted SCID mice glomerular lesions that were
histologically similar to the lesions induced in the F
1mice (Fig.
4e, f, i, and j). The presence of xenotropic viral gp70, along
with IgG and complement, was demonstrated in glomerular
lesions of SCID mice transplanted with either one of the two
pathogenic hybridoma clones (Fig. 4f and j), suggesting the
deposition of gp70 IC.
On the other hand, an IgG2a-producing anti-gp70
hybrid-oma clone, 36D1.1, did not induce significant nephritic lesions
in SCID mice. Since differential measurement of the
concen-trations of IgG produced from transplanted hybridoma cells
was impractical in immunocompetent (BALB/c
⫻
MRL/
⫹
)F
1mice, serum concentrations of hybridoma-derived IgG were
determined by single radial immunodiffusion in SCID mice. At
the time the transplanted SCID mice were killed for
histopath-ologic examination, average serum concentration of IgG2a in
hybridoma 36D1.1-bearing mice was 6.7 mg/ml, while that of
IgG3 in hybridoma 12H5.1-bearing mice was 5.3 mg/ml. In
some SCID mice transplanted with hybridoma 36D1.1 cells,
higher serum concentrations of IgG2a such as 16.6 mg/ml were
observed.
Induction of gp70 deposition and glomerular pathology by
injecting purified MAb.
To further exclude the possibility that
the glomerular lesions were induced by products of the
hybrid-oma cells other than Ig, IgG3 molecules purified from culture
supernatants of hybridoma cells 12H5.1 and 51D1.1 were
in-jected i.v. into syngeneic (BALB/c
⫻
MRL/
⫹
)F
1mice. A single
injection of purified 12H5.1 induced minimal glomerular
pa-thology; however, when purified 12H5.1 IgG3 (0.25 mg/mouse)
was injected for 3 consecutive days and the kidneys were
ex-amined 2 days after the final injection, diffuse granular
depo-sition of retroviral gp70 in the glomeruli was observed by
immunohistochemical staining (Fig. 5c and d) along with IgG.
No gp70 deposition was observed when control anti-SRBC
IgG3 was injected in the same manner (Fig. 5e). Histologic
changes characterized by PAS-positive depositions in the
mes-angial area were also observed in all the mice injected with
purified 12H5.1 IgG (Fig. 5g) but not in those injected with
purified anti-SRBC IgG (Fig. 5f). Repeated injection of
puri-fied 51D1.1 on the same schedule resulted in slight expansion
of mesangial areas and minimal deposition of gp70. However,
when purified 12H5.1 and 51D1.1 were mixed and injected for
3 consecutive days as described above, apparently more severe
glomerular pathology characterized by edema and
PAS-posi-tive deposits in the mesangial areas and some capillary walls
was observed (Fig. 5h).
Thus, these results directly indicate that at least some
mono-clonal anti-gp70 autoantibodies induce, in the absence of other
cellular products, glomerular deposition of gp70 and renal
pathology.
Differences in glomerular pathology in mice expressing high
and low levels of serum gp70.
To further examine the
possi-bility that injected anti-gp70 autoantibodies were involved in
the formation of immune complexes with serum gp70, purified
MAb 12H5.1 was injected i.v. into three different strains of
mice that are known to express high or low levels of serum
gp70. NZW mice have been shown to express the highest level
of serum gp70 among several different strains tested (14), while
B6 mice express a very low level of gp70 in their sera (15).
These differences in the amount of expressed serum gp70 were
also confirmed by Western blotting, along with the expression
of a relatively large amount of serum gp70 in (BALB/c
⫻
MRL/
⫹
)F
1mice (Fig. 6a). Although NZW and B6 mice are
not syngeneic to BALB/c and MRL backgrounds in which the
hybridoma cells were produced, mouse IgG3 constant regions
contain extremely limited polymorphisms (32), and no
sero-logically definable IgG3 allotypes have been reported. Thus,
induction of anti-allotypic immune responses by injecting
pu-rified IgG3 molecules is very unlikely.
When IgG3 molecules purified from culture supernatants of
12H5.1 or control N.S-7 hybridoma cells were injected for 3
consecutive days as described above, all of the 10 NZW and 8
(BALB/c
⫻
MRL/
⫹
)F
1mice injected with 12H5.1 IgG3
de-veloped focal but significant glomerular pathologies
character-ized by thickening of the capillary walls, cell proliferation, and
inflammatory cell infiltration (Fig. 6b and c). On the other
hand, significant pathologic changes were not observed in the
B6 mice injected with purified 12H5.1 IgG3 (Fig. 6d). Control
N.S-7 IgG3 did not induce significant glomerular lesions in any
of the three strains of mice. Furthermore, no deposition of
on November 9, 2019 by guest
http://jvi.asm.org/
gp70 was demonstrated in the kidneys of B6 mice after
injec-tion of purified 12H5.1 IgG3.
DISCUSSION
In this study, we established from unmanipulated MRL/
lpr
lupus mice hybridoma clones that secrete MAbs reactive with
[image:7.612.81.525.71.493.2]the endogenous xenotropic viral
env
gene products. The MAbs
were selected both for reactivity with CV-1 cells expressing the
xenotropic viral
env
cDNA and for lack of reactivity with the
same cells expressing the influenza virus HA gene. Specificities
of the established MAbs were further confirmed by Western
blotting and immunofluorescence assays using CV-1 cells
ex-pressing chimeric
env
genes between NZB xenotropic virus and
FIG. 4. Representative kidney pathology of hybridoma-transplanted mice. (a) (BALB/c⫻MRL/⫹)F1mouse transplanted with 8.653 fusion partner cells. PASstaining,⫻70. (b) (BALB/c⫻MRL/⫹)F1mouse transplanted with hybridoma cells 12H5.1. PAS staining,⫻70. Note the extreme expansion of the glomeruli compared
to those in panel a, which shows normal glomeruli at the same magnification. Sizes of tubules and of the nuclei of tubular epithelial cells are not different in panels a and b, but glomeruli are markedly enlarged in panel b. Granular deposition of fibrin in the affected glomeruli was also shown when phosphotungstenic acid-hematoxylin staining was applied (not shown). (c) Immunofluorescence staining with FITC-conjugated anti-mouse IgG of a fresh-frozen section taken from a representative (BALB/c⫻MRL/⫹)F1mouse transplanted with hybridoma cells 12H5.1. Use of FITC-conjugated anti-mouse C3 resulted in a similar pattern of staining.
(d) Electron micrograph showing an affected glomerulus of a representative (BALB/c⫻MRL/⫹)F1mouse transplanted with hybridoma 12H5.1. Cells occupying the
capillary lumina (❉) are filled with numerous electron-dense granules. Arrows indicate subendothelial deposits along the basement membrane. Bar⫽2m. (e) SCID mouse transplanted with hybridoma cells 12H5.1. PAS staining,⫻140. (f) Immunoperoxidase staining of a fresh-frozen section prepared from a SCID mouse at 3 days after transplantation of hybridoma 12H5.1. Purified MAb 24-9 (23) was biotinylated to detect the presence of xenotropic viralenvgene products. (g) (BALB/c⫻
MRL/⫹)F1mouse transplanted with hybridoma cells 37C4.1 showing typical wire loop lesions. PAS staining,⫻175. (h) Electron micrograph of the kidney from a
(BALB/c⫻MRL/⫹)F1mouse transplanted with hybridoma cells 37C4.1. Bar⫽2m. Note the dense subendothelial deposits consistent with light microscopic wire
loops along the basement membrane. (i) SCID mouse transplanted with hybridoma cells 37C4.1. PAS staining,⫻140. (j) Dense linear deposition of mouse C3 in a representative glomerulus from a SCID mouse transplanted with hybridoma 37C4.1. Similar deposition of mouse IgG and xenotropic viral gp70 in the affected glomeruli was also demonstrated in fresh-frozen sections of the transplanted SCID mice. (k and l) (BALB/c⫻MRL/⫹)F1mice transplanted with one clone of hybridomas 51D1.1
and 59C4.1, respectively. PAS staining,⫻140. Note PAS-positive deposition and expansion of the mesangial areas in panel k and cell proliferation (arrowheads) and occlusive changes (arrow) of capillaries in panel l. Lesions similar to those in panel l were observed in the mice transplanted with hybridoma 60A5.1 (not shown).
on November 9, 2019 by guest
http://jvi.asm.org/
Friend SFFV. About one-half of the gp70-reactive MAbs
es-tablished from MRL/
lpr
mice lost reactivity when a part of the
xenotropic viral
env
gene was replaced with the corresponding
portion of SFFV
env
, thus confirming the presence of antigenic
epitopes within the xenotropic viral gp70. On the other hand,
[image:8.612.141.464.68.584.2]some other MAbs similarly established from MRL/
lpr
mice
were reactive to both the xenotropic viral and SFFV
env
gene
products. These latter MAbs, exemplified by clone 51D1.1,
tended to show reactivities to several protein bands other than
gp85 that were common to uninfected Mv1Lu mink cells and
FIG. 5. Photomicrographs showing gp70 deposition in glomeruli and pathology induced in mice injected with purified anti-gp70 MAb. (a [⫻85] and b [⫻175]) Representative frozen sections taken from a female MRL/lprmouse showing granular deposition of gp70 in glomeruli; immunoperoxidase staining with MAb 24-6. (c [⫻85] and d [⫻175]) Representative frozen sections taken from a (BALB/c⫻MRL/⫹)F1mouse injected with purified 12H5.1 IgG3; immunoperoxidase staining withMAb 24-6. Note that all four glomeruli seen in panel c (arrows) exhibit gp70 deposition. Deposits of gp70 seem to localize along mesangial cells (d). (e) Representative frozen section taken from a control (BALB/c⫻MRL/⫹)F1mouse injected with purified N-S.7 IgG3. No gp70 deposition was observed. (f) Representative glomeruli
of a control (BALB/c⫻MRL/⫹)F1mouse injected with purified N-S.7 IgG3; PAS staining,⫻175. (g) Representative glomerular pathology induced by injection of
purified 12H5.1 IgG3 in (BALB/c ⫻MRL/⫹)F1 mice; PAS staining,⫻175. Note expansion of mesangial areas with PAS-positive deposits (arrowhead). (h)
Representative glomerular pathology induced in (BALB/c⫻MRL/⫹)F1mice by injecting a mixture of purified 12H5.1 and 51D1.1 IgG3; PAS staining,⫻350. Note
expansion of mesangial spaces between the capillaries and subendothelial hyaline deposits (arrowhead).
on November 9, 2019 by guest
http://jvi.asm.org/
NZB-AR cells chronically infected with an NZB xenotropic
virus in Western blotting. Possible cross-reactivity of these
gp70-reactive antibodies with normal cellular components and
its potential roles in the development of autoimmune lesions,
described as molecular mimicry (6), might be worth pursuing.
Glomerular lesions were induced in non-autoimmune mice
by transplanting single clones of hybridoma cells producing
gp70-reactive Abs. At least 7 of the 16 separate hybridoma
clones established from unmanipulated MRL/
lpr
mice induced
histopathologically significant glomerular lesions, and
deposi-tion of xenotropic viral gp70 along with IgG and C3 was
dem-onstrated in the lesions induced by transplantation of the two
representative hybridoma clones into SCID mice. Direct
in-volvement of the anti-gp70 MAb, rather than possible
second-ary host immune responses to the transplanted hybridoma cells
including anti-idiotypic Ab production, in the induction of
glo-merular pathology was demonstrated by successful induction in
SCID mice of glomerular lesions that were similar to those
induced in (BALB/c
⫻
MRL/
⫹
)F
1mice.
It has been shown that a single i.p. injection of pristane
induces in non-autoimmune BALB/c mice production of
anti-nuclear ribonucleoprotein and anti-Su autoantibody
produc-tion and proliferative glomerulonephritis (27, 28). However,
the development of autoantibody production and nephritis
took months after pristane injection (27). On the other hand,
our mice were killed and examined within 5 weeks after a
single pristane injection, and thus it is unlikely that the
injec-tion of pristane alone was responsible for the development of
glomerular lesions in the hybridoma-transplanted mice. In fact,
control mice transplanted with the fusion partner cells or a
nonproducer clone of hybridoma cells established from MRL/
[image:9.612.85.520.72.410.2]lpr
mice after an injection of the same pristane dose showed
only minimal pathologic changes in the kidneys (Fig. 4 and
Table 2). Reproduction of severe glomerular pathology in
SCID mice that should not produce any Ab in response to
pristane injection (Fig. 4) also supports the notion that
pristane-induced autoantibody production is not the major
pathogenetic factor in this transplantation model. It should be
noted that possible production of anti-gp70 autoantibodies in
the above-described pristane-induced model of nephritis has
not been examined. Thus, the presence of the pristane-induced
model neither contradicts nor supports possible pathogenicity
FIG. 6. Differences in serum gp70 expression and glomerular pathology induced after injection of purified 12H5.1 IgG3 in NZW, (BALB/c⫻MRL/⫹)F1, and B6mice. (a) Results of Western blotting assays showing the expression of serum gp70 in three different strains of mice. Sera were diluted 1:20 into SDS sample buffer without a reducing reagent and boiled for 5 min; 10l of each boiled mixture was loaded into a well of 7.5% polyacrylamide gel. Plasma from a 4 month-old female MRL/lprmouse (Lpr), which should contain a large amount of gp70–anti-gp70 immune complexes, was used as a positive control. As reported previously (14, 15), NZW mice expressed a high level of serum gp85 (gp70 plus p15E), gp70, and a degradation product gp45 (arrowheads), while their expression in B6 mice was low. (BALB/c⫻
MRL/⫹)F1mice (F1) expressed an intermediate level of serum gp70. Mr, biotinylated markers, with positions indicated in kilodaltons at the left. (b to d) Representative
photomicrographs taken from kidney sections of NZW (b), (BALB/c⫻MRL/⫹)F1(c), and B6 (d) mice injected with purified anti-gp70 IgG3, 12H5.1; hematoxylin
and eosin staining,⫻300. Note apparent thickening of the capillary walls (arrowheads) and inflammatory cell infiltration (arrow) in panel b and marked increase in glomerular cellularity and evident neutrophilic infiltration in panel c.
on November 9, 2019 by guest
http://jvi.asm.org/
of anti-gp70 autoantibodies. It is also possible, however, that
production of some cytokines, especially IL-6, either from the
hybridoma cells or from host tissues in response to pristane
injection and/or hybridoma transplantation, might have
con-tributed to the development of glomerular pathology. The
slight increase in the index of glomerular pathology in mice
transplanted with 8.653 myeloma cells (Table 2) might be
ex-plained by this mechanism. However, representative clones of
the anti-gp70 MAb did induce glomerular deposition of gp70
and significant pathology in non-autoimmune mice when
in-jected as purified IgG, clearly eliminating possible
pathoge-netic effects of cellular products other than Ig.
Possible involvement of gp70–anti-gp70 immune complexes
in the induction of the currently described Ab transfer models
was indicated by the demonstration of gp70 deposition in
af-fected glomeruli along with IgG and C3 (Fig. 4 and 5). This
finding was supported by the demonstration of differences in
glomerular pathology induced by injection of purified
anti-gp70 MAb 12H5.1 in NZW and B6 strains of mice that are
known to express high and low serum levels of gp70,
respec-tively (Fig. 6). Thus, mice expressing high levels of serum gp70
developed apparently more severe glomerular pathology after
injection of 12H5.1 IgG3, while B6 mice expressing minimal
serum gp70 did not develop glomerular lesions even when the
same amount of purified anti-gp70 IgG3 was injected. These
results support the possibility that injected anti-gp70 MAb
produced immune complexes with serum gp70 before being
deposited into kidney glomeruli. Further studies including
measurements of serum immune complexes are required to
correlate possible production of gp70–anti-gp70 immune
com-plexes and the development of glomerulonephritis.
It is noteworthy that anti-gp70 IgG3-producing hybridoma
clones induced histologically evident glomerular lesions at an
apparently higher frequency than IgM- and IgG2a-producing
clones did (Table 2). Since it is difficult to differentially
mea-sure the amount of IgG produced from transplanted
hybrid-oma cells in immunocompetent (BALB/c
⫻
MRL/
⫹
)F
1mice,
concentrations of hybridoma-derived IgG were determined for
a limited number of hybridoma clones in transplanted SCID
mice. Average concentrations of serum IgG were in the same
range among the mice transplanted with a representative
IgG2a-producing hybridoma cells and those bearing
represen-tative IgG3-producing cells. Although hybridoma-derived Ab
concentrations were not measured in every transplanted
ani-mal, it is unlikely that IgM- and IgG2a-producing hybridoma
cells, but not IgG3-producing ones, selectively lose their
Ab-producing ability soon after transplantation. Therefore, IgG3
anti-gp70 MAbs may have higher pathogenic potentials than
IgM and IgG2a anti-gp70 MAbs. In fact, the importance of the
IgG3 isotype in the induction of glomerular lesions has been
demonstrated in spontaneous and induced models of MRL/
lpr
mice (7, 34). The importance of IgG3 isotype in the
pathogen-esis of mouse SLE was also demonstrated in a recent study (25)
in which transgenic expression of IL-4 protected (NZW
⫻
C57BL/6.
Yaa
)F
1lupus mice from fatal glomerulonephritis in
association with a lack of IgG3 and strong reduction in the
serum levels of gp70 IC. It has been suggested that IgG3 MAbs
of specific yet undefined physicochemical properties might
in-duce glomerular lesions in non-autoimmune mice when
pro-duced from transplanted hybridoma cells, regardless of their
antigenic specificity (9, 33, 34). One can argue that an
ex-tremely high serum concentration of IgG3 would be achieved
when hybridoma cells were transplanted, and cryoprecipitating
activity of IgG3 molecules induced the observed glomerular
lesions. However, it should be noted that hybridoma clones
secreting MAbs with strong cryoprecipitating activity were
clearly distinguished from noncryogenerating or less
cryogen-erating anti-gp70 clones through the screening procedure and,
thus eliminated from the present study, because the former
actually caused fine granular precipitates throughout the
bot-toms of culture wells after overnight incubation with target
cells at 4°C. In addition, none of the mice transplanted with our
anti-gp70 Ab-producing hybridomas, either of IgG3 or another
isotype, developed purpuric skin lesions, a typical
manifesta-tion of cryoglobulinemia (7, 11). These findings, along with the
demonstration of gp70 deposition in the induced glomerular
lesions (Fig. 4 and 5) and differences in the pathogenicity of
injected anti-gp70 MAbs in strains of mice expressing high and
low levels of serum gp70 (Fig. 6), strongly suggest that cognate
binding of anti-gp70 MAbs to serum gp70 is mainly responsible
for their ability to induce glomerular pathology.
Because gp70 was expressed from a cloned cDNA in our
experiments, and different isolates of endogenous mouse
retro-viruses are readily available, these anti-gp70 MAbs might
be-come very useful in identifying Ab-binding epitope structures
and in analyzing the ontogenic origins of
autoantibody-produc-ing cells. Our preliminary analyses usautoantibody-produc-ing chimeras between
NZB xenotropic viral and SFFV
env
genes has shown that
one-half of the anti-gp70 autoantibody clones including the highly
pathogenic 12H5.1 recognize epitopes located within the
N-terminal 150 amino acids of the xenotropic viral gp70. Amino
acid sequences are rather homologous in this region between
NZB xenotropic virus and SFFV (21, 29, 42). The major
dif-ference consists of an insertion of four consecutive amino acids
in NZB xenotropic viral gp70, in addition to several scattered
amino acid substitutions. Construction of recombinant vaccinia
viruses that express gp70 minigenes and use of synthetic
oli-gopeptides may lead to the identification of epitope structures
recognized by pathogenic anti-gp70 MAbs in the near future.
ACKNOWLEDGMENTS
We thank M. Nose for providing SCID mice and scientific advice,
M. P. Gorman for reviewing the manuscript, and J. Nishio, H.
Shi-waku, E. Kondoh, and Y. Akahoshi for technical assistance.
Some of the recombinant vaccinia viruses described in this report
were constructed during M. Miyazawa’s stay at the Rocky Mountain
Laboratories, Hamilton, Mont., under the financial support of B.
Chesebro. This work was supported in part by grants from the
Minis-tries of Education, Science and Culture and of Health and Welfare of
Japan and from the Cell Science Foundation.
REFERENCES
1.Andrews, B. S., R. A. Eisenberg, A. N. Theofilopoulos, S. Izui, C. B. Wilson, P. J. McConahey, E. D. Murphy, J. B. Roth, and F. J. Dixon.1978. Sponta-neous murine lupus-like syndromes. Clinical and immunopathological man-ifestations in several strains. J. Exp. Med.148:1198–1215.
2.Chesebro, B., W. Britt, L. Evans, K. Wehrly, J. Nishio, and M. Cloyd.1983. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of Friend MCF and Friend ecotropic murine leukemia virus. Virology127:134–148.
3.Chesebro, B., and K. Wehrly.1976. Studies on the role of the host immune responses in recovery from Friend virus leukemia. I. Antiviral and antileu-kemia cell antibodies. J. Exp. Med.143:73–84.
4.Cohen, P. L., and R. A. Eisenberg.1991.Lprandgld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu. Rev. Immu-nol.9:243–269.
5.Elder, J. H., F. C. Jensen, M. L. Bryant, and R. A. Lerner.1977. Polymor-phism of the major envelope glycoprotein (gp70) of murine C-type viruses: virion associated and differentiation antigens encoded by a multi-gene fam-ily. Nature267:23–28.
6.Fijinami, R. S., and M. B. A. Oldstone.1985. Amino acid homology and immune response between the encephalitogenic site of myelin basic protein and virus: a mechanism of autoimmunity. Science230:1093–1095. 7.Gyotoku, Y., M. Abdelmoula, F. Spertini, S. Izui, and P.-H. Lambert.1987.
Cryoglobulinemia induced by monoclonal immunoglobulin G rheumatoid factors derived from autoimmune MRL/MpJ-lpr/lprmice. J. Immunol.138: 3785–3792.
on November 9, 2019 by guest
http://jvi.asm.org/
8.Hara, I., S. Izui, and F. J. Dixon.1982. Murine serum glycoprotein gp70 behaves as an acute phase reactant. J. Exp. Med.155:345–357.
9.Itoh, J., S. Takahashi, M. Ono, T. Yamamoto, M. Nose, and M. Kyogoku. 1994. Nephritogenic antibodies in MRL/lpr lupus mice: molecular charac-teristics in pathological and genetic aspects. Tohoku J. Exp. Med.173: 65–174.
10. Iwashiro, M., T. Kondo, T. Shimizu, H. Yamagishi, K. Takahashi, Y. Mat-subayashi, T. Masuda, A. Otaka, N. Fujii, A. Ishimoto, M. Miyazawa, M. N. Robertson, B. Chesebro, and K. Kuribayashi.1993. Multiplicity of virus-encoded helper T cell epitopes expressed on FBL-3 tumor cells. J. Virol.67: 4533–4542.
11. Izui, S., T. Berney, T. Shibata, T. Fulpius, L. Fossati, and R. Merino.1994. Molecular and cellular basis for pathogenicity of autoantibodies. Tohoku J. Exp. Med.173:15–30.
12. Izui, S., J. H. Elder, P. J. McConahey, and F. J. Dixon.1981. Identification of retroviral gp70 and anti-gp70 antibodies involved in circulating immune complexes in NZB⫻NZW mice. J. Exp. Med.153:1151–1160.
13. Madaio, M. P., J. Carlson, J. Cataldo, A. Ucci, P. Migliorini, and O. G. Pankewycz.1987. Murine monoclonal anti-DNA antibodies bind directly to glomerular antigens and form immune deposits. J. Immunol.138:2883–2894. 14. Maruyama, N., and C. O. Lindstrom.1983. H-2-linked regulation of serum
gp70 production in mice. Immunogenetics17:507–521.
15. Maruyama, N., C. O. Lindstrom, H. Sato, and F. J. Dixon.1983. Serum gp70 production regulated by a gene on murine chromosome 7. Immunogenetics 18:365–371.
16. Miyazawa, M., S. Mori, G. J. Spangrude, J. B. Wolfinbarger, and M. E. Bloom.1994. Production and characterization of new monoclonal antibodies that distinguish subsets of mink lymphoid cells. Hybridoma13:107–114. 17. Miyazawa, M., J. Nishio, and B. Chesebro.1992. Protection against Friend
retrovirus-induced leukemia by recombinant vaccinia viruses expressing the
gaggene. J. Virol.66:4497–4507.
18. Miyazawa, M., J. Nishio, M. Kyogoku, and B. Chesebro.1992. Host genetic control of immune responses to molecularly cloned Friend leukemia virus antigens, p. 177–184.InT. O. Yoshida and J. M. Wilson (ed.), Molecular approaches to the study and treatment of human diseases. Elsevier Science Publishers, B.V., Amsterdam, The Netherlands.
19. Miyazawa, M., M. Nose, M. Kawashima, and M. Kyogoku.1987. Pathogen-esis of arteritis of SL/Ni mice. Possible lytic effect of anti-gp70 antibodies on vascular smooth muscle cells. J. Exp. Med.166:890–908.
20. Naparstek, Y., and H. P. Plotz.1993. The role of autoantibodies in autoim-mune disease. Annu. Rev. Immunol.11:79–104.
21. O’Neill, R. R., C. E. Buckler, T. S. Theodore, M. A. Martin, and R. Repaske. 1985. Envelope and long terminal repeat sequences of a cloned infectious NZB xenotropic murine leukemia virus. J. Virol.53:100–106.
22. Portis, J. L., and F. J. McAtee.1983. Monoclonal antibodies derived during graft-versus-host reaction. II. Antibodies detect unique determinants com-mon to many MCF viruses. Virology126:96–105.
23. Portis, J. L., F. J. McAtee, and M. W. Cloyd.1982. Monoclonal antibodies to xenotropic and MCF murine leukemia viruses derived during the graft-versus-host reaction. Virology118:181–190.
24. Robertson, M. N., M. Miyazawa, S. Mori, B. Caughey, L. H. Evans, S. F. Hayes, and B. Chesebro.1991. Production of monoclonal antibodies reacting with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, elec-tron microscopy, and Western blotting. J. Virol. Methods34:255–271. 25. Santiago, M.-L., L. Fossati, C. Jacquet, W. Mu¨ller, S. Izui, and L. Reininger.
1997. Interleukin-4 protects against a genetically linked lupus-like autoim-mune syndrome. J. Exp. Med.185:65–70.
26. Santiago, M.-L., C. Mary, D. Parzy, C. Jacquet, X. Montagutelli, R. M. E. Parkhouse, R. Lemoine, S. Izui, and L. Reininger.1998. Linkage of a major quantitative trait locus toYaagene-induced lupus-like nephritis in (NZW⫻
C57BL/6)F1mice. Eur. J. Immunol.28:4257–4267.
27. Satoh, M., A. Kumar, Y. S. Kanwar, and W. H. Reeves.1995. Anti-nuclear antibody production and immune-complex glomerulonephritis in BALB/c mice treated with pristane. Proc. Natl. Acad. Sci. USA92:10934–10938. 28. Satoh, M., and W. H. Reeves.1994. Induction of lupus-associated
autoanti-bodies in BALB/c mice by intraperitoneal injection of pristane. J. Exp. Med. 180:2341–2346.
29. Shigemoto, K., S. Kubo, Y. Itoh, G. Tate, S. Handa, and N. Maruyama.1992. Expression and structure of serum gp70 as an acute phase protein in NZB mice. Mol. Immunol.29:573–582.24.
30. Smith, G. L., B. R. Murphy, and B. Moss.1983. Construction and charac-terization of an infectious vaccinia virus recombinant that expresses the influenza hemagglutinin gene and induces resistance to influenza virus in-fection in hamsters. Proc. Natl. Acad. Sci. USA80:7155–7159.
31. Stoye, J. P., and J. M. Coffin.1987. The four classes of endogenous murine leukemia virus: structural relationships and potential for recombination. J. Virol.61:2659–2669.
32. Sun, L., M. J. Luce, K. Ren, H. Ha, and P. D. Burrows.1995. Identification of polymorphism in the constant region of IgG3: the missing mouse allotype. Int. Immunol.7:337–341.
33. Takahashi, S., J. Itoh, M. Nose, M. Ono, T. Yamamoto, and M. Kyogoku. 1993. Cloning and cDNA sequence analysis of nephritogenic monoclonal antibodies derived from an MRL/lpr lupus mouse. Mol. Immunol.30:177– 182.
34. Takahashi, S., M. Nose, J. Sasaki, T. Yamamoto, and M. Kyogoku.1991. IgG3 production in MRL/lpr lupus mice is responsible for development of lupus nephritis. J. Immunol.147:515–519.
35. Theofilopoulos, A. N., and F. J. Dixon.1985. Murine models of systemic lupus erythematosus. Adv. Immunol.37:269–390.
36. Tsao, B. P., F. M. Ebling, C. Roman, N. Panosian-Sahakian, K. Calame, and B. H. Hahn.1990. Structural characteristics of the variable regions of im-munoglobulin genes encoding a pathogenic autoantibody in murine lupus. J. Clin. Investig.85:530–540.
37. Tsurudome, M., A. Yamada, M. Hishiyama, and Y. Ito.1990. Monoclonal antibodies against the nucleoprotein of mumps virus: their binding charac-teristics and cross-reactivity with other paramyxoviruses. Acta Virol.34: 220–227.
38. Vlahakos, D. V., M. H. Foster, S. Adams, M. Katz, A. A. Ucci, K. J. Barrett, S. K. Datta, and M. P. Madaio.1992. Anti-DNA antibodies form immune deposits at distinct glomerular and vascular sites. Kidney Int.41:1690–1700. 39. Vogt, A., S. Batsford, and T. Morioka.1994. Nephritogenic antibodies in
lupus nephritis. Tohoku J. Exp. Med.173:31–41.
40. Vyse, T. J., C. G. Drake, S. J. Rozzo, E. Roper, S. Izui, and B. L. Kotzin.1996. Genetic linkage of IgG autoantibody production in relation to lupus nephri-tis in New Zealand hybrid mice. J. Clin. Investig.98:1762–1772.
41. Vyse, T. J., and B. L. Kotzin.1998. Genetic susceptibility to systemic lupus erythematosus. Annu. Rev. Immunol.16:261–292.
42. Wolff, L., E. Scolnick, and S. Ruscetti.1983. Envelope gene of the Friend spleen focus-forming virus: deletion and insertions in 3⬘gp70/p15E-encoding region have resulted in unique features in the primary structure of its protein product. Proc. Natl. Acad. Sci. USA80:4718–4722.
on November 9, 2019 by guest
http://jvi.asm.org/