CopyrightC1977 American Society for Microbiology Printed in U.S.A.
Cyclic AMP Regulation
of Mammary
Tumor Virus
Production
JASON YANG* AND S. NANDICancer Research Laboratory, University of California, Berkeley, California 94720
Receivedforpublication 13 August 1976
Addition of dibutyryl cyclic AMP (cAMP) and agents (isoproterenol and epinephrine) that stimulate the activities of adenylate cyclase enhance the stimulation of mammary tumor virus (MTV) production two- to threefold by glucocorticoidin short-term primary cultures of mammary tumors. This cAMP potentiation seems to depend on the stimulated level of MTV production by
glucocorticoid alone, which increases MTV production 5- to 10-fold over basal level butvaries greatly in absolute terms. When the stimulated level by
gluco-corticoid alone is suboptimal, cAMP seems to restore the sensitivity of the cells
to the stimulatory effect of glucocorticoid to its maximum.
Inbred strains of mice that differ greatly in ment and differentiation of normal mammary
the spontaneous incidence of mammary tumors glands is not known, but its involvement is seem to have comparable levels of mammary suggested by the observation that cAMPrises
tumor virus (MTV) sequence in their somatic continuouslyinmammarytissuesuntil the end cellular DNA (15, 21; but see 11). That the of pregnancyand then fallsprogressivelytoits
regulation of expression may account for differ- lowest value by the 16th day of lactation (8, 19).
ences intumor incidence and/or virusproduc- Wenowreportthat cAMPmayhavea regula-tion among different strains and tissues is sug- tory role onthe production ofMTV in short-gested by the high correlation between the termprimarytumorcell cultures.
mammary tumorincidence indifferent strains (A portion of these data hasbeenpresented
and the MTV expressioninmilk (12, 13, 23) as atthe 67thAnnualMeetingof the Association wellas intheir tissues (12, 22). Hormones have of Cancer Research, May 1976, Toronto, Can-been implicated in MTV production both in ada).
vivoandinvitro. Therefore, anunderstanding Primary cultures were derived from sponta-ofthe hormonal regulation of MTV production neous tumorsthat developed in MTV-infected maybe useful for determining the role of hor- multiparous BALB/cfC3H female mice and monesinmammarygland carcinogenesis. It is were prepared according to the procedure de-important, however, to delineate the direct ef- scribedbyMcGrath (10).Dissociated cellswere
fects of various hormones in the absence of plated in either 35- or 60-mm petri dishes in
secondary effects caused by hormone interac- Dulbecco modified Eagle medium containing
tions invivo. Cell culture studies have shown 5%fetal calf serum, 100 Uofpenicillinperml, that thehormones such as glucocorticoids, in- and100
gg
ofstreptomycin perml,at aseedingsulin, estrogen, and prolactin which are in- density of5 x 105 cells/cm2, exceptfordensity
volvedin the development and differentiation experiments where differentseeding densities ofnormal mammary glands are also implicated were used. After 24 to 48 h, media were inthe regulation of viral expression. Both glu- changed to Dulbecco modified Eagle medium cocorticoids and insulin have been shown to supplementedwith various testhormone
com-increase MTV expression in primary tumor cell binations. Serum-free media was generally cultures (2, 6, 10, 28) as well as in mammary used, except under situations of low seeding
tumor celllines (4,14,17). Recently, it has been density,topreclude stimulatoryeffects of other shown that estrogen has a synergistic effect trophichormones in serum.Anyvariationfrom
with glucocorticoid in primary tumor cell cul- the above procedure will bementioned in the tures from BALB/cfC3H mice (29) and that appropriate section. Hydrocortisone,
dexa-prolactinalso has asimilareffect withglucocor- methasone, insulin, dibutyryl (db) cAMP,
L-ticoid in normal mammary primary cell cul- epinephrine, and DL-isoproterenol sulfatewere tures from BALB/cfC3H (J. Enami, J. Yang, obtained from Sigma.
andS.Nandi,manuscript in preparation). The Areversetranscriptaseassaywasperformed
exactrole ofcyclicAMP(cAMP)inthedevelop- accordingtotheprocedureofDicksonetal. (2),
815
on November 10, 2019 by guest
http://jvi.asm.org/
except that a 0.04 absorbancy unit at 260 nM fold, resultingin a 10- to 30-fold increase over
(A260)ofriboadenylate:deoxythmidylate and 10 basal level. The addition of db cAMP alone did ,Ci of[3H]ITPwerereplaced by0.04A260units not stimulate MTVproductionoverbasal level. of ribocytidylate:deoxyguanylate (rC:dG) (PL Six different time course studies of MTV pro-Biochemicals) and 5 ,uCi of [3H]GTP (New duction in cultures treatedwithhydrocortisone England Nuclear, 9 Ci/mmol), respectively. alone, with db cAMP alone, with both, and
Radioimmunoassay of MTVwascarriedout ac- withneither (control) are shown inFig. 1. The
cording to the procedure described by Cardiff concentration of hydrocortisone used in the (1). Rabbitanti-MTV serum used inthe assay present set of experiments elicits maximal
was prepared and adsorbed as previously de- stimulation of MTV production. Further in-scribed (3). The method ofLowryetal. (9)was creasesin theconcentration do not enhance the usedforthe determination ofprotein content, production significantly inoursystem (2).
using bovine serum albumin as a standard. The stimulated level of MTV production at
Cell number was determined by hemocytom- this concentration of hydrocortisone is ex-etercounting. tremelyvariabledependingonthetumors,and
Underconditionswhere 0.5 ,ugofhydrocorti- the synergistic effect of db cAMP with
hydro-sonealone per mlcan stimulate MTV produc- cortisonedid notalwaystakeplace (Fig. 1).Our
tion 5- to 10-fold, the addition of 0.5 mM db observation is that when the stimulated level cAMPcan furtherenhance thisprocess 2- to 3- due to hydrocortisone is already high (i.e.,
A B C D E F G
3 6
2
2 J
2 3 4 1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 1 2 3 4 1 2 3 4 S
D A Y
FIG. 1. Sixseparatetimecoursestudies (A throughF)ofMTVproduction inculturesseededat5 x105
cellslcm2 and treatedwith 0.5,ug of hydrocortisonealoneperml(O),0.5 ugofhydrocortisoneperml,and0.5 mM db cAMP (0),anddbcAMP alone orwithoutadditives ( ). Gshows MTVproductionin cultures treated with5pgofdexamethasone aloneperml (O),5pgofde-xamethasonepermland0.5mMdb cAMP (-),and db cAMP aloneorwithout additives( ).MTVproductionwasmonitoredby duplicate determina-tion(whichdiffered byno morethan10%S)ofreversetranscriptaseactivity fromapproximately5mloftissue culturefluidpooled froma24-h incubationofduplicate60-mmortriplicate35-mmdishes.The tissue culture
fluidwasclarifiedby9,000 xgcentrifugation for10min, andthesupernatantwascentrifuged for30 minat
160,000 xg. Theresulting pelletwasresuspendedin 100piof buffer(0.01MTris-hydrochloride, pH7.4,
0.14 MNaCI, 0.04% bovine serum albumin) and disrupted by theaddition ofa 50-p1sample of0.1 M dithiothreitol and 50piof0.2%Nonidet P-40.Samples(50p1)werethenaddedto75p ofreaction mixture
consisting of50mMTris-hydrochloride.,pH8.3,40mMKCI,10mMMgCl,,0.04
A26N0
unitsof rC:dG,and 5,uCi of[3H]GTP. After incubation at37°C for 50 min., the reactions were stopped by addition of10% trichloroacetic acid with 0.1 M sodium pyrophosphate and carrier DNA (0.4 mg). Theprecipitate was collectedin 25mmWhatmanGFCfilters,washed with trichloroaceticacid, dried,andplacedinvial with 5 mlofscintillationcocktail(Omnifluor,4glliter oftoluene)forcounting. Undertheseconditions,the reaction islinear withanincreasingadditionofsucrosegradient-purifi'edMTVup toapproximately2
pug
ofprotein correspondingtoabout 3 x105cpm.-ThepresenceoftypeC viruswasmonitoredby replacing10mMMgCl,with 0.5 mMMnCl,,andnoevidencefor significanttypeC virusproductionwasnoted.
on November 10, 2019 by guest
http://jvi.asm.org/
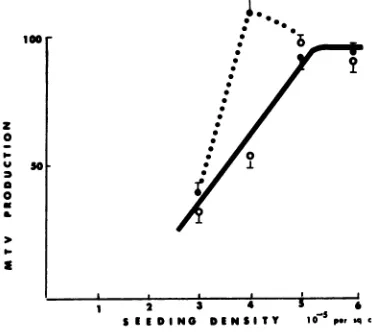
[image:2.501.76.455.264.481.2]greater than approximately 30,000 cpm of re- Thisdensity dependencemaysimplyreflect the verse transcriptase activity under our assay variable level of MTV productionwith different
condition), potentiation by db cAMP usually seeding density, since cAMP seems to act only didnot take place. Finally, the synergistic ef- when the stimulation elicited by glucocorticoid fect of db cAMP can also be observed using 5 ,ug alone is below a certain level as mentioned ofdexamethasone per ml as also shown in Fig. previously. Sincethe stimulated level of MTV 1. Aspreviously reported (2), the stimulation of productionwasfairlyhighinculturesseededat
MTV production by hydrocortisone or dexa- the highest density, isoproterenol presumably methasone at a concentration of 0.5 ,ug/ml is only elicited its effect in cultures seeded at a comparable and is at its maximum in our sys- lower density where the level of stimulation
tem. was not atits maximum. However, cAMP
po-That the increased intracellular level of tentiationwill takeplaceevenathigh seeding
cAMPmay beresponsible for theenhancement density (5 x 105 cells/cm2) provided that the issuggested by the use of8-adrenergic agonist stimulated level
by
glucocorticoid
alone is be-(isoproterenol) that stimulates adenylate cy- low 30,000 cpm reverse transcriptase activity clase. Db cAMP can be replaced with isoproter- underourassaycondition(seealso Fig. 1).enol resulting in a similar enhancement of Ithas been reported that reverse transcrip-MTVproduction. Figure 2 shows this enhance- tase from Roussarcomaviruscanbe
phospho-ment during peak production, as well as its rylated, resulting in a two- tofivefold increase dependence on the initial seeding density of the in DNA synthetic activity (7). Since cAMP is cells. As reported by several groups (2, 6, 28) known to exert itseffects through protein ki-and also shown in Fig. 2, the stimulation of nase, theeffect of cAMP described above could MTVproduction due to glucocorticoid alone is haveresulted from an increase in the
specific
dependent on the seeding density. Simultane- activity ofreverse transcriptaseand not to an
ousaddition of nanomolarisoproterenol results increase in the number of released virions. in the enhancement of MTV production, but Therefore, radioimmunoassay accordingto the only in cultures seeded at a certain density. procedure ofCardiff (1)wasused todetermine whether or notthe effect of cAMP iscorrelated
I with an increase in the number of released
4... virions. The
displacement
of the curves(ob-ool -*0* tained withmedia from cultures treated with
dexamethasone alone andincombination with
isoproterenol)
suggest that the response is duetoanactual increase in the number of released virions.
O : / Underourculture conditions
(high
cellden-| ./°
sity
and presence ofglucocorticoid),
there isSiZ formation of domes. The effect of cAMP on
o domes has been examined but no consistent
pattern has been seen. However, domes are occasionallynoted inthe presence of cAMP at
lowcelldensity which usually does notsupport
dome formation. In addition, there was occa-; wj 4 5 ,A sional
decrease
in thesizeof domesin response S IIDING
DENSITY10-
psq m tocAMPinglucocorticoid-treated
high
cellden-sity cultures in which there are numerous FIG. 2. Relative MTVproductionas afunction of domes.
initial seeding density showing density dependence Thesynergistic effect between glucocorticoid ofcAMP potentiation of MTV stimulation due to andcAMP has been observed in a number of glucocorticoid. Cultures seeded at various density different systems. Thepermissiveeffect of glu-were monitoredforMTVproduction in responseto1 cocorticoid on the action of cAMP has been
pgof dexamethasonealoneperml(0)and in combi- known for some time, and the pleiotypic re-nationwith10-9Misoproterenol (-).Bothcultures sponses exerted by these agentsare very simi-contained10
pg
of insulin per ml.MTVproduction lar(20,24).Inaddition,they
actsynergistically
atitspeak (day3) isplottedasa functionofinitial inthe inductionof many enzymes, most
nota-seeding density. MTVproductionin cultures seeded m
the
aminotransferase mospho-atdifferent density is standardized on the basis of bly tyrosineaminotransferase (5) and phospho-finalproteincontentand cell number. 100% in the enolpyruvate carboxykinase (25). Secondly,plot represents approximately 23,000 cpm, and the transformedortumorcellshavelower levels of barsrepresent variability between duplicate dishes. intracellularcAMPingeneral(16, 18), andthe
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.501.44.230.355.521.2]100 00 ofsacrifice. Thepotentiation by cAMP seems to
bedependentonthelevel of stimulation elicited
by
glucocorticoid
alone. Under conditions in which glucocorticoid alone canstimulate MTVproductionfive-totenfold above that ofcontrol,
db cAMPoragentsthat stimulate the activities §so * \a\w \ \ \ of
adenylate cyclase
can further enhance theo production two- tothreefold, provided the level
of stimulation by glucocorticoid alone is less
X 0\\°\ than approximately 30,000 cpm reverse
tran-QK
scriptase activity under our condition (orap-proximately
0.8,ug
of viralprotein
in 5 ml ofmedia
pooled
fromduplicate
60-mm ortripli-J! . ,cate 35-mm dishes). Therefore, even if the cells
001 0.1 I 0.1 1 are not producing MTV at its maximum,
whether due to seeding atsuboptimal density D ll U T I O N ordue to their poorMTV-producingcapability, thesensitivity of the cells to the effectof
gluco-FIG. 3. Two separate radioimmunoassays of cotcica- ersoe oismxmmb h MTVin medium from cultures containing 5 pgof
addtion
ob
cAMPore
ctecoaxmine
tha
dexamethasone alone per ml (0), 5pgofdexametha-addtilon
of dbcAMP
orcatecholamines
that soneper ml and 10-7 M isoproterenol (0), and isopro-stimulate
adenylate cyclase
activities.terenol alone or no additives (U). Both cultures also Finally, our report adds one moreto the ex-contained 10 pgof insulin and 5% fetal calf serum. isting list of effects of cAMP on virus replica-MTVwas pelleted from approximately 5 ml of media tion, some of which are stimulated, some in-as described for Fig. 1 and resuspended in buffer hibited, and others unaffected (reviewed in 26). 0.01 MTris-hydrochloride, pH 7.4, 0.14 M NaCl). It has been observed that cAMP can amplify Various dilutions of this concentrated virus were replication of RNA tumor virus-like particles used for inhibition of radio-immunoprecipitation. associated with Chinese hamster ovary cells Fifty microliters of variousdilutions were mixed with (26) andincreaseviralparticles associated with equal volume of anti-MTV and incubated for 2 h at and cels
(27)l
palsociases
th 37°C. Fifty microliters of['25I]MTV (about 5,000 leukemic cells (27). cAMP also increases the cpm) was then added, mixed, and incubated for an incidence of somatic cell hybrids which synthe-additional 2 h at 37°C. Finally, 50M1
of goat anti- sizeEpstein-Barrvirusearly antigensand cap-rabbit serum was added and incubated at 370C for1 sid antigens (30). On the other hand, cAMP handat 4°C overnight. The optimum conditions of inhibits replication of adenovirus type 2 in thesecondary system (i.e., concentration ofgoat anti- three fibroblastic cell lines (26). It has very rabbit that assured maximum precipitation of rabbit little effect on the replication of murine leuke-immunoglobulin) and the 50% end-point titer (i.e., mia virus in Kirsten sarcoma virus-trans-the concentration of anti-MTV that precipitates 50% formed 3T3 and itfailstoinducemurine leuke-of input count) were determined for each batch of[125I]MTV. The specific activity of [125I]MTV was
mia
virusin AKR mouse embryo cells (26). Itis usually between 107 to 108cpm/mg of viralprotein. possible that the varying effect of cAMP in The results are normalized to 100% value. The assays virus replication may have been due to varia-(except for control cultures done in duplicates) were tion inthe level ofviralproductionatthetime done intriplicates. The range of counts were at most of cAMP addition as well as variation in cell 10% (usually about5%o) of average counts and the density, since our studies suggest that cAMP range between two samples being compared did not seems to be effective only when the viruspro-overlap.
duction
issuboptimal.
addition of db cAMP resultsinthesynthesisof We thankC. Dicksonfor consultationon reverse tran-differentiatedproducts (26). scriptase assayand R. D. Cardiffand T. S. Prattfor
con-sultationonradioimmunoassay. Wealsoacknowledge help Inaccordance with theabove,wehave shown from J. Puma during the early part of theinvestigation. that cAMPmay have aregulatory role onthe Our sincere thanksalsogoes to J. Enamifor continuous production of MTV inshort-term primarytu- interest and helpfuldiscussion throughoutthe entireperiod mor cell cultures. The picture that seems to ofinvestigation.
mor Thiswork wassupportedby Public Health Service grants
emerge from our observation concerningMTV CA 05388 and CA09041,awarded by the NationalCancer production may be summarizedasfollows. The Institute, and an Abraham Rosenberg ResearchFellowship
sensitivity of the cellstotheenhancing effectof from the Universityof California, Berkeleyto JasonYang.
glucocorticoid on MTV production is variable
dependingonthe tumors,possibly duetothein LITERATURE CITED
vivo hormonal statusof the animalatthetime 1. Cardiff, C. D. 1973. Quantitationofmouse mammary
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.501.66.255.47.236.2]tumor virus(MTV) virions byradioimmunoassay. J. research, vol. 19. Academic Press Inc., New York. Immunol. 111:1722-1729. 17. Ringold, G., E. Y.Lasfargues, J. M. Bishop, and H. E. 2. Dickson, C., S. Haslam, and S. Nandi. 1974. Conditions Varmus.1975. Production of mouse mammary tumor foroptimal MTV synthesis in vitro and the effect of virus bycultured cells inthe absenceand presence of steroid hormones on virus production. Virology hormones: assay by molecular hybridization.
Virol-62:242-252. ogy65:135-147.
3. Dickson, C., J. P. Puma,andS. Nandi. 1975. Intracel- 18. Ryan, W. L., and M. L.Heidrick. 1974. Role of cyclic lular synthesis of mouse mammary tumor viruspoly- nucleotides incancer,p. 81-116. In P.Greengard and peptides: indication of a precursor glycoprotein. J. G. A. Robison (ed.), Advances in cyclic nucleotide Virol. 16:250-258. research, vol. 4. Raven Press, New York.
4. Fine, D. L., J. K. Plowman, S. P.Kelly, L.0. Arthur, 19. Sapag-Hagan, M., and A. L. Greenbaum. 1974. Adeno-and E. A. Hillman. 1974. Enhanced production of sine3':5'-monophosphate and hormone interrelation-mouse mammary tumor virus in dexamethasone- ship in themammary gland of the rat during preg-treated5-iododeoxyuridine-stimulated mammary tu- nancy and lactation. Eur. J.Biochem. 47:303-312. morcell culture. J.Natl. CancerInst. 52:1881-1886. 20. Thompson, E. B., and M. E. Lippman. 1974.
Mecha-5. Gelehrter, T. D. 1973. Mechanisms of hormonal induc- nism of action of glucocorticoids. Metabolism 23:159-tionof enzymes.Metabolism22:85-100. 202.
6. Kimball, P. C., M. Boehm-Truitt, G. Schochetman, 21. Varmus, H. E., J. M. Bishop, R. C.Nowinski, and N. and J. Schlom. 1976. Characterization of mouse mam- H. Sarkar. 1972. Mammary tumor virusspecific nu-mary tumor viruses from primary tumor cell cul- cleotide sequences in mouse DNA. Nature (London) tures.I.Immunologic and structural studies.J.Natl. New Biol.238:189-191.
CancerInst. 56:111-117. 22. Varmus, H. E., N. Quintrell, E. Mederiros, J. M. 7. Lee, S. G., M. V. Miceli, R. A.Jungmann, andP. P. Bishop, R. C. Nowinski, and N. H. Sarkar. 1973. Hung. 1975. Proteinkinaseanditsregulatoryeffect Transcription of mouse mammary tumor virus genes on reverse transcriptase activity of Rous sarcoma intissues from high and low tumorincidence mouse virus. Proc.Natl.Acad. Sci.U.S.A. 72:2945-2949. strains. J. Mol. Biol. 79:663-679.
8. Louis,S. L.,andR. L.Baldwin. 1975.Changesinthe 23. Verstraeten, A. A., R. van Nie, H. G. Kwa,and P.C. cyclic 3',5'-adenosine monophosphatesystem inrat Hageman. 1975. Quantitative estimation ofmouse mammarygland during lactationcycle.J.Dairy Sci. mammary tumor virus antigensby
radioimmunoas-58:861-869. say.Int. J. Cancer 15:270-281.
9. Lowry,0.H., N. J.Rosebrough,L. A.Farr, andR. J. 24. Wicks, W. D. 1974. The mode of action of
glucocorti-Randall. 1951. Proteinmeasurementwith the Folin coids, p. 211-141. In H. V. Rickenberg (ed.), MTP phenol reagent. J. Biol. Chem. 193:265-275. Internationalreview of science.Biochemistryof hor-10. McGrath, C. M. 1971. Replication of mammarytumor mones, vol. 8. UniversityPark Press,Baltimore.
virus in tumorcell cultures:dependenceonhormone- 25. Wicks, W. D., C. A. Barnett, and J. B. McKibbin. 1974. inducedcellular organization. J. Natl. Cancer Inst. Interaction between hormones and cyclic AMP in 47:455-467. regulating specific hepatic enzyme synthesis. Fed. 11. Michalides, F., G. Vlahakis,and J. Schlom. 1976. A Proc.33:1105-1111.
biochemicalapproachtothestudyofthe transmission 26. Willingham, M.C.1976.CyclicAMP and cellbehavior of mouse mammary tumor viruses inmousestrains incultured cells,p.310-363. InG. H.BourneandJ. RHIand C3H.Int. J.Cancer. 18:105-115. F. Danielli (ed.), International review ofcytology, 12. Noon, M. C., R. G.Wolford, and W.P. Parks. 1975. vol. 44.AcademicPressInc., New York.
Expressionof mouse mammarytumorviralpolypep- 27. Yang, T. J., andN. S. Wang. 1974. Dibutyryl cyclic
tidesinmilks andtissues. J. Immunol. 115:653-658. AMP:increase in viralparticlesassociatedwith mor-13. Parks, W. P., R.S. Howk, E. M. Scolnick,S. Orosz- phologic changes in leukemiacells. Exp.Mol.Pathol.
land,and R. V. Gilden.1974.Immunochemicalchar- 20:147-153.
acterization oftwo major polypeptidesfrom mouse 28. Young, L.J.T.,R. D.Cardiff,and R. L.Ashley. 1975. mammarytumor virus. J.Virol. 13:1200-1210. Long-term primary culture of mouse mammary tu-14. Parks,W.P., J. C. Ransom, H. A.Young, andE. M. mor cells: production ofvirus.J. Natl. CancerInst.
Scolnick. 1975.Mammarytumorvirusinductionby 54:1215-1221.
glucocorticoids. Characterization of specific tran- 29. Young, L. J. T., R. D. Cardiff, andT. S. Pratt.1976. scriptionalregulation.J. Biol. Chem.250:3330-3336. Stimulation of mouse mammary tumor virus replica-15. Parks, W.P.,andE. M.Scolnick.1973.Murinemam- tionby
estradiol-17,t.
In Vitro 12:315.mary tumorcell clones with varyingdegreesof virus
tiobesrmadioJ.7E.
InVitro
1 .Rexpression.Virology55:163-173. 30. Zimmerman, J. E., Jr., R. Glaser, and F. Rapp. 1973. 16. Pastan, I., and G.S. Johnson. 1974. CyclicAMPand Effect of dibutyryl cyclic AMP on the induction of the transformationoffibroblasts, p. 303-329. In G. Epstein-Barr virus in hybrid cells. J. Virol. 12:1442-Kleinand S. Weinhouse (ed.), Advances incancer 1445.
on November 10, 2019 by guest
http://jvi.asm.org/
![FIG. virus was monitored by replacing 10 mMwithistrichloroaceticml(-),,uCi linear C virus production was x of of (which and db M 0.5 wasscintillation with fluid [3H]GTP](https://thumb-us.123doks.com/thumbv2/123dok_us/1550296.107570/2.501.76.455.264.481/monitored-replacing-mmwithistrichloroaceticml-linear-virus-production-wasscintillation-fluid.webp)
![FIG.3.asFiftyMTVsone0.01MTVdexamethasonecontainedterenol[125I]MTV.37°C.equaladditionalusedcpm)10%rabbittherabbittheofVariousimmunoglobulin)rangedone(excepthTheusually and described input Twoseparateradioimmunoassaysof in medium from cultures containing 5 p](https://thumb-us.123doks.com/thumbv2/123dok_us/1550296.107570/4.501.66.255.47.236/asfiftymtvsone-mtvdexamethasonecontainedterenol-equaladditionalusedcpm-rabbittherabbittheofvariousimmunoglobulin-rangedone-excepththeusually-twoseparateradioimmunoassaysof-containing.webp)