HIV-1 Entry in SupT1-R5, CEM-ss, and Primary CD4
T Cells
Occurs at the Plasma Membrane and Does Not Require Endocytosis
Nikolas Herold,* Maria Anders-O¨ ßwein, Bärbel Glass, Manon Eckhardt,* Barbara Müller, Hans-Georg Kräusslich
Department of Infectious Diseases, Virology, University Hospital Heidelberg, Heidelberg, Germany
ABSTRACT
Cytoplasmic entry of HIV-1 requires binding of the viral glycoproteins to the cellular receptor and coreceptor, leading to fusion of viral and cellular membranes. Early studies suggested that productive HIV-1 infection occurs by direct fusion at the plasma membrane. Endocytotic uptake of HIV-1 was frequently observed but was considered to constitute an unspecific dead-end path-way. More recent evidence suggested that endocytosis contributes to productive HIV-1 entry and may even represent the pre-dominant or exclusive route of infection. We have analyzed HIV-1 binding, endocytosis, cytoplasmic entry, and infection in T-cell lines and in primary CD4ⴙT cells. Efficient cell binding and endocytosis required viral glycoproteins and CD4, but not the coreceptor. The contribution of endocytosis to cytoplasmic entry and infection was assessed by two strategies: (i) expression of dominant negative dynamin-2 was measured and was found to efficiently block HIV-1 endocytosis but to not affect fusion or productive infection. (ii) Making use of the fact that HIV-1 fusion is blocked at temperatures below 23°C, cells were incubated with HIV-1 at 22°C for various times, and endocytosis was quantified by parallel analysis of transferrin and fluorescent HIV-1 uptake. Subsequently, entry at the plasma membrane was blocked by high concentrations of the peptidic fusion inhibitor T-20, which does not reach previously endocytosed particles. HIV-1 infection was scored after cells were shifted to 37°C in the pres-ence of T-20. These experiments revealed that productive HIV-1 entry occurs predominantly at the plasma membrane in SupT1-R5, CEM-ss, and primary CD4ⴙT cells, with little, if any, contribution coming from endocytosed virions.
IMPORTANCE
HIV-1, like all enveloped viruses, reaches the cytoplasm by fusion of the viral and cellular membranes. Many viruses enter the cytoplasm by endosomal uptake and fusion from the endosome, while cell entry can also occur by direct fusion at the plasma membrane in some cases. Conflicting evidence regarding the site of HIV-1 fusion has been reported, with some studies claiming that fusion occurs predominantly at the plasma membrane, while others have suggested predominant or even exclusive fusion from the endosome. We have revisited HIV-1 entry using a T-cell line that exhibits HIV-1 endocytosis dependent on the viral glycoproteins and the cellular CD4 receptor; results with this cell line were confirmed for another T-cell line and primary CD4ⴙ T cells. Our studies show that fusion and productive entry occur predominantly at the plasma membrane, and we conclude that endocytosis is dispensable for HIV-1 infectivity in these T-cell lines and in primary CD4ⴙT cells.
H
uman immunodeficiency virus type 1 (HIV-1) is an envel-oped retrovirus that enters target cells by fusion of viral and cellular membranes. Productive entry is mediated by specific in-teractions of the viral envelope (Env) glycoproteins with the cel-lular receptor CD4 (1) and one of two coreceptors (CXCR4 or CCR5) (2,3). The HIV-1 Env protein is synthesized as a precursor cleaved into the surface glycoprotein gp120/SU and the trans-membrane glycoprotein gp41/TM during transport to the cell sur-face (4). A low number of 7 to 14 gp120/gp41 trimers are incor-porated into the virion membrane during HIV-1 assembly (5). Much is known about the molecular interactions of Env with its receptors leading to specific recognition, conformational changes, and subsequent membrane fusion (for a review, see references6and7). The actual site of membrane fusion has remained contro-versial, however. Both direct fusion at the plasma membrane (e.g., in ecotropic murine leukemia virus [8]) and fusion via an endo-somal pathway (e.g., in avian leukosis virus [9]) have been shown to constitute possible modes of entry for other retroviruses. Stud-ies on HIV-1 provided evidence for both of these pathways being the predominant or exclusive route of productive infection, but the site of HIV-1 entry has not been unequivocally clarified to date.
Most early studies concluded that productive HIV-1– cell
fu-sion occurs at the plasma membrane, while endocytosis represents a dead-end pathway leading to virion degradation via the lyso-somal route (10–12). This conclusion was based on three main observations: (i) HIV-1 fusion and entry are pH independent (13,
14) and therefore do not require endosomal acidification, (ii) ex-pression of HIV-1 Env on the cell surface of CD4⫹cells allows cell-to-cell fusion, indicating that direct fusion at the plasma membrane is possible (1), and (iii) the endocytosis signal in the cytoplasmic domain of CD4 is dispensable for HIV-1 infection (15), arguing against a need for receptor endocytosis.
Further-Received28 May 2014Accepted15 September 2014
Published ahead of print24 September 2014
Editor:R. W. Doms
Address correspondence to Hans-Georg Kräusslich, hans-georg.kraeusslich@med.uni-heidelberg.de.
* Present address: Nikolas Herold, Department of Medical Virology, National Reference Center for Retroviruses, University Hospital of Frankfurt, Frankfurt, Germany; Manon Eckhardt, Department of Cellular and Molecular Pharmacology, UCSF School of Medicine, San Francisco, California, USA.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
doi:10.1128/JVI.01543-14
on November 7, 2019 by guest
http://jvi.asm.org/
more, unspecific endocytosis independent of the CD4 receptor was observed in many cell lines and primary cells, presumably leading to lysosomal degradation in these cells (10,14,16). Some early studies suggested that endocytosis contributes to productive HIV-1 entry (17,18), however, and this hypothesis was supported by subsequent reports showing that pharmacological inhibition of endosomal acidification could enhance HIV-1 infection in re-porter cell lines (e.g., HeLa-, HEK293T-, and HOS-derived cell lines [19,20]). Furthermore, blocking clathrin- and dynamin-2 (Dyn-2)-dependent endocytosis strongly reduced HIV-1 infec-tion in a HeLa-derived cell line (21,22), suggesting a substantial contribution of clathrin-mediated endocytosis to productive HIV-1 entry, at least in this cell line. A later report by Miyauchi et al. (23) even suggested that HIV-1 fusion occurs exclusively from endosomes, while plasma membrane-associated virions remain trapped at the stage of membrane hemifusion and do not contrib-ute to productive infection at all; these results were reported for HeLa-derived and lymphoid cell lines.
The described results provided conflicting evidence for either the plasma membrane or the endosome being the main portal of entry for HIV-1. This suggests that both pathways may contribute to HIV-1 infection, possibly on the basis of the target cell. Further-more, while HIV-1 can clearly enter cells via endocytosis, it is not clear whether this requires the viral receptor and coreceptor(s) in relevant target cells. Many studies have shown Env- and CD4-independent endosomal HIV-1 internalization, but most of these studies were conducted in artificial model cells, mostly HeLa-de-rived cell lines (for reviews, see references24to26). Furthermore, HIV-1 Env has been reported to interact with various plasma membrane components that could mediate endosomal uptake of the particle. These include heparan sulfate proteoglycans (HSPGs)
(27–33), lectins like DC-SIGN (34), and the integrin␣47(35).
On the other hand, there is also evidence for CD4-mediated HIV-1 endocytosis, at least in the case of cell-to-cell spread through the virological synapse (36–39).
Here, we present evidence that endocytosis occurs in an Env-and CD4-dependent manner in SupT1-R5 T cells, but this path-way contributes little if anything to productive HIV-1 entry. Mak-ing use of the differential temperature requirements for HIV-1 fusion and endocytosis, we preincubated cells and HIV-1 at a tem-perature that allows endocytotic uptake of the virus but not fu-sion. Subsequent addition of the peptidic fusion inhibitor T-20, which does not reach preformed endosomal compartments, fol-lowed by a temperature shift, efficiently blocked HIV-1 fusion and infection. Thus, productive entry appears to occur predominantly at the plasma membrane in SupT1-R5 cells, and this result was confirmed for CEM-ss and primary CD4⫹T cells. We conclude that endocytosis is dispensable for HIV-1 infection in these cell types.
MATERIALS AND METHODS
Cell culture.HEK293T cells were maintained in Dulbecco’s modified
Eagle medium (DMEM) supplemented with 10% fetal calf serum (Bio-chrom), 50 U/ml of penicillin, and 50g/ml of streptomycin (Gibco, Invitrogen). CEM-ss cells (40) were cultured in RPMI 1640 medium with GlutaMAX (Gibco, Invitrogen) with the same supplements. SupT1-R5 cells stably expressing exogenous CCR5 under puromycin selection (a kind gift from Robert Doms, University of Pennsylvania) were cultured in RPMI 1640 medium with GlutaMAX (Gibco, Invitrogen) with the same supplements indicated above and 0.3g/ml puromycin (Sigma-Aldrich). Peripheral blood mononuclear cells (PBMCs) were purified from the
buffy coats of healthy blood donors by centrifugation through a Ficoll gradient (Amersham Biosciences) according to standard procedures, and CD4⫹T cells were enriched by magnetic bead negative selection accord-ing to the manufacturer’s protocol (CD4⫹T cell isolation kit; Miltenyi Biotec). Alternatively, CD4⫹T cells were directly enriched from the buffy coats of healthy blood donors using the RosetteSup human CD4⫹T cell enrichment cocktail according to the manufacturer’s protocol (Stemcell Technologies, Vancouver, Canada). CD4⫹T cells were stimulated for 3 days using 100 IU/ml interleukin-2 (Biomol) and 2g/ml phytohemag-glutinin (PHA; Sigma) and cultured in RPMI 1640 medium with Gluta-MAX (Gibco, Invitrogen) with the supplements described above.
Plasmids.The proviral HIV-1 plasmid pNL4-3 (41), pNL4-3.Env(⫺),
and the Env expression vector pCAGGS.NL4-3-Xba (42), here designated pEnv.NL4-3, were described previously. The Env expression vector pCAGGS.NL4-3R5, encoding an R5-tropic Env glycoprotein that differs from EnvNL4-3by seven point mutations within the V3 loop (42), was
kindly provided by S. Pöhlmann (German Primate Center, Göttingen, Germany). An expression plasmid for the envelope glycoprotein of vesic-ular stomatitis virus (VSV-G) under the control of the cytomegalovirus promoter (pM3) was provided by D. von Laer (University of Innsbruck, Innsbruck, Austria). The empty expression vector pEnv.mock was con-structed by deleting the Env-coding sequence from pEnv.NL4-3 using XhoI/EcoRI. Plasmid pEnvCD4(⫺) was constructed on the basis of pEnv.NL4-3 by exchanging an Acc65I/XhoI fragment for the correspond-ing fragment from the subviral plasmid pNL-A1(CD4⫺) carrying a dele-tion of the CD4 binding domain (43). Coreceptor binding-deficient pEnv.CoR(⫺) was constructed on the basis of pEnv.NL4-3 by exchanging an Acc65I/XhoI fragment for the corresponding fragment from pHenv319GE, an Env expression vector carrying a G-to-E mutation within the V3 loop at position 319 (44). Plasmid pEnv(Fus)V2E was con-structed on the basis of pEnv.NL4-3 by exchanging an AleI/Acc65I frag-ment for the corresponding fragfrag-ment from pcDNA-gp41V2E-TR752 (a kind gift from V. Bosch, DKFZ Heidelberg), carrying a V2E mutation in the gp41 fusion peptide (45). The expression vectors pEnv.5002 (plasma) and pEnv.4051 (plasma), carrying an X4-tropic or an R5-tropicenvgene, respectively, directly isolated from patient plasma (46), were kindly pro-vided by R. Swanstrom (University of North Carolina, Chapel Hill, NC, USA). Plasmids pNLC4-3.Env(⫺), pNLC4-3.Env(⫺).mCherry, and pNLC4-3.Env(⫺).eGFP were constructed on the basis of the previously described plasmids pNLC4-3, pNLC4-3.mCherry, and pNLC4-3.eGFP, respectively (30,47), by exchange of an AgeI/XhoI fragment against the corresponding fragment from subviral plasmid PA3 (30), in which an NdeI site at nucleotide 6401 of the NL4-3 sequence was filled in with Klenow polymerase to generate a frameshift in theenvopen reading frame leading to a premature stop of translation.
Expression vectors for wild-type (wt) Dyn-2 [Dyn-2(wt)] fused with enhanced green fluorescent protein (eGFP) [pDyn-2(aa)-GFP] and the Dyn-2 K44A variant fused with eGFP [pDyn-2(aa)K44A-GFP] have been described previously (48). pcDNA3.1(⫹)-Dyn-2-wild-type-mCherry and pcDNA3.1(⫹)-Dyn-2-K44A-mCherry (23) were kindly provided by G. Melikyan (Emory University, Atlanta, GA, USA). The Dyn-2 sequences from these plasmids were excised using HindIII and EcoRI and cloned into pmCherry-N1 (Clontech) in order to exchange the ampicillin resis-tance marker for a kanamycin (Kana) resisresis-tance marker, obtaining pDyn-2-wt-mCherryKana and pDyn-2-K44A-mCherryKana, respectively; this
was done to avoid the background in the Vpr.-lactamase (Vpr.BlaM) fusion assay. Plasmid pMM310 (49) was kindly provided by N. Landau (New York University, New York, NY, USA). Plasmid peGFP.Vpr (50) was a kind gift of T. Hope (Northwestern University, Chicago, IL, USA).
Transfection procedures and virus stocks. HEK293T cells were
transfected using polyethyleneimine (Sigma-Aldrich) according to stan-dard procedures in 10-cm tissue culture dishes (Falcon; BD Bioscience). To produce fluorescently labeled virions, 7.5g of pNLC4-3.Env(⫺) was cotransfected with 7.5g of pNLC4-3.Env(⫺).mCherry and with 2.5g of the respective Env expression vector. For production of Vpr. -lacta-HIV-1 Entry at the Plasma Membrane
on November 7, 2019 by guest
http://jvi.asm.org/
mase-containing particles (HIV-1BlaM), cells were transfected with 7.5g
of pNLC4-3.Env(⫺), 1.5g of pMM310, and 7.5g of the respective Env expression vector. For production of eGFP.Vpr-containing particles, cells were transfected with 15g of either pNLC4-3 or pNL4-3 and 1.5g of peGFP.Vpr. For production of infectious HIV-1 pseudotyped with the respective Env variant, cells were transfected with 15 g of pNL4-3.Env(⫺) and 1.5g of the respective Env expression vector. Media were harvested 48 h after transfection and purified through a 20% (wt/wt) sucrose cushion.
For electroporation of SupT1-R5 cells, 1.5⫻107cells were transferred
to electroporation buffer (20 mM HEPES, 135 mM KCl, 2 mM MgCl2, pH 7.6). Electroporation was performed using a Bio-Rad Gene Pulser Xcell system and 0.4-cm electroporation cuvettes (Invitrogen) in the presence of 15 to 40g of the plasmids encoding fluorescent fusion proteins of Lifeact, Dyn-2(wt), and dnDyn-2(K44A), respectively, using an exponen-tial decay protocol at a voltage set between 250 to 300 V and a capacitance set to 1,000F. After electroporation, cells were resuspended in RPMI 1640 medium. For nucleofection, 5⫻105cells were washed in
phosphate-buffered saline (PBS) and resuspended in 100l cell Nucleofection solu-tion V (Amaxa) in the presence of 4g plasmid DNA. Program O-17 of the Nucleofector 2b device (Amaxa) was used. Imaging analyses were carried out at 18 h postelectroporation, unless indicated differently.
Virus binding and endocytosis.Particle concentrations in purified
preparations of fluorescently labeled HIV derivatives were estimated on the basis of CA quantification using quantitative immunoblotting (LI-COR Odyssey). For virus binding, 106SupT1-R5 cells were incubated
with particles corresponding to⬃100 ng CA for 3 h at 16°C in CO2
-independent medium (Gibco, Invitrogen) and fixed with 3% paraformal-dehyde (PFA) for 30 min at 16°C, and cell nuclei were counterstained with Hoechst 33258 (Sigma-Aldrich). Cells were imaged by wide-field micros-copy (Zeiss Axiovert 200M), and the number of particles bound per cell was determined in maximum projections ofz-sections comprising the complete cell volume using a custom-made semiautomated ImageJ plug-in.
To determine HIV-1 endocytosis, cells were distributed in 8-chamber glass-bottom slides (Lab-Tek, Nunc) at a density of 3⫻105cells per well
and immediately incubated with viral particles (corresponding to⬃1g CA) for the times indicated below at 37°C or 22°C. Subsequently, cells were fixed for 20 min with 4% PFA at room temperature. Fixed cells were stained with phalloidin conjugated to either Atto488 or Atto633 (Atto-Tec), Dy415 (Dyomics), or tetramethylrhodamine-isothiocyanate (Mo-lecular Probes, Invitrogen), washed with PBS, and analyzed using a spin-ning-disk confocal microscope (SDCM; ERS-6; PerkinElmer). Whole cells were imaged with a slice spacing of 150 nm for deconvolution (Huy-gens, Scientific Volume Imaging) or 400 nm and were reconstructed in three dimensions using Volocity analysis software (Improvision, PerkinElmer). The interior of the cell was defined by the extension of the stained actin cortex. Individual particles were scored as being internalized in the cell when they were found within the three-dimensional limits of the actin cortex.
For colocalization studies, SupT1-R5 cells were incubated with eGFP.Vpr containing viral particles for 3 h at 37°C prior to fixation in 4% PFA for 20 min and subsequent permeabilization with 0.05% digitonin (Sigma-Aldrich). Staining was performed using Fab fragments of human anti-Env 2G12 or a polyclonal rabbit anti-CD4 serum (a kind gift from V. Bosch), Atto565-labeled mouse anti-human Fab, and Alexa 647-labeled goat anti-rabbit IgG.
To quantify clathrin-dependent endocytosis, 106SupT1-R5 cells were
incubated for 7 min with Alexa 488- or Alexa 647-transferrin (Tfn; Invit-rogen) at 37°C. Subsequently, surface-bound Tfn was removed by an acid wash (a wash for 1 min at pH 2.5 with 0.5 M NaCl, 0.2 N acetic acid), cells were fixed, and fluorescence signals were measured by flow cytometry.
For inhibition experiments with AMD-3100 (Sigma-Aldrich) or maraviroc (MVC; ViiV Healthcare), cells were preincubated for 30 min at 37°C with the respective compound at a concentration of 10M prior to
addition of virus. For experiments with the fusion inhibitor T-1249 (76) (kindly provided by S. Urban, University of Heidelberg, Heidelberg, Ger-many), cells were preincubated for 30 min at 37°C at a concentration of 250 nM. T-20 (enfuvirtide; Roche) was used at a concentration of 50M and was added at the time points indicated below for the different exper-iments. For analysis of peptide uptake, C-terminally Atto488 labeled T-20 and Atto488-labeled Tat peptide (as a control) were produced and kindly supplied by C. Kaufmann and W. Mier (University Hospital Heidelberg, Heidelberg, Germany) and were used at a concentration of 50M. For experiments using soluble CD4 (sCD4; Gibco, Invitrogen), virus was pre-incubated with sCD4 at a concentration of 10g/ml for 15 min at 37°C prior to addition to cells.
Vpr.-lactamase entry assay.Virus cell entry was measured using a
previously described -lactamase HIV fusion assay (51). SupT1-R5, CEM-ss, or activated primary human CD4⫹T cells (2⫻105to 3⫻105)
were incubated with the amounts of particles carrying the Vpr. -lacta-mase fusion protein indicated below for the times indicated below at 22°C or 37°C in the presence or absence of T-20 using a V-bottom 96-well plate (Costar; Corning). Cells were washed with PBS and incubated in the dark at room temperature with 2M CCF2-AM (Invitrogen), prepared ac-cording to the manufacturer’s instructions, in CO2-independent medium
(Gibco, Invitrogen) containing 20l/ml probenecid (Invitrogen). Cells were washed, fixed, and analyzed by flow cytometry, using a LSR-II or FACSVerse flow cytometer (Becton Dickinson). CCF2 fluorescence was excited at 405 nm, and emission maxima at 447 nm (CCF2blue) and 520 nm (CCF2green) were recorded using appropriate filter settings. Data were analyzed using FlowJo software (TreeStar).
Infectivity assays.To determine HIV-1 infectivity, 2⫻105to 106
SupT1-R5, CEM-ss, or activated primary human CD4⫹T cells per well were distributed into V-bottom 96-well plates (Costar; Corning). Subse-quently, cells were incubated with different amounts of HIV-1 variants for different periods of time at 37°C or 22°C. T-20 (50M) (52), AMD-3100 (2M) (53), or MVC (2M) (54) was added at the time points indicated below. If compounds were added prior to infection, preincubation with the compound was performed for 1 h. For temperature shift experiments, medium was removed after the times indicated below, fresh medium con-taining 50M T-20 was added, and incubation was continued for 48 h at 37°C. Subsequently, cells were fixed (4% PFA for 60 min at room temper-ature) and stained for intracellular CA using fluorescein isothiocyanate-conjugated anti HIV-1 CA antibody KC57 (Beckman Coulter) and Perm/ Wash buffer (BD Bioscience). Analysis was performed by flow cytometry. For temperature shift experiments in the presence of inhibitors of endo-somal acidification, cells were preincubated with 100 nM bafilomycin A1 (Sigma-Aldrich) or 10 mM ammonium chloride (Sigma-Aldrich) for 1 h prior to addition of virus, and further incubation was performed in the presence of the same drugs.
Ethics statement.Buffy coats which otherwise would have been
dis-carded were obtained from routine whole-blood preparations from healthy donors who signed an informed consent.
RESULTS
HIV-1 endocytosis is Env and CD4 dependent in SupT1-R5 cells.To directly study HIV-1 binding and uptake by imaging, it is crucial to discriminate between specific, receptor-mediated events and nonspecific uptake. The T-cell line SupT1-R5 was chosen for use in this study because it is efficiently infected by both R5- and X4-tropic HIV-1 strains and displays low surface expression of HSPGs (55). Initial experiments established that uptake of labeled HIV-1 particles can be detected in SupT1-R5 cells by fluorescence microscopy. Cells were incubated with fluorescently labeled HIV-1 particles carrying MA.mCherry, MA.eGFP, or eGFP.Vpr. After fixation, the F-actin cytoskeleton was stained using fluores-cent phalloidin conjugates. This allowed identification of cell boundaries as the dense layer of cortical F-actin in all three
on November 7, 2019 by guest
http://jvi.asm.org/
sions using a spinning disk confocal microscope (SDCM) (Fig. 1A
toC). Viral particles appeared as diffraction-limited dots and could be visually classified as being bound to the cell surface or being internalized, i.e., being located within the boundaries of the actin cortex.
Next, we wanted to determine whether Env and the viral recep-tor and coreceprecep-tor are functionally required for HIV-1 attach-ment and endocytosis in SupT1-R5 cells. For this, we performed experiments using fluorescent HIV-1 particles carrying Env pro-teins with different coreceptor tropisms or carrying mutations in the receptor or coreceptor binding site, as well as particles lacking Env. Particle attachment to the cell surface was quantified follow-ing virus bindfollow-ing at 16°C to prevent endocytosis (56). Cells were imaged in a wide-field microscope, and the number of particles bound was determined in maximum projections covering the complete cell volume. Cell binding efficiency was significantly lower for viruses lacking HIV-1 Env than isogenic viruses carrying EnvNL4-3(Fig. 2A). Impaired attachment was also observed for HIV-1 particles carrying Env with a deletion in the CD4-binding domain, while mutation of the coreceptor binding V3 loop in gp120 had no significant influence on virus cell binding (Fig. 2A). Thus, stable attachment of HIV-1 to SupT1-R5 cells requires the
viral receptor CD4 with some contribution of nonspecific bind-ing, while the coreceptor appears to have no role in this process.
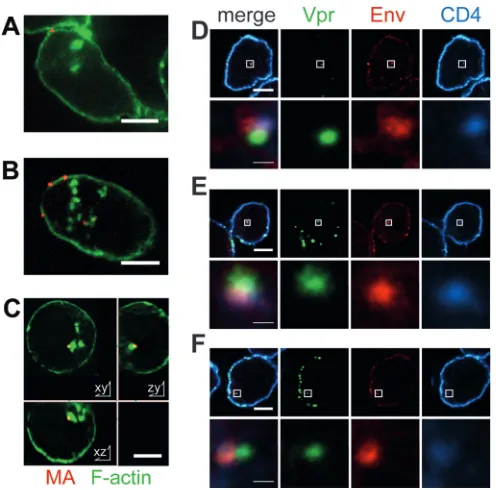
To assess particle endocytosis, SupT1-R5 cells were incubated with fluorescent HIV-1 carrying different Env proteins, and fluo-FIG 1Microscopic analysis of HIV-1 uptake into SupT1-R5 cells. (A to C)
SupT1-R5 cells were incubated with HIV-1MA.mCherryparticles either lacking (A) or carrying (B and C) EnvNL4-3for 3 h, fixed with 4% PFA, and stained with phalloidin-Atto488. z-stacks covering whole cell volumes were recorded by SDCM; individual z-slices (red, MA.mCherry; green, phalloidin-Atto488) through representative cells are shown. On the basis of the image series re-corded, all particles were visually scored to be either bound or internalized with respect to the cortical F-actin stain. (D to F) SupT1-R5 cells were incu-bated with HIV-1eGFP.Vpr(green) for 3 h, fixed, permeabilized, and immuno-stained using Env 2G12 Fab fragments (red) and a polyclonal rabbit anti-CD4 serum (cyan). Three representative examples of individual cells are shown. The top row of each panel shows individual slices of a z-stack covering the whole cell volume. The bottom rows show enlargements of the area indi-cated by the white squares in the corresponding top row. Thick bars, 5m; thin bars, 1m.
0 20 40 60 80 100 0 1 2 3 4
*** ***
A
B
0 20 40 60 80 100
0 20 40 60 80 100 0 20 40 60 80 100
Endocytosis [%
]
VLPs / cell
EnvNL4-3 + T-1249 EnvFus(V2E)
EnvNL4 -3 EnvNL4-3 Env(
-)
EnvCoR(-) EnvCD4( -)
EnvNL4-3 Env(-) En vNL4-3
Env(-) EnvNL4-3
Env + s CD4
NL4-3 EnvCD4
(-)
EnvNL4-3Env CoR(-)
Env + AMDN L4-3
EnvJR-FL
Env + MVCJR-FL
* ***
***
**
ns ns
ns *
C
D
***
SupT1-R5 CD4+ T cells
ns
Endocytosis [%
]
Endocytosis [%] Endocytosis [%]
E
FIG 2Contribution of CD4 binding, coreceptor binding, and fusion to cell attachment and endocytotic uptake of HIV-1 particles in SupT1-R5 cells. (A) Cell attachment. SupT1-R5 cells were incubated for 3 h at 16°C with HIV-1MA.mCherrylacking Env [Env(⫺)] or carrying wild-type Env
NL4-3or a CD4- or a coreceptor binding-deficient variant [EnvCD4(⫺)and EnvCoR(⫺), respectively]. Cells were imaged by wide-field microscopy, and the number of particles bound per cell was determined on the basis of maximum projections. Mean values and SDs for 25 individual view fields (corresponding to a total of ⬃900 particles) from one representative experiment are shown. VLPs, virus-like particles. (B to E) Uptake by endocytosis. Cells were incubated for 3 h with HIV-1MA.mCherryparticles pseudotyped with the indicated Env variants, fixed, and stained with phalloidin-Atto488. z-stacks covering whole cell volumes were recorded by SDCM, and particle uptake was quantified as described in the legend toFig. 1. The proportion of cells scoring positive for one or more internalized particles is shown. More than 150 cells corresponding to 10 ran-domly selected view fields from at least two independent experiments were analyzed for each condition. Dotted lines, values obtained for particles lacking a viral Env protein. (B) Internalization of particles carrying or lacking EnvNL4-3 in SupT1-R5 or activated primary human CD4⫹T cells. (C) Requirement for CD4. SupT1-R5 cells were incubated with HIV-1mCherryeither carrying EnvNL4-3preincubated in the presence or absence of sCD4 or carrying EnvCD4(⫺). (D) Requirement for a coreceptor. The internalization of HIV-1mCherrycarrying X4-tropic (Env
NL4-3; black bars) or R5-tropic (EnvJR-FL; white bars) Env variants was assessed in the absence or presence of specific coreceptor antagonists (AMD-3100 [AMD] for CXCR4 or MVC for CCR5). EnvCoR(⫺), a variant with a mutation in the coreceptor binding V3 loop of EnvNL4-3. (E) Effect of inhibition of membrane fusion. Internalization of HIV-1MA.mCherrycarrying Env
NL4-3was quantitated in the presence or absence of the fusion inhibitor T-1249, and the results were compared to those for particles carrying a fusion-incompetent Env mutant [EnvFus(V2E)]. For all experiments, mean values and SDs are shown.Pvalues were determined by a two-tailed Stu-dent’sttest. ns, not significant; *,Pⱕ0.05; **,Pⱕ0.01; ***,Pⱕ0.001.
HIV-1 Entry at the Plasma Membrane
on November 7, 2019 by guest
http://jvi.asm.org/
[image:4.585.318.524.63.364.2] [image:4.585.40.288.65.310.2]rescent particles within the F-actin boundary were counted using a series of confocal optical sections (z-stacks) covering the whole cell volume. Most cells (⬃75%) incubated with HIV-1 carrying either X4-tropic or R5-tropic Env displayed internalized particles
(Fig. 2BandD), with an average of⬃2.5 particles per cell and no
significant difference between the two Env variants being found. In contrast, incubation with particles lacking HIV-1 Env yielded internalized particles in only⬃10% of cells (Fig. 2B), and a single particle per cell was observed in positive cells. A significant differ-ence between viruses carrying or lacking Env proteins was also observed for primary CD4⫹T cells (Fig. 2B). The overall particle uptake was lower in this case, however, and the differences were less pronounced.
The potential role of receptor and coreceptor binding of HIV-1 Env for viral endocytosis was analyzed using the binding mutants described above as well as specific inhibitors. For compound treat-ment, virus or cells were preincubated with the respective sub-stance (soluble CD4 [sCD4] for virus, AMD-3100 or MVC for cells), followed by infection for 3 h and SDCM to detect intracel-lular particles. Either preincubation with sCD4 or a deletion in the CD4 binding site of gp120 abolished specific HIV-1 endocytosis, reducing uptake to a level close to the background for an Env-negative [Env(⫺)] control (Fig. 2C). Since these results indicated CD4-dependent HIV-1 endocytosis in this cell line, we analyzed whether particles in endosomes were associated with CD4. Cells were incubated with fluorescent HIV-1 as described above and were subsequently fixed, permeabilized, and stained for Env and CD4. Immunofluorescence analysis clearly re-vealed fluorescent intracellular particles that stained positive for the Env glycoprotein and were associated with a CD4 signal
(Fig. 1DtoF). Thus, endocytosis of HIV-1 in SupT1-R5 cells
depends on the CD4 receptor, and endocytosed virions appear to remain engaged with CD4.
The role of coreceptor binding was analyzed using fluorescent HIV-1 carrying an X4-tropic Env variant with a point mutation in the coreceptor binding site. Alternatively, coreceptor binding was inhibited by preincubation of cells with antagonists. Neither mu-tation of the coreceptor binding site nor treatment with the core-ceptor antagonists caused a decrease in particle endocytosis (Fig. 2D). A slight increase of internalization was observed upon AMD-3100 treatment. Effective inhibition of coreceptor binding was validated using a-lactamase reporter-based HIV-1 fusion assay (51), revealing a complete block of fusion for viruses carrying a mutation in the coreceptor binding site or with pharmacological inhibition of coreceptor binding (data not shown). Thus, corecep-tor binding is dispensable for HIV-1 endocytosis but not for cyto-plasmic entry in SupT1-R5 cells. As expected, blocking of mem-brane fusion by mutation of the fusion peptide or by treatment with the fusion inhibitor T-1249 also did not decrease HIV-1 en-docytosis in these cells but led to a slight increase of endocytosed particles in the case of T-1249 treatment (Fig. 2E).
Dyn-2 is required for HIV-1 endocytosis but dispensable for cytoplasmic entry and infection.Having established that endo-cytotic uptake of HIV-1 into SupT1-R5 cells is Env and CD4 de-pendent, we wanted to investigate its functional relevance. The most straightforward approach, previously employed to address related questions, would be to test the effect of pharmacological inhibition of clathrin-mediated endocytosis on HIV-1 cell entry and infection. Dynasore (57), pitstop-2 (22), and dyngo-4a (58) have all been described to be specific inhibitors of
clathrin-medi-ated endocytosis. Control experiments measuring inhibition of Tfn endocytosis and cytotoxic effects in parallel indicated, how-ever, that efficient inhibition of clathrin-mediated endocytosis oc-curred only at cytotoxic concentrations of these compounds in SupT1-R5 and primary CD4⫹T cells (data not shown).
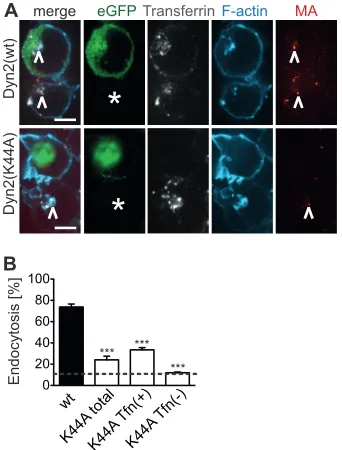
We therefore made use of a dominant negative (dn) mutant of Dyn-2 [dnDyn-2(K44A)] to block clathrin-mediated endocytosis. SupT1-R5 cells were transfected with plasmids carrying eGFP-tagged wild-type Dyn-2 [Dyn-2(wt)] or mutant Dyn-2, followed by incubation with fluorescent HIV-1 carrying wt EnvNL4-3. Tfn-Alexa 647 was added for the last 5 min of incubation to control for inhibition of endocytosis. Cells were fixed and stained for actin, and four-color z-stacks of whole cells were recorded by SDCM.
Figure 3Adisplays individual z-slices for
eGFP–Dyn-2(wt)-ex-pressing cells (top) and eGFP– dnDyn-2(K44A)-exeGFP–Dyn-2(wt)-ex-pressing cells (bottom). The eGFP signal served to identify cells expressing Dyn-2(wt) or dnDyn-2(K44A). Cells displaying a disrupted F-actin cytoskeleton were excluded from the analysis to minimize con-founding nonspecific cytotoxic effects. The intracellular Tfn-Alexa 647 signal allowed validation of the dnDyn-2(K44A) effect and stratification of dnDyn-2(K44A)-expressing cells into a pop-ulation that showed complete inhibition of Tfn uptake [Tfn(⫺)]
A
wt
K44A tota
l
K44A Tfn(+)
K44A Tfn(-) 0
20 40 60 80 100
Dyn2(wt)
Dyn2(K44A
)
*
TransferrinF-actin MA
*
merge eGFP
Endocytosis [%
]
B
*
*** *** ***
FIG 3Effect of dnDyn-2(K44A) on endocytotic uptake of HIV-1 into SupT1-R5 cells. (A) SupT1-R5 cells transiently expressing eGFP–Dyn-2(wt) (top, green) or eGFP– dnDyn-2(K44A) (bottom, green) were incubated with HIV-1MA.mCherry(red) for 3 h at 37°C, fixed, and stained with phalloidin-Dy415 (Dyomics) (cyan). Tfn-Alexa 647 (white) was added 5 min prior to fixation. z-stacks covering whole cell volumes were recorded by SDCM as described in Materials and Methods. Individualz-slices through representative cells are shown. White asterisks, untransfected cells; white arrowheads, inter-nalized HIV-1 particles. Bars, 5m. (B) Quantitation of particle uptake by endocytosis. The graph shows the percentage of eGFP–Dyn-2(wt)- or eGFP– dnDyn-2(K44A)-expressing cells carrying one or more internalized HIV-1 particles; dnDyn-2(K44A)-expressing cells were further stratified into cells displaying residual Tfn uptake [K44A Tfn(⫹)] and cells where Tfn uptake was blocked [K44A Tfn(⫺)]. Mean values and SDs for 10 randomly chosen view fields from two independent experiments (corresponding to a total of⬎50 cells) are shown.Pvalues were determined by a two-tailed Student’sttest. ***,
Pⱕ0.001. Dotted line, values obtained for particles lacking a viral Env protein.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:5.585.337.508.68.293.2]and a population that exhibited residual Tfn uptake [Tfn(⫹)]. Internalized virus particles (Fig. 3A, white arrowheads) were de-tected in both eGFP-positive cells and untransfected cells (aster-isks) but were strongly reduced in dnDyn-2(K44A)-expressing cells. Quantitative analysis revealed that⬃75% of all Dyn-2(wt)-expressing cells carried internalized HIV-1 particles (Fig. 3B), similar to the situation for untransfected SupT1-R5 cells (Fig. 2B). In contrast, endocytosed HIV-1 was detected in only⬃25% of all dnDyn-2(K44A)-expressing cells. The degree of inhibition of HIV-1 endocytosis correlated with the efficiency of inhibition of transferrin uptake: ⬃35% of dnDyn-2(K44A)-expressing cells with residual Tfn uptake exhibited intracellular HIV-1 particles, while only 12% of those classified to be negative for Tfn uptake showed intracellular HIV-1 (Fig. 3B). The latter proportion was similar to the unspecific endocytosis observed for Env(⫺) parti-cles (represented by a dashed line inFig. 3B; compareFig. 3B
and2B).
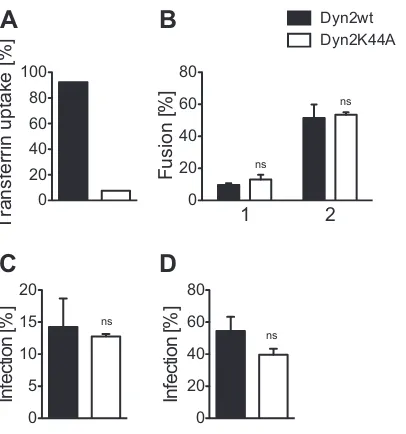
To determine the functional relevance of the observed Env-, CD4-, and Dyn-2-dependent endocytosis for cytosolic entry of HIV-1 in SupT1-R5 cells, we employed an established entry assay detecting the activity of-lactamase (BlaM) incorporated into HIV-1 particles as a Vpr.BlaM fusion protein (51). Cytosolic de-livery of active BlaM upon virus-cell fusion can be detected by a shift in the emission maximum of a fluorogenic BlaM substrate following cleavage. Since eGFP fluorescence would interfere with this readout, mCherry-tagged fusions of Dyn-2(wt) and dnDyn-2(K44A) were used for these experiments. Inhibition of endocy-tosis was verified by incubation of mCherry–Dyn-2-expressing cells with Tfn-Alexa 488. Expression of mCherry– dnDyn-2(K44A) sig-nificantly reduced transferrin uptake to⬃8% of the control level, while expression of mCherry–Dyn-2(wt) had no effect (Fig. 4A). Cells expressing either mCherry–Dyn-2(wt) or mCherry– dnDyn-2(K44A) were incubated for 3 h with different amounts of HIV-1BlaMreporter particles, followed by CCF2 staining and multi-color flow cytometry. Quantitative evaluation of three independent experiments performed with two different reporter virus prepara-tions showed no significant effect of dnDyn-2(K44A)-mediated inhi-bition of endocytosis on the proportion of entry-positive cells (Fig. 4B). Similar entry rates were observed for the Dyn-2(wt)- and dn-Dyn-2(K44A)-positive populations, independent of the virus dose (from 2.5% to 80% BlaM-positive cells;Fig. 4Band data not shown). Thus, Dyn-2-dependent HIV-1 endocytosis is dispensable for HIV-1 fusion and cytosolic entry in SupT1-R5 cells.
To analyze whether expression of Dyn-2(wt) or dnDyn-2(K44A) affects productive HIV-1 infection, we infected SupT1-R5 cells previously transfected with the respective expres-sion vector with HIV-1NL4-3(Fig. 4C). Infectivity was scored by flow cytometry, determining the proportion of CA-positive cells in the Dyn-2-mCherry-positive (and thus transfected) popula-tion. Cell viability was monitored in parallel by 7-amino-actino-mycin D staining, which showed no significant difference between Dyn-2(wt)- and dnDyn-2(K44A)-expressing cells (data not shown). Quantitative analysis of three independent infection ex-periments, each performed with several different virus prepara-tions, revealed no significant difference in HIV-1 infection be-tween cells expressing dominant negative Dyn-2 and control cells
(Fig. 4C). Similar results were observed for HIV-1 pseudotyped
with the G glycoprotein of vesicular stomatitis virus (VSV-G) (Fig. 4D). The latter result appears to be counterintuitive, since pseu-dotyping with VSV-G is suggested to reroute viral entry to the
endocytotic pathway. No effect of dnDyn-2(K44A) on infection by VSV-G-pseudotyped HIV-1 was also observed in our previous work (21), and other studies reported a marginal 2- to 3-fold reduction of infectivity of VSV-G pseudotypes by dnDyn-2(K44A) (59,60). Accordingly, Dyn-2 appears to be largely dis-pensable for the entry of VSV-G-pseudotyped HIV-1.
Productive HIV-1 entry occurs primarily at the plasma membrane in SupT1-R5, CEM-ss, and primary CD4ⴙT cells. The fact that Dyn-2-dependent endosomal uptake of HIV-1 could be blocked without any effect on cytoplasmic entry or infection suggested that endocytosis is dispensable for productive HIV-1 entry in SupT1-R5 cells. However, entry via Dyn-2-independent endocytosis was not excluded by our experiments, and the results observed for VSV-G pseudotypes also suggested alternative path-ways. To directly determine the contribution of the plasma mem-brane versus endosomal fusion to HIV-1 infection in different cells, we made use of the differential temperature requirements of endocytosis and HIV-1 Env-mediated fusion. Previous studies have shown that HIV-1 fusion is virtually abolished at tempera-tures below 23°C (61,62), while endocytosis is retained at temper-atures above 16°C (63,64). Incubation of HIV-1 particles with
0 5 10 15 20
0 20 40 60 80
B
C
Dyn2wt Dyn2K44A
D
0 20 40 60 80 100
0 20 40 60 80
Transferrin uptake [%]
Fu
s
io
n
[%
]
Infection [%
]
Infection [%
]
ns
ns
ns
ns
A
1 2
FIG 4Effect of dnDyn-2(K44A) on HIV-1 cytoplasmic entry and infectivity. SupT1-R5 cells transiently expressing mCherry–Dyn-2(wt) or mCherry– dn-Dyn-2(K44A) were tested for Tfn uptake (A), HIV-1 cell entry (B), and HIV-1 infection (C, D). Analysis was performed by multicolor flow cytometry. (A) Cells were incubated with Tfn-Alexa 488 for 7 min prior to acid wash and fixation, as described in Materials and Methods. Bars indicate the proportion of mCherry-expressing cells positive for Tfn-Alexa 488. (B) Cytoplasmic entry by membrane fusion. Cells were incubated with BlaM reporter virions for 5 h. Virus entry in individual cells was detected using a Förster resonance energy transfer-based-lactamase assay, as described in Materials and Methods. Bars indicate the proportion of mCherry-expressing cells that displayed virus entry for two independent experiments applying low (panel 1) or high (panel 2) virus doses corresponding to estimated fusion levels of 10% and 50%, respec-tively, as determined by titration. (C, D) Productive infection. Cells were in-fected with HIV-1NL4-3(C) or HIV-1 pseudotyped with VSV glycoprotein G (D) and fixed. Infection was scored by staining for intracellular CA. Bars indi-cate the proportion of mCherry-expressing cells positive for virus infection from one representative experiment performed in triplicate. For all experi-ments, mean values and SDs are shown.Pvalues were determined by a two-tailed Student’sttest. ns, not significant.
HIV-1 Entry at the Plasma Membrane
on November 7, 2019 by guest
http://jvi.asm.org/
[image:6.585.317.520.64.281.2]target cells for several hours at temperatures below 23°C leads to a so-called temperature-arrested state (TAS), where virions remain susceptible to inhibition by coreceptor antagonists and do not undergo membrane fusion (61,62).
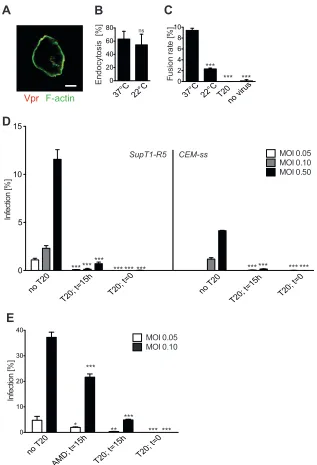
First, we investigated whether HIV-1 endocytosis occurred at 22°C. Incubating SupT1-R5 cells with fluorescent HIV-1 for 5 h at 22°C or 37°C revealed intracellular particles in both cases, but endocytosis was reduced 3-fold at the lower temperature (Fig. 5A
andB). Similar results were obtained for Tfn, which showed a 2-fold reduction in uptake after incubation for 1 h at 22°C com-pared to the level of uptake after incubation at 37°C (data not shown). Analysis of the temperature dependence of HIV-1 fusion performed in parallel confirmed that incubation for 5 h at 22°C did not permit fusion, while efficient cell entry was observed at 37°C (Fig. 5C). Based on these observations, we developed an experimental approach (Fig. 5D) where SupT1-R5 cells were in-cubated with HIV-1 for 5 h at 22°C to allow endocytosis without fusion. During the last hour of incubation at 22°C, a high concen-tration of the non-cell-permeant (65) peptidic fusion inhibitor T-20 was added. Subsequently, the temperature was switched to 37°C. T-20 does not reach previously established endosomal com-partments (66,67) and can thus inhibit only the fusion of virus particles remaining surface accessible up to the time of T-20 ad-dition. A lack of cell entry of T-20 was confirmed microscopically for a T-20 peptide labeled with Atto488 at its C terminus using an equally labeled HIV-1 Tat peptide as a control (data not shown). The proportion of HIV-1 entry and infection resistant to T-20 addition after prior incubation of cells and virus without T-20 at 22°C should thus reveal the contribution of endosomal uptake to HIV-1 infection if the T-20 concentration is sufficient to block all cell surface entry. In contrast, the small-molecule drugs AMD-3100 and MVC should also reach previously endocytosed viruses and should thus inhibit all HIV-1 entry events.
SupT1-R5 cells were incubated at 22°C with the X4-tropic HIV-1NL4-3or with an R5-tropic derivative, followed by addition of inhibitor and a temperature shift as depicted in the schematic in
Fig. 5D. Medium was changed 5 h after the temperature shift, and
T-20 was added to all samples at this time to prevent multiple rounds of infection. Cells were fixed and stained for CA at 48 h after the temperature shift. Approximately 8% of cells were in-fected by either the X4- or the R5-tropic HIV-1 variant under these conditions, and infection by both variants was completely abrogated when T-20 was added from the beginning (Fig. 5Eand
F). Importantly, the same negative result was obtained when T-20 was added 4 h after incubation at 22°C. No residual infectivity was detected, although significant endocytosis of fluorescent HIV-1 occurred at this temperature (Fig. 5B,E, andF). Incomplete inhi-bition was observed for the coreceptor antagonists when infection was with a virus targeting the respective coreceptor (Fig. 5Eand
F). This could be due to insufficient drug concentrations but is also consistent with the observation that a subset of viral particles in the TAS becomes resistant to coreceptor antagonists over time (62).
The full sensitivity of HIV-1 infection to T-20 inhibition after prior incubation of cells and virus at 22°C for 4 h in the absence of T-20 suggested that endocytosis may not contribute to productive fusion in this cell line. Alternatively, endocytosed HIV-1 particles may have been inactivated by the low endosomal pH over time, thus obscuring their potential for productive entry. To determine whether a lack of infection was due to exposure to a low pH, the
experiment described above was repeated in the presence or ab-sence of compounds preventing endosomal acidification. SupT1-R5 cells were infected with HIV-1 in the presence or ab-sence of ammonium chloride or bafilomycin A1. While bafilomy-cin A1 reduced infection by a factor of⬃2, ammonium chloride did not affect productive infection at 37°C (Fig. 5G, left), consis-tent with the fact that HIV-1 entry is pH insensitive (13,14). Incubation with HIV-1 at 22°C in the presence or absence of these compounds, followed by addition of T-20 and a temperature shift, also revealed no difference (Fig. 5F), indicating that acid exposure cannot explain the lack of infection of SupT1-R5 cells by previ-ously endocytosed HIV-1.
To investigate whether the HIV-1 entry pathway depends on the T cell line used, we performed similar experiments in CEM-ss cells. This T cell line had been used in a previous study that sug-gested exclusive HIV-1 entry via endocytosis, with plasma mem-brane entry apparently blocked at the stage of hemifusion (23). Incubation of CEM-ss cells with HIV-1NL4-3for 4 h at 22°C, fol-lowed by addition of T-20 and a temperature shift (as depicted schematically inFig. 5D), again led to an almost complete loss of infection (Fig. 5G, right), indicating that endocytosis is also dis-pensable for productive HIV-1 entry in CEM-ss cells. As was ob-served for SupT1-R5 cells, addition of ammonium chloride had no effect on HIV-1 infectivity and did not restore infection in CEM-ss cells treated with T-20 after preincubation with virus at 22°C (Fig. 5G, right).
The observation that HIV-1 infection was completely lost, while fluorescent particle endocytosis was reduced only 3-fold, indicated that endocytosis may not contribute significantly to HIV-1 infection. These results do not rule out a threshold effect, however, where a certain amount of endocytosis could be needed to score positive in the infection assay and the reduction of particle uptake would not linearly correlate with infection. To address this point, we extended the total period of incubation at 22°C from 5 h to 16 h. Endocytosis of fluorescent HIV-1 in SupT1-R5 cells was similar at 37°C and 22°C for this longer incubation time (Fig. 6A
andB). Incubation for 16 h at 22°C led to a significant increase in HIV-1 fusion compared to that with 5 h of incubation (compare
Fig. 5Cand6C), indicating that the TAS is not stable over time and
may thus be better called a temperature-delayed state. Impor-tantly, the amount of fusion at 22°C indicated inFig. 6C consti-tutes the sum of all fusion events of particles that escaped TAS during the 16-h period before the temperature shift, irrespective of their subcellular localization. This number of fusion events thus provides a baseline for the events that were induced by the tem-perature shift in the following infection experiment.
The contribution of endocytosis to productive HIV-1 infection was determined by incubating SupT1-R5 cells (Fig. 6D, left) or CEM-ss cells (Fig. 6D, right) with different concentrations of HIV-1 for a total of 16 h at 22°C. T-20 was added after 15 h, and the temperature was shifted to 37°C after one additional hour at 22°C (see the schematic inFig. 5D). Addition of T-20 from the start of the experiment completely abrogated infectivity in all cases, while addition of T-20 after 15 h of incubation at 22°C reduced infectivity to a residual level of⬍10% of that for the respective control (Fig. 6DandE). This phenotype was indepen-dent of the infectious dose. Infectivity was reduced only 2-fold or less upon addition of AMD-3100 after 15 h at 22°C, indicating that ⬎50% of HIV-1 particles had already proceeded from TAS to a coreceptor-engaged state during this period (Fig. 6E). This result
on November 7, 2019 by guest
http://jvi.asm.org/
FIG 5Contribution of internalized HIV-1 to productive infection after preincubation at a temperature not permissive for fusion. (A, B) Quantitation of particle uptake. Cells were incubated with infectious HIV-1eGFP.Vprparticles (red) at a multiplicity of infection of 0.3 for 5 h at the indicated temperature, fixed, and stained with phalloidin-Dy415 (Dyomics) (green). z-stacks covering whole cell volumes were recorded by SDCM, and particle uptake was quantitated as described in the legend to Fig. 1. (A) Individual z-slice through a representative SupT1-R5 cell. Bar, 5m. (B) Proportion of SupT1-R5 cells containing one or more internalized particles. More than 50 cells, corresponding to 15 randomly selected view fields, were analyzed for each condition. (C) Cytoplasmic entry by membrane fusion. SupT1-R5 cells were incubated for 5 h with BlaM reporter virions at the indicated temperature in the presence or absence of T-20 and analyzed using the BlaM assay. Bars indicate the proportion of entry-positive cells from one representative experiment performed in triplicate. (D to G) Contribution of endocytotic uptake to HIV-1 infectivity in SupT1-R5 and CEM-ss cells. (D) Schematic illustration of the temperature shift experiment. See the text for details. PM, plasma membrane; t, time. (E, F) Effect of entry inhibitors added after incubation at 22°C prior to a shift to 37°C. SupT1-R5 cells were incubated with HIV-1NL4-3(E) or HIV-1NL4-3R5(F) at a multiplicity of infection of 0.1 for 4 h prior to addition of AMD-3100 (AMD), MVC, or T-20. Cells were further incubated at 22°C for one more hour and subsequently shifted to 37°C. Samples in which T-20 was present throughout the experiment (T-20, time zero [t⫽0]) were included as controls. Infection was scored by immunostaining for intracellular CA followed by flow cytometry. Bars indicate the results for infected cells from one representative experiment performed in triplicate. (G) Effect of endosomal acidification inhibitors on infection. SupT1-R5 cells (left) or CEM-ss cells (right) were preincubated with NH4Cl, bafilomycin A1, or dimethyl sulfoxide (DMSO) for 1 h, as indicated. Subsequently, HIV-1NL4-3was added at a multiplicity of infection of 0.2 in the continued presence of acidification inhibitors for 4 h prior to addition of T-20. Samples in which T-20 was present throughout the experiment (T-20, time zero) were included as controls. Infection was scored by immunostaining for intracellular CA and flow cytometry. Bars indicate the results of virus infection from one representative experiment performed in triplicate. For all experiments, mean values and SDs are shown.
Pvalues were determined by a two-tailed Student’sttest. *,Pⱕ0.05; **,Pⱕ0.01; ***,Pⱕ0.001.
on November 7, 2019 by guest
http://jvi.asm.org/
also suggested that HIV-1 infectivity was not severely affected by the extended preincubation. The infection events observed fol-lowing addition of T-20 after a 15-h preincubation at 22°C and a subsequent temperature shift represent the sum of particle fusions that occurred during the low-temperature incubation period
(fu-sion both from the plasma membrane and from the endosome) and of fusion events from previously endocytosed HIV-1 particles after the temperature shift. Infectivity after preincubation at the lower temperature, T-20 addition, and the subsequent tempera-ture shift was reduced⬎10-fold (Fig. 6DandE), whereas fusion 0
5 10 15
MOI 0.05 MOI 0.10 MOI 0.50
no T20
T20; t=15h T20; t= 0
no T2 0
T20; t=15 h
T20; t= 0
SupT1-R5 CEM-ss
*** *** ***
*** *** *** *** *** *** *** 0
20 40 60 80
0 2 4 6 8 10
A
C
D
***
*** ***
F
u
si
o
n
ra
te
[%
]
Endocytosis [%]
37° C
22° C
37°C 22° C
T20 no viru
s
Vpr F-actin
ns
Infection [%
]
0 10 20 30 40
no T2 0
AMD; t=15h T20; t=15h
T20; t=0
Infection [%
]
E
*** ***
*
***
** *** MOI 0.05 MOI 0.10
B
FIG 6Contribution of internalized HIV-1 to productive infection after prolonged preincubation at a temperature not permissive for fusion. (A, B) Quantitation of particle uptake. SupT1-R5 cells were incubated with infectious HIV-1eGFP.Vpr(red) at a multiplicity of infection of 0.3 for 16 h at the indicated temperature, fixed, and stained with phalloidin-Dy415 (Dyomics) (green). z-stacks covering whole cell volumes were recorded by SDCM, and particle uptake was quantitated as described in the legend toFig. 1. (A) Individual z-slice through a representative SupT1-R5 cell. Bar, 5m. (B) Proportion of SupT1-R5 cells containing one or more internalized particles. Seventy-five cells from 18 randomly selected view fields were analyzed for each condition. (C) Cytoplasmic entry by membrane fusion. SupT1-R5 cells were incubated for 16 h with BlaM reporter virions at the indicated temperature in the presence or absence of T-20 and analyzed using the BlaM assay. Bars indicate the proportion of entry-positive cells from one representative experiment performed in triplicate. (D, E) Contribution of endocytotic uptake to HIV-1 infectivity. SupT1-R5 cells (D, left, and E) or CEM-ss cells (D, right) were incubated with HIV-1NL4-3at the indicated multiplicity of infection (MOI) for 15 h at 22°C prior to addition of AMD-3100 (AMD) or T-20, as indicated. Cells were further incubated at 22°C for one more hour prior to a shift to 37°C. Samples in which T-20 was present throughout the experiment (T-20; time zero) were included as controls. Infection was scored by immunostaining for intracellular CA and flow cytometry. Bars indicate the level of virus infection from one representative experiment performed in triplicate. For all experiments, mean values and SDs are shown.Pvalues were determined by a two-tailed Student’sttest. ns, not significant; *,Pⱕ0.05; **,Pⱕ0.01; ***,Pⱕ0.001.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:9.585.134.451.64.529.2]during the 16 h of incubation at 22°C was reduced only⬃5-fold
(Fig. 6C). Thus, cytoplasmic entry during the period of
preincu-bation could completely explain the residual infectivity.
While T-cell lines recapitulate many features of HIV-1 target cells, it is important to validate the findings in natural target cells of HIV-1. We thus performed similar experiments in primary hu-man CD4⫹T cells. Cells from three different donors were either infected with HIV-1NL4-3, incubated with HIV-1BlaMat 37°C in the presence or absence of T-20, or incubated with the virus for 4 h or 15 h at 22°C, followed by addition of T-20 and a temperature shift after one more hour at 22°C. Viral entry by membrane fusion was almost completely abolished when T-20 was added at the time of virus addition or 4 h after addition of virus and was reduced to⬍20% of the level for the control when particles were allowed to enter at 22°C for 15 h, followed by addition of T-20 (Fig. 7AandC). Similar observations were made for productive HIV-1 infection. Addition of T-20 at the time of infection abolished HIV-1 infectivity, and addi-tion of T-20 after 4 h or 15 h of incubaaddi-tion at 22°C reduced HIV-1 infection by a factor of 30 (Fig. 7BandD). Notably, the reduction of infectivity was even more pronounced than the reduction of mem-brane fusion in this case. No difference in infectivity was observed when cells were infected in the presence or absence of ammonium chloride, excluding a detrimental effect of prolonged exposure to low pH of endosomally entering viruses (Fig. 7E).
Besides cell-type dependent differences, HIV-1 entry may also be influenced by the viral Env proteins, and we thus expanded our analyses beyond the commonly used laboratory-adapted strain HIV-1NL43. To this end, we produced HIV-1 pseudotypes by cotransfection of anenv-defective proviral plasmid with expres-sion vectors carryingenvgenes directly amplified from the plasma of well-characterized HIV-1-infected patients. These primaryenv
sequences give rise to highly T-cell-tropic viruses (requiring high CD4 surface levels on the target cell), circulate in patients at high levels, and are thought to be the predominant species mediating interindividual HIV transmission. Hence, they should better re-flect the contribution of endocytosis to HIV infection that occurs under physiological rather than laboratory-adapted conditions. To determine a potential difference dependent on coreceptor us-age, we performed these experiments with two well-characterized Env proteins: the X4-tropic Env5002 and the R5-tropic Env4051 (46). HIV-1BlaM particles pseudotyped with either Env5002 or Env4051were incubated with primary CD4⫹T cells from two dif-ferent donors for difdif-ferent periods of time in the presence or ab-sence of T-20. Addition of T-20 from the beginning abrogated membrane fusion and infection in all cases (Fig. 7FtoI). Mem-brane fusion and viral infectivity were also reduced to background levels for both Env pseudotypes when T-20 was added after virus and cells had been incubated for 4 h at 22°C (Fig. 7FandG). These results indicate that endocytosis is also dispensable for infection of primary CD4⫹T cells with HIV-1 particles carrying primary T-cell-tropic Env proteins using either CXCR4 or CCR5 as the core-ceptor. Membrane fusion was reduced only 3-fold compared to the level of fusion in the control incubation at 37°C when virus and cells were incubated for 15 h at 22°C (Fig. 7H), indicating that the TAS is much less tight for these primary envelopes. Impor-tantly, however, addition of T-20 after incubation for 15 h at 22°C of primary CD4⫹T cells with HIV-1 pseudotyped with either Env5002or Env4051almost completely abrogated infectivity (Fig.
7I), indicating that entry from endosomal compartments is
dis-pensable for productive infection of HIV-1 carrying primary en-velope proteins.
DISCUSSION
The site of HIV-1 entry has remained controversial, despite nu-merous studies over the past 25 years, and evidence for both ex-clusive plasma membrane fusion and exex-clusive endosomal fusion has been reported. Here, we show Env- and CD4-dependent, core-ceptor-independent endocytosis of HIV-1 via a Dyn-2-dependent pathway in SupT1-R5 cells. Blocking this entry route had no effect either on cytosolic delivery of a virion-associated reporter enzyme (as an indicator of fusion) or on HIV-1 infection, however. Furthermore, allowing endocytosis for extended periods of time at a temperature where HIV-1 fusion cannot occur, followed by addition of a cell-impermeant, peptidic fusion inhibitor and a shift to a fusion-permis-sive temperature, indicated that endocytotic entry contributes little, if anything, to HIV-1 infection in this cell line. The same result was observed for the T-cell line CEM-ss, which was previously reported to exhibit exclusively endosomal entry of HIV-1 (23). Furthermore, en-docytotic entry was also dispensable for HIV-1 infection of primary CD4⫹T cells, independently of whether laboratory-adapted or pri-mary envelopes were used.
Expression of dnDyn-2(K44A) in HeLa-derived reporter cells had previously been shown to cause a 50 to 80% decrease in HIV-1 entry (21), while we observed no such reduction, despite an al-most complete block of Tfn uptake, in SupT1-R5 cells. HIV-1 endocytosis was abrogated by dnDyn-2(K44A) in this cell line, indicating that neither Dyn-2-dependent nor Dyn-2-independent endocytosis contributed significantly to cytoplasmic entry and productive HIV-1 infection. Unaltered HIV-1 fusion and infec-tion, despite a block of Tfn uptake and virus endocytosis, clearly showed that fusion from within the endosome is not necessary for cytosolic entry of and infection by HIV-1. This finding does not exclude the possibility of a contribution of this route to productive viral entry, however. Such a contribution could be supported by the observed trend toward a higher number of endosome-associ-ated virions when fusion was blocked. Conceivably, viral fusion may occur either at the cell membrane or from within the endo-some, and the relative contribution of either route could be influ-enced by the endocytotic activity of the respective cell and the fusogenicity of the respective virus (26). Thus, the same virus could fuse primarily at the plasma membrane if endocytosis rates were low or from within the endosome when endocytosis activity would be high. Blocking endocytotic entry by dnDyn-2(K44A) would then shift HIV-1 fusion entirely to the plasma membrane, while endocytotic entry could still contribute to productive entry under normal conditions. From this part of the study, we there-fore conclude that endocytosis is not required for HIV-1 entry in SupT1-R5 cells and infection is equally efficient in the absence of HIV-1 endocytosis.
The conclusion that endocytosis is not only dispensable for HIV-1 entry and infection but also contributes little, if anything, to these processes in SupT1-R5, CEM-ss, and CD4⫹primary T cells is based on a second set of experiments. Making use of the different temperature dependences of HIV-1 fusion and endocy-tosis, we showed that virus particles endocytosed at a temperature that is not permissive for HIV-1 membrane fusion contribute little to viral entry and infection upon a subsequent shift to a fusion-permissive temperature. The peptidic fusion inhibitor T-20 was added to the culture prior to the temperature shift, thus blocking HIV-1 Entry at the Plasma Membrane
on November 7, 2019 by guest
http://jvi.asm.org/
0 5 10 15 20 25
Fu
s
io
n
[%
]
*** *** 37°C 22°
C T20
0 2 4 6
Infection [%
]
*** ***
no T2 0
T20; t=15 h
T20; t=0
no T2 0
T20; t=15h T20; t= 0
0 2 4 6 8
0 2 4 6
0 1 2
*** *** 37°
C
22°C T20
*** ***
no T2 0
T20; t=4hT20; t= 0
Fu
s
io
n
[%
]
Infection [%
]
Infection [%
]
*** *** *** ***
E
0 1 2
0 1 2 3
0 1 2
0 1 2
0 1 2 3
0 2 4 6
0 1 2 0 1 2
no T20
T20; t=4h T20; t= 0 37°C 22°
C
T20
no T20
T20; t=15h T20; t= 0 37°
C
22°C T2 0
5002 4051
Fu
s
io
n
[%
]
Fu
s
io
n
[%
]
Infection [%
]
Infection [%
]
F
*** *** *** ***
*** *** *** ***
*** ***
*** ***
***
*** *** ***
*** *** *** ***
*** *** *** ***
*** *** *** ***
*** *** *** ***
G
H
NL4-3 NL4-3 + NH4Cl
I
FIG 7Contribution of internalized HIV-1 to productive infection of primary human CD4⫹T cells. Data for primary CD4⫹T cells from five different donors are shown. (A, B) Donor 1; (C, D) donor 2; (E) donor 3; (F to I, top) donor 4; (F to I, bottom) donor 5. (A, C). Cytoplasmic entry by membrane fusion of wild-type HIV-1NL4-3. Cells were incubated for either 5 h (A) or 16 h (C) with BlaM reporter virions at the indicated temperature in the presence or absence of T-20 and analyzed using the BlaM assay. Bars indicate the proportion of entry-positive cells from one representative experiment performed in triplicate. (B, D) Contri-bution of endocytotic uptake to HIV-1NL4-3infectivity in primary CD4⫹T cells. Cells were incubated with virus at a multiplicity of infection of 1 for 15 h at 22°C prior to addition of T-20. Cells were incubated for one more hour at 22°C prior to a shift to 37°C. Samples in which T-20 was present throughout the experiment (T-20; time zero) were included as controls. Infection was scored by immunostaining for intracellular CA and flow cytometry. (E) Effect of ammonium chloride on HIV-1 infection in primary CD4⫹T cells. Cells were incubated with HIV-1NL4-3in the presence or absence of 10 mM NH4Cl at a multiplicity of infection of 0.2 for 15 h at 22°C prior to addition of T-20. Cells were further incubated at 22°C for one more hour prior to a shift to 37°C. Samples in which T-20 was present throughout the experiment (T-20, time zero) were included as controls. Infection was scored by immunostaining for intracellular CA and flow cytometry. (F, H) Cytoplasmic entry by membrane fusion of HIV-1 particles pseudotyped with envelope glycoproteins directly derived from infected patients. Cells were incubated for either 5 h (F) or 16 h (H) with BlaM reporter virions pseudotyped with either the X4 T-cell-tropic Env5002or the R5-tropic Env4051at the indicated temperature in the presence or absence of T-20 and analyzed using the BlaM fusion assay. (Top and bottom) Infection of cells from two different donors, respectively, that were analyzed in parallel. Bars indicate the proportion of entry-positive cells from one representative experiment performed in triplicate. (G, I) Contribution of endocytotic uptake to infectivity of HIV-1 pseudotyped with envelope glycoproteins directly derived from HIV-infected patients in primary CD4⫹T cells. Cells were incubated at 22°C with NL4-3Env5002or NL4-3Env4051at a multiplicity of infection of 1 for either 4 h (G) or 15 h (I) prior to addition of T-20. Cells were incubated for one more hour at 22°C prior to a shift to 37°C. Samples in which T-20 was present throughout the experiment (T-20, time zero) were included as controls. Infection was scored by immunostaining for intracellular CA and flow cytometry. For all experiments, mean values and SDs are shown.
Pvalues were determined by a two-tailed Student’sttest. ***,Pⱕ0.001.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:11.585.120.468.33.546.2]all subsequent fusion events by cell surface-accessible virions. T-20 can bind to the outer leaflet of the plasma membrane but is not internalized and thus does not reach preformed endosomes (66,67). HIV-1 fusion and infection were largely or completely abrogated when T-20 was added after prolonged preincubation of virus and cells at the lower temperature, despite the presence of many previously internalized HIV-1 particles that were no longer susceptible to T-20. A lack of endosomal fusion in this case cannot be attributed to reduced endocytosis—as would be possible for dnDyn-2(K44A)—since equal amounts of particles were found in the endosomal compartment when incubation was performed at either temperature for 16 h and internalization was reduced only 3-fold after 5 h of incubation at 22°C compared to the level after incubation at 37°C. HIV-1 entry and infection were completely abrogated, however, when T-20 was added after incubation for 4 h at 22°C and a subsequent temperature shift. We conclude, there-fore, that the previously endocytosed particles contribute little to productive HIV-1 entry under these conditions. A loss of infectiv-ity due to prolonged exposure to the low pH in the endosomal pathway would be an alternative explanation for this result, but this possibility was excluded after the same experiment was per-formed in the presence of compounds that block endosomal acid-ification. Theoretically, endosomal HIV-1 entry could also be blocked if subsequently added T-20 could reach preformed endo-somes containing HIV-1 particles. This would require the transfer of T-20 across the plasma membrane and uptake into a preformed endosomal compartment, but these actions are not compatible with previous observations that T-20 is an extracellular peptide that is incapable of crossing a lipid bilayer (68) and that needs to be present at the time of virus addition to exhibit activity (23,65–67). Furthermore, our own experiments with fluorescently labeled T-20 also showed no cellular uptake of this highly negatively charged polypeptide.
Our observations are clearly incompatible with the suggested exclusive endosomal entry of HIV-1 reported in another study (23). These authors used double-labeled HIV-1 derivatives in an imaging-based approach and reported productive fusion to occur exclusively from an endosomal route, with plasma membrane-attached virus being arrested at the stage of hemifusion. Hemifu-sion would not be sufficient for cytosolic entry of a reporter en-zyme or for productive infection, however, and we thus conclude that complete fusion has occurred in our system. A major differ-ence between the current study and most previous reports is the cell type used. Most of the previous studies have been performed in adherent cell lines mostly derived from HeLa and HOS cells, but studies have also been performed in U937 cells (19–21). Blocking endocytosis in these cell types consistently led to a reduction of HIV-1 entry and infection, as reported by different groups (19– 21), and endocytosis thus appears to yield productive HIV-1 entry and infection in these reporter cell lines. Considering that CD4⫹T cells are the predominant target for HIV-1in vivo, the plasma membrane fusion observed in the current study should more ac-curately reflect the situation in HIV-1-infected patients, however, at least for transmission of cell-free virus. The two previous studies reporting HIV-1 entry and infection via the endosomal route in a T cell line used CEM (or CEM-ss) cells (18,23), and we therefore included CEM-ss cells in our analysis as well. No difference be-tween SupT1-R5 and CEM-ss cells was observed, however, and our results are thus inconsistent with the reported predominant or exclusive endosomal entry of HIV-1 in these cells. Moreover, our
results were very similar for infection of primary human T cells with HIV-1 carrying either laboratory-adapted or primary enve-lopes, clearly supporting the plasma membrane as the predomi-nant entry site in relevant target cells, at least in tissue culture.
Current techniques do not allow the number of fusion events on a per cell basis to be directly correlated with the number of endocytosed particles. Accordingly, a potential (minor) contribu-tion of endocytosis to HIV-1 entry could not be quantitatively evaluated. Nevertheless, it is counterintuitive that easily detectable Env- and CD4-dependent endocytosis via a Dyn-2-dependent pathway, with endosomal HIV-1 particles apparently retaining receptor engagement, contributes so little to productive HIV-1 entry. Many viruses enter cells via endocytosis, and this is gener-ally believed to have two advantages: (i) endosomal acidification can be used as a pH cue for triggering the viral fusion protein, and (ii) uptake via the endosome overcomes the barrier of cortical actin and brings the incoming virus closer to the cell center and the nuclear membrane. The first aspect is clearly not relevant for HIV-1 since viral entry is pH independent. The second aspect could be advantageous since T cells have a tight cortical cytoskel-eton and the viral capsid, or components thereof, needs to even-tually reach the nuclear membrane. However, the results obtained here indicate that incoming HIV-1 can efficiently overcome the cortical cytoskeletal barrier without the need for endocytosis. This conclusion is compatible with studies reporting that HIV-1 may employ Env-induced signaling to overcome an actin-based entry restriction (69,70). Furthermore, reverse transcription occurs in the cytoplasm and peaks⬃8 h after virus infection in T cell lines (71), indicating that there is no need for the rapid translocation of incoming particles to the nuclear pore. While these aspects may explain why HIV-1 endocytosis is not needed, it is still surprising that receptor-engaged HIV-1 in the endosome does not signifi-cantly contribute to productive entry. A possible explanation would be inhibition of fusion for HIV-1 particles entering via receptor-mediated endocytosis by a cellular restriction mecha-nism in T cells. Cellular membrane proteins, including interferon-inducible transmembrane protein (IFITM-1), IFITM-2, and IF-ITM-3, have been reported to interfere with viral fusion from the endosome for other viruses (72–74), and it will be of interest to determine the presence and potential function of these proteins at the membrane of HIV-1-containing endosomes in T-cell lines. Cell type-dependent recruitment of such fusion inhibitors could explain the difference in results observed in the present study with T-cell lines and primary T cells and those observed in previous studies with reporter cell lines. One should also emphasize that the current report analyzed HIV-1 infection by cell-free virus, and a contribution of endocytotic uptake to HIV-1 transmission by cell-to-cell spread (75) remains possible.
In summary, the results shown here clearly identify the plasma membrane to be the predominant portal of entry for cell-free HIV-1 infection of T-cell lines and primary CD4⫹T cells. We suggest that different results observed in previous studies (19–21,
23) may largely be explained by the use of adherent reporter cell lines.
ACKNOWLEDGMENTS
We acknowledge Ulrike Engel and the Nikon Imaging Center Heidelberg for the microscopes used in this study, image deconvolution, and advice. We are grateful for the technical support by Steffen Schmitt and the Flow Cytometry Core Facility of the German Cancer Research Center. We HIV-1 Entry at the Plasma Membrane
on November 7, 2019 by guest
http://jvi.asm.org/
thank Monika Langlotz for assistance at the Cell Sorting Facility of the Center for Molecular Biology Heidelberg. We thank Oliver Keppler for scientific advice and technical support. We tha