Vol. 33, No. 1 JOURNAL OFVIROLOGY,Jan.1980,p.152-166
0022-538X/80/01-0152/15$02.00/0
Protein-Protein
Interactions Within Paramyxoviruses
Identified
by
Native
Disulfide
Bonding
or
Reversible Chemical
Cross-Linking
MARY ANN K. MARKWELL* ANDC. FRED FOX
Departmentof Microbiologyand the Parvin Cancer ResearchLaboratories, Molecular Biology Institute,
University
of California,
LosAngeles,
California
90024Analysis of native disulfide-bonded protein oligomers in paramyxoviruses showed thatsomeviral proteinsareconsistentlypresent as covalent complexes. InisolatedSendai virus the hemagglutinating proteinHN is present in homodi-meric and homotetrahomodi-mericforms, and the minor nucleocapsid protein P exists
partlyas amonomerandpartlyas adisulfide-linkedhomotrimer. Similar disul-fide-linkedcomplexeswereobserved in Newcastle disease virus (strainHP-16), in which HNexistsas ahomodimnerandsomeof the majornucleocapsid proteinNP existsas ahomotrimer. Noncovalent intermolecular interactionsbetween proteins were studied with the reversible chemical cross-linkers dimethyl-3,3'-dithiobis-propionimidate and methyl
3-[(p-azidophenyl)dithio]propionimidate,
which con-tain disulfide bridgesand a 1.1-nmseparation between their functional groups. Thesameresultswereachieved with bothreagents.The conditions ofpreparation, isolation, and storage of the viruses affected the protein-protein interactions observed upon cross-linking. Homooligomers ofthe glycoprotein F, the matrix protein M, and the major nucleocapsidprotein NP were produced in both Sendai and Newcastle disease viruses after mild cross-linking of all viralpreparations examined, but NP-M heterodimner formation in both viruseswas mostprevalent in early harvest preparations that were cross-linked soon after isolation. TheabilityofNPand Mtoformaheterodimneruponcross-linkingindicatesthat the matrixproteinlayerlies in closeproximity (within1.1nm)tothenucleocapsidin the newly formedvirion. Some noncovalent intermolecularproteininteractions inSendai and Newcastle disease viruses, i.e., those leadingtotheformationof F, NP, and Mhomooligomers uponcross-linking, are more stabletovirus storage thanothers, i.e., thoseleading tothe formation ofan NP-M heterodimerupon
cross-linking. The storage-induced loss of the ability of NP and M to form a heterodimer isnotaccompanied byany apparentloss ofinfectivity.Thisindicates thatsomespacial relationshipswhich formduringvirusassemblycanalter after
particleformation andare not essential for theensuing stagesof the infectious process.
Sendai virus and Newcastle disease virus (NDV), members of the family
Paramyxoviri-dae,arecomposedofan outerlipoprotein
enve-lope and aninternal helical nucleocapsid. The
envelopewhich consistsofalipid bilayer,
exter-nal glycoprotein spikes, and an inner layer of carbohydrate-free Mprotein is acquired by the
virusesin theprocessofbuddingfrom the host
cell surface(29).At present,littleis known about thespatialrelationshipsofthe viralproteinsin thematurevirusparticleand therolesthatthese relationshipsmayplayin viralassembly and in theensuingstages of the infectious process.
Ev-idence available from selective extraction (34)
and phenotypic mixing experiments (20)
sug-gests that M isinvolvedintransmembrane
rec-ognitions between the spike and nucleocapsid
proteins. Direct interactionsofMwith the gly-coproteins or nucleocapsid proteins have not
been demonstrated in paramyxoviruses, but
have beendemonstrated invesicular stomatitis
virus withreversiblecross-linkingagents (7).
Our studiesweredesignedto testthe
hypoth-esis that M exists in close proximity to the interior nucleocapsid proteins andthe exterior spikeproteinsinparamyxoviruses.A
secondary
objective was to probe for information on thespatial relationships of the individual proteins
within the viral membrane andnucleocapsidof
the maturevirusparticle by analyzing the pro-tein complexes present as a result of native disulfide bondingorformedbychemical
cross-linking.
The two cross-linking agents chosenforthis
152
on November 10, 2019 by guest
http://jvi.asm.org/
study both contain disulfide bridges and a
1.1-nmseparationbetweentheirfunctionalgroups.
Thehomobifunctional reagent dimethyl-3,3'-di-thiobispropionimidate (DTBP) is a
water-solu-ble imidoester with a half-life in the minute
range atslightly alkaline pH (26). The
hetero-bifumctional reagent methyl 3-[(p-azidophen-yl)dithio]propionimidate (PAPDIP)contains an
imidoester groupandalipophilicaryl azide
por-tion. Theimidoestergroupof DTBPorPAPDIP
reactswiththeprimary amine of a protein(Fig.
1,reaction 1). With DTBP (Fig. 1) the second
step (Fig. 1, reaction2) isthe sametypeof
site-specificreaction asthe first.
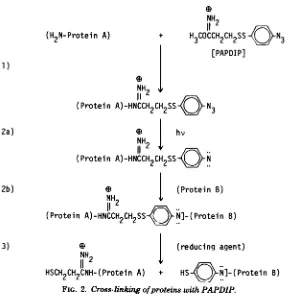
With PAPDIP (Fig. 2), reaction of the
imi-doester group with protein (Fig. 2,reaction 1) is
done in the dark to preventsimultaneous
acti-vation of the secondgroup. Thesecond group of PAPDIP, an aryl azide, is activated by pho-tolysis to the nitrene (Fig. 2, reaction 2a).
Ni-trenes donot require aspecific reactive group.
Theycaninsert atN-H and atC-H bonds in
proteins (Fig.2,reaction 2b) and have half-lives
in themillisecondrange(26).Protein cross-links
formed with either DTBP or PAPDIP can be reversedby reductive cleavageoftheirdisulfide bridges (Fig. 2, reaction 3).
(Parts of this workhave been described briefly before [16; M. A. K. Markwell, Fed. Proc. 37:
1773, 1978].)
MATERIALS AND METHODS
Virus. Sendai (also known as hemagglutinating
(H2N-Protein A)
1)
2)
3)
virusofJapan, HVJ) and NDV(strainHP-16)viruses werepropagated in 11-day-oldembryonatedchicken eggsincubated at38°C. Allantoic fluid was harvested at 22h(earlyharvest)or48h(lateharvest)
postinfec-tion. Viruswaspurifiedundersterile conditionsbya modificationof theprocedure of Samson and Fox (30); this modificationsubstantiallyshortenedthe time re-quired for purification. Centrifuge tubes and bottles weresterilized byovernight exposuretoethyleneoxide (Anprolene, H. W. Andersen Products, Inc.). Linear gradients were formed overnight by diffusionof se-quentiallayers(incrementsof5%)of sterile solutions of sucrose or Renografin. All procedures were per-formedat0 to4°Cunless otherwisespecified.Clarified (50,000g-min) allantoic fluidwaslayeredontopofa two-stepgradientconsistingof 20%(wt/vol) and 65% sucrose in 0.01 M Tris.hydrochloride-0.1 M NaCl-0.001 Mdisodium EDTAatpH7.4 (TSE).The gra-dientwas centrifugedfor 30 minat 19,000rpmin a Sorvall SV-288 verticalrotor. Virus concentrated at
the 20/65%sucrose interfacewasdiluted withTSE,
and bandedsequentiallyin linear 40 to 65% sucrose and 6to25% iodine equivalentRenografin gradients
bycentrifugationfor 1 hat19,000rpmin the vertical
rotor.The viruswaspelletedbycentrifugationfor 90 minat75,000xg inafixed-anglerotor, and thepellet
wassuspendedinTSEat aproteinconcentration of 5
mg/ml and used within 3 days or stored frozen at
-70°C. Thedye-bindingassayforproteins(2) under-estimated viralproteins bytwo- tothreefold inpurified
viral samples. Protein wasthereforeroutinely
meas-ured by a modification of the Lowry procedure for membrane andlipoproteinsamples(18).
Celis. Monolayercultures ofbabyhamsterkidney
(BHK-21) celLsweregrowninamediumconsistingof
10%tryptose phosphate broth, 10%fetal calfserum,
NH2 NH2
11
I1I
+ H3COCCH2CH2SSCH2CH2COCH3
[DTBP]
NH, ' , NH2
(Protein A)-HNCCH2CH2SSCH2CH2COCH3
(H2N-Protein B)
0
e
NH2 NH2
11
eif
11
(Protein
A)-HN`CCH2CH
2SSCH2CH2CNH-
(Protein B) (reducing agent)e D
NH2 ' NH2
HSCH2CH2CNH-(Protein
A) +HSCH2CH2CNH-(Protein
B)
FIG. 1. Cross-linkingofproteins with DTBP.
on November 10, 2019 by guest
http://jvi.asm.org/
154 MARKWELL AND FOX
(H2N-Protein A)
NH2
11Cii)N3
H3COCCH2CH2SS
PAPDIP]
IDNH2
(Protein A)-HNCCH2CH2SS N3
NH hv
(Protein A)-HNCCH2CH2SS-N
e j (Protein B)
NH2
(Protein A)-HNCCH2CH2SS
iQN]-(Protein
B)Q | (reducing agent)
NH
[image:3.504.115.409.65.361.2]HSCH2CH2CNH-(Protein A) + HS(N]-(Protein B)
FIG. 2. Cross-linking ofproteinswith PAPDIP. and 80% minimum essential medium from (GIBCO
Laboratories,GrandIsland, N.Y.).Thecells wereused for virusadsorption studies within 24 hofattaining confluency.
lodination and cross-linking conditions. The externallydisposedproteinsof thevirusenvelopewere
iodinated with 1,3,4,6-tetrachloro-3a,6a-diphenylgly-coluril (chloroglycoluril, available as Iodo-gen from
Pierce ChemicalCo.) under conditionsoptimizedfor surface-specific labeling (17).Portionsofintact virus (100jigofprotein)werereactedfor 10minat0°Cwith 0.5 mCi ofNaI251 (carrier free,Amersham Corp.) in
thepresenceof10,Agofchloroglycoluril.The iodinated virus preparation wastransferred from the reaction vesseltoatesttubecontaining25,umolof carrierNaI in TSEand usedimmediatelyforcross-linkingstudies. Interioras well asexternallydisplayed proteinsof the viruswereiodinatedbyanonvectorial
chlorogly-colurilmethod which includesdetergenttodisruptthe virus (17).Controlandcross-linkedsampleswere sol-ubilized in2% sodium dodecylsulfate (SDS) by
im-mersion in boilingwater for 2minand then reacted withNa'25I in thepresenceofchloroglycolurilas
de-scribed above. Thesampleswereusedforgel
electro-phoresisafter the addition of 25,umolof carrierNal. Paramyxoviruses were cross-linked with DTBP (Pierce Chemical Co.) by the procedure described previouslyfor NDV(21)exceptthat thenative disul-fidebondsofthe viruswerenotreduced before
cross-linkingandthecross-linkingreactionoccurredat0°C. The viralsuspension(100ugofproteinin 25plof 0.2
MtriethanolaminehydrochlorideatpH 8.5)wasmade
3 mM in DTBP and incubated for 30minat0°C. The reactionwasterminatedbythe additionof ammonium acetateandfreshlyprepared N-ethylmaleimide, both
atafinalconcentration of 50mM,andincubatedfor
30minatroomtemperature.Sampleswerethen made 2% in SDSandimmersedfor2mininboilingwaterin preparationforgel electrophoresisornonvectorial io-dination.
Thesameinitialincubation conditionwasused with theheterobifunctional cross-linkingreagent PAPDIP (5), but in the darktopreventphotolysisof thearyl azidegroup.Afterthe30-min incubationin ammonium acetate and N-ethylmaleimide, the virus was
sedi-mented for 90minat75,000x gthrough20%sucrose
ontoa65%sucroseshelf,and thePAPDIP-derivatized virus that concentrated attheinterface wasdivided into two samples. In the first sample, the second reactivegroupof PAPDIPwasactivatedbyexposure
to a UV lamp (Blak-ray UVL-21, peak of 366 nm,
Ultra-violetProducts, Inc.) for 3minatadistance of
5 cm from the sample. The rest of the PAPDIP-derivatizedviruswasdiluted with minimum essential
mediumtoaproteinconcentration of 0.5mg/mland
allowedtoadsorbto BHK confluentmonolayersfor 30 min at 4°C. Themonolayers were washed three
times with ice-cold minimum essential medium to
removeunattachedvirusand thenexposedtoUVlight for 3min.
Fractionation ofBHK cells and cross-linked Sendaivirus. The cellularpreparationwasenriched
for virus cross-linkedtoplasmamembranebya mod-ification ofthe zinc ion method used to isolate the 1)
2a)
2b)
3)
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
surface membranes of L cells (38). BHK cells were
scraped with a rubber policeman from three 10-cm
dishes into 15mlof 0.025 MTris.hydrochloride-0.14 M NaCl atpH7.4andcentrifugedat500 xgfor 15 min. Thepelletwas gently suspendedin 3 ml of 50
mMTris-hydrochlorideatpH7.4containing1mgof
soybean trypsin inhibitor per ml. Thecellswere
al-lowed toswell for 15min at roomtemperature. An equal volume of 1 mM ZnCl2 was added, and the
suspension was incubated foran additional 10 min.
Thesuspension wascooledto4°C and homogenized with50to60strokes ofatypeB, tight Dounce pestle. Thehomogenatewaslayeredontopofatwo-step(20
and65%sucroseinTSE) gradient and centrifuged for
30minat19,000rpminaSorvall SV-288verticalrotor.
The membranefraction which concentratedatthe20/ 65% sucrose interface was collected, diluted 10-fold
with TSE, and centrifugedat 6,000 x gfor 15min. The resulting pelletwas solubilized in 0.5 M
Tris-hydrochloride-2% SDS atpH 6.8forgel
electropho-resis.
Paramyxovirus preparations cross-linked with either DTBPorPAPDIPweresubdividedinto
enve-lope and nucleocapsid fractions by the selective
ex-traction procedures of Scheid andChoppin (31) and RaghowandKingsbury(28)butsubstitutingNonidet P-40(NP-40) (Shell Oil Co.)for Triton X-100. Suspen-sions of the cross-linked virus(2.5mgofproteinin 2.5
ml)weredialyzed against1MNaCl in 0.01 M sodium phosphate buffer at pH 7.2. NP-40 wasadded to a
final concentration of2%(vol/vol). The virus
prepa-rations wereincubated for 2 hat roomtemperature
(21to 22°C), then iodinatedfor 10additional minat
roomtemperaturewith 2.5 mCi ofNa251Iand 25ytgof chloroglycoluril. CarrierNaI (1.25 mmol) wasadded
to the iodinated virus. The viralsuspension was
cen-trifuged for 30 min at 10,000 x g to separate the solubilized membrane proteins HN,F, and M from the insoluble nucleocapsid fraction. The pellet was
sus-pendedin 2 ml of 1 MNaCl in 0.01 M sodium phos-phate buffer at pH 7.2 and extracted for 30min at
room temperature to selectively remove the minor
nucleocapsid proteins (28). Thesuspensionwas
cen-trifugedfor 90minat75,000xg.Theresultingpellet, containingNPcomplexedwith viralRNA,was solu-bilizedin 0.5M Tris hydrochloride-2% SDSatpH6.8 forgelelectrophoresis.
Gel electrophoresis in SDS. Reagents for gel electrophoresiswerepurchasedfromEastman Kodak
Co. SDS (lauryl, sequanal grade)wasobtainedfrom Pierce Chemical Co.SDS, glycerol,andbromophenol blue were added to samples for electrophoresis to
achieve final concentrations of2, 10,and0.008%,
re-spectively, in a sample buffer of 0.0625 M
Tris-hy-drochloride, pH6.8.Reducedsamples contained 5% 2-mercaptoethanol. The molecularweightmarkers mol-luschemocyanin, erythrocyte spectrinbands 1and2, Escherichia coli,-galactosidase, phosphorylasea,
bo-vine serum albumin, ovalbumin, soybean trypsin
in-hibitor, myoglobin, and lysozyme were used in
con-structingcalibrationcurvesfor the 5to12.5%gradient gels.Hemocyaninwasthe gift ofP. S. Linsley,
Uni-versity ofCalifornia, Los Angeles. Spectrin bands 1
and2wereisolatedasreferenced (24).
,B-Galactosid-ase,phosphorylasea,lysozyme, andtrypsininhibitor
werepurchased fromWorthington BiochemicalCorp.,
bovine serum albumin, and myoglobin were from Sigma Chemical Co., and ovalbumin was from Schwarz/Mann.
Theprocedure for two-dimensionalgel electropho-resis isamodificationof theone-dimensionalLaemmli system(13) and has beenpreviously described foruse with erythrocyte ghosts (17). Samples were electro-phoresed in the first dimension without mercaptanon a 1-mm-thick 5 to 12.5% (wt/wt) acrylamide linear gradient resolving gel.The first-dimensional slab gel
was thensliced intoitsrespectivelanes,incubatedfor 15 min in reducing solution (0.0625 M Tris.hydro-chlorideatpH6.8,3% SDS,and 3%2-mercaptoethanol
or 5 mMdithioerythritol), andaffixed with 1.5% aga-rose inreducing solutionontoanother5to12.5%slab gel (1.25 mm thick) for the second dimension. To improve the resolution between the F, and NP pro-teins ofNDV, a7.5 to10%gradient gelwasused in thesecond dimension.
Gelswerefixed,stained forprotein,destained,and
soaked in the aqueous solutions as described in the outline which follows. The gelswere gently agitated on ashakerat roomtemperaturefor the stated times
intightlysealed containers. At least 500 ml of solution
pergelwasusedfor each step. (i) Fixingsolution was composed of 25%isopropanol-10%acetic acid(60 min).
(ii)Stainingsolution consisted of 0.1%Coomassie
bril-liantblue R-250, 25%isopropanol,and 10% acetic acid (30 to60min).(iii)Destainingsolutionwascomposed 5% methanol-7% acetic acid to which were added several whiteplastic foamplugs (diSPo plugs, Scien-tificProducts)toadsorb the staineluted during over-night destaining. (Gels can be soaked forat least 1 week withoutlosingdyefrom the protein bands.) (iv) Soaking solution Awas10%methanol-2%glycerol(3 h).(v)Soaking solution Bwas20%methanol-2% glyc-erol (1h).ThegelswerethentransferredtoWhatman no. 1 chromatography paper and dried under house vacuumovernightatroomtemperature.Kodak single-coatedSB-5X-rayfilmwasused forautoradiography. To determine the percent distribution of radiolabel
incorporated byindividual viralprotein species,
Coo-massie brilliant blue-stained spotswere cutfrom the driedgeland counted inaPackard gamma scintillation spectrometer.
RESULTS
Resolution ofSendai viral proteins. The
majorpolypeptide speciespresent in Sendai
vi-rusand theirlocationwithinthevirionare
pre-sented in Table1. Identification of each species
is based onapproximate molecular weight,
ac-cessibility tosurface-specific labeling,and
frac-tionationby selective extractionprocedures. F2
hasbeen shown to existin disulfide linkage to
F1.to formthe active Fprotein (33). The
glyco-proteins HN and F arethe only viral components
readily accessible to surface-specific iodination
(15, 17). During sequential selective extraction
procedures themembrane proteinsHN, F, and
M(fraction 1) andminornucleocapsid proteins
L and P(fraction2) arestrippedfrom the virus,
on November 10, 2019 by guest
http://jvi.asm.org/
156 MARKWELL AND FOX
TABLE 1. Proteins of Sendai virus Accessi- Selective
Poly- Molwt biltyto
extrac-peptide'
(XlO_3
b Positioninvirion surface- tion frac-peptidea(x10)~~~~specific tionld
________ ~~~~labeling ____
L 215 Nucleocapsid _ 2
P 78 Nucleocapsid _ 2
HN 70 Membrane exterior + 1
NP 60 Nucleocapsid _ 3
F, 47 Membraneexterior + 1
M 34 Membranematrix 1
F2 15 Membrane exterior + 1
aNomenclature forparamyxovirus proteins
descrbed
byScheidandChoppin(32).
bApparentmolecular weights of Sendai viral proteins were calculated from molecular weight standards (see Fig. 3) coe-lectrophoresed with the viral proteins on 5 to12.5%gradient slabgels.
cAportion of intact virus (100 pgofviral protein) was
reacted for 10 min at 0°C with 0.5 mCi ofNalnI in the presence of 10pugofchloroglycolurilasdescribed in the text. dSendai viralpreparationswere subjectedto sequential selective extraction steps to fractionate the virus into its components asdescribedinthetext.FractionI, supernatant fraction of the 2% NP-40-1 M NaCl in 0.01 M sodium phos-phate extraction step; fraction 2,supematant fraction of the 1M NaCl in 0.01 M sodium phosphateextraction step; fraction 3,pelletof the 1 M NaCl in 0.01 M sodiumphosphate extrac-tionstep.
leaving NPcomplexedtoviral RNA(fraction3). Because of the greatly differing sizes of the
polypeptides,agelsystemwasdevelopedwhich
couldbeusedtoapproximatemolecularweights
ofpolypeptidesand theircomplexesover awide
range ofvalues. The Laemmli SDS gel system
(13)wasmodifiedtoincludea5 to12.5%linear gradient resolving gel (17). Molecular weight standards from 14,000to290,000were runwith eachslabgeltoconstructmolecularweight cal-ibrationcurves(Fig. 3).
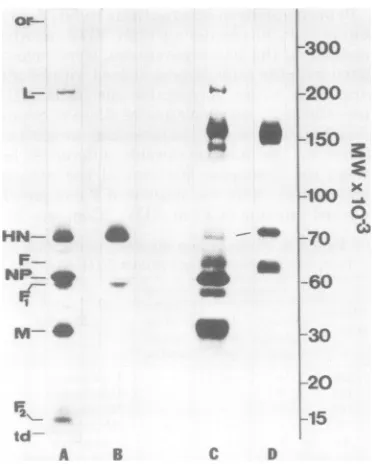
By using the5 to 12.5% gradient gelsystem, all theproteinsof thereduced, SDS-solubilized
viruswere resolved (Fig. 4A). The high degree ofintegrity of the viral preparation is demon-stratedby the fact thatonlythe external mem-brane polypeptides HN, F1,and F2are labeled
bysurface-specificiodination of the intact virus
(Fig. 4B).Thissurface-specifictechnique proved usefulindistinguishingbetween NP and F which have similar electrophoretic mobilities after SDS solubilization from the nonreduced virus (Fig. 4C and D).
NativedisulfidebondinginSendai virus. Reduction of Sendai virusdestroys allits biolog-ical activities:infectivity, cell fusion, hemagglu-tination, neuraminidase, andhemolysis (23, 25, 33). Therefore, the polypeptides which
partici-pate innative disulfidebondingwereexamined.
Ozawa et al. (25) had previously reported the
presence of disulfide-linked
high-molecular-weight oligomers of HN and P in egg-grown
Sendaivirus (Z strain), butwereunable to ac-curately estimate their size. Under nonreducing conditions, threehigh-molecular-weight species inadditiontoLwereobservedonthe5 to12.5% gels (Fig. 40). Two of these(273,000and140,000 molecular weight) were accessible to surface-specific labeling; the third (238,000 molecular weight)was not(Fig. 4D).
Two-dimensional slab gel electrophoresis sys-tems facilitated identification of these species.
Polypeptidechains existinginnativedisulfideor cross-linkedcomplexes will exhibitagreater
mo-lecular weight inanonreducing firstdimension than in a reducing second dimension. They
therefore appear as spots below the diagonal
formed by polypeptideswhich have equal
mo-lecularweightsin both dimensions.Thespecies migrating at 273,000 and 140,000 in the first
(nonreducing) dimension migrated as a single polypeptide of 70,000 in the second (reducing) dimension(Fig. 5).Themigrationcharacteristics and theaccessibility tosurface-specific labeling
(Fig. 4D) indicated that thesespecieswere
tetra-meric anddimeric forms of the membrane
gly-300F HEMOCYANIN
200-1501
In
I0
x
3
<,r
I-0
w 1oo0
7
50
PHOSPHORYLASEA
\ VINESERUM
OLALBUMIN
t OVALBUMIN
30-20- SOYBEAN TRYPSINi
LYSOZYME-20 40 60 80 100
%MOBILITY
FIG. 3. SDS-polyacrylamide gel electrophoresis of molecularweightstandards. A standardconsisting of mollusc hemocyanin (290,000), erythrocyte spectrin bandsIand2(240,000 and 215,000),E.coli
Il-galac-tosidase (130,000),phosphorylasea (100,000), bovine serumalbumin(68,000), ovalbumin (43,000), soybean
trypsininhibitor(21,500), myoglobin(17,200), and
ly-sozyme(14,300) waselectrophoresedon a 5 to12.5%
acrylamidelineargradientgel.
J. VIROI,.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.504.63.258.52.204.2] [image:5.504.267.457.322.567.2]coprotein HN. The homotrimeric form of HN was notobserved. The incompletereduction of the HN tetramer ofSendaivirus to the dimeric form suggests that notall the disulfide linkages
_ _ am 300
_ - 200
_ I _ .150
MO 0
- 100
-70:
4 a - * 60 !
C D E F
FIG. 4. Comparisonof reduced, nonreducea, and
cross-linkedproteinsofSendai virus by
one-dimen-sional gelelectrophoresis.A100)-pgsample ofreduced egg-grownSendai virus waselectrophoresedon a 5
to12.5%acrylamide linear gradient gel and stained
with Coomassie brilliant blue (A). Samples of the
sameviruspreparationwithout reducing agent (C)br
aftercross-linkingwith 3 mM DTBP (E) are shown.
Theautoradiographs ofsamples A, C, and E which
had beensurface-specifically labeledby the
chloro-glycolurilmethodfor'2Iarerespectively shown in B,
D, and F. Lettersdesignatethepositionsof the major Sendai viral proteins and their complexes, theorigin
(or)ofthegelandthetracking dye (td)front.
haveequivalent sensitivity to reduction and that the tetramer maybe formed from thelinkage of dimers during viral assembly. When the first-dimensional gelwasincubated inreducing solu-tion(0.0625MTris-hydrochlorideatpH6.8, 3% SDS, and 3% 2-mercaptoethanol or 5 mM dithi-oerythritol) for 30min instead of the usual 10 mi, total reductionwasachieved in the second dimension.
In theisolated nonreduced virionmostof HN existed in disulfide-linked dimeric and tetra-meric forms (54 and 38%, respectively, of the total radioactivity incorporated by HN); only8% was detected in monomeric form. In the nine different batchesof egg-grown Sendai virus ex-amined, the percentage of HN existing as a dimeror tetramervariedbyless than 5%. For-mation of the disulfide bond by autooxidation
duringsolubilizationasreportedfor H-2 (9)can
probably be excluded, since treatmentof virus withfreshly prepared50mMN-ethylmaleimide before solubilization did not alter the electro-phoreticprofileof the viralproteins.
The third high-molecular-weight
disulfide-bondedspeciesmigrated at amolecular weight
of238,000 in the first dimensionandcomigrated
with P monomer (78,000 molecular weight) in the second dimension. This and its lack of
ac-cessibility tosurface-specific labeling indicated that itwas atrimericform of minornucleocapsid
protein P. Approximately equal amounts of P
1stD-nonroduced
X MWM x1-3
b 300:or~ ° X0
t2001
1501 S *
70-*
3
60.]
a 45j,;
304 9
201
td'
A
FIG. 5. Two-dimensionalelectrophoretic analysisofproteins and theirnative disulfide complexespresent
inegg-grownSendai virus.Approximately50pgofviral protein which had beeniodinated in thepresenceof
SDSwerefractionatedinthefirst dimension withoutreducingagenton a5to12.5%polyacrylamideslabgel.
Thefirst-dimensionalgelwasslicedintoitsrespective lanes,reduced with2-mercaptoethanol, and affixed
ontoanother 5to12.5%slabgel for theseconddimension. Theautoradiograph of the gel isshownonthe left,
and itscorresponding schematic diagramisshownontheright. RefertoTable2for identificationofproteins
and their nativedisulfide complexes.
or
L
p
HN _ _
F NP-m
M _
F2 td
A 8
0
O
c
P tomer
aW N
Orw _mer
FQ1
p_
F N
r--B
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.504.50.245.120.248.2] [image:6.504.107.396.390.589.2]158 MARKWELL AND FOX
existed in the monomeric andtrimeric forms(49 and 51%, respectively), and this distribution var-iedby less than5% among thedifferentbatches ofSendai virus examined. Disulfide-linked hom-odimers ofPwerenotobserved.
Reduction of the disulfide-linked F protein (60,000molecular weight)toitscomponent poly-peptides F1 (47,000 molecular weight) and F2 (15,000 molecular weight) isclearly definedon the two-dimensional 5 to 12.5% gradient slab gels. Several minor proteins were sometimes
seen on orbelow thediagonal. These differed in
intensity frombatch tobatch ofegg-grown virus andcould be cellular contaminantsor proteoly-sisproducts of the viral proteins.Asummaryof theproteins and their native disulfidecomplexes
presentin egg-grown Sendai virusis presented
inTable2.
Cross-linking of Sendai virus. Noncova-lent protein-protein interactionswereelucidated with reversible chemical cross-linkers. Intact, nonreduced preparations of Sendai virus were cross-linkedasdescribedinMaterialsand Meth-ods with either DTBP or PAPDIP. Both
re-agents contain disulfide bridges and a 1.1-mn
separation between functional groups. The in-dividualcomponents of cross-linked complexes formed with these reagents appeared as new
spots below the diagonal on two-dimensional
[image:7.504.64.259.383.577.2]gels.
TABLE 2. Proteins and their native
disulfide
complexes present in egg-grown Sendai virusPresent in: Access-Molwt Nonre- Re- ible to
(X1O')a ore e surface-
Identity'
duced duced specific gels gels
labelingb
273 + + HNhomotetramer
238 + _ - P homotrimer
215 + + - L monomer
140 + - + HNhomodimer
78 + + - Pmonomer
70 + + + HNmonomer
60 + - + Fmonomer
(F1+ F2oligomer)
60 + + - NPmonomer
47 - + + F1monomer
34 + + - Mmonomer
15 + + F2monomer
aMolecularweights ofSendai viralproteinswere
calculated from molecularweightmarkers(seeFig.3)
coelectrophoresed on5 to 12.5% slabgelsinthefirst
(nonreduced) and second (reduced) dimension. bA100-,ugportion of intact viruswasreactedfor10 minat0°Cwith0.5mCi ofNaI261inthe presenceof10 ,ug ofchloroglycolurilasdescribed inthetext.
'Theindividual components ofproteincomplexes wereidentifiedbytheir molecularweight,accessibility
tosurface-specificiodination,and selective extraction
procedures.
J. VIROL.
Experiments were performed to ensure that the off-diagonal spots arosefromthe reductive cleavage ofcomplexes stabilized by native disul-fide bondsorbydisulfide-containing cross-links. No off-diagonal spots were observed with control
or cross-linked samples, when reducing
condi-tions (3%2-mercaptoethanol or5 mM
dithioe-rythritol) or nonreducing conditionswereused in both dimensions of the two-dimensional gel. Additionalexperimentsweredesignedto estab-lish that theappearanceofnew high-molecular-weight bandswasthe direct result of cross-link-ingby the chemicalreagentintroduced. All the
newcomplexes formed in the presence ofDTBP
orPAPDIPwerereductivelycleavedbyboth
2-mercaptoethanolanddithioerythritol.A10-min incubation ofDTBP or PAPDIP with 20mM ammoniumacetatebefore the addition of virus prevented theappearanceofnew
high-molecu-lar-weight complexes. These complexes were alsonotformed when viralsampleswere irradi-ated for3 minwith UVlightin the absence of PAPDIP. When PAPDIP-derivatizedviruswas solubilized in 2% SDS before photolysis, no
cross-linking was observed.Therequirementfor
photolysis eliminated the possibility of
cross-linking by monofunctional imidoesters (3,26,36)
or by DTBPproduced from PAPDIP through
an exchange reaction: 2PAPDIP - DTBP +
4,4'-dithiobisphenylazide.
Disulfide reagents (R-SS-R) such as DTBP and PAPDIP are susceptible to SH-SS inter-change (26).Duringincubation of suchreagents with virusatpH 8.5 anexchangereaction (pro-tein-SH + R-SS-R protein-SS-R + R-SH)
can occurbetween thereagentdisulfide and free
sulfhydrylgroupsofviralproteins.The
require-mentforphotolysistoobtaincross-linkingwith
PAPDIP suggests that significant amounts of
e
NH2
11
protein-SS-CH2CH2COCH3 had not been
formed bymercaptan-disulfide interchange
be-tweenprotein andreagent. Also, incubationof
viral proteins with 50 mM N-ethylmaleimide
beforecross-linkingdid notalterthe
cross-link-ing results.Thesecontrols indicatethat none of
the cross-linkedproductswasformedby SH-SS
interchange.
Chemical cross-linked homodimers of F
and NP. DTBP treatment ofSendaivirus
prep-arations stored frozen for 3 to 12 months at
-70°C before cross-linking produced the
one-dimensionalpatternseeninFig.4E. Under the
mild cross-linking conditions used, little or no protein remained at the origin ofthe
first-di-mensional gel. The sameresultswereobtained
whencross-linkingwasperformedat 0, 11, 22, or
on November 10, 2019 by guest
http://jvi.asm.org/
37°Cover arangeof DTBPconcentrations(0.5
to 10 mM) or when PAPDIP (0.1 to 0.5 mM)
was used as the cross-linking reagent. A new
band(120,000molecular weight) appeared in the
one-dimensional profile as the result of
cross-linking.Thiscomplexwasselectively labeledby surface-specific iodination, indicating the pres-enceof HNorF (Fig. 4F).
Two-dimensional analysis indicatedthat the 120,000-molecular-weight bandcontains NP,Fl,
andF2 (Fig. 6A).From molecular weight consid-erationsalone, the band could be explained by the formation ofaheterodimerof the two 60,000-molecular-weightproteins NP and F or by their
homodimers. Thefact that theF1spot at
molec-ular weight47,000containedmoreradioactivity than the NP spot at molecular weight 60,000 suggested the homodimer explanation. Also, a species just below the diagonal is visible, mi-grating atmolecularweight 120,000 in the first
MWx1cr3 Ott) 00 OtLo
N.- - t- IDl
C
a
a a,
STORED VIRUS
0 0
0
GQoj
t)(
NPdin,ner a
0
Fdimer Mdimer
0 0
A B
MWxio-3
0 0 0 0
Ott)Ln
08-o O 0O ° e 00m
.o
.-0
aS
4
00 00
00
0 0
NP-M dimer
oC; e
20
15 0
td
C D
FIG. 6. Reversible cross-linkingofproteins inegg-grownSendaivirus by DTBP. Sendai virns (100pgof protein) wastreated with 3 mM DTBPfor30minat0to4°C.Thesamepreparation of earlyharvestSendai
viruswasusedinFig.4A and4C,but the stored virusof4Awaskept frozen3monthsat-70°Cbefore
cross-linking. Thefresh virus of4C waskept at 4°Cand usedfor cross-linking experiments within 3days of isolation. Cross-linkedproductswere iodinated inthepresence ofSDSand analyzed by two-dimensional
polyacrylamide electrophoresisasdescribed inFig.5.
ionreduced
0
1stD-n4
-0
0
n or
v 300
t 200 150
100
I?
O 70
x 60
45 30 20 15 td
Kb
300 200 150
FRESHVIRUS
100
0
70-3 60
45
30
Ab a
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.504.108.394.182.594.2]160 MARKWELL AND FOX
dimension and molecular weight 97,000 in the second dimension. From its molecular weight and accessibility to surface-specific iodination (datanotshown), itappearedtobeahomodimer of F1, whichwas nottotallyreducedtoits mono-mer form. The presence ofan F1 homodimer
supports the homodimer explanation of the
120,000-molecular-weight species.The presence ofsomeHNin tetrameric and dimeric forms in the second dimension further indicates incom-plete reduction of the first-dimensional gel. A 30-min ratherthan the usual10-minincubation of thefirst-dimensional gelinreducing solution completely reduced the F1 homodimeric and HN tetrameric and dimericforms.
To obtain conclusive evidence of the identity of the 120,000-molecular-weight complex(es),
cross-linked viral preparations were extracted firstwith 2% NP-40 in1MNaCl, then with1M NaCl aloneasdescribedinMaterials and Meth-ods. The extractedpellet consisted ofNP mono-mer (60,000 molecularweight) and homodimer (120,000molecular weight) (Fig. 7A).A 120,000-molecular-weightcomplex containingonlyFwas found in the NP-40-NaCl supernatant fraction (datanotshown). No evidence forNP-F heter-odimer formation was observed in any of the extracted fractions.Therefore, the
120,000-mo-lecular-weightspeciesactuallyconsisted oftwo
1st0-nonreduced
u
0
a
L
ff
different types of protein complexes: an NP homodimer and an F homodimer which comi-grated.
Chemicalcross-linkedhomodimers ofM. Two additional products of cross-linking were detected as off-diagonal spots on two-dimen-sionalgels. These migratedatmolecularweights
67,000and63,000in thefirstdimensionand thus
wereobscured by the F and NP bandson one-dimensionalgels.Inthesecondreducing dimen-sion, they both migrated at molecular weight
34,000.Bothspecieswereinaccessibleto
surface-specific labeling. Their molecularweights in the first dimensionplus comigrationuponreduction with the monomer of the M protein strongly suggested thattheyarehomodimersof M. When cross-linked preparations of Sendai viruswere extracted with 2% NP-40 in 1 M NaCl as de-scribed in Materials and Methods, the 67,000-and63,000-molecular-weiiht sDecieswerefound in the supernatant fraction along with the M
monomer. These selective extraction
experi-mentsconfirmed their identificationas M
hom-odimers. Thetwodifferent molecular weightsin the firstdimensionmaybe dueto adifferencein theiraxesofcross-linkingorthe 63,000-molecu-lar-weight species may have intramolecular as wellasintermolecular cross-links.
Additional species formed by chemical
MWx1O-3
0
x3o
0 O ° ° C
4 r
v-300 200 150
90 60
:E.
_e 4
0 0",
__ 4
-M
20 15 toi
A B
FIG. 7. Selective extractionofcross-linked Sendai virus. The stored(A)andfresh (B) virussamplesthat had beencross-linkedfor Fig. 6A and Cwereextractedfor2hat roomtemperaturein 2%NP-40-1 MNaCi
in0.01 Msodiumphosphate buffer atpH 7.2and then iodinated in the extraction buffer. The insoluble
nucleocapsidfractionwas thenfurtherextractedwith 1MNaCi. Theresulting pelletwasanalyzedby
two-dimensionalpolyacrylamidegelelectrophoresisasdescribed inFig.5.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:9.504.123.410.371.599.2]cross-linking. Production of homodimers of
NP, F, and Mupon cross-linkingwas observed
with six independent batches of late harvest
Sendai virus and three independent batches of
early harvest Sendai virus. No differences in
cross-linking productswerenoted betweenearly
and late harvests after 3to12 months of storage
at -70°Corin the cross-linked complexes
pro-ducedbyDTBP and PAPDIP. Evidence for the
formation of small amounts of M trimeric
(105,000 molecular weight), F tetrameric
(245,000 molecular weight), NP trimeric (178,000
molecular weight) and tetrameric (250,000
mo-lecular weight) forms was found when the gel
which produced the autoradiograph shown in
Fig. 6Awasexposed for several weeks (datanot
shown).
Effect of storage on cross-linking:
for-mation of an NP-M heterodimer upon
cross-linking of freshly harvested
prepa-rations. When freshly harvested (not frozen,
storedno morethan 3daysat4°C) Sendai virus
preparationsweresubjectedtocross-linking,an
additional bandwasobservedinthefirst
dimen-sion at molecular weight 97,000 (data not
shown). This bandwas morepredominant in the
twoearly harvest preparations examined than in
thetwolate harvest preparationsgrowninthe
samebatches ofeggsastheearlyonesbut
har-vested 26 h later. Reduction of the 97,000-mo-lecular-weightcomplex inthesecond dimension
producedtwospotswhichcomigrated with NP
(60,000 molecular weight) and M (34,000
molec-ular weight), indicating the possible formation
ofaNP-Mheterodimer (Fig. 6C).
A selective extraction method was used to
demonstrateconclusivelythatthe
97,000-molec-ular-weight species was aheterodimer and not
homooligomers of NP and M with similar
elec-trophoretic mobilities. Cross-linked
prepara-tions were extracted with2% NP-40 plus 1 M
NaCl and then with 1 M NaCl as previously
described in Materials and Methods. The
insol-ublepelletcontained the NPmonomerand
hom-odimer plus the NP-M heterodimer (Fig. 7B).
The monomer and homooligomers of M were
notpresentinthepellet,havingbeen previously
extracted into the 2% NP-40-1 MNaCl
super-natantfraction.
Theability of NP and Mtoforma
heterodi-merupontreatmentwithcross-linkingreagents
diminisheduponfreezing and prolongedstorage
of the sample. The same preparation ofearly
harvest Sendai viruswasused inFig. 6A and C,
but the sample in 6Awasfrozenand storedfor
3 monthsat-70°C beforecross-linking. In Fig.
6C the amount of NP-M heterodimer formed
wasabout thesame astheamountof NP
hom-odimerformed. InFig. 6Aonly NP homodimer formation wasobserved.
Cross-linking of Sendai virus was also per-formed after its adsorption to host cells. Both freshly harvested and stored Sendai virus prep-arationswerederivatized in the dark with PAP-DIP and then adsorbedtoBHKmonolayers as described in Materials and Methods. Unad-sorbed virus and excess cross-linking reagent were removed before photolysis. Cross-linking ofstored Sendai viruspreparations adsorbedto BHKcellsproduced thesameviralprotein com-plexes as seen in Fig. 6A, i.e., homodimers of
NP, F, andM.Cross-linkingof thefreshly
har-vestedvirus preparation adsorbed tohost cells additionallyproduced the NP-M heterodimeras inFig. 4C. These results indicate that PAPDIP-derivatized virus retained itsabilitytoadsorbto host cells and that the cross-linked products
obtained with isolated viruswerealso obtained with virus adsorbedto hostcells. No new band formed togivean indication of cross-linking of
HNor F tohost membraneproteins.
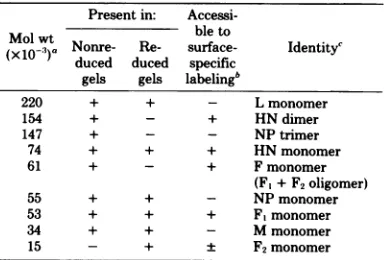
Protein-protein interactions inNDV. Pro-tein-protein interactions within NDV, another member of the paramyxoviruses, were investi-gated with thesameapproachused with Sendai virus. The major polypeptide species of NDV have thesamenomenclatureasthoseofSendai virus but differslightlyinmolecular weight (see Table 3). The most noticeable differences
be-tween the Coomassie patterns of the reduced
viral proteinsweretheabsence of P and greatly reduced amount of F1 in NDV. (Compare Fig.
TABLE 3. Proteins and their native disulfide complexes present in egg-grown NDV (HP-16)
Present in:
Accessi-Molwt bleto
(xlO-)a Nonre- Re- surface- Identityc duced duced specific
gels gels labelingb
220 + + - Lmonomer
154 + - + HNdimer
147 + - - NP trimer
74 + + + HNmonomer
61 + - + Fmonomer
(Ft+F2oligomer)
55 + + - NPmonomer
53 + + + F,monomer
34 + + - Mmonomer
15 - + F2monomer
Molecularweightsof NDV viralproteinswerecalculated from molecularweight markers(seeFig.3)coelectrophoresed on5to 12.5% slabgelsin the first(nonreduced)andsecond (reduced) dimension.
bA 100-ug portion ofintactviruswasreacted for10minat
00Cwith0.5mCi ofNa'251Iinthe presence of 10 yg of chloro-glycolurilasdescribed inthetext.
'The individual components ofprotein complexes were identifiedbytheirmolecularweight, accessibilityto surface-specificiodination,and selective extractionprocedures.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:10.504.249.441.432.562.2]162 MARKWELL AND FOX
4A to Fig. 8A.) Although NP and
F,
have similar electrophoretic mobilit wereeasily distinguished by
surface-sj
beling (Fig. 8B). Most of the radiol incorporated into the major glycopro and therest wasincorporatedintoFl.'resultswereobtainedfor NDV (L.Kan lactoperoxidase-catalyzed iodination (
NDV, unlike F2 of Sendai virus, inc(
little radioiodine.
Under nonreducing conditionsthe F observedjust above NP (Fig. 8Cand ]
F1wasalsopresent inthenonreduced
tion. Two bandswerepresent in the
hig
ular-weight region of the nonreduced80). The one at molecular weight 154 accessibletosurface-specificiodinatior
atmolecular weight 147,000 was not ( Two-dimensional analysis (Fig. 9A) thatthe154,000-molecular-weightspec homodimer of glycoprotein HN. Most
HN was present in the dimeric form;
or--L!
HN Q
F-F,
2
m....t
"I
soJ. VIROL.
ofNDV (5%) was present as a monomer. The
147,000-ties, they molecular-weight specieswasidentifiedas
hom-pecific
la- otrimerof NP. Most(92%) of NP existed in thelabel was monomeric form in thenonreduced virus, buta itein HN, significant amount (8%) formed a
disulfide-The same linked homotrimer inthe sixviral preparations
isas)with examined.Noevidence forahomodimer form of
15). F2 of NP wasobserved. The 5 to 12.5% gradient gel
Drporated employed in these studies resolved the
homo-oligomersof HN andNP into twodiscretebands,
bandwas but on a 7.5%gel such as thatused previously
D). Some for cross-linking studies in this laboratory (21,
prepara- 37), the two homooligomers comigrated at an
gh-molet-
apparentmolecular weight of140,000.gel (Fig. DTBP-induced cross-linking of freshly
har-4,000 was vested (notfrozen,storedno morethan3 days
i;theone at400)
preparations
of NDVproducedthetwo-(Fig.
8D). dimensional pattem observed in Fig. 9C. Theindicated same results were obtained when cross-lilking
-ieswas a wasperformedat0, 11, 22,or
370C
over arange(95%) of of DTBP concentrations(0.5to10mM)orwhen the rest PAPDIP (0.1to0.5mM) wasusedasthe
cross-linkingreagent.Thehomodimersof F, NP, and Mproducedby cross-linkingwereidentified by theirmolecularweight and accessibilityto
sur--300 face-specific labeling (data not shown). NP-M
heterodimer formation was furtherverified by -200 selective extraction
experiments
describedpre-viously
foridentification of the NP-Mheterodi--150 mer in Sendai virus. There wasslightly greater K heterodimerformation inearlyharvest than in > late harvest preparations ofNDV, but the
dif-__1Q(
X ference betweenearlyandlate harvestwas noto
nearly
so dramatic aswith Sendai virus. NDV_70
C whichwasstoredfor3monthsormore at-70°C beforecross-linkingshowedlittleor noevidence 60 of NP-M heterodimerformation.DISCUSSION 30
20
15
[image:11.504.74.260.294.526.2]A kvi D
FIG. 8. Comparison ofreduced and nonreduced
proteins ofNDVbyone-dimensionalgel electropho-resis. A 100-pug sample ofreduced egg-grownNDV
waselectrophoresedon a5 to 12.5%lineargradient resolving geland stained with Coomassie brilliant
blue (A). A sample ofthe same viruspreparation withoutreducingagent is shown in C. The
autora-diographs of samples A and C which have been
surface specifically labeled by the chloroglycoluril methodfor125Iare shown inB andD, respectively. Letters designate thepositions of the major viral
proteins. RefertoTable 3for identificationofproteins andtheir nativedisulfide complexes.
Theprotein-proteininteractions identified in these studiesexpandourconceptual knowledge
of the molecular architecture of the paramyxo-viruses. Analysis of native disulfide bondingin isolated egg-grown viruses demonstrates that someof the proteinsof Sendai virusandNDV consistentlyform covalentcomplexes. Thesize
of thecomplexand the percentage of the
partic-ular protein found in complex form appear to
depend on the infecting virus rather than the
hostcell. The HN ofegg-grownSendaivirus is
found mostly in tetrameric anddimeric forms,
withonlyasmallamountpresentas a monomer.
Thissamedistributionof HNincomplexeswas
observedwith Sendai virusproducedbyMDBK
cells(unpublished data,MarkwellandFox).The
HN ofegg-grownNDV,strainHP-16,ispresent
mostly as adisulfide-linked dimer,but the HN of arelatedstrain ofNDV,L-Kansas,ispresent
on November 10, 2019 by guest
http://jvi.asm.org/
1stD-nonreduced
's MWx1,03
a
i 2001
120-1
>ci
cp 75;t
3 60' '
451
30-1
A MWx1Oe
O-
9
8v
IC-
, rne 8 8 aI
a
~6 i
@0 9
C
O
o0
NMd mr,
rd°*-d
(S?
__i
N-dOr _~:bo
FIG. 9. Two-dimensionalanalysis ofnativedisulfideandchemically cross-linkedprotein complexes of
egg-grownNDV. Approximately50pgof viral protein from untreated (A)orDTBP-cross-linked (C) viruswere
iodinated in thepresenceofSDS andthenfractionatedin thefirstdimension withoutreducingagentona5
to12.5%polyacrylamideslabgel. Thefirst-dimensional gelwasslicedinto itsrespective lanes,reduced with 2-mercaptoethanol, andaffixedonto7.5to10% slabgel forthe second dimension. Theautoradiograph ofthe gelis shownontheleft,and itscorrespondingschematicdiagramis shownontheright.
onlyas amonomerwhenproducedin
embryo-nated eggs (unpublished data, Markwell and
Fox).To what extentthesenativedisulfide
link-ages influence the number of HN polypeptide
chains which assembletoformthe HNspike is
notcurrentlyknown.
At theonsetofthesestudies, itwasrecognized
that thevalidityofthecross-linking results
ob-tained would depend to a large extent on the
integrity of the virus preparations used. From
hemolysis studies,Homma et al. (10)concluded
that Sendai virusobtained from harvestsjustat the end of theone-stepgrowth cycle (early
har-vests) hadmoreintactenvelopesthanvirus
ob-tained from late harvests. Acomparisonofearly
and late harvest preparationswastherefore in-cluded in thepresentstudies.Use of the SV-288
vertical rotor greatly shortened the time
re-MP -0
trimer: p
201
15;
td
a
3004
200-15011
120-_ 75
x
60-30 20H,
15 tdK
D
on November 10, 2019 by guest
http://jvi.asm.org/
[image:12.504.98.401.56.498.2]164 MARKWELL AND FOX
quired for viruspurificationandproduced prep-arations of virus thatweremoreintactasjudged bysurface-specific iodination than theprevious procedure (30) with swinging bucketrotors and a number of pelleting steps. Sterile, purified virus preparations that were stored for longer than3dayswerefrozenathigh protein concen-trations (5 mg/ml) at -70°C and thawed only
once, immediately before use. The importance
of native disulfide bondingin Sendaivirus has beenemphasizedbyreportsof the loss of infec-tivity,cellfusion,hemagglutinin, neuraminidase, andhemolyticactivitiesupon treatmentof Sen-dai virus with reducing agents (23, 25, 33).
Therefore,viruspreparationsused inthepresent
cross-linking studies were not pretreated with
reducing agent asdescribed inpreviousreports (21,37), andcross-linkingwasperformed under conditionsdesignedtopreservenative disulfide bonding. Moreover, under the mild cross-linking conditions used in thisstudy,littleor noprotein
remained atthe origin of thefirst-dimensional gel. This indicates that we are looking at the
initial products of cross-linking and that the
cross-linking process has only miniimally per-turbed the nativestructureofthe virion.
The sameproductswereformed when
cross-linking was induced with DTBP or PAPDIP.
Both reagents have an 1.1-nm separation
be-tween theirfunctionalgroups (4, 26),andboth
diffuse across the viral membrane as demon-stratedbytheirability toformhomodimers of NP. The imidoester group of both reagents is
specificfor primary amino groups (26); the
ni-trenegenerated by photolysis of the arylazide
group of PAPDIP does not require a specific
reactive group. Recent evidence suggests that theimidoesters and nitrenespreferentially inter-actwith extrinsic membranecomponents(1, 11). Ourstudies indicate that theprotein-protein interactions identifiedbyreversiblecross-linking
areinfluenced bythe conditionsofproduction, isolation, and storage of these
membrane-enve-lopedviruses. Some of the
interactions,
namelytheabilitytoformhomooligomersofF, NP, and Mupon cross-linking, appeartobevery stable
andcanbe observed inpreparations that have
beenstoredat-70°Cforaslongas2years. This typeof interaction has beenpreviously reported
for theparamyxoviruses (22, 28).Other
interac-tionssuchasthosebetween NP and Marebest
observed in preparations with the greatest de-gree of biological integrity, i.e., early harvest
preparationsisolated bytheverticalrotor pro-cedure and cross-linked immediately after iso-lation. This type of interaction has not been demonstratedpreviously by cross-linkingin
par-amyxoviruses.The
ability
of NP and Mtoform aheterodimerappears tobe thereflection ofastable complex rather than a transient one formed momentarily by diffusionof components for a numberofreasons. Comparable complex formation was demonstrated at 37 and 0°C,
wherediffusionis severely limited, and also with
aphotoactivated reagent which yields a reactive
species witha lifetimeon the order of millisec-onds (6).
This report of NP-M heterodimerformation
in paramyxoviruses demonstrates that the M
protein layer is in close proximity (1.1 nm or less) to thenucleocapsid and could serve as its recognition siteduring viralassembly. The pro-gressive loss of the ability of NP and M to form a heterodimer as the virus is stored without a corresponding loss in infectivity suggests that thisspatial relationship alters afterparticle for-mation and is not necessary for the ensuing
stagesof the infectiousprocess. Thisstructural
changeis notsimplydue to freezing and thawing. Viral preparationsthawed after 1 week of
stor-age at-70°C still formedsome NP-M
hetero-dimer upon treatment with cross-linking
re-agents.Rather, itappearsthat thepreparations
age upon storage even at-70°C. Theprogressive
lossof the interaction betweenNP and M may reflect eitherageneral breakdownof weak, non-covalent interactions between proteinsor inac-tivation ofparticular binding sites between the nucleocapsid and membranecomponentsof the virus. A recent electron microscopic study of Sendai virus (12) indicates that as the virions ageinovo(late harvest preparations)orinvitro, the nucleocapsid becomes irregularlyfolded and detaches from the viral envelope.
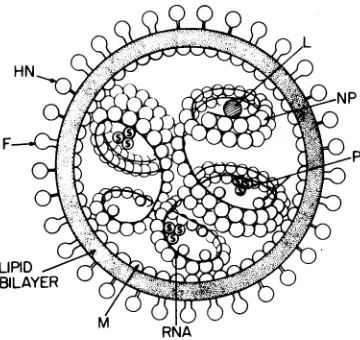
F-FIG. 10. Astructural modelofprotein-protein in-teractions within theSendaivirion.RefertoTable2
and textfor description of protein complexes
pro-ducedbynativedisulfidebondingorreversible
chem-icalcross-linking.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:13.504.277.457.432.602.2]Figure10depictsourmodel of the topograph-ical organization of proteins within the para-myxoviruses,specifically Sendai virus, basedon theprotein-proteininteractions discussed in this
paperandcurrentknowledgeofparamyxovirus
structure. The glycoproteins HN and F form
separatespikesontheoutersurface of the
mem-brane envelope. Each spike appears to be an
oligomer whose component polypeptides asso-ciatethrough noncovalent interactionsor disul-fide bonding or both. Shimizuet al. (35) have
separated two types ofdumbbell-shaped spikes
(HN+ and HN-) from detergent-solubilized
Sendai virus that differed in their biological,
serological, morphological, and chemical prop-erties.
Theglycoproteins ofmembrane-enveloped
vi-rusesappear to associate with thelipid bilayer
through a relatively small, predominantly
hy-drophobic peptide (14). Cross-linking studies with SemlikiForestvirus (8) and vesicular sto-matitis virus(7)suggestthat in these lipid-con-taining viruses the anchorpeptide tailspansthe bilayer. The selective extractionexperiments de-scribed in this paper suggest that HN and F of Sendai and Newcastle disease viruses are
embeddedinthelipid bilayer. However,no evi-dence for transmembranecross-linking between the glycoproteins andM wasfound for thetwo paramyxoviruses examined in this study, and previous reportsfrom this laboratory of trans-membranecross-linking betweenHNandNP in NDV (HP-16) (21, 37) wereshowntobea mis-interpretation of data due to the inadequate resolution of the two-dimensional gel system used. The lackofcross-linking between HN or
FandMbyno meanseliminates the possibility
thatthe glycoproteins span the bilayer. Cross-linkingreagents with different site specificities than those usedinthisstudy might demonstrate this topographicalrelationship.
Our cross-linking data indicate that the nu-cleocapsidlies inclose proximitytothe M pro-teinlayerinfreshly harvested paramyxoviruses.
The ease of extraction of P and L from the
nucleocapsidsuggests a moresuperficial location
in or weaker associationwiththe nucleocapsid
forthese twoproteinsthan the
firmly
attachedNP. Both thePprotein ofSendaivirus and the
NPprotein of NDV (HP-16) exist as monomers
and as disulfide-linked homotrimers. A
func-tionalrole(s) for these trimers is not known.
Cross-linking of intact virus revealed little
more about the organizationof the
paramyxo-virus nucleocapsid beyond the relationship of
NP as nearestneighbor to itself. No cross-linking
of PorLwhich are alsopart of the transcriptive
apparatus of Sendai virus (19) was observed.
Whether P and L interact with NP ordirectly
with viral RNAorboth is stillopen toquestion. Cross-linking of isolated Sendai virus nucleocap-sids with DTBP,tetranitromethane,ordimethyl suberimidate has been previously reported to produce homooligomers of NP andto convertP andL tohigh-molecular-weightcomplexes (27,
28).In ourstudy,cross-linkingof intact virus led
toformation ofverysmallamountsof NP hom-otrimerandhomotetramer, butnot to high-mo-lecular-weight complexes of PorL.Thismaybe
duetothe presenceof the viral membrane which
could either limittheaccessibilityof
cross-link-ing reagents to the nucleocapsid or subtly
change thestructure of thenucleocapsid by as-sociating with it during the processof viral as-sembly.
ACKNOWLEDGMENTS
Wegratefully acknowledge the assistance of Rochelle Weiss inpreparing the egg-grownSendai virus and of Tokichi
Mi-yakawafor synthesizing thecross-linking reagent PAPDIP used in thisstudy. We also wish to thank Debi P.Nayak and Joseph T. Seto for theirhelpfulcommentsontheproposed structural model of Sendai virus.
This workwassupportedby Public Health Research grant GM-18233 from the National Institute of General Medical Sciences and by a grant from the Muscular Dystrophy Asso-ciation,Inc. M.A.K.M.is therecipient of postdoctoral fellow-ship grant DRG 118-FT from the Damon Runyon-Walter Winchell Cancer Fund.
LITERATURE CITED
1. Bayley,B., and J. R. Knowles. 1978. Photogenerated reagents for membrane labeling. I. Phenylnitrene formed within thelipidbilayer. Biochemistry 17:2414-2423.
2. Bradford,M. M.1976.Arapid and sensitive method for the quantitation ofmicrogram quantities of protein utilizing the principle of protein-dye binding. Anal. Bio-chem.72:248-254.
3. Crain,R.C.,andG. V. Marinetti.1978.Cross-linkingof membranephospolipid and protein using putative mon-ofunctional imidoesters. Chem.Phys. Lip.21:195-204. 4. Das, M., and C. F. Fox.1977.Specific radiolabeling of a
cellsurface receptor forepidermalgrowthfactor. Proc. Natl. Acad. Sci. U.S.A. 74:2790-2794.
5.Das, M., and C. F. Fox. 1978.Molecular mechanism of mitogen action:processing of receptor induced by epi-dermalgrowthfactor. Proc.Natl. Acad.Sci. U.S.A.75: 2644-2648.
6. Das, M.,andC. F. Fox.1979.Chemicalcross-linking in biology. Annu. Rev.Biophys. Bioeng. 8:165-193. 7. Dubovi,E.J., and R. R.Wagner.1977.Spatial
relation-ships of the proteins of vesicular stomatitis virus: in-duction of reversible oligomers by cleavable protein cross-linkers and oxidation. J. Virol.22:500-509. 8. Garoff, H.,and K. Simons. 1974.Location of thespike
glycoproteins inthe Semliki Forestvirus membrane. Proc.Natl. Acad. Sci. U.S.A. 71:3988-3992.
9. Henning, R., R. J. Milner, K. Reske, B. A. Cun-ningham, and G.M.Edelman. 1976. Subunit struc-ture, cell surface orientation, and partial amino-acid sequencesofmurinehistocompatibility antigens. Proc. Natl.Acad.Sci. U.S.A. 73:118-122.
10. Homma,M.,K.Shimizu,Y. K.Shimizu,and N. Ish-ida. 1976.OnthestudyofSendaivirus hemolysis. I. Complete Sendai virus lacking in hemolytic activity. Virology71:41-47.
11. Ji,T. H. 1979. Theapplication of chemical crosslinking
on November 10, 2019 by guest
http://jvi.asm.org/
166 MARKWELL AND FOX
for studiesoncell membranes andthe identification of surface receptors.Biochim. Biophys. Acta 569:39-69. 12. Kim, J.,K.Hama, Y. Miyake, and Y. Okada. 1979. Transformation of intramembrane particles of HVJ (Sendai virus) envelopes from aninvisible to visible formonaging of virions. Virology 95:523-535.
13. Laemmli, U. K. 1970. Cleavage of structural proteins
during the assembly of thehead of bacteriophage T4. Nature (London)227:680-685.
14. Lenard, J.1978.Virus envelopes and plasmamembranes. Annu. Rev.Biophys. Bioeng. 7:139-165.
15. Li,J.K.-K., and C. F. Fox.1975.Radioiodinationof the
envelope proteins ofNewcastledisease virus. J. Supra-mol. Struct. 3:51-60.
16. Markwell, M. A. K., and C. F. Fox.1978.Protein-protein interactions within the Sendai virion. J. Supramol. Struct.(Suppl. 2),p.261.
17. Markwell, M. A. K., and C. F.Fox. 1978. Surface-specific iodination of membrane proteins of viruses and eucaryoticcellsusing
1,3,4,6-tetrachloro-3a,6a-diphen-ylglycoluril.Biochemistry17:4807-4817.
18. Markwell, M. A. K., S.M.Haas, L. L. Bieber,and N. E. Tolbert.1978.Amodification of theLowry
proce-dureto simplify protein determination in membrane andlipoprotein samples. Anal. Biochem. 87:206-210. 19. Marx, P. A., A. Portner, and D. Kingsbury. 1973. Sendai virion transcriptase complex:polypeptide
com-positionand inhibitionbyvirionenvelope proteins.J. Virol. 13:107-112.
20.McSharry,J.J.,R. W.Compans,and P. W.Choppin. 1971.Proteinsof vesicular stomatitis virus and of phe-notypicallymixed vesicular stomatitis virus-simian
vi-rus5 virions. J. Virol. 8:722-729.
21. Miyakawa, T.,L. J.Takemoto,and C. F. Fox. 1976. Supramolecular organizationofproteinsinNewcastle diseasevirus,p.485-497. In D.Baltimore,A. S.Huang, and C. F. Fox (ed.),Animalvirology.Academic Press Inc., NewYork.
22. Nagai, Y.,T.Yoshida,M.Hamaguchi,M.Iinuma,K. Maeno, and T. Matsumoto. 1978. Cross-linking of Newcastle disease virus(NDV) proteins.Arch. Virol. 58:15-28.
23. Neurath,A.R.,S. K.Vernon, R.W.Hartzell,and B. A. Rubin. 1973. Virocidalcleavageofdisulphidebonds within structuralproteinsofSendaivirus. J. Gen. Virol. 19:9-20.
24. Nicolson,G.L.,and R. G. Painter. 1973. Anionic sites ofhumanerythrocytemembranes. II. Antispectrin-in-ducedtransmembraneaggregationof thebindingsites forpositively charged colloidalparticles. J. Cell Biol. 59:395-406.
J. VIROL.
25. Ozawa,M., A.Asano,and Y.Okada.1976.Importance of interpeptide disulfide bond in a viral glycoprotein with hemagglutination and neuraminidase activities. FEBS Lett. 70:145-149.
26. Peters, K., and F. M. Richards. 1977. Chemical cross-linking. Annu. Rev. Biochem. 46:523-551.
27. Raghow, R.,D. W. Kingsbury, A. Portner,and S.
George.1979.Topography of a flexible ribonucleopro-tein helix:protein-protein contacts in Sendai virus nu-cleocapsids. J. Virol.30:701-710.
28. Raghow,R., and D. W.Kingsbury.1976.The molecular organization of Sendai virus nucleocapsids, p. 471-484. In D. Baltimore, A. S. Huang, and C. F. Fox (ed.), Animalvirology. Academic Press Inc., New York. 29. Rott,R.,and H. D. Klenk.1977.Structure andassembly
of viral envelopes, p. 47-81. In G. Poste and G. L. Nicolson (ed.), Virus infection and the cell surface. North-Holland Publishing Co., New York.
30. Samson, A.C.R.,and C. F. Fox.1973.Precursor protein forNewcastle disease virus. J. Virol. 12:579-587. 31. Scheid, A., and P. W. Choppin. 1973. Isolation and
purification of theenvelope proteins of Newcastle dis-easevirus. J. Virol. 11:263-271.
32. Scheid, A.,and P. W.Choppin.1974.Identification of biological activities of paramyxovirus glycoproteins. Ac-tivation ofcell fusion, hemolysis, and infectivity by proteolytic cleavage ofaninactive precursor protein of Sendai virus.Virology 57:475-490.
33. Scheid,A., and P. W. Choppin. 1977. Two disulfide-linkedpolypeptide chains constitute the active F pro-tein of paramyxoviruses.Virology 80:54-66.
34. Shimizu,K.,and N. Ishida. 1975. The smallest protein ofSendai virus: its candidate function of binding nu-cleocapsidtoenvelope. Virology 67:427-437. 35. Shimizu, K., Y. K. Shimizu, T. Kohama, and N.
Ish-ida. 1974. Isolation and characterization of two distinct typesof HJV(Sendaivirus) spikes. Virology 62:90-101. 36. Sweadner,K.J. 1977.Crosslinking and modification of Na,K-ATPase by ethyl acetimidate. Biochem. Biophys. Res. Commun.78:962-969.
37. Takemoto,L. J., T.Miyakawa,andC.F.Fox. 1977. Analysis of membrane protein topography of Newcastle disease virus and cultured mammalianfibroblasts, p. 605-614.In J. P.Revel,U.Henning,and C. F. Fox(ed.),
Cellshapeand surface architecture. Alan R.Liss,Inc.,
New York.
38. Warren, L., and M. C. Glick. 1969. Isolation of the surface membranes of tissue culturecells, p.66.InK. Habel and N. P. Salzman (ed.), Fundamental tech-niques invirology.Academic PressInc.,New York.
on November 10, 2019 by guest
http://jvi.asm.org/