Copyright ©1972 AmericanSociety forMicrobiology Printed inU.S.A
Control
of
Interferon
Synthesis:
Effect of
Diethyl-aminoethyl-Dextran
on
Induction
by
Polyinosinic-Polycytidylic Acid
JAN VILCEK, SANDRA L. BARMAK, AND EDWARD A. HAVELL
Departmentof Microbiology, New York UniversitySchool of Medicine, New York, New York 10016
Received for publication24July1972
Interferonproductionincultures ofrabbit kidney cells (RKC) stimulated with 10
to250,igofpolyinosinic-polycytidylic acid (polyI polyC) permlpeakedat3to4
hraftertheexposureofcellstoinducerand rapidly declined thereafter. On the other hand, RKC stimulatedwithpoly I poly C (10or2 jig/ml) inthepresenceof
diethyl-aminoethyl (DEAE)-dextran (100or20 jig/ml,respectively) producedaprotracted
interferonresponse, with the release of interferon continuing for over24 hr. The
kinetics of interferonproduction inRKC stimulated with lower concentrations of
the mixture ofpolyI polyCandDEAE-dextranwere similartotheresponse
pro-ducedbypolyI-polyCalone(10to250,ug/ml). Only theresponsesthatterminated earlywereparadoxically enhanced bytreatmentwithlowdoses ofactinomycinD or
withcycloheximide.Cellsstimulated with 50 Mgof poly I polyC/mni showed
hypo-responsiveness to a secondinterferoninduction with poly I-poly Cwhen restimu-lated 7 hrafter primaryinduction. Thishyporesponsiveness could beovercome by
restimulatingwithhigherconcentrations ofthe polyI polyC-DEAE-dextran
com-plex. Theresults are compatible with thehypothesis that the early termination of
interferonproductionandhyporesponsivenesstorepeated inductionwith polyI* poly
C areduetoa cellularrepressor exerting negative controlon interferonsynthesis,
and that the increased cellular uptakeofpoly I -poly C in thepresence of
DEAE-dextranmay effectively neutralizethe repressor. These results also suggested that the often observed different kineticsandthevaried effects of inhibitors ofribonucleic
acidorprotein synthesisoninterferonresponsesin variouscellsand in cells
stimu-lated with different inducers (suchaswith virusesascompared withpolynucleotides)
need notimply the existenceoffundamentally different mechanisms ofinterferon production.
Polyinosinic-polycytidylic acid (polyI-poly C)-induced interferon
production
in rabbit kidneycells(RKC)hasbeenwidely
employed
asamodelsystem for the study of cellular control
mecha-nismsoperating in interferon
synthesis.
Thesys-tem ischaracterized byarapid synthesis and
re-lease ofinterferon, whichpeaksin3 to4hrafter
the exposure of cells to the inducer. Interferon
production declines rapidly thereafter; concur-rently with the cessation of the interferon
re-sponse, cells develop a state of hyporesponsive-ness to
repeated
induction (3, 14,15).
Judicioustreatmentof induced cells withsome
inhibitors of ribonucleic acid (RNA) or protein synthesishas beenfoundtointerferewith the
nor-mal shut-off process of interferon synthesis and
to result in a
paradoxical
enhancement of inter-feronproduction.These resultssuggestedthattheearlytermination ofinterferon
production
is dueto the synthesis of a regulatory protein (repres-sor). Theparadoxical enhancement ofinterferon production by some metabolic inhibitors is
thoughtto bethe result ofa
preferential
inhibi-tion of repressorsynthesisoverinterferon
synthe-sis
(12,
14).Similar experiments with some other types of
cell culture, such as with diploid human cell strains (8, 10; E. A. Havell and J. Vilcek, Bac-teriol. Proc., p. 195, 1971), largely upheldthe
re-sults and the conclusions of the experiments in RKC. However,poly
I.
polyC-induced interferonproduction in cultures ofmouse Lcells (11,
14)
and chickembryo cells(9) couldnotbe enhanced
bytreatmentwith metabolicinhibitors, seemingly
suggesting thatthecontrolof interferon
synthesis
614
on November 10, 2019 by guest
http://jvi.asm.org/
CONTROL OF INTERFERON SYNTHESIS
in those lattercells is somehow different from the controlexertedin RKC.
The present study wasinspired by the fortuitous observation that interferon production in RKC stimulated by the complex of poly I- poly C and
diethylaminoethyl (DEAE)-dextran neither dis-played the early shut-offphenomenonnor could it be paradoxically enhanced by treatment with metabolicinhibitors. Further experiments offered anexplanation of theseobservations that is fully compatible with the hypotheses advanced earlier onthemechanism ofinterferon induction by
poly-nucleotides in RKC(lOa, 14, 16). Moreover, this study suggested that the differences in the re-sponse of poly I-poly C-induced interferon
pro-ductiontometabolicinhibitors, such as observed between RKC and some other types of cells, may not necessarily be due to fundamental
dis-similarities in the underlyingmechanisms of the control of interferon synthesis, and could be causedby less essential differences in the mode of
deliveryanduptake ofthe inducer.
MATERIALS AND METHODS
Cell cultures. Cultures of RKC were prepared by trypsinization of kidneys from 2- to 4-week-old rabbits.Allexperiments were done in secondary RKC cultures grown to confluency in 60-mm petri dishes (Falcon Plastics, Los Angeles, Calif.) at 36 C in a
humidified incubator provided with 5% CO2. Eagle
minimal essential medium (MEM) with 10% heated fetalcalfserum or2% gammaglobulin-freefetalcalf
serum was employed as growth medium or
main-tenancemedium, respectively.Alltissueculture media andserawerepurchasedfromGrandIslandBiological
Co., GrandIsland, N.Y.
Interferon induction. RKC cultures were washed
oncewithEarlebalanced salt solution(EBSS)and in-cubatedat36Cwith theappropriateconcentration of
poly
I.-poly
C (suppliedby theAntiviral Substances Program, National Institutes of Health, Bethesda,Md.) orof the mixture ofpolyI-poly C and DEAE-dextran (molecular weight, 2 X 106, Pharmacia, Uppsala,Sweden),diluted inwarm(37C)
phosphate-bufferedsaline (PBS; 0.13 MNaCl, 7 mmphosphate, 0.9mimCaC12,and 0.5 mMMgCl2-6H20; pH 7.4). The mixture of poly I-poly C (10 sg/ml) and DEAE-dextran(100,g/ml) in PBSwasfirstincubated for 30 minat37Cinawaterbath; furtherdilutions of this mixture were prepared in warm (37 C) PBS as
re-quired. RKC were incubated with 1 ml of the ap-propriate inducer for 30 or 60 min, as indicated, andwerethoroughlywashedwith EBSS thereafter.
Interferon titrations. A modification of the
semi-micromethod described by Armstrong (1) was
em-ployed.IndividualwellsofMicro Test II tissueculture plates (FalconPlastics,LosAngeles,Calif.)werefirst
filledwith50Aliters of serum-free MEM, bufferedwith
0.155%sodiumbicarbonate,6.6mm N-2-hydroxyethyl-piperazine-N'-2'-ethanesulfonic acid, and 3.3 mm
tricine to pH 7.6, and containing 100 units ofpenicillin, 100jugofstreptomycin, 1001Agofgentamicin, and 2.5 ,ug ofFungizone(amphotericin B) per ml. Portions of 25 or 50jliters of the interferon samples were then added to the first well. Series of two- or threefold dilu-tions were then prepareddirectly in the microplates by serially transferring 25 or 50;&literswith anEppendorf automaticmicropipette (Brinkmann Instruments Inc., Westbury, N.Y.). Each well was then seeded with 40,000 RKC(obtainedbytrypsinizationofprimary or secondarycultures) in100uliters of MEM containing buffers and antibiotics as indicated above, plus7.5% heated gammaglobulin-free fetal calf serum. After 18 hr ofincubation at 36 C in an atmosphere of 5% C02, each well was inoculated with 1,000plaque-forming
units (PFU) of the Indiana-type vesicular stomatitis virus in 50 ,Aliters of serum-free MEM with additions asabove. Severalwells on eachplate served as virus controls and cell controls.
Thetitrationswerescoredmicroscopically24to 48 hr after virus inoculation, when the virus controls showed
completedestruction by the virus. Thehighestdilution of thetitratedsamplecausingan atleast 50% protec-tion ofcells was considered the end point. In most
experiments,eachsample was titrated in duplicate. An internal laboratory rabbit interferon standard was
included with each titration. This internal standard had been calibrated against areference rabbit inter-feron standard prepared by thelaboratoryof Monto Ho, University of Pittsburgh, Pittsburgh, Pa., and received from the Reference Reagents Branch, Na-tional Institutes of Health, Bethesda, Md. The calibra-tion of the internal standard against the reference standard was done by the plaque assay (16) with the
useof2ml of each interferon dilutionperplate. All
interferontiters were corrected tothis standard. Thus,
although the actual volume of diluted interferon samples employed in the microplate assay was 100
,Iiters, all interferon yields are expressed per 2 ml.
Disregarding the volume used in the assay, the
sen-sitivityof themicroplateassay wasabouthalf that of the plaque assay. The internal laboratory standard calibratedtocontain 1,000referenceunits/2ml in the
plaque assayaveraged about 500 units in the micro-plate assay.
Chemicals. Cycloheximide (Acti-dione) was ob-tained from TheUpjohnCo.,Kalamazoo, Mich.,and actinomycin DwaspurchasedfromCalbiochem, Los
Angeles,Calif.
RESULTS
Kinetics of interferon production in cultures stimulated with various concentrations of poly
I-poly C and of the poly I -poly C-DEAE-dextran mixture. Interferonproduction in RKC cultures stimulated with various concentrations of poly
I-polyC,rangingfrom 10 to 250 ,ug/ml, peaked
at 3 to 4hr after theexposure of cells to the in-ducer and declined rapidly thereafter. Although
RKCstimulated with higher doses ofpolyI-poly Cproducedmoreinterferon,thekinetics of
inter-feron release werenot strikingly different.
How-615 VOL. IO, 1972
on November 10, 2019 by guest
http://jvi.asm.org/
616 VILCEK, BARMA
ever,cultures of thesamebatchof RKCexposed
toamixture of poly I * poly C and DEAE-dextran (10 and 100
Ag/ml,
respectively) produced amarkedly different type ofresponse: the rateof interferonproduction continuedto riseuntil5 hr after induction andremained high throughout the duration of the experiment (Fig. 1).
Todeterminewhether theprotracted interferon
response wascharacteristic of thepolyI-poly
C-DEAE-dextranmixture, or whether it was
essen-tiallyafunction of the dose ofinducertaken up by cells, RKC werestimulated withvarious dilu-tionsof themixtureofpolyI*polyCand
DEAE-dextran. A mixture of 10 and 100 jug of poly
I*poly C andDEAE-dextran perml,respectively, producedaresponsesimilartotheoneobserved with thesamedoseofinducer inthepreceding
ex-periment.Afivetimeslower concentration of this
mixture also produced a protracted response, but
the rate ofdecline of interferon
production
was faster than with thehigher
dose.Cellsstimulated witha25-fold dilution of theoriginal poly
I*poly
C-DEAE-dextran
mixtureproduced
a responsewhichwasquite similartothat
produced by poly
I-poly C uncomplexed with DEAE-dextran, in
that itreached a peak between 3 and4hr after induction andrapidly
declined
thereafter(Fig.
2).
That theprotracted interferonresponse was not
theresult ofadirect action of DEAE-dextranper se on RKC cultures was shown by the results summarized in Fig. 3. RKC cultures were first treatedwithpolyI *polyCfor1hr,and thenwere
washed and incubated for 30 min with various concentrations of DEAE-dextran alone. Such
separate treatment with DEAE-dextranfailed to
alter the kinetics of interferon
production
charac-teristicof polyI*polyCalone.PolyI *poly C is knowntoformacomplexwith
1 2 3 4 56 7
HOURS
[image:3.495.93.437.286.585.2]8 9 23 30
FIG. 1. Kineticsof interferon production inrabbit kidneycells stimulated with various concentrations ofpoly
I-polyCorwithamixtureofpolyI-poly C and DEAE-dextran.Fourgroupsof cultureswere incubated for Ihr
withtheindicatedconcentrationsof inducer,washedfivetimesthereafter, and replenishedwith 2ml ofmaintenance
medium.Atthe indicated intervals,fluidswerecollected,andthe cultureswerewashedoncewith EBSS antd imme-diately replenishedwithwarm (37C) freshmaintenancemedium. Interferontitersweredetermined inthepooled
fluids from individualgroups.If the interval betweentwosamplingswas morethan Ihr, the determined interferon
yield (units/2 ml)wasdividedbythe number of hoursthat hadelapsedfrom the collection of the precedingsample.
1,2801
640+
3201
E
cmJ
I-tn
z
0
-a I
w
z
w
w
1601
80s
404
201
101
5
S 2.5
on November 10, 2019 by guest
http://jvi.asm.org/
CONTROL OF INTERFERON SYNTHESIS
6401
320
1
i i
i i I Ii , K E
1 2 3 4 5 6 7 8 9 10 23 31
[image:4.495.67.430.78.383.2]HOURS
FIG. 2.Kineticsofinterferonproduction in rabbit kidney cellsstimulated withvarious concentrations ofthe
poly I-polyC-DEAE-dextran mixture. Fourgroupsofcultureswereincubatedfor30 min with the indicated
con-centrationsofthepolyI-poly C-DEAE-dextranmixtureorforI hrwithpoly I-poly C(250 jig/ml). Other
pro-cedureswere asintheexperimentshowni inFig.1.
DEAE-dextran (4). The formation of such a
complex isapparently responsible forthegreatly
enhanced cellularuptakeofpoly I-polyCinthe presenceof DEAE-dextran(2, 5). Theprotracted interferonproductioninRKCculturesstimulated
with high doses of the poly I-poly C-DEAE-dextran mixture is thus likely to be due to the moreefficientuptake ofthe inducerbycells.
Effects ofactinomycinD and cycloheximide on
interferonproduction stimulated by polyI-poly C
or poly I poly C-DEAE-dextran mixtures. RKC
cultureswerestimulatedeitherwithpolyI* polyC
(50
,g/ml)
or with the mixture ofpolyI polyCand DEAE-dextran (10 and 100 ,ug/ml,
respec-tively). Immediately after a 30-min incubation with theinducer,groupsof cultures weretreated with various concentrations ofactinomycinDor
cycloheximide. Theyieldof interferonwas
deter-mined in culture fluids collected 24 hr after
induc-tion.Actinomycin D,atconcentrations of0.1and 0.3
Ag/ml,
increased theyieldof interferonstimu-lated withpolyI polyCalone. No such increase
wasnotedin RKCstimulated with themixtureof
polyI-polyCandDEAE-dextran treated with the same doses of actinomycin D. An even more
significant difference was found in the effects of
cycloheximide on the two interferon responses:
the interferon yield in cultures stimulated with polyI.polyCalonewasincreased about fivefold,
but itwas decreased in cells exposedtothe poly
I-poly C-DEAE-dextranmixture(Table 1).
Cultures stimulated with decreasing
concentra-tions ofthe polyI-polyC-DEAE-dextran mixture
produced decreasing yields ofinterferon, but at
the sametime interferon production became
in-creasingly sensitive to paradoxical enhancement
byactinomycinDorcycloheximide (Table 2).
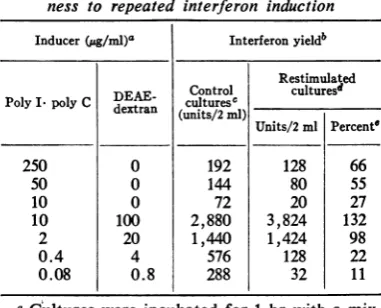
Hyporesponsivenesstorepeatedinterferon induc-tionin cultures restimulated with various dosesof
poly I poly C and of the poly I poly C-DEAE-dextran mixture. It was shown that RKC
stimu-lated with poly I poly C (50 jug/ml) produced
decreased interferon yields when restimulated with thesamedoseof inducer. Such hyporespon-160 *
801
cmJ
-z
I
0
z
0
Iie
a:
I-z
40
20
101
617 VOL. 10, 1972
on November 10, 2019 by guest
http://jvi.asm.org/
640
.
0
3201
A160
80.1
40Q
20
10+
5.
1
2
3
4
5
6
7
8
9
10
23
HOURS
FIG. 3. Effect ofDEAE-dextran,addedtorabbitkidneycellsafterinduction withpolyI-poly C, onthe kinetics
of interferon production.Cultureswereincubatedfor Ihr withpoly I-polyC(250 pAg/ml)and washedfivetimes
thereafter. Threegroups werethen treatedfor 30min with the indicatedconcentrationsofDEAE-dextran while
onegroup receivednofurthertreatment.Otherprocedureswere asin theexperimentshown inFig.1.
TABLE 1. Effectsof actinomycinDandcycloheximideoninterferon productionstimulated with
poly I-poly Cor amixtureofpoly I-poly Cand DEAE-dextran Interferonyieldb
Treatmenta PolyI-poly Cc Poly I-poly C +DEAE-dextrand
Units/2ml Percent' Units/2ml Percent' None... 87 (100) 8,100 (100) Actinomycin D,0.1
,pg/ml
... 218 250 8,100 100ActinomycinD,
0.3,pg/ml
... 150 172 4,050 50Actinomycin D,
1.0,pg/ml
... 16 18 2,025 25Cycloheximide, 1.0,pg/ml. ... 486 558 4,050 50
Cycloheximide, 10.0
pg/ml
... ... 486 558 1,620 20aThe indicated concentrations ofinhibitors were added immediately after a 30-min exposure to
inducer. ActinomycinDwasleft on the cultures for 30 min; thereafter, thecultures were washed twice and replenished with drug-free maintenance medium. Cycloheximide was maintained in the cultures throughout the duration ofexperiment.
bInculture fluids collected 24 hr after exposure to inducer. Fluids from cycloheximide-treated
cul-tures weredialyzed priortointerferon assay.
cCultureswereincubated with 50 ug of polyI-polyC/ml for 30 min and washed five times thereafter.
dCultureswereincubated with a mixture of poly I- polyC(10,g/ml)andDEAE-dextran (100,g/ml)
for 30 min and washed five times thereafter. e Percentageof control.
jg
/ml
SYMBOL
DEAE-DEXTRAN
*
0
a 4
D
20
100
E
cm
1-z
D
0
I
cr
w
OJ
L I
z
618
be .
IDA
#4 i
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.495.61.476.55.382.2]CONTROL OF INTERFERON SYNTHESIS
TABLE 2. Effects of actinomycin D andcycloheximide onlinterferon production stimulated with various concentrations of poly I- poly C andDEAE-dextran
Interferonyieldb Inducer(.g/ml)a
ActinomycinDc
Cycloheximide,10Ag/mld
Polyl-polC dextran Control 0.3
ug/ml
0.1jug/ml
PolyI-polyC DEAE- (units/2ml) 0. /m01pgm
dextran
Units/2ml Percent' Units/2 ml Percent Units/2 ml Percent'
10 100 8,747 2,916 33 4,374 50 1,458 16
2 20 1,458 972 66 972 66 1,458 100
0.4 4 162 972 600 2,187 1,345 2,916 1,800
0.08 0.8 18 162 900 1,458 8,100 2,916 16,200
a
Cultures
wereincubated for 30 min with a mixture of the indicated concentrations ofpoly I- poly Cand DEAE-dextran and washed four times thereafter. b Inculture fluids collected 24 hr after exposure to inducer.
cTreated with the indicated concentrations for 30 min immediately after the exposure to inducer.
Thereafter, thecultureswere washed twiceandreplenished with drug-free maintenance medium.
dCycloheximide was added immediately after the 30-min exposure of cultures to inducer and was
maintained in the culture fluids throughout the duration of the experiment. Prior to titrating the inter-feron content, the harvested fluids were dialyzed to remove the cycloheximide.
ePercentage of control.
siveness was shown todevelop concurrently with thecessation ofinterferon production and to last forabout48 hrafterthe first exposureofcellsto the inducer (3, 15; S. L. Barmak, Ph.D. thesis, NewYork University,1972).
Intheexperimentsummarized in Table3,RKC
werefirststimulated withpoly I polyC (50,ug/
ml)and wererestimulated7hrlaterwithvarious doses of poly I-polyC either complexed or un-complexed with DEAE-dextran. The interferon yields obtained on the second stimulation were
compared with yields from control, previously unstimulated culturesexposedtothe samedoses of inducer. The
degree
of hyporesponsiveness decreased with increasing doses ofpoly
I-polyCused for restimulation. Hyporesponsiveness was notdetectedincultures restimulated with2or 10
jig
ofpoly
I-poly
C per ml inthe presence ofa 10-foldexcessofDEAE-dextran.DISCUSSION
The kinetics ofinterferonproductionin RKC
stimulated withthepolyI-poly C-DEAE-dextran complex weredifferent from those observed with poly I-
poly
C alone. Stimulation with various concentrations of thepolynucleotide
uncom-plexed with DEAE-dextran produced an inter-feron response thatpeakedat3to4hrafterinduc-tion and declined quiterapidlythereafter. Onthe other hand, cells stimulated with 2 or 10Ag of
polyI
poly
C per ml in the presenceofa 10-foldexcess of DEAE-dextran
produced
a muchpro-tracted response, with interferon
being
releasedintothe culture fluid formorethan 24 hr.
Asnoted earlier (12,14),interferon
production
stimulated withpoly I poly C was paradoxically enhanced by treatment with certain concentra-tions of actinomycin D or by incubating stimu-lated cultures in the presence of cycloheximide.
Onthe other hand, the production ofinterferon stimulated with high concentrations of the poly
I-polyC-DEAE-dextran complex was suppressed by similar treatment withactinomycinDor cyclo-heximide.
These findings raised the question of whether interferon induction by the polyI *poly C-DEAE-dextrancomplexwasperhaps qualitatively
differ-entfromtheinterferon response elicitedby poly
I *polyCalone.Furtherexperimentsrevealedthat
interferon production in response to lower
con-centrations ofthe
poly
I-poly C-DEAE-dextran complex showed similar characteristics to inter-feronproduction after thestimulation with polyI-polyCalone: it ceased early after inductionand it could be paradoxicallyenhancedbytreatment
withactinomycinD orcycloheximide.
The findings supportthe previously suggested notionthat arelationshipexists among theearly
shut-offof interferon
production,
theparadoxicalenhancement ofinterferonsynthesisbymetabolic inhibitors, and the hyporesponsiveness to re-peated interferon induction by
poly
Ipoly
C(15).Theearlycessation of interferonproduction
has been attributed to a labile repressorprotein
that exerts a negative controlon interferon
syn-thesis.Theparadoxicalenhancement of interferon
productionby metabolicinhibitors is the result of
protractedreleaseof interferonand is believedto
be duetopreferentialinhibition of repressor syn-thesis over interferon synthesis (12, 14).
Hypo-VOL. l0, 1972 619
on November 10, 2019 by guest
http://jvi.asm.org/
TABLE3. Effect of stimulation with various concen-trations of poly I-poly C and of the polyI-poly
C-DEAE-dextran mixture on hyporesponsive-ness to repeated interferon induction
Inducer(ug/ml)" Interferonyieldb Restimulated Control culturesd PolyI. polyC DEAE- culturesc_________
dextran (units/2ml)
Units/2 ml Percente
250 0 192 128 66
50 0 144 80 55
10 0 72 20 27
10 100 2,880 3,824 132 2 20 1,440 1,424 98
0.4 4 576 128 22
0.08 0.8 288 32 11
a
Cultures
were incubated for 1 hr withamix-ture of the indicated concentrations of poly
I.polyCand DEAE-dextran and washed fivetimes thereafter.
IInculture fluids collected 24 hr after exposure to inducer.
cAt 7 hr prior to stimulation, these cultures
were incubated with PBS for 1 hr, washed, and then incubated in maintenance medium until
stimulation.
dAt 7 hrpriorto restimulation, thesecultures
were incubated with poly I poly C (50 ,ug/ml)
for 1 hr, washed three times, and replenished withmaintenancemedium;6 hrlater,thecultures
werewashedtwice and restimulated by incubating with theappropriateinducerfor1hr. Thereafter, the cultures were washed four times and replen-ished with maintenance medium. Culture fluids collected7hrafterprimary stimulationcontained 192 units of interferon per 2 ml. The interferon
yields givenare aftersubtraction of the residual
amount of interferon (16units/2 ml)produced by
control unrestimulated cultures during the same experimentalperiod.
e Percentageof control.
responsiveness to repeated induction by poly I-poly C, which appears concurrently with the
cessation of interferon production (15), is also
likely to be attributable to the action of this repressor. The repressorapparently controls inter-feronproduction by suppressing itssynthesisat a
post-transcriptional level, mostlikely bydirectly
bindingtotheinterferon messenger RNA (16). A
similarmechanism was earlier implicated in the
control of steroidhormone-inducedenzyme syn-thesis in animalcells(13).
Ithasbeensuggested that the first step in
inter-feron induction by polynucleotides involves the
binding and neutralization ofthe repressor pro-tein by the inducer (lOa). According to thisas yetspeculative notion,a basallevel ofrepressor
is thought to be continuously synthesized in
uninduced cells; the rate of repressor synthesis would be stepped up after interferon induction
andtheincreasedrepressor concentration would inturn resultin the cessationof interferon syn-thesis and hyporesponsiveness to repeated in-duction.
The observations made in this study could be explained by the increased cellular uptake of inducer, resulting in a more efficient binding of
therepressor incells stimulated with higher con-centrations of the poly I-poly C-DEAE-dextran
complex. Efficient neutralization ofthe repressor
could account for the lack of theearly shut-off, as well as for the failure to increase interferon
production by treatment with metabolic inhibi-tors. As long as the cellular concentration of
inducer is sufficient to neutralize the repressor,
interferon synthesis could continue,and inhibition of repressor synthesis by metabolic inhibitors would not be expected to promote interferon
production. The overcoming of
hyporesponsive-ness to repeated induction by higher
concentra-tionsofthepoly I. poly C-DEAE-dextran complex
could be explained on the same basis: by the
uptake ofenough inducer to neutralize the in-creased concentrationofrepressorbelieved to be present inhyporesponsive cells.
Wehave alsoconsidereda somewhatdifferent explanation forthedissimilarresponsesproduced by poly I poly C or low concentrations ofthe
poly
I.poly C-DEAE-dextran mixtures ascom-paredtothe responseobtained with high doses of
thecomplexedinducer. It was shown earlier that
primarymousekidney cell culturesarecomposed ofheterogeneous cell populationswhichdifferin
theirresponse to stimulation with poly I polyC orNewcastledisease virus(11).It cannotbe ruled outthat RKC culturesmay also havecontained heterogeneous cellpopulations, with one type
of
cellsthat could be stimulatedtoproducetheearly
response, andanother celltype characteristically producing a later and protracted interferon re-sponse that could be stimulated only with high doses ofthepolyI-poly C-DEAE-dextran
com-plex. However, experiments similar to the ones
describedinthis paper,performed in culturesofa
serially passaged human diploid cell strainandof
acontinuous line of RKC (RK 13)which are not
composed of markedlyheterogeneous cell
popula-tions, yieldedresults quite comparable to those in RKC (E. A. Havell, L. Mozes, and J. Vildek, unpublisheddata). Thus, cell heterogeneity does not seem to be a likely basis for the observed
differencesin theinterferonresponses.
That the presence of DEAE-dextran greatly
enhances therateof cellular uptakeof poly I-poly Cwasdemonstratedby other investigators (2, 5).
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.495.61.252.104.258.2]CONTROL OF INTERFERON SYNTHESIS
Whether the polycation actually increases the amountof inducer that penetrates across the cell membrane or only increases its rate of attachment to thecell surface has not been determined. It is also not clear what role, if any, the increased
resistance of the poly I-poly C-DEAE-dextran
complex to nuclease degradation (4, 6) plays in
promoting induceruptake and interferon
produc-tion.
Earlier studies failed to demonstrate paradoxi-calenhancement of polyI polyC-induced inter-feron production by inhibitors ofRNA orprotein synthesis in cultures ofL cells (11, 14) or chick embryo cells (9), and it was concluded that the control of interferon production in these cells is probably different from the control in RKC.
However, asthese studiesemployed high
concen-trations of the poly I-poly C-DEAE-dextran complex, the differences may, in fact, not have
been due to fundamental dissimilarities in the control of interferon synthesis but, rather, to
different conditions of induction. Ourresultsalso suggest thattheobserved differencesin the kinet-ics, andinthedegree of suppression or
enhance-ment by various metabolic inhibitors, of the interferon responses produced by viruses or by polyI polyC (7, 9, 15, 17) neednotimplythat
fundamentally different mechanisms operate in
interferon production stimulated by viruses or isolatedpolynucleotides.
ACKNOWLEDGMENTS
Wethank Fermina Varacalli and JuanCasanova for skilled technicalassistance.
This investigation was supported by Public Health Service grant AI-07057 and contract NIH-NIAID-70-2169 from the National Institute ofAllergyand InfectiousDiseases andby a
grant from the IrwinStrassburgerMemorialMedical Foundation. J. V.istherecipient ofPublic Health Service Career Develop-mentaward 5-K4-AI-38784. S. L. B. wassupported byPublic HealthServicetraininggrantGM-01290.
LIIERATURECITD
1. Armstrong, J. A. 1971. Semi-micro, dye-binding assayfor rabbit interferon.AppI.Microbiol. 21:723-725.
2. Bausek,G.H., andT.C.Merigan.1969. Cellinteraction with
a synthetic polynucleotide and interferon production in
vitro.Virology 39:491-498.
3. Billau, A. 1970. The refractory state after induction of inter-feron with double-stranded RNA. J. Gen. Virol. 7:225-232. 4. Carter, W. A., and P. M. Pitha. 1971. Structural requirements of ribopolymers for induction of human interferon: evi-dence forinterferon subunits, p. 89-105. In R. F. Beers and
W. Braun (ed.), Biological effects of polynucleotides. Springer-Verlag, New York.
5.Colby, C., and M. J. Chamberlin. 1969. The specificity of interferon induction in chick embryo cells by helical RNA. Proc. Nat. Acad. Sci. U.S.A. 63:160-167.
6. Dianzani, F., S. Gagnoni, and P.Cantagalli. 1970. Studies on the potentiating effect of DEAE-dextran on interferon production induced by double-stranded synthetic poly-nucleotides. Ann. N.Y. Acad.Sci. 173:727-735.
7. Finkelstein, M. S., G. H. Bausek, and T. C. Merigan. 1968. Interferon inducers in vitro: difference in sensitivity to inhibitors of RNA and protein synthesis. Science 161:465-466.
8. Ho, M., Y. H. Tan, and J. A. Armstrong. 1972. Accentuation of production of human interferon by metabolic inhibitors. Proc. Soc. Exp. Biol. Med. 139:259-262.
9. Long, W. F. and D. C. Burke. 1971. Interferon production by double-stranded RNA:a comparison of induction by reovirustothatbyasynthetic double-stranded polynucleo-tide. J. Gen. Virol. 12:1-11.
10. Myers,M. W., and R. M. Ftiedman. 1971. Potentiation of humaninterferon production by superinduction. J. Nat. CancerInst. 47:757-764.
10a. Ng, M. H., and J. Vilcek. 1972. Interferons: physico-chemical properties and control of cellular synthesis, p. 173-241. In C. B. Anfinsen, J. T. Edsall, and F. M. Richards (ed.),Advances in protein chemistry, vol. 26. Academic Press Inc., New York.
11. Stewart,W.E.,II, L. B. Gosser, and R. Z. Lockart, Jr. 1971. Distinguishing characteristics of the interferon responses ofprimary and continuous mouse cell cultures. J. Gen. Virol. 13:35-50.
12. Tan,Y.H., J.A.Armstrong, and M.Ho.1971.Accentuation
ofinterferon production by metabolic inhibitors and its dependenceonproteinsynthesis. Virology 44:503-509. 13. Tomkins,G.M.,T. D.Gelehrter, D.Granner,D.Martin,
Jr.,H. H.Samuels, andE. B.Thompson. 1969. Control of specificgeneexpressioninhigher organisms. Science166:
1474-1480.
14.Vilcek, J. 1970. Metabolicdeterminants oftheinduction of interferon bya synthetic double-stranded polynucleotide in rabbitkidney cells.Ann.N.Y. Acad.Sci.173:390-403. 15. Vilcek, J. 1970. Cellularmechanisms of interferonproduction.
J. Gen. Physiol. 56:76s-89s.
16. Vilcek, J., and M.H.Ng. 1971.Post-transcriptionalcontrol
ofinterferonsynthesis. J. Virol. 7:588-594.
17.Youngner, J. S., and J. V. Hallum. 1968. Interferon produc-tioninmiceby double-strandedpolynucleotides: induction
orrelease?Virology 35:177-179.
VOL. 10, 1972 621