0022-538X/86/120888-07$02.00/0
Copyright© 1986, AmericanSocietyforMicrobiology
Phosphorylation Downregulates the DNA-Binding Activity of Simian
Virus
40 T
Antigen
DANIEL T. SIMMONS,* WENSHI CHOU,AND KENTRODGERS
School of Life andHealth Sciences, University of Delaware, Newark, Delaware 19716
Received 23 June1986/Accepted 11 August1986
Proteolytic fragments of simian virus 40tumor (T)antigen and T antigen thatwasdephosphorylated with alkaline phosphatase bound between 1.5to2 timesmoreorigin-containing simian virus40 DNA than did intact
Tantigen in DNA saturation experiments. Kineticexperiments showed that thesetreatmentsalsoenhanced the rateatwhich T antigen boundtotheDNA. The enhanced bindingofT-antigenfragmentscorrelated with the
generation of DNA-binding fragments that lackedtheNH2-terminal region. Dephosphorylation ofT antigenin
vitro resulted in the removal of phosphate groups from the NH2-terminal region as well as from the
COOH-terminal region. Totest theeffects of dephosphorylation onthesize of the protein,
immunoaffinity-purffied T antigenwassubjectedtosedimentation with and without priortreatmentwith alkaline phosphatase. Most of the purified protein sedimented as a monomer and no significant effect was observed after
dephosphorylation, indicatingthatthe enhanced DNA-binding activitywasprobablynotduetotheuncovering ofadditional binding sites buried specifically in oligomerizedTantigen.Taken together, these results indicate
thatin vivo phosphorylation ofthe NH2-terminal region (residues 106 to 124) decreases the binding of the proteintotheDNAorigin. Theeffectisreversed by in vitrodephosphorylationorbyproteolysiswhichremoves thehighly phosphorylated NH2-terminalarmof the polypeptide. Wesuggestthat phosphorylationinactivates
oneoftwodistinctDNA-binding activities onthe polypeptide chain perhaps corresponding totwoseparate
regions in T antigen.
Simian virus 40 (SV40) codes foraphosphoprotein(tumor [T] antigen) which is involved intheinitiation of virusDNA replication in infected monkey cells (37). In addition, this protein isrequired for the regulation of early (19,24)and late (3, 12)geneexpression ininfected cells. Ithas been demon-strated that therearethreesitesaroundthe DNAreplication
origin which can bindto T antigen in vitro (29, 39, 40). T antigen has thegreatestaffinityto site1,which is closestto theearly region (19, 39, 40). This is followed bybinding to sites2and then 3. Bindingtosite2isrequired for initiation of DNAreplication(20,30-32),whereasbindingtosites 1, 2, and
possibly
3 is necessary for the regulation of viralgeneexpression (10, 19, 20, 24, 25,29).
The DNA-binding region of the SV40 T antigen maps betweenresidues131and371(5, 21, 35).Itis clearfrom the work of several investigators (7, 8, 27), thatnewly madeT antigen binds significantly better to theorigin of SV40 DNA than does olderTantigen. Theexplanation for this observa-tion is not known for certain, although it is known that T
antigen becomes oligomerized (2, 6, 9, 15, 16, 22, 23) and phosphorylated (13, 26, 38, 42) with time after it is made in thecell.OligomerizedTantigenbindstoDNAless well than the monomerform(4, 7, 8, 27).
TheDNA-binding region ofthe polypeptide chain isnot phosphorylated (35). Most of the phosphate groups in T antigenarelocatedupstreamof thisregion between residues 106 and 124(13, 26,42). Wehave previously shownthat, in infected cells, phosphate groups are added and deleted at
these sites in sequential steps (34). The rates of these phosphate transfer reactionshavebeen measured (34) and, overall, phosphorylation proceeds with kinetics that are roughlycompatible withthe rateatwhich theDNA-binding activity of newly made Tantigenin the cell decreases(27).
*Correspondingauthor.
However, the function of these phosphorylation events is unknown.
We demonstrate here that proteolytic fragments of T
antigen that lack the potentially phosphorylated NH2-terminalregion(residues1 to130)bind about twiceasmuch
origin-containing DNA than intact T antigen at saturating
amounts ofDNA. Dephosphorylation of intact T antigen leads to a similar increase in DNA-binding activity. De-phosphorylation of purified T antigen has no effect on its size, indicating that its abilitytobindmoreDNAisnotdue totheexposureofDNA-binding regionsburied inan
oligo-merized form of the protein. It is suggested that in vivo
phosphorylation ofthe NH2-terminal region affects DNA-binding by abolishing one of two DNA-binding activities associated with newly made (unphosphorylated) T-antigen
monomers.
MATERIALS AND METHODS
Labeling of cells andimmunoprecipitation. BSC-1monkey
cellswereinfected with SV40at amultiplicityof10 PFU per cell.Cellswerelysedat38to40hpostinfectionwithasmall volume of Nonidet P-40 (NP-40) lysis buffer (20 mM Tris hydrochloride, pH 8.0, 500 mM NaCl, 1% Nonidet P-40)
containing80,ugofaprotininand800,ugofleupeptinper ml. Priortocentrifugation,thelysatewasdiluted with3volumes ofNP-40lysisbufferlackingNaCl. Inlabeling
experiments,
the cells were labeled with
L-[35S]methionine
(50 to 125,uCi/ml)or
[32P]H3PO4
(0.5to 1.0mCi/ml)inasmall volume of Eagle medium lacking unlabeled methionine orphos-phates, respectively, and containing 2% dialyzed fetal bo-vine serum.
Immunoprecipitation ofTantigenwas performedas pre-viously described (14), using serum from hamsters with SV40-induced tumors (anti-T serum) oranti-T monoclonal antibodies. The immunecomplexes were precipitated with fixed protein A-bearing Staphylococcus aureus. After the 888
on November 10, 2019 by guest
http://jvi.asm.org/
bacteriawere washed, the bacterialpellets were suspended inelectrophoresissample buffer (36)orinasolution contain-ing trypsin or alkaline phosphatase.
Immunoaffinity purification of T antigen. T antigen was purified from infected monkey cells by chromatography on Sepharose columns containing covalently bound PAb419 monoclonal antibodies (11), essentially as described by Simanis and Lane (33). The protein was eluted at high pH and immediately neutralized as described before (33); how-ever, it was not dialyzed to preventlosses in DNA-binding activity. It was stored frozen at -80°C insmall aliquots.
Partial proteolysis. Partial trypsinization of immuno-precipitated T antigen was performed by suspending the S.
aureus pellet in a small volume ofN-tosyl-L-phenylalanine chloromethyl ketone-trypsin (Worthington Diagnostics, Freehold, N.J.) (1 to200 ,jg/ml)in0.05 M(NH4)2CO3-0.1%
NP-40 (pH 8.6). Digestionwas carriedout at0°C for30min. Thereactionwasterminatedbytheaddition of aprotinin(0.1 to 1.0 mg/ml depending on the concentration of trypsin used).
Partialtrypsinization of immunoaffinity-purified T antigen was performed with various concentrations of trypsin (1 to 625,ug/ml)afteradjustingtheprotein solutionto 0.1 MNaCl, 0.1% NP-40, and 100,g of lactoglobulinper ml. Digestion was carried out for 30 min at 0°C and terminated with
aprotinin (0.1 to 1.0 mg/ml).
DNAfragment-binding assay. 32P-labeled SV40 DNA was cleaved withBstNI, and the labeled fragments were sepa-ratedon a2%agarosegel. FragmentG, which containsthe
replication originand thethreeT-antigen-binding sites, was recoveredfrom thegel by electroelution. To test the
DNA-binding activity of immunoprecipitated T antigen, the la-beled DNA fragment was added directly to the S. aureus
suspensioninDNA-binding buffer(0.02 MNaH2PO4,0.15 M
NaCl, 0.002 M dithiothreitol, 0.0001 M EDTA, 0.05% NP-40, 3% dimethyl sulfoxide, pH 6.8) (17). The binding reactionwascarriedoutfor various periods of time at room temperature.CalfthymusDNA(25,g/ml)wasaddedtostop thereaction and the bacteriawerepelleted in thecold. The S.aureus waswashed twice in wash buffer (0.01MTris,pH
8.0,0.15 MNaCl,0.5% NP-40) (17), andtheradioactivity in thepelletwasquantitated by liquid scintillationcounting.
The DNA-binding activity of solubilized T antigen or
fragments was determined with labeled DNA fragment G
essentially as described byMcKay (17)and Simmons (35).
Briefly,the protein-DNA complexwas immunoprecipitated with hamster anti-Tserumand S. aureus, and theamount of radioactiveDNAin theprecipitatewasquantitated by liquid scintillation counting.
Acrylamidegel electrophoresis.Acrylamide gel electropho-resis of sodium dodecyl sulfate-denatured T antigen or
fragmentswas performedaspreviously described(36). Alkaline phosphatase treatment. Immunoprecipitated T
antigenwas dephosphorylated by suspendingthe S. aureus
pelletin0.02 MTris(pH8.0)-0.005MMgCl2-0.01%bovine albumin (1) containing various concentrations of alkaline phosphatase(type VII;SigmaChemicalCo., St. Louis, Mo.) per milliliter. The sampleswere incubatedat room
temper-aturefor30min.
Immunoaffinity-purified Tantigen was dephosphorylated essentially as described above after adjusting the protein solutionto pH8.0with 1 M Tris base and addingMgCl2 to 0.005Mand bovine albuminorlactoglobulinto 100 ,ug/ml.
Sucrose gradient centrifugation. Immunoaffinity-purified labeled T antigen that had been treated or not treated with alkalinephosphatasewassubjectedtocentrifugation in 15 to
30% sucrosegradientscontaining0.01 MTris (pH
7.4)-0.14
M NaCI-0.001 M dithiothreitol. The gradients were centri-fuged for 17 h at 28,000 rpm and4°C inaSpincoSW41 rotor. About 33 fractions were collected and countedby scintilla-tion counting. Sedimentation coefficients were estimated from the sedimentation of Escherichia colirRNA.
RESULTS
Enhanced DNA-binding activity of T-antigen fragments. The relative DNA-binding activity of T antigen and T-antigen fragments was investigated by using both im-munoprecipitated and immunoaffinity-purified T antigen.
The protein was isolated from infected monkey cells and immunoprecipitatedwithhamsteranti-T serum asdescribed above. Alternatively, T antigen was purified by immunoaf-finity by using Sepharosebeads containing covalently bound PAb419 antibody. PAb419 is directed against the amino-terminal region of large T antigen and also recognizes small T antigen(11).
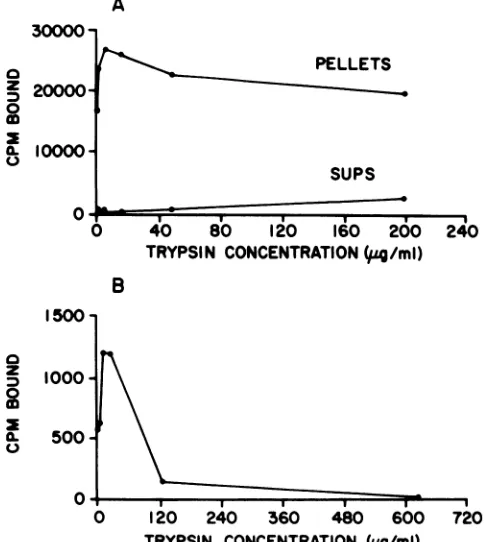
The immunoprecipitated or immunoaffinity-purified pro-tein was cleavedwith various concentrations of trypsin, and the resultingfragments were tested for their ability to bindto an origin-containing SV40 DNA fragment (Fig. 1). The fragments of immunoprecipitated T antigen tested consisted of those that remained associated with the S. aureuspellet after proteolysis as well as those that were solubilized by the enzyme treatment (Fig. 1A).Inagreement with our previous findings (35), the released fragments did not have significant amounts ofDNA-binding activity until high concentrations of trypsin were used (Fig. 1A). Itis clear from Fig. 1Athat theDNA-binding activity of the bound fragments increased and then decreased as more trypsin was added. The same qualitative pattern was obtained with immunoaffinity-purified T antigen (Fig. 1B). The fragments generated with lowconcentrations of trypsin bound about 1.5 to 2 times as much DNA as intact T antigen (Fig. 1). These binding reactionswere carried out for only 10min and did not reach equilibrium (see below). Control reactions of precipitates, usingnonimmune serum, had very low levels (<100 cpm) of bound radioactivity(not shown).
Fragments of T antigen also bound about twofold more BstNI fragment G than intact T antigen in reactions contain-ing the unseparated mixture ofSV40BstNI fragments (not shown). This demonstratedthat theincreased binding activ-itywasoriginspecific (17).
We (34) and others (18, 26) have previously shown that when T antigen is immunoprecipitated with anti-T serum, trypsin proteolysis solubilizes an NH2-terminal fragment which contains the sequence from residues 1 and 130 (28). Trypsinization of immunoaffinity-purified T antigen gener-ates the same fragment (35). In the case of immunoprecipit-atedTantigen,the other fragments generated by proteolysis remainassociated with the S. aureus after digestion with low and moderateconcentrations of trypsin (34, 35). To identify theproteinfragments generatedfromprecipitated T antigen under each trypsinization condition used in the experiment described in Fig. 1A, 35S-labeled T antigen was immu-noprecipitated and cleaved with trypsin, using identical conditions.Thefragments released into thesupernatantsand those that remained associated with the bacterial pellets were analyzed byacrylamide gel electrophoresis (Fig. 2).
Figure 2shows that at concentrations of trypsin as low as 1 ,ug/ml, immunoprecipitated T antigen was cleaved into a 17,000-dalton (17K)fragment, which was released from the immunoprecipitate, and into several fragments (76K, 46K,
on November 10, 2019 by guest
http://jvi.asm.org/
and several at about 30K) which remained associated with theprecipitate. Previous work (35) has shown that the 17K fragment is the NH2-terminal fragment (residues 1 to 130) and that the 76Kfragmentrepresentsthe remaining portion of the polypeptide chain (residues 131 to 708). The 46K fragment originates from amino acid residues 131 to about 517 and the 30K fragments are derived from the COOH-terminal region (35). Digestion of T antigen with higher concentrations of trypsin resulted in the cleavage of the 76K fragmentto smallerpeptides (Fig.2), aspreviously observed (35). At the highest concentration of trypsin used (200 jxg/ml), the resistant fragments that remained associated with the pellet were those of46K, 37K, and about 30K. Tryptic peptide mapping of the 46K and 30K fragments generated under these conditions has shown (35) that they arederived from approximately residues131 to 517and131 to371,respectively (the'COOH-terminal 30Kfragmentsare degraded with thisamountof trypsin). The origin of the37K fragment isnotknown for sure,but since theNH2-terminal region of the76Kfragmentis highly resistanttoproteolysis, itmost likelyrepresents afragment mapping from residues 131 toabout 418.
The threeresistantfragments (46K, 37K, and 30K) and the larger 76K fragment have DNA-binding activity (35). They
A
30000-0
Z
20000-0
a
L
10000-0
IOO150
0 2
Z
1000-X.
500-0O
PELLETS
SUPS
40 80 120 160 200 241
TRYPSI N CONCENTRATION
(u4/ml)
B
120 240 560 480 600
TRYPSIN CONCENTRATION (pg/ml) FIG. 1. DNA-binding activity ofT-antigen fragments. SV40 T
antigenwaspurified by immunoprecipitation (A)orimmunoaffinity (B) from SV40-infected BSC-1 cells and cleaved with various
concentrations of trypsin. The immunoprecipitates were centri-fuged, and thefragments released into thesupernatantsaswellas those that remained associated with the pellets were tested for
DNA-binding activity (A). Immunoaffinity-purified Tantigen was alsotested forDNA-binding activity (B). Bindingwasperformed by incubatingthesamplesfor 10minat23°Cwithanexcessof labeled SV40DNA BstNIfragment G,which containsthe viralreplication originand the threeT-antigen-bindingsites. Theamountof labeled
fragmentG boundtotheprecipitatesortosolubilizedTantigenor fragmentswasdeterminedbyscintillationcounting.
2 3 4 5 6 7 8 9 10 11
-
TAg
-
76K
-
46K
-37K
_
-30K
_
.k
_ 00 _i 40
a_I
_a ..
-
17K
FIG. 2. T-antigen fragments generated by partial proteolysis.
SV40-infected BSC-1 cellswerelabeled with[35S]methioninefor1 h, and theTantigenwasimmunoprecipitated with hamster anti-T serum.Samplesweretreated with variousconcentrations oftrypsin
for 30 minat0C, and the S.aureus waspelletedbycentrifugation.
LabeledT-antigen fragments in the supernatants and in thepellets
wereanalyzed by sodiumdodecyl sulfate-acrylamide gel electropho-resis andautoradiography. Lanes: 1, uncleaved T antigen; 2, 4, 6, 8, 10,fragments released into the supernatantsby 1, 5, 15, 50, and 200 ,ug of trypsin per ml, respectively; 3, 5, 7, 9, 11, fragments
associated with the pellets aftertreatmentwith 1,5, 15, 50, and 200
pLgof trypsin per ml, respectively. Molecular weights were esti-matedbycomparisonwith themigrationof markerproteins.
all contain the DNA-binding domain of T antigen which maps within residues 131 to 371 (5, 21, 35). It is clear from the above experiment that the fragments retained in the precipitates atall concentrations oftrypsin used had more DNA-bindingactivitythan theintactpolypeptide chain (Fig. 1A). The increase in DNA-binding activity appeared tobe correlated with the loss ofthe NH2-terminal 17Kfragment
from the polypeptide. This deduction is clearest for the fragments generated with low (1to 5 ,ug/ml) concentrations oftrypsin (Fig. 1Aand2). Similar conclusions could bemade from the analysis of fragments derived from T antigen
purifiedbyimmunoaffinity(notshown), althoughin thiscase the products ofdigestion are much more complex. In Fig.
1B, the measuredDNA-binding activityoffragments gener-ated fromimmunoaffinity-purified Tantigendecreased
sub-stantiallyathighconcentrations oftrypsin.Theexplanation
for this appearsto be that smallfragmentsofTantigen are inefficiently precipitated in the assay. On the other hand,
smallfragmentsthatarealreadyinaprecipitatedformcanbe measuredaccuratelyforDNA-bindingwithoutasubstantial lossinactivity (Fig. 1A).
The most directinterpretation of these results is that the amino-terminal region imposes a constraint on the DNA-binding region lying further downstream. Removal ofthis constraint allows the polypeptidetobindmore DNA. Two explanations for thisarepossible. One is thatremovalofthe NH2-terminal regionexposes otherDNA-bindingsites either onthe same molecule or onpreviously inactive molecules. Second, it is possible thatremoving the constraint simply
allows the same binding region tobind to the DNA faster (i.e., it now has ahigher affinityforDNA). To
distinguish
between thesepossibilities, DNA-binding kinetic and satu-ration experiments were performed. Kinetic experiments
were performed with an excess of DNA, using im-munoprecipitatedTantigenthatwaseitherintactor
digested
immailL
.1
4 :f
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.612.347.541.74.241.2] [image:3.612.69.312.335.606.2]20000
-
15000-a z 0 co 10000 0.
5000
0
0 20 40 60
TIME (MIN)
FIG. 3. Kinetics of DNA binding of T antigen and T-antigen fragments. SV40 T antigen was immunoprecipitated with anti-T serum and a sample was cleaved with 5 ,ug of trypsin per ml. Uncleaved T antigen and the fragments associated with the S. aureus after thetrypsin digestion were reacted with an excessof labeled DNAfragment Gfor variousperiods of timeat23°C. The reactions were stopped by the addition ofcalfthymus DNA fol-lowed by rapidcentrifugation topellet thebacteria. LabeledDNA boundtothepelletswasquantitated by scintillationcounting. withtrypsin at aconcentration of5 ,ug/ml (Fig. 3). Figure3 shows that fragments of T antigen did indeed bind to the DNAfaster than intactTantigen. From the initial slopesof thetwo curves,it is estimated that the differencein kinetics of the two reactions is about threefold. DNA saturation experiments wereperformed by incubatingtheproteinwith increasingamounts ofDNAfor2h to eliminate differences inbinding kinetics (Fig. 4). These experiments showed that, at saturation, the fragments bound almost twice as much DNA as the intact protein, indicating that other DNA-binding sites do become exposed by the trypsin treatment.
24000
20000
c
16000
z
3
0
m
12000-
a-(
f^)
0
fragments
intact
TAg
0
2
4
6
8DNA
ADDED
(pJ)
FIG. 4. Saturation binding ofDNAto TantigenandT-antigen fragments. SV40TantigenandT-antigen fragmentswereprepared accordingtothelegendtoFig. 3andreacted with variousamounts
oflabeledfragmentG for 2 hat23°C.Labeled DNA boundtointact T antigen (0) and fragments (0) was quantitated by scintillation counting.
The additional binding sites are probably responsible for the
more rapid binding of T fragments to DNA (Fig. 3). We conclude, therefore, that the enhanced binding of fragments toorigin DNA cannot be explained simply byanincrease in the affinity of the fragments to the DNA.
Dephosphorylation of SV40 T antigen increases its DNA-binding activity. To determine if the constraint on DNA binding by the amino-terminal region is duetothe presence ofphosphate groups in that region, T antigen was dephos-phorylated in vitro, using alkaline phosphatase, and tested for DNA-binding activity. To first test the activity of the enzyme onphosphorylated T antigen, in vivo 32P-labeled T antigen was immunoprecipitated with PAb416 and then treated with various concentrations of alkaline phosphatase (Fig. 5). PAb416 wasused instead of anti-T serum because anti-T serum-precipitated T antigen was not readily dephosphorylated by alkaline phosphatase (not shown). Fig-ure 5 (lanes 1 to 5) shows that the enzyme effectively removed most of the labeled phosphate groups from the
protein at thehighest concentrations used(160U/ml). Figure 5(lanes6 to10)also shows that the enzyme removed most of thephosphates from the NH2- and COOH-terminal regions of the protein. In this part of the experiment, alkaline phosphatase-treated T antigen was subjected to partial proteolysis, using10 ,ugoftrypsinperml. The76Kfragment, whichcontainstheCOOH-terminal region (35), andthe 17K fragment, which includes the NH2-terminal region, had both been dephosphorylated with high concentrations of the enzyme. A similar experiment with 35S-labeled T antigen showed that thepolypeptidechain was notdegradedduring thephosphatase reaction(not shown).
The kinetics of DNA binding were then measured as before, using intact and partially dephosphorylatedTantigen (Fig. 6). Figure 6 shows that dephosphorylated T antigen
2 3
4
5
6 7
8 9
10
O o
-
TAg
76K
_~~~~1
K
FIG. 5. Dephosphorylation ofTantigen with alkaline phospha-tase.SV40-infectedBSC-1 cells werelabeledwith[32P]H3PO4for 1 h,and Tantigenwasimmunoprecipitated with PAb416 monoclonal antibody. The S.aureuswastreated with various concentrations of alkalinephosphatase for30min at23°C. The bacteria were pelleted by centrifugation and washed, and afraction of each sample was incubated withtrypsinat aconcentration of10 pJmlfor30minat 0°C. Uncleavedandcleavedsampleswereanalyzed byacrylamide gel electrophoresisandautoradiography. Lanes: 1-5, UncleavedT antigen treated with 0, 20, 40, 80,and 160 Uofalkaline phosphatase perml, respectively; 6-10,Tantigen treated with 0, 20, 40, 80, and 160 U of enzyme per ml and cleaved withtrypsin.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.612.102.237.76.219.2] [image:4.612.321.542.433.620.2] [image:4.612.77.271.443.674.2]bound to origin-containing SV40 DNA almost twice as fast asintact T antigen. This represents a somewhat lower level ofincreased activitycomparedwith what was obtained with proteolytic fragments (Fig. 3), perhaps because of incom-pletedephosphorylation. Although it is much more difficult to perform kinetic experiments of this type with soluble immunoaffinity-purified T antigen, we obtained about a 2.5-foldincrease in DNA-binding activity (in a 10-min bind-ing reaction) when free T antigen was dephosphorylated. This compares favorably with the increase observed after proteolysis of immunoaffinity-purified T antigen (Fig. 1B).
Toshow whethertheincreasesinDNA-bindingactivity of fragments and of dephosphorylated T antigen were due to the same or different effects, T antigen was first dephos-phorylated andthentrypsinized and the resultingfragments were tested for DNA-binding activity. In this experiment, we also observed a 2.5-fold increase in DNA-binding activ-ity, indicatingthat the neteffecton the DNA-bindingregion was the same (i.e., the effects were not additive).
Figure7 shows that partially dephosphorylated T antigen bound slightly higher than 1.5-fold more DNA than un-treatedTantigenatsaturatingamountsof DNA. Theresults areshownfor immunoaffinity-purified Tantigen,andalmost identical results were obtained when T antigen was im-munoprecipitated with PAb416 instead. DNA-binding reac-tions carriedout inthe presence of competitor calfthymus DNA showed that the enhanced DNA-binding activity of dephosphorylatedTantigenwasorigin specific(notshown).
The data indicate that DNA binding was affected about equally by the removal of the NH2-terminal region of T antigen as by dephosphorylation. Since the NH2-terminal arm is highly phosphorylated (Fig. 5), it is most likely that dephosphorylation ofonly thatregionwould have the same effect.
Thereare atleasttwo ways toexplainourobservationsin termsofadditional sites forDNAbinding(seeabove).First, it ispossible that trypsinizationand dephosphorylation of T antigencause abreakdown in thequaternary structureofthe protein such that previously inaccessible DNA-binding re-gionsarenowexposed.Inthis first model, onlysomeofthe monomers in an oligomerized form ofT antigen would be active. The second possibility isthat each monomer mole-cule hastwoDNA-binding activities, oneof which is abol-ishedby in vivo phosphorylation of the protein chain.In this secondmodel, trypsinizationorinvitro dephosphorylation of the polypeptide chain would reverse the process and
15000
-z
D 10000-0
m
A)-
5000-]
o Dv- /
M 0
0~~~~~
0
° 600 /
t 400 /
200 .
0 2 4 6 8 l
DNA
ADDEDN(/I)
FIG. 7. Saturation binding of DNA to untreated and alkaline phosphatase (a.p.)-treated Tantigen. T antigen was purified from infected BSC-1 cells by immunoaffinity. A sample was treated with alkaline phosphatase (80 U/ml) for 30 min at 23°C. Untreated (0) andenzyme-treated (0) T antigens were then reacted with various amounts of labeled SV40 DNA fragment G, and thebinding was quantitated as described for Fig. 4.
switchon the secondDNA-binding activity. Todistinguish between the twomodels, immunoaffinity-purified labeled T antigenwastreated withalkalinephosphataseand subjected to velocity sedimentation on sucrose gradients. Figure 8 shows that untreated T antigen purified by this procedure was mostly in a monomer form, even though the labeling conditions approximated steady state (16-h labeling).
400-a z 0 0
(a)
300*
200-100
-/a.p.
//-a~-.p.
0 10 20
TIME (MIN) 30
FIG. 6. Kinetics of DNA binding of untreated and alkaline phosphatase (a.p.)-treated T antigen. T antigen was immuno-precipitatedfrom infected BSC-1 cells withPAb416, andasample wastreated with 160 U of alkalinephosphatase perml.Kinetics of DNA bindingwere measured for untreated and enzyme-treatedT
antigenasdescribed forFig. 3.
0
16S
I
0
I
10 20
FRACTION NUMBER 4S
I
30
FIG. 8. Sedimentation of untreated and alkaline
phosphatase-treated T antigen. Infected BSC-1 cells were labeled with
[35S]methionine for 16 h, and the T antigen was purified by
im-munoaffinity. A samplewas treated with alkalinephosphatase (80 U/ml)for30minat23°C.Untreated andenzyme-treatedTantigens were subjected to sedimentation in 15 to 30o sucrose gradients.
Fractions were counted by scintillation counting. Sedimentation coefficientswereestimatedbycomparisonwith thesedimentationof
rRNAs. Symbols: 0, control Tantigen; 0, alkaline phosphatase-treated Tantigen.Thedirection of sedimentationwasfromrightto left.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.612.359.530.75.259.2] [image:5.612.335.554.431.631.2] [image:5.612.121.258.557.674.2]Acrylamide gel analysis
showed that themajority
(about80%)
oftheradioactivity
in thesample
wasinlarge
Tantigen (the remainder was mostly in small Tantigen).
It seems likelythat the conditions usedfor elutingthe protein from theimmunosorbent
column breakupT-antigen oligomersto monomersandre-oligomerization doesnot occur.The alka-linephosphatasetreatmenthad little ifanyeffectonthesizeof the
protein (Fig.
8). Hence, we believe that the second mechanism is much morelikely.DISCUSSION
The results presented here showed that fragments of T
antigen
that aremissing
theNH2-terminal region
bound almosttwiceasmuchorigin-containing
SV40DNAasintact Tantigen, using saturatingamounts ofDNA. This suggests that there is a physical or chemical constraint on DNAbinding
in the intact protein. Experiments with alkalinephosphatase-treated T antigen indicated that the constraint is probably duetothe presence ofphosphategroupsatthe
amino-terminal
region.
Thus, phosphorylationof thatregionappears to downregulate the DNA-binding activity of T
antigen.
Thefunctionof the polypeptidechainat oraround the replication origin islikely to be affectedbyphosphory-lation as aconsequence.
Basedontheaboveobservations,wesuggestthat invitro
dephosphorylation of T antigen exposes additional
DNA-binding sites, thereby allowing itto bind more DNA. It is
important
to notethat anincreasein the DNAaffinity
ofabinding
site in dephosphorylated T antigen simply cannotexplain
the DNAsaturation results(Fig. 7);additionalDNA-binding
sitesmust beinvoked.Since T antigen exists as an oligomer in the cell, it was
possible
that theseadditionalbinding
sitesoriginated from subunits whichwere inactive in the oligomerizedmolecule. In thisexplanation,
dephosphorylation (or fragmentationwithtrypsin)causestheoligomertobreakupandexpose the buried
DNA-binding
sites. However, we showed(Fig.
8)that the
majority
of the immunoaffinity-purified T antigen was ofmonomer sizeto startwith and thatdephosphoryla-tion had no impact on its sedimentation coefficient.
Dephosphorylation
ofimmunoaffinity-purifiedTantigen did,on the other
hand,
have an effect on its DNA-bindingactivity.
Thesimplest
and most directexplanation of these results isthatTantigenhastwoorigin-binding activities,one of which is abolished by phosphorylation. In vitrodephosphorylation
of the molecule with alkaline phospha-tase orfragmentation oftheprotein with trypsinexpresses the secondbinding activityinthismodel. Insupportofthis, weshowedthatremovalof thehighly phosphorylated NH2-terminal arm of the molecule resulted in about a twofold increase in the number ofDNAmolecules that boundtotheremaining portions
ofthepolypeptidechain(Fig. 1A, 2,and4).
Thetwo
DNA-binding
activities couldrepresent twosep-arate
regions
of thepolypeptide chain,
eachofwhich could bind to the origin region independently. Alternatively, thetwoDNA-bindingactivities could beduetothesameregion of the
polypeptide
chainbut present in differentmolecules. Ineithercase,oneof the activities would bephosphorylationsensitive,
andthe other would beinsensitive. Inthe second case,different classes of molecules expressing eitherDNA-binding activity
would exist presumably due todifferentialphosphorylation.
This modelis, however, difficultto recon-cile with the data that the large majority of T antigenbecomes
similarly
phosphorylated in thecell(34).If thetwo-binding-regionmodel is correct, wherearethese regions located in the polypeptide? Our previous mapping studiesplaced the portion of the protein withDNA-binding activitytoresidues131to371(35).Recent studiesbyPaucha et al. (21) showed that there is a cluster mapping around residues 147 to 166 which is important for DNA-binding activity. Analysis (41) of the region between residues 131 and 371 forhomologywith this cluster revealed only these two regions of related sequences: 142 Leu-Leu-Ser-Phe-Leu-Ser-His-Ala-Val-Phe 151; 217 Thr-Phe-Ser-Phe-Leu-Ile-Cys-Lys-Gly-Val 226. Althoughthe homologybetween the two regions is weak, Paucha et al. (21) showed that a mutant with a different amino acid sequence between 220 and 223 (Phe-Leu-Ile-Cys replaced by Arg-Asn-Ser-Gly) makesa Tantigen that doesnotbind DNA, whereasmutants that have residues 179 to 180 or 189 to 193 replaced with different sequences are positive. Given that widely spaced mutations affect DNA-binding activity, the tertiarystructure ofthe molecule in that region is bound to be critical for properDNA-binding function, and it remains tobe seen if the above sequences correspond to different DNA-binding domains.
Previous work fromourlaboratory (34) has shown thatT antigen becomes phosphorylated inastepwisemannerin the cell. The process involves at least three different phosphorylated forms of the protein. Interestingly, the poly-peptide undergoes several phosphorylation and dephos-phorylation steps as it maturesto its stable form (form C) (34). The functionof these various forms is unknown, butthe results presented here suggest that the generation of the stable form C is related to the formation of a T-antigen molecule that has lost a DNA-binding activity. Conse-quently, each intermediate in thephosphorylation pathway mayinteract with theDNAdifferently.
What biological significance wouldtwo DNA-binding ac-tivitieshave? One attractive possibility is that each onehas a different function during SV40 infection. One of the DNA-binding activities could be involved in DNA replica-tion, for example, with the otherone servinga role in the regulation of early or late gene expression or both. It is assumed thatoneof these functions would be inactivated by phosphorylation, possibly to ensure that only newly made T-antigen moleculesareused. Itmaybe possibleto testthis
possibility with in vitro replication and transcription sys-tems.
ACKNOWLEDGMENTS
This workwassupported by Public Health Service grant CA36118 from the NationalCancer InstitutetoD.T.S.
We thank Florence Schmieg and Louis Levinger for helpful
discussions.
LITERATURECITED
1. Baumann, E. A., and R. Hand. 1982. Phosphorylation and dephosphorylation alter thestructureof D2 hybrid T antigen. J. Virol. 44:78-87.
2. Bradley, M. K., J. D. Griffin, and D. M. Livingston. 1982. Relationship of oligomerizationtoenzymaticandDNA-binding properties of the SV40 largeTantigen. Cell 28:125-134. 3. Brady, J. N.,J.B. Bolen,M.Radonovich,N.Salzman,and G.
Khoury. 1984. Stimulation ofsimian virus 40 late expression by simian virus 40 tumor antigen. Proc. Natl. Acad. Sci. USA 81:2040-2044.
4. Burger, C.,and E.Fanning. 1983.Specific DNA binding activity of T antigen subclasses varies among different SV40-transformed cell lines. Virology 126:19-31.
5. Clark, R., K. Peden, J. M. Pipas, D. Nathans, and R. Tjian.
on November 10, 2019 by guest
http://jvi.asm.org/
1983. Biochemical activities of T-antigen proteins encoded by simian virus 40 A gene deletion mutants. Mol. Cell. Biol. 3:220-228.
6. Fanning, E., B. Nowak, and C. Burger. 1981. Detection and characterization of multiple forms of simian virus 40 large T antigep. J. Virol. 37:92-102.
7. Fanning,E., K.-H. Westphal, D. Brauer, and D. Corlin. 1982. Subclasses of simian virus 40 large T antigen: differential binding of two subclasses of T antigen from productively infected cells to viraland cellular DNA. EMBO J. 1:1023-1028. 8. Gidoni, D., A.Scheller, B.Barnet, P. Hantzopoulos, M. Oren, and C. Prives. 1982. Different forms of simian virus 40large tumor antigen varying in their affinities for DNA. J. Virol. 42:456-466.
9. Greenspan, D. S., and R. B.Carroll. 1981. Complex of simian virus 40 large tumor antigen and 48,000 dalton host tumor antigen. Proc. Natl. Acad. Sci. USA 78:105-109.
10. Hansen, U., D. G. Tenen, D. M. Livingston, and P. A. Sharp. 1981.Tantigenrepression of SV40 early transcription from two promoters. Cell 27:603-612.
11. Harlow, E., L. V.Crawford, D. C. Pim, and N. M. Williamson. 1981.Monoclonalantibodiesspecific for simian virus 40 tumor antigens. J. Virol. 39:861-869.
12. Keller, J. M., and J. C. Alwine. 1984. Activation of the SV40 late promoter: direct effects in the absence of viral DNA replication. Cell 36:381-389.
13. Kress, M., M.Resche-Rigon, and J. Feunteun. 1982. Phosphor-ylation pattern of large T antigens in mouse cells infected by simian virus 40 wild type or deletion mutants. J. Virol. 44:116-133.
14. Levitsky, K., K. Chandrasekaran, P. T. Mora, and D. T. Sim-mons. 1983.Immunoaffinity chromatography of a cellulartumor antigen from mouse neuroblastoma cells. Int. J. Cancer 32:597-602.
15. Livingston,D.M.,I.C.Henderson, andJ.Hudson. 1974.SV40 Tantigen: partial purification andproperties. Cold Spring Har-bor Symp. Quant. Biol. 39:283-289.
16. McCormick, F., and E. Harlow. 1980. Association ofamurine 53,000 dalton phosphoprotein with simian virus 40 large T antigen intransformedcells. J. Virol. 34:213-224.
17. McKay,R. 1981. Binding of simian virus40 T antigen-related proteintoDNA. J. Mol. Biol. 145:471-488.
18. Morrison, B., M. Kress, G. Khoury, and G. Jay. 1983. Simian virus 40 tumor antigen: isolation of the origin-specific DNA-binding domain. J. Virol. 47:106-114.
19. Myers, R. M., D. C.Rio, A. K. Robbins, and R.Tjian. 1981. SV40 geneexpression is modulated by thecooperativebinding ofTantigentoDNA. Cell 25:373-384.
20. Myers,R.M., and R. Tjian. 1980. Construction andanalysis of simian virus 40origins defective intumorantigenbinding and DNAreplication. Proc. Natl. Acad. Sci. USA 77:6491-6495. 21. Paucha, E.,D.Kalderon,R.W.Harvey,and A. E. Smith.1986.
Simian virus 40originDNA-bindingdomainonlargeTantigen.
J. Virol.57:50-64.
22. Potter, C. W.,B.C.McLauglin,andJ. S. Oxford.1969. Simian virus 40-induced T and tumor antigens. J. Virol. 4:574-579. 23. Prives, C., Y. Beck,D.Gidoni, M. Oren,and H.Shure. 1979.
DNA binding and sedimentation properties of SV40 tumour
antigenssynthesized in vivo and in vitro. Cold Spring Harbor Symp. Quant. Biol. 44:123-130.
24. Rio, D., A.Robbins, R. Myers, and R. Tjian. 1980.Regulation of simian virus 40 early transcription in vitro by a purified tumor antigen. Proc. Natl. Acad. Sci. USA 77:5706-5710.
25. Rio, D., and R. Tjian. 1983. SV40 T antigen binding site mutations thataffect autoregulation. Cell 32:1227-1240. 26. Scheidtmann, K.-H., B. Echle, and G. Walter. 1982. Simian
virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J. Virol.44:116-133. 27. Scheicdtmann, K. H., M. Hardung, B. Echle, and G. Walter.
1984. DNA-binding activity of simian virus 40 large T antigen correlates with a distinctphosphorylation state. J. Virol. 50:1-12.
28. Schwyzer, M., R. Weil, G. Frank, and H. Zuber. 1980. Amino acid sequence analysis of fragments generated by partial proteolysis from large simian virus 40 tumour antigen. J. Biol. Chem. 255:5617-5634.
29. Shalloway, D., T. Kleinberger, and D. M. Livingston. 1980. Mapping of SV40 DNAreplication origin region binding sites for the SV40 T antigen by protection against exonuclease III digestion. Cell 20:411-422.
30. Shortle,D.R.,R. F.Margolskee, and D. Nathans. 1979. Muta-tionalanalysis of the simian virus 40 replicon: pseudorevertants ofmutantswithadefectivereplication origin.Proc.Natl. Acad. Sci. USA 76:6128-6131.
31. Shortle, D.,and D. Nathans. 1979. Mutants ofSV40 with base substitutions at the origin of DNA replication. Cold Spring
HarborSymp. Quant. Biol.43:663-668.
32. Shortle,D.,andD.Nathans.1979.Regulatorymutantsofsimian virus 40: constructed mutants with base substitutions at the originofDNAreplication.J.Mol. Biol. 131:801-817.
33. Simanis, V.,and D. P.Lane. 1985.An immunoaffinity
purifica-tion procedure for SV40 large Tantigen. Virology 144:88-97. 34. Simmons, D. T. 1984. Stepwise phosphorylation of the
NH2-terminal region of the simian virus 40 large Tantigen. J. Biol. Chem. 259:8633-8640.
35. Simmons,D.T.1986.DNA-bindingregionof thesimian virus40 tumorantigen.J. Virol.57:776-785.
36. Simmons,D.T.,M.A.Martin,P. T.Mora,andC.Chang.1980. Relationshipamong Tauantigensisolated from various lines of simianvirus40-transformed cells. J. Virol. 34:650-657. 37. Tegtmeyer, P. 1972. Simian virus 40 deoxyribonucleic acid
synthesis:theviralreplicon. J. Virol. 10:591-598.
38. Tegtmeyer, P.,K.Rundell,andJ.K.Collins. 1977. Modification of simian virus 40proteinA. J.Virol. 21:647-657.
39. Tjian,R.1978.Protein-DNA interactionsattheoriginofsimian
virus 40 DNA replication. Cold Spring HarborSymp. Quant. Biol.43:655-662.
40. Tjian, R. 1978. Thebindingsite ofSV40 DNA foraT
antigen-relatedprotein. Cell 13:165-179.
41. Tooze, J. 1981. DNAtumorviruses. Molecularbiologyoftumor
viruses, 2nd ed., revised. ColdSpring Harbor Laboratory, Cold Spring Harbor,N.Y.
42. VanRoy,F., L.Fransen,and W. Fiers.1981. Phosphorylation patterns oftumorantigens in cells lyticallyinfected or
trans-formedbysimian virus 40. J. Virol. 40:28-40.
on November 10, 2019 by guest
http://jvi.asm.org/
![FIG. 5.gelper0°C.incubatedbytase.h,antigenantibody.alkaline160 and Dephosphorylation of T antigen with alkaline phospha- SV40-infected BSC-1 cells were labeled with [32P]H3PO4 for 1 T antigen was immunoprecipitated with PAb416 monoclonal The S](https://thumb-us.123doks.com/thumbv2/123dok_us/1368934.90248/4.612.77.271.443.674/incubatedbytase-antigenantibody-alkaline-dephosphorylation-alkaline-infected-immunoprecipitated-monoclonal.webp)
