Dissertation on
I
NFECTIONS IN
C
HILDREN WITH
D
IABETES
M
ELLITUS
Submitted to
The Tamilnadu Dr.M.G.R. Medical University in partial fulfillment of the requirement
for the award of degree of
MD BRANCH VII
PAEDIATRIC MEDICINE
INSTITUTE OF CHILD HEALTH AND HOSPITAL FOR CHILDREN
MADRAS MEDICAL COLLEGE
CHENNAITHE TAMILNADU DR. M.G.R. MEDICAL UNIVERSITY CHENNAI, TAMILNADU
CERTIFICATE
Certified that this dissertation entitled “INFECTIONS IN CHILDREN WITH
DIABETES MELLITUS” is a bonafide work done by Dr. VIDYA KRISHNA, Post Graduate, Department of Paediatric Medicine, Institute of Child Health and Hospital for Children, Madras
Medical College, Chennai during the academic year 2004-2007.
Prof. SARADHA SURESH, M.D., Ph.D.,
Addl. Professor of Paediatrics,
Institute of Child Health and Hospital for Children,
Madras Medical College, Chennai.
Prof. R. KULANDAI KASTHURI, M.D., D.C.H.,
Director and Superintendent (I/C), Institute of Child Health and Hospital for Children,
Madras Medical College, Chennai.
Prof. KALAVATHI PONNIRAIVAN, B.Sc., M.D.,
Dean,
ACKNOWLEDGEMENT
I express my sincere thanks to Prof. Dr. KALAVATHI PONNIRAIVAN, B.Sc., M.D.,
Dean, Madras Medical College for allowing me to conduct the study using the available
facilities.
I whole-heartedly thank Prof. Dr. R. KULANDAI KASTHURI, M.D., D.C.H., Director
and Superintendent (I/C), Institute of Child Health and Hospital for Children for her invaluable
help and guidance.
I feel greatly indebted to Prof. Dr. SARADHA SURESH, M.D., Ph.D., Addl. Professor of Paediatrics, Institute of Child Health and Hospital for Children, for her help and guidance.
I also thank our former Director and Superintendent
Prof. MANGAYARKARASI SENGUTTUVAN,for her guidance in doing this study.
I thank Dr. D. GUNASINGH, Dr. C. SUBBULAKSHMI, Dr. LUKE RAVI CHELLIAH and Dr. V. POOVAZHAGI for their comments and suggestions in this study.
I am indebted to all the children with diabetes mellitus and their parents without whom
this study would not have been possible.
Lastly I want to express my gratitude to my mother, who has been the source of constant
CONTENTS
SL.NO. TITLE PAGE NO.
1. INTRODUCTION 1
2. IMMUNOPATHOLOGY OF INFECTIONS IN DIABETES MELLITUS
5
3. LITERATURE REVIEW 18
4. STUDY JUSTIFICATION 27
5. OBJECTIVES OF THE STUDY 28
6. STUDY METHODOLOGY 29
7. RESULTS 37
8. SUMMARY OF THE STUDY 63
9. DISCUSSION 65
10. CONCLUSION 69
11. ANNEXURE – I DATA COLLECTION FORMS
1. INTRODUCTION
Diabetes mellitus comprises of a group of common metabolic disorders that share the phenotype of hyperglycemia. It is the most common endocrine – metabolic disorder affecting both children and adults. The worldwide prevalence of type 1 and type 2 diabetes mellitus is increasing worldwide, with especially type 2 diabetes mellitus rising more rapidly both in children and adults due to the recent epidemic of obesity and also due to lifestyle changes. In 2000, the prevalence of diabetes mellitus was estimated to be 0.19% in people <20years and 8.6% in people >20 years1.
Magnitude of the problem in India:
Though this disease has a low incidence in our country of only 0.1 per 100,000 population the magnitude of the problem is indeed huge considering the chronicity of the illness, its effect on growth and development and long-term complications on the various organ systems causing considerable morbidity and mortality. The disease also brings about with it a change in lifestyle for the young diabetics with the need for daily exogenous insulin therapy, blood glucose monitoring and dietary changes. Due to the same reasons, diabetes mellitus imposes a great drain on the economy.
In India, Government health expenditure accounts for just 2% of the monetary budget and 0.8% of the Gross Domestic Product (GDP) (World Bank Development indicators). The per capita expenditure on health care is only 6.4% of the average global figure, while India accounts for 23.5% of the world’s disability- adjusted life years lost due to diabetes4. Given the very limited resources available, the main thrust of health care provision is on the eradication of communicable diseases. There are also services provided by private medical practitioners for those who can afford the cost.
reported that the median direct cost to the family of an individual with type 1 diabetes is US$310(range US$45 –1936). The percentages of family income spent on diabetes care were 59%, 32%, 18% and 12% in low, middle, upper-middle and upper socio economic groups, respectively. Thus, the disease has its effects not only on the growth, development and emotional aspects of a child; it also carries the risk of long term complications with its associated morbidity and mortality with a significant effect on the economy as well.
Our experience:
The Diabetic clinic at the Institute of Child Health and Hospital for Children was started in the year 1999 and has about 300 registered patients. The services provided at the clinic include:
a) Monitoring of blood glucose and insulin therapy b) Monitoring of glycemic control
c) Growth monitoring
d) Monitoring for complications:
• Injection site assessment for atrophy / hypertrophy / abscess
• Annual ophthalmologic review
• Periodic monitoring of urine microalbuminuria for risk of diabetic nephropathy.
• Evaluation of hands, feet and peripheral pulses for signs of neuropathy or peripheral vascular disease.
• Evaluating for associated autoimmune disorder like thyroiditis in suspected cases.
Immunopathology of
infections in diabetes
2. IMMUNOPATHOLOGY OF INFECTIONS
IN DIABETES MELLITUS
Diabetes Mellitus is a common chronic metabolic disorder affecting both children and adults. It is characterized by chronic hyperglycemia with disturbances of carbohydrate, protein and fat metabolism resulting from defects in insulin action or insulin secretion or both. It can have long-term effects on the various organs of the body like the eye, kidneys, heart, peripheral vessels and nerves.
The disease was first mentioned in the Eber’s papyrus as early as 1500 B.C The discovery of insulin by Banting et al. was a significant breakthrough in the history of diabetes. They were followed by many such researchers, who have helped us to understand this disease better. And hence there has been a shift of terms from the older ‘Non insulin dependent diabetes mellitus’ and ‘Insulin dependent mellitus’ to the newer ‘type 1 diabetes mellitus’ and ‘type 2 diabetes mellitus’.
Type 1 Diabetes Mellitus:
Type 1 Diabetes Mellitus is a T cell mediated autoimmune disease involving
Epidemiology:
Type 1 diabetes mellitus, the most common form of diabetes mellitus encountered in childhood, accounts for approximately two thirds of all cases of diabetes mellitus in children2.
The incidence and prevalence of Type 1 DM varies dramatically around the world, with more than 400 fold variation in the incidence in reporting countries2. Type 1 DM is uncommon in India, China with an incidence of only 0.1 / 100,0002. It is more common in Finland & Sardinia with an incidence of 50 cases per 100,000 population per year2. The incidence of Type 1 DM is increasing throughout the world especially in nations with a previous low incidence of autoimmune diabetes. It is predicted that the overall incidence of Type 1 DM will be 40% higher in 2010 than in 19973. Also the disease has a younger age of onset now than earlier3.
Complications of Diabetes Mellitus:
Diabetes Mellitus is a chronic metabolic disorder characterised by both acute and chronic complications.
Acute complications of Diabetes Mellitus:
Diabetic Ketoacidosis:
It is an important complication of childhood diabetes mellitus and the most frequent diabetes – related cause of death in children. It is a syndrome characterised by hyperglycemia, ketosis and acidemia. Diabetic Ketoacidosis (DKA) in diabetes mellitus can be the initial presentation, an acute metabolic compensation or the cause of mortality. In established diabetics, it can be precipitated by infections, intercurrent illness or by omission of insulin.
Chronic complications of Diabetes Mellitus:
These include retinopathy, cataracts, hypertension, nephropathy, neuropathy, coronary artery disease, peripheral vascular disease etc., These occur due to the effects of hyperglycemia or insulinopenia on the various tissues and can be prevented by proper glycemic control as was established by the Diabetes Control and Complications Trial (DCCT) 6.
Infections are an important complication of Diabetes Mellitus and they can be commonly encountered in children, whereas the other complications may be delayed in their presentation till adolescence or early adulthood.
Associated complications:
Infections and Diabetes Mellitus:
It has been a time-honoured concept that the incidence of infections is higher in persons with diabetes mellitus and that such infections in the diabetic person result in complications and death more frequently than would be anticipated in otherwise healthy individuals7, 8.
Older studies, upon which much of this information is based, focus particularly on infections of the urinary tract, respiratory tree and the extremities and derive their data from autopsy cases. However, in these studies, the degree to which infection at these sites actually contributed to the cause of death is frequently not clear, and control groups were typical lacking9.
Later studies, while documenting excess mortality among patients with diabetes, ascribed it largely to cardiovascular diseases rather than to uncontrolled infection10, 11. For example, pneumonia did not cause an increase in mortality rate over that in age and sex matched controls10, 11.
The increased frequency and severity of infection in diabetes mellitus have been ascribed to the incompletely defined abnormalities in cell-mediated immunity and phagocyte function, hyperglycemia and diminished vascularisation. Hyperglycemia is proposed to aid the colonisation and growth of a variety of organisms like Candida and other fungal species1.
Diabetes Mellitus and Host Defence:
Application of immunological techniques in the laboratory has facilitated early diagnosis of functional defects in the immune system. The World Health Organisation (WHO) has included diabetes in its classification of secondary immunodeficiency diseases13. These secondary immunodeficiencies, unlike primary immunodeficiencies, can be resolved if their underlying cause (e.g. a tumour or steroid treatment) is eliminated.
The immune system is a system designed to fight infections while maintaining homeostasis, thus avoiding chronic inflammatory processes and autoimmune disease. It comprises two arms, one recognising molecular patterns and the other recognising variable molecular details. These two components of the immune system are called innate and adaptive, respectively.
basis for vaccine design. The innate response on the other hand does not have the ability to remember a previous challenge and therefore has no immunological memory. There is a close relationship between innate and adaptive immunity, and changes in the former could provoke inappropriate responses in the latter. Since changes in innate immunity have been reported in type 1 diabetes, it is possible that these changes predispose not only to the disease but also to infections14. There is no evidence that the immune response to infections is altered in subjects at risk of type 1 or type 2 diabetes. The evidence supports the concept that hyperglycemia per se or the metabolic abnormality of diabetes is sufficient to explain the impaired immune response in patients responding to infections15.
Polymorphonuclear Cells:
Polymorphonuclear (PMN) granulocytes represent the host’s first defence against bacterial agents. In diabetic patients these cells show functional alterations in chemotaxy and under some circumstances also in phagocytosis15,16.
Chemotaxy:
Cells from diabetic patients have a reduced chemotaxy especially when the diabetes is poorly controlled15. Monocytes in diabetic patients show a decreased cellular response to a chemokine, vascular endothelial growth factor α, because of a downstream signal transduction defect17.
Phagocytosis:
There are two phases of phagocytosis 1) Adhesion 2) Ingestion of foreign particles into intra cytoplasmic vacuoles. The energy required for this process is supplied by ATP produced during anaerobic glycolysis. Phagocytosis may be impaired in patients with long standing diabetes16,18 and there is evidence for PMN functional impairment19, 20. In general terms the metabolic disturbances associated with diabetes are probably important in impairing the function of immune effector cells.
Killing Activity:
Lymphocytes Population and their Functions:
Type 1 DM is associated with alterations in some lymphocyte sub population. Several studies have reported a reduction in total number of T-lymphocytes and more specifically the number of CD4 T cells causing a subsequent reduction in CD4/CD8 ratio22,23,24. This defect could due to either decreased insulin levels or decreased insulin activity or both25. However, optimisation of metabolic control is accompanied by normal lymphocyte transformation and normalization of levels of T lymphocytes sub populations26.
Immunoglobulins:
Serum Immunoglobulins levels (IgG and IgA) have been reported to be reduced diabetic patients compared to normal subjects27,28. However the antibody response in the diabetic population for example to pneumococcal polysaccharide is normal29.
Complement:
Table 1: Predisposing factors for infections in diabetes mellitus12.
Primary Factors
Granulocyte adherence, chemo taxis and phagocytic dysfunction Myeloperoxidase deficiency
Complement pathway defects
Cytokine-mediated (e.g. interleukin-1, tumour necrosis factor)
Secondary Factors
Ketoacidosis
Use of intra vascular access lines Antibiotic misuse / resistance Frequent hospitalisation Peripheral vascular disease Neuropathy
Gastro paresis, reflux and aspiration Indwelling urinary catheters
A number of factors make certain tissues in diabetic patients particularly prone to infections. The four most important elements are an underlying susceptibility to infections, vascular disease, nerve damage and hyperglycemia14. Hyperglycemia may predispose the diabetic patient to bacterial and fungal infections. Neuropathy can 1) alter the pressure distributions contributing to ulceration and as a result infection of the feet32 and 2) lead to autonomic involvement of the bladder with urine retention which predisposes to bacteriuria32. Vascular insufficiency and tissue hypoxia allow the growth of anaerobic organisms and limit host defense mechanisms14.
The complex deterioration of both large and small blood vessels in diabetes can result in reduced peripheral circulation, relative hypoxia and as a result a predisposition to proliferation of anaerobic bacteria33. Furthermore hypoxia can modify the oxygen dependent function of PMN granulocytes34. Finally, reduction in antibiotic absorption in diabetic patients with microangiopathy might lead to persistence of infections14.
Diabetes Mellitus and Specific Infections:
Table 2:Classifications of Infections in Diabetes
A) Common infections with increased incidence in diabetic patients 1) Urinary tract infections
2) Respiratory tract infections 3) Soft tissue infections
B) Infections predominantly occurring in diabetic patients 1) Malignant otitis externa
2) Rhinocerebral mucormycosis 3) Necrotizing fasciitis
4) Fournier’s gangrene
5) Emphysematous cholecystitis and pyelonephritis 6) Infections in diabetic foot
C) Micro-organisms strongly associated with infections in diabetic patients 1) Candida species
2) Group B streptococcus 3) Klebsiella species 4) Hepatitis C
D) Infections resulting from Iatrogenic causes 1) Insulin injection
Infections of the urinary tract, respiratory tract and soft tissues occur with increased frequency in the diabetic population12.
1. Urinary Tract Infections:
Urinary tract infection (UTI) is a frequently encountered problem in diabetic patients. The probable causes for increased risk of UTI in diabetic patients include:
a) Increased use of urinary catheters35
b) Presence of diabetic autonomic neuropathy, which causes increased residual urine volume, vesicoureteric reflux and recurrent upper UTIs36, 37
c) Coexistent vaginitis, cystocoele and rectocoele12.
Upper urinary tract is more commonly involved in diabetic patient. A poor response to therapy may be due to complications, which may include, papillary necrosis or perinephric abscess. Emphysematous pyelonephritis is rare, necrotising infection of the renal parenchyma and perirenal tissue with gas formation, mostly caused by E.coli12.
2. Respiratory Tract Infections:
four times more in diabetics than in non – diabetics38. There is a predilection for lower lobes and the disease is more aggressive in poorly controlled diabetes38. The lungs in patients with diabetes show histopathologic alterations due to accumulation of non- enzymatic glycosylation end products (NGEs) of tissue proteins in the connective tissue and this manifests as functional abnormalities: reduced lung volumes, reduced pulmonary diffusion capacity and elastic recoil38. Again, the alteration in chemo tactic, phagocytic and bactericidal activity of PMNs predisposes to infections.
3. Skin and Soft Tissue Infections:
Skin is the body’s largest and thinnest organ and is protected from infections by virtue of many factors:
• Intact stratum corneum
• Dry and acidic microenvironment
• Antibacterial effects of lipids, free fatty acids and IgA
• Endogenous micro flora and
• Mast cells
Diabetics are more prone to infections of skin and soft tissue due to:
• PMNL dysfunction
• Increased rates of skin colonization with Staphylococcus aureus39
They are especially prone to specific infections like necrotising fasciitis, Fournier’s gangrene, Rhinocerebral Mucormycosis, malignant otitis externa etc12. Infections of the foot are seen in adults due to neuropathy, peripheral vascular disease and hyperglycemia.
Micro-organisms Strongly Associated with Infections in Diabetes:
Certain micro-organisms appear to cause infections in patients with diabetes at a disproportionately high rate. A higher incidence of underlying diabetes has been noted in patients with Klebsiella infections such as bacteremia, liver abscess, thyroid disease and endophthalmitis40-43. Although an increased incidence of Staphylococcal infections has been noted, a careful recent review did not confirm the same39.Among enteric pathogens, Campylobacter and Salmonella enteritidis have been reported with increased frequency in patients with diabetes44,45.There is a strong association of diabetes with chronic Hepatitis C virus (HCV)46.
Oropharyngeal candidiasis is a well-documented complication of uncontrolled diabetes mellitus12.
Iatrogenic infections:
3.
LITERATURE REVIEW
Infections have all along been considered to occur with greater frequency and severity in patients with diabetes mellitus51. This was because at the turn of the century, many diabetic patients died of overwhelming infections. However, subsequently the introduction of insulin dramatically altered the status and today non – communicable diseases like cardiovascular diseases are the leading cause of death in diabetic patients as was found by Sasaki et al.10 and Kessler II.11 in two independent studies on mortality in diabetic patients. So, it is considered that the association between infections and diabetes mellitus needs a more critical re- evaluation.
Urinary Tract Infections and Diabetes Mellitus:
Urinary tract infections (UTI) are a common problem in diabetes. Asymptomatic bacteriuria occurs with a higher frequency in diabetics: a study by Geerlings et al54 demonstrated 26% prevalence in diabetic women, compared to 6% in controls. Studies have failed to demonstrate significant differences in epidemiological, clinical and microbiological features of UTI in patients with or without diabetes mellitus except for a higher frequency of catheterization and difficulty in eradicating infection in the former group55.
Upper urinary tract involvement may be up to five times more frequent tin diabetics than non-diabetics12. Bilateral kidney disease is also more frequent56. Complications like pyelonephritis, renal abscess and papillary necrosis are more common. Cortical and peri-renal cysts are more frequent in the diabetic population and 25% are caused by staphylococcal septicemia.
E.coli is the most common bacterial pathogen causing urinary infection in patients with diabetes, other organisms being Klebsiella pneumoniae and Proteus mirabilis12. Pseudomonas aeruginosa should be suspected if there is a history of recent instrumentation or hospitalization12.
diabetic patients but the number was significantly less than in the control group. The number of diabetic patients with Klebsiella as community-acquired infection was significantly more than in the control group. In nosocomial UTI in the diabetics, Klebsiella preponderance was noted. They concluded that patients with diabetes had increased susceptibility to Klebsiella as a pathogen in both community acquired and nosocomial UTI.
Respiratory Infections and Diabetes Mellitus:
streptococci, Legionella and Influenza60. These were observed by Koziel et al60. in their study on pulmonary complications of diabetes mellitus: pneumonia. Lipsky et al61 observed in Type 2 diabetics that up to 30% of diabetics are nasal carriers of Staphylococcus aureus compared to 11% of the healthy individuals. This is a major organism in both community-acquired and nosocomial pneumonia. Gram-negative organisms are acquired by aspiration, hematogenous spread or contaminated equipment. Aspergillus species, Coccidiodes immitis and Cryptococcus neoformans can cause primary pneumonia in the diabetic host.
Tuberculosis and Diabetes Mellitus:
Diabetics have a risk for increased incidence of tuberculosis and have a more advanced disease at presentation12. Reider et al64 reviewed the epidemiology of tuberculosis in diabetes mellitus based on three large surveys from 1950s and suggested that the relative risk of tuberculosis in individuals with diabetes is 2-3.6 times that in those without diabetes. In a study from Papua New Guinea65, the frequency of occurrence of tuberculosis in diabetic patients was found to be 11 times the expected rate in the general population. There does not seem to be an increased prevalence of patients in developed countries14. There is however, evidence for increased rate of both tuberculosis in diabetes mellitus patients and new diabetes mellitus in tuberculosis patients in Africa66.
Skin and Soft Tissue Infections and Diabetes Mellitus:
Diabetic patients have an increased frequency of deep soft tissue infections, both bacterial and fungal.
Bacterial Infections:
There is no clear evidence that diabetic patients are more prone to Staphylococcal infections than control subjects14. However, Smith JA et al67 reported in the Lancet that there is evidence for increased nasal carriage of Staphylococcus aureus in diabetic patients, especially those treated with insulin. Patients with well-controlled diabetes do not have an increased risk for infection post operatively14. Cluff et al68 in their study on staphylococcal bacteremia found that the mortality in staphylococcal bacteremia and diabetes is higher than in those without the disease.
Fungal Infections:
direct swab technique, found that a level of HbA1C of 12% or greater correlated
with yeast colonization.
Mucormycosis relates to a group of fulminant infections caused by Phycomycetes. Infection with these organisms is rare as there is strong innate immunity. The increased risk in diabetics is evident only in those who are debilitated, for example with diabetic ketoacidosis14. Batra et al72. have reported that about 75% of the patients with mucormycosis have diabetes mellitus. In such patients, the natural inhibitory activity of the patient’s serum against the Phycomycetes is lost but can return with insulin treatment.
Diabetic Ketoacidosis and Infections:
Various studies have been done in adults to find the incidence of Diabetic Ketoacidosis (DKA) in new onset and established cases, the most common precipitating factor in DKA and to find the cause of mortality in DKA.
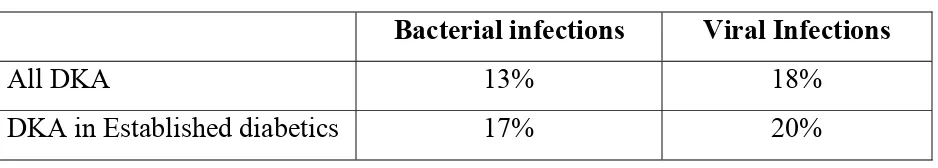
Infection has been found to be the most common precipitating factor of DKA in the various studies done in adults. In many case series in adults in the last 20 years, infection was the most common precipitating factor in about 28-43%. However, insulin omission is considered to be the most frequent cause of DKA in children with known diabetes73.
were present in only 13% and viral infections in18%. Among the subgroups of children with established diabetes, bacterial infections were present in 17% and viral infections in 20%.
Table 3: DKA study – Chiang et al74
Bacterial infections Viral Infections
All DKA 13% 18% DKA in Established diabetics 17% 20%
A study was done by Matoo et al75 at Chandigarh among 143 cases of DKA in the age group 8-70 years. 33.5% was new onset DKA and infection was the most common precipitating factor (30%). Omission of insulin was the cause in 20%. Mortality rate was 23.7%.
Table 4: Summary of DKA studies
Reference Episodes
(n)
Infection (%)
Poor insulin compliance
(%)
New onset diabetes
[image:34.612.72.543.498.648.2]Infections and Glycemic Control:
Some studies have failed to prove a causal relation ship between hyperglycemia and infections like the study by Wheat LJ36 published in the Diabetes Care in the year 1980. However, some authors like Moutschen et al15 suggest that hyperglycemia or the metabolic abnormality is sufficient to explain the immune response in patients responding to infections. They have reported that cells from diabetic patients have reduced chemotaxy, especially when diabetes is poorly controlled. Gin H et al21. reported that the bactericidal capacity of the Polymorphonuclear leucocytes, which is decreased in the presence of high blood glucose, normalizes following intensive insulin regime. So, there is evidence both for and against the fact that poor glycemic control predisposes to infections in diabetes mellitus.
4. STUDY JUSTIFICATION
5. OBJECTIVES OF THE STUDY
1) To identify the incidence of infections in children with Type 1 Diabetes Mellitus.
2) To determine the type and severity of infections.
3) To evaluate the risk factors causing infections in diabetic children.
6. STUDY METHODOLOGY
Study Design:
This study was carried out with a Cohort study design.
Setting:
The study was conducted at the Institute of Child Health and Hospital for Children, Madras Medical College, a Tertiary care Children’s Hospital in Chennai.
Study Period:
It was done during the period of January 2005 – September 2006
Study Population:
Inclusion Criteria:
For Group 1: Children with Diabetes Mellitus in the age group of 0 – 12 years and registered at the Diabetic clinic at Institute of Child Health and Hospital for Children and on regular follow up and willing to participate in the study.
For Group 2: Age and Sex matched children free of Diabetes Mellitus and willing to participate in the study.
Exclusion criteria:
• Children with severe PEM (Grade III and Grade IV PEM)
• Children with malignancies like leukaemia, lymphoma
• Children on immunosuppressive drugs or steroids for any reason.
• Children with Renal failure, nephrotic syndrome or any known immunodeficiency states, sickle cell anaemia and recurrent wheeze.
Sample size:
Manoeuvre:
The study was conducted at the Institute of Child Health and Hospital for Children between the period of January 2005 and September 2006. The cases were enrolled for the study from the Diabetic clinic at the Institute.
Though 300 patients are registered with the clinic many patients collect their drug (insulin) from a neighbouring government hospital and come only for assessment of glycemic control and complications. The others come for a review once in every 6-8 weeks. So, it was decided to enrol patients who lived in and around Chennai and collected drugs from the clinic and were on a regular follow up. Enrolment was done from April 2006 – June 2006. About 170 children who were on regular follow up were selected and data regarding age of onset, family history of diabetes mellitus, previous hospitalisations and other relevant medical history were collected and anthropometry recorded.
the principal investigator. The parents were also encouraged to contact the principal investigator in case of intercurrent infections or hospitalisations.
At the time of monthly follow up, data was collected regarding any intercurrent illness – its type and also severity – i.e., whether it required hospitalisation or not. The children were thoroughly examined and anthropometric measurements were recorded. All infections in the Group 1 were appropriately investigated with complete blood count, peripheral smear, urine routine, urine culture and sensitivity, radiological investigation, culture of body fluids & serological tests. The children were screened for HBsAg after obtaining consent from the patients. For the assessment of glycemic control, HbA1C levels were
evaluated once in every 4 months i.e., at 4, 8 and 12 months into the study.
hospitalisations. Appropriate investigations were done for these children when they developed infections. They were not tested for HBsAg. Mantoux and Chest X ray were not done. HbA1C was also not done in this group, as it is not relevant.
Both the groups were investigated based on the signs and symptoms of infection only. There was no difference in the intensity of the investigations done in either group.
Any documented infection based on clinical and/or lab investigations was considered as an outcome measure. List of expected infections included Urinary tract infections, Pneumonia, Impetigo, cellulitis, furunculosis and other soft tissue infections, Fungal infections of skin and mucosal membranes, Supportive otitis media, Tonsillitis, Sinusitis, fever > 5 days without or localising symptoms , etc., Simple URI i.e. fever < 5 days duration with or without cough and rhinitis, fever without localising focus of infection and acute watery diarrhoea with a few loose stools lasting for less than three days, all of which did not warrant antimicrobial or antimalarial therapy were documented but were not considered as an outcome. The detailed definitions for infections considered as outcome in this study are as follows:
Urinary Tract Infection:
examination showing pyuria with or without urine culture and sensitivity positivity was considered as UTI.
Pneumonia:
Features such as fever, cough, breathlessness, chest pain, preceded by symptoms of upper respiratory infection with clinical evidence in the form of tachypnea, respiratory distress, cyanosis, adventitious breath sounds with increased total WBC count in the range of 15 – 40,000 cells/cu .mm with polymorphic or lymphocytic predominance and a positive CRP, confirmed by radiological evidence of pneumonia in the form of pneumonitis, consolidation, bronchopneumonia with or without pleural effusion and empyema.
Pharyngitis / Tonsillitis:
Fever, sore throat, headache, change of voice, dysphagia, with congestion of the tonsils with exudates, enlarged tender anterior cervical lymph nodes supported by increased total WBC count, with polymorphic predominance and a positive CRP confirmed by positive throat swab culture.
Sinusitis:
for 3 – 4 days, supported by radiological evidence of infection of the sinuses in the form of opacification, mucosal thickening or presence of air fluid levels.
Otitis Media:
History of fever, ear pain, irritability, ear discharge supported by otoscopic evidence of infection in the form of two of the three abnormalities 1. White, yellow of amber or blue colour of the tympanic membrane. 2. Opacification of the membrane and 3. Decreased or absent mobility.
Pyogenic Infections of Skin:
Including impetigo, abscess, follicullitis, hydradenitis, cellulitis and ecthyma were diagnosed based on that clinical examination findings, Gram stain and culture positivity of the blister fluid or a moist plaque if present.
Candidiasis:
Clinical evidence of candidal infection as oral thrush and genital involvement as itching, pain, dysuria, vulvar erythema, with cheesy exudates, paronychia and onychomycosis with laboratory evidence of candida in the scrapings.
Septicemia:
Any other infection encountered was diagnosed as per the clinical features and confirmed by laboratory evidence. The end point for the diagnosis was based on laboratory evidence in the form of culture positivity wherever feasible. However in certain conditions where lab evidence was not possible always (for e.g. Impetigo) definite clinical features were taken as the end point for infection.
Diabetic Ketoacidosis:
All the children admitted with diabetic ketoacidosis during the study period (January 2005 – June 2006) were evaluated. Detailed history regarding the clinical features and duration, compliance of insulin and features suggestive of infection were taken. Clinical examination for assessing the hydration status, circulatory status and to identify the focus of infection was done. Baseline investigations included complete blood count, blood sugar, blood urea, serum creatinine and serum electrolytes, urine routine with ketones, urine culture and sensitivity, non-enteric culture and chest X ray. Special additional investigations like culture of body fluids were done as required.
7. RESULTS
A total of 112 diabetic children were enrolled in the study. The same number of age and sex matched children who were not the siblings of the study children were identified from the Immunisation OPD and School Health Cell of The Institute of Child Health and Hospital for Children, Chennai were enrolled as the comparison group. Both the groups of children were followed up for a period of one year from the time of enrolment. They were reviewed every month for the presence of infections and the details were documented. The data was analysed using SPSS software Version 11.0 for Windows.
The results of analysis are presented as follows:
1.Socio - demographic characteristics
2.Incidence, type and severity of infections
3. Risk factors for infections in diabetic children
7.1.Socio- demographic Characteristics:
These are presented as follows: Age and Sex Nutritional status
Age and Sex Distribution of the Study Populations
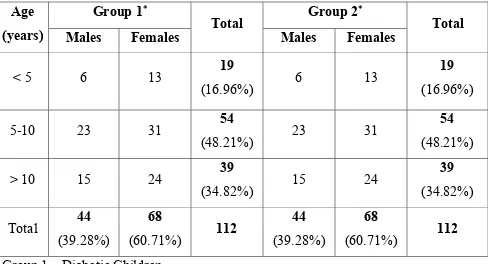
[image:51.612.70.558.199.463.2]The age and sex distribution of the study populations is depicted in the following table.
Table 1: Age and Sex Distribution of the Study populations
Group 1∗ Group 2∗
Age
(years) Males Females Total Males Females Total
< 5 6 13 19
(16.96%) 6 13
19
(16.96%) 5-10 23 31 54
(48.21%) 23 31
54
(48.21%) > 10 15 24 39
(34.82%) 15 24
39
(34.82%) Total 44
(39.28%)
68
(60.71%) 112
44
(39.28%)
68
(60.71%) 112 Group 1 – Diabetic Children
Group 2 – Non Diabetic children
design and their age and sex distribution were similar to the diabetic children as revealed by the table.
Nutritional Status:
The nutritional status of the study populations is presented as weight, height and Body Mass Index (BMI) percentiles .
Weight for Age Centiles Distribution:
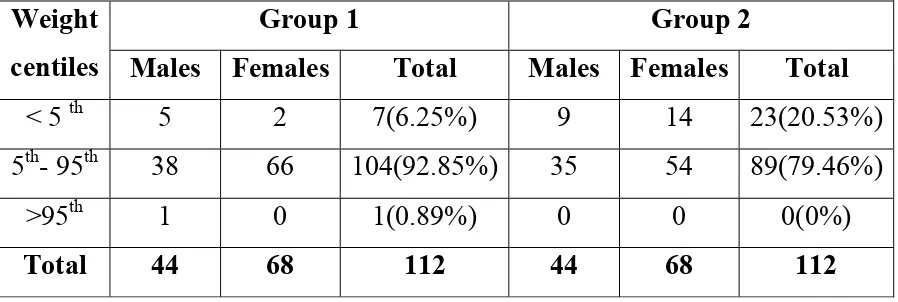
[image:52.612.67.516.327.478.2]The following table shows the weight for age centiles of the study groups.
Table 2: Weight for Age Centiles Distribution of Group 1 and Group 2:
Group 1 Group 2
Weight
centiles Males Females Total Males Females Total
< 5 th 5 2 7(6.25%) 9 14 23(20.53%) 5th- 95th 38 66 104(92.85%) 35 54 89(79.46%)
>95th 1 0 1(0.89%) 0 0 0(0%)
Total 44 68 112 44 68 112
Height for Age Centiles Distribution:
[image:53.612.69.520.165.332.2]The following table shows the height for age centiles of the study groups.
Table 3: Height for Age Centiles Distribution of Group 1 and Group 2
Group 1 Group 2
Height
centiles Males Females Total Males Females Total
< 5 th 4 4 8(7.14%) 9 17
26(23.21%)
5th- 95th 39 63 102(91.07%) 35 51 86(76.78%) >95th 1 1 2(1.78%) 0 0 0 (0%)
Total 44 68 112 44 68 112
BMI for Age Centiles Distribution:
[image:54.612.63.534.199.376.2]The following table shows the BMI for age centiles of the study groups.
Table 4: BMI for age centiles distribution of Group 1 and Group 2
Group 1 Group 2
BMI
centiles Males Females Total Males Females Total
Normal 36 61 97(86.6%) 34 48 82(73.21%) Underweight 8 6 14(12.5%) 8 17 25(22.32%) Overweight - - - 2 2 4(3.57%) Obese 0 1 1(0.89%) 0 1 1(0.89%) Total 44 68 112 44 68 112
Family History of Diabetes Mellitus:
[image:55.612.65.512.226.388.2]The family history of diabetes mellitus in the children with diabetes mellitus is depicted below.
Table 5: Family History of Diabetes in Group 1
Relation Male Female Total
Mother 0 1 1
Father 1 4 5
Siblings 0 1 1
Other relatives 12 7 19
Total 13 13 26
Diabetic Age:
[image:56.612.69.510.182.309.2]This is analysed as diabetic age less than two years and more than two years.
Table 6: Diabetic Age Group:
Number of children Diabetic Age
Males Females Total
<2 years 18(40.9%) 27(39.7%) 45(40.17%) >2 years 26(59.09%) 41(60.29%) 67(59.82%)
Total 44 68 112
7.2. Incidence of Infections:
In this section , the incidence of infections in the study populations is discussed. Simple URI, Fever without localizing signs lasting less than five days and acute
[image:57.612.72.549.293.497.2]watery diarrhea lasting less than three days were not considered for analysis. The following table shows the number of infected children in both the groups.
Table 7: Number of children with infections in Group 1 and Group 2
Group 1 (N = 112)
Group 2 (N =112) No. of children who had infections
n % n % p – value*
Total no. of children with infections 56 50.5% 46 41.1% 0.23 Skin infections 51 45.5% 23 20.5% 0.000
Urinary tract infections 7 6.3% 0 - 0.014
Respiratory Infections 7 6.3% 22 19.6% 0.005 *Chi-square test
Number and Type of Infections in the Study Populations:
The table below shows the number and type of infections in both the groups.
Table 8: Type of Infections in Group 1 and Group 2:
Infections Group 1 Group 2
Respiratory Infections:
Pneumonia 1 1
Sinusitis 1 2
Pharyngotonsillitis 2 16
ASOM 3 3
Urinary tract infections 8 0
Skin and soft tissue infections:
Bacterial Infections 77 21 Fungal Infections 13 2
Sepsis 0 0
TOTAL 107 45
were infections of the skin and soft tissue. Here again, bacterial infections (46.66%) were commoner than fungal (4.44%). A total of eight Urinary tract infections (7.4%) were seen in the diabetic group and none in the control group (0%) during the one-year follow up. There were 22 (48.88%) infections of the respiratory tract in Group 2 as against 7(6.5%) in Group 1.
This table shows that diabetic children had more number of infections when compared to the normal children. Also, infections of the skin and soft tissue and urinary tract were more commonly seen in the diabetic children.
None of the diabetic children tested positive for HBsAg. Though three children had Mantoux positivity, none required treatment with Anti tuberculous therapy.
Infections of the Skin and Soft Tissue:
[image:61.612.80.530.167.462.2]Below is shown, the type of skin infections in both the groups.
Table 9: Type of Skin infections in Group 1 and Group 2:
Skin Infections Group 1 Group 2
Bacterial infections:
Impetigo 31 (34.4%) 14 (60.86%) Cellulitis 10 (11.11%) 2 (8.7%) Abscess 18 (20%) 0 (0%) Furunculosis 14 (15.56%) 5 (21.73%) Nail infections 4 (4.44%) 0 (0%)
Fungal infections:
Dermatophytosis 3 (3.33%) 2 (8.7)% Candidal Infections 10 (11.11%) 0 (0%)
Total 90 23
from one of the five injection site abscesses (n=1, 20%). and ten of the thirteen abscesses at other sites (n=10,76.9%). Totally 11 cultures (61.1%) were positive of the 18. The rest of the cultures (38.89%) were sterile. Looking at fungal infections in Group 1, Candidal infections were the commonest (n=10,76.92%) and Dermatophytosis constituted the rest (n=3,23.07%).
The commonest bacterial infection in Group 2 was impetigo (n=14,66.67%). The number of furunculosis and cellulitis seen were five (23.8%) and two (9.52%) respectively. All fungal infections were Dermatophytal infections (n=2,100%). No Candidal infection was seen.
Infections of the Urinary Tract:
[image:62.612.160.461.492.595.2]The organisms causing urinary tract infections in Group 1 is shown below. There were no urinary tract infections in Group 2 during the study period.
Table 10: Organisms causing urinary tract infections in Group 1
Organism Number
E.coli 4(50%) Klebsiella 3(37.5%) Pseudomonas aeruginosa 1(12.5%)
Total 8
Severity of Infections:
7.3 Risk Factor Analysis for Infections in Children with Diabetes Mellitus:
Risk factor analysis was done for children with diabetes mellitus based on the following:
1.Socio-demographic factors:
• Age
• Sex
• Diabetic age and
• Nutritional status (BMI).
2. Glycemic control (Mean HbA1C)
3. Presence of Diabetic Ketoacidosis (DKA)
Analysis of Risk Factors for Total Infections :
[image:65.612.73.519.166.472.2]The risk factor analysis for total infections in diabetic children is discussed below.
Table 11: Analysis of risk factors for total infections
Total Infections
Yes No
N %
n % n % p-value*
Age
< 5 years 19 17.0 10 52.6 9 47.4 5 - 10 years 54 48.2 27 50.0 27 50.0
> 10 years 39 34.8 19 48.7 20 51.3 0.96
Sex
Male 44 39.3 21 47.7 23 52.3
Female 68 60.7 35 51.5 33 48.5 0.85
Diabetic age
<=2 years 45 40.2 20 44.4 25 55.6
> 2 years 67 59.8 36 53.7 31 46.3 0.44
Nutrition: Normal Under weight Overweight Obese 97 14 - 1 86.6 12.5 - 0.9 49 6 - 1 50.5 42.9 - 100.0 48 8 - - 49.5 57.1 - - 0.52
*Chi-square test
correlation with sex. There were 45 children with diabetic age less than 2 years, of whom 20(44.4%) had infections .36 of the 67 children (53.7%) with diabetic age more than 2 years had infections. This again was not statistically significant. Of the 97 children with normal nutrition, 49 (50.5%) had infections and 48 (49.5%) had no infections. Six of the14 underweight children (42.1%) had infections and eight (57.1%) did not. The one obese child (100%)had no infection. No statistical significance was noted.
Glycemic Status and Occurrence of Total Infections:
[image:66.612.73.483.423.573.2]The influence of glycemic status on the occurrence of total infections is shown below.
Table 12: Mean HbA1C Level and Occurrence of Total Infections
Occurrence of Total Infections All diabetic
children Had Infection No infection
p-value
Mean
HbA1C 11.7 ± 3.1 13 ± 2.9 10.5 ± 2.8 0.00+
+ Two sample t-test
Mean HbA1C in the study population was 11.7 ±3.1. It was higher in the infected
Analysis of Risk Factors for Skin Infections:
[image:67.612.71.543.164.586.2]The risk factor analysis for skin infections in diabetic children is discussed below.
Table 13: Analysis of Risk Factors for Skin Infections:
Skin Infections
Yes No
N %
n % n %
p-value*
Age
< 5 years 19 17.0 10 52.6 9 47.4 5 - 10 years 54 48.2 25 46.3 29 53.7
> 10 years 39 34.8 16 41.0 23 59.0 0.70
Sex
Male 44 39.3 19 43.2 25 56.8 Female 68 60.7 32 47.1 36 52.9 0.70
Diabetic age
<=2 years 45 40.2 19 42.2 26 57.8 > 2 years 67 59.8 32 47.8 35 52.2 0.70
Nutrition: Normal Under weight Overweight Obese 97 14 - 1 86.6 12.5 - 0.9 44 6 - 1 45.4 42.9 - 100.0 53 8 - - 54.6 57.1 - - 0.54
*Chi-square test
(43.2%) and 32 of 68(47.1%) female diabetics had skin infections, which again failed to show any statistical significance. Diabetic age again, did not seem to affect the incidence of skin infections as 19 of 45 (42.2%)children with diabetic age <2 years had skin infections as against 32 of 67 (47.8%)children with diabetic age >2 years. Of the 97 children with normal nutrition, 44 (45.4%) had skin infections whereas 53 (54.6%) did not. Six of the 14 underweight children (42.1%) had skin infections and eight (57.1%) did not. The one obese child (100%) was infected. No statistical significance was noted.
Glycemic Status and Skin Infections:
[image:68.612.92.512.454.593.2]The influence of glycemic status on the occurrence of skin infections is shown below.
Table 14: Mean HbA1C Level and Skin Infections
Skin Infections All Diabetic
Children Had Infection No infection
p-value+
Mean
HbA1C 11.7 ± 3.1
13.1 ± 2.8 10.6 ± 2.9 0.00
+ Two sample t-test
The glycemic status however did affect the occurrence, with greater HbA1C
Analysis of Risk Factors for Respiratory Infections:
[image:69.612.72.501.192.613.2]The risk factor analysis for respiratory infections in diabetic children is discussed below.
Table 15: Analysis of risk factors for Respiratory infections in Group 1:
Respiratory Infections Yes No
N %
n % n % p-value*
Age
< 5 years 19 17.0 1 5.3 18 94.7 5 - 10 years 54 48.2 4 7.4 50 92.6
> 10 years 39 34.8 2 5.1 37 94.9 0.89
Sex
Male 44 39.3 5 11.4 39 88.6 Female 68 60.7 2 2.9 66 97.1 0.11
Diabetic age
<=2 years 45 40.2 2 4.4 43 95.6
> 2 years 67 59.8 5 7.5 62 92.5 0.70
Nutrition: Normal Under weight Overweight Obese 97 14 - 1 86.6 12.5 - 0.9 7 - - - 7.2 - - - 90 14 - 1 92.8 100.0 - 100.0 0.56 * Chi-square test
analysed statistically. 5 males (11.4%)and two females (2.9%) had respiratory tract infections .Sex again, did not have any effect on the incidence of respiratory infections. 2 children (4.4%) with diabetic age less than 2 years and 5 children (7.5%)with diabetic age more than 2 years had respiratory infections with no statistical significance. Of the 97 children with normal nutrition, 7(7.2%) had respiratory infections whereas 90 (92.8%) did not. None of the underweight or obese children were infected. No statistical significance was noted.
Glycemic Status and Respiratory Infections:
[image:70.612.85.512.426.599.2]The influence of glycemic status on the occurrence of respiratory infections is shown below.
Table 16: Mean HbA1C level and Respiratory infections
Respiratory Infections
All diabetic
children Had
Infection No infection
p-value+
Mean
HbA1C 11.7 ± 3.1 12.7 ± 3.7 11.7 ± 3.1 0.40
+ Two sample t-test
Mean HbA1C also showed no difference between the diabetic children with
Analysis of Risk Factors for Urinary Tract Infections:
[image:71.612.72.544.196.602.2]The risk factor analysis for urinary tract infections in diabetic children is discussed below.
Table 17: Analysis of Risk Factors for Urinary Tract Infections :
Urinary Tract Infections
Yes No
N %
n % n % p-value+ Age
< 5 years 19 17.0 2 10.5 17 89.5 5 – 10 years 54 48.2 2 3.7 52 96.3
> 10 years 39 34.8 3 7.7 36 92.3 0.52
Sex
Male 44 39.3 2 4.5 42 95.5 Female 68 60.7 5 7.4 63 92.6 0.70
Diabetic age
<=2 years 45 40.2 5 11.1 40 88.9
> 2 years 67 59.8 2 3.0 65 97.0 0.12
Nutrition Normal Under weight Overweight Obese 97 14 - 1 86.6 12.5 - 0.9 7 - - - 7.2 - - - 90 14 - 1 92.8 100.0 - 100.0 0.56 * Chi-square test
age was noted. There were two males (4.5%) and five females (7.4%) with no statistical significance for sex. Five of the children had diabetic age less than two years (11.1%) and two had a diabetic age more than two years (3%). This was also not found to be significant statistically. Of the 97 children with normal nutrition, 7(7.2%) had urinary tract infections whereas 90 (92.8%) did not. None of the underweight or obese children were infected. No statistical significance was noted.
Glycemic Status and Urinary Tract infections:
[image:72.612.72.480.411.519.2]The influence of glycemic status on the occurrence of urinary tract infections is shown below.
Table 18: Mean HbA1C level and Urinary tract infections Urinary Tract Infections All diabetic
children Had Infection No infection valuep- +
Mean
HbA1C 11.7 ± 3.1 10.8 ± 2.9 11.8 ± 3.1 0.40
+ Two sample t-test
The mean HbA1C was 10.8 ± 2.9 in the diabetic group with urinary tract
Infections and Diabetic Ketoacidosis:
[image:73.612.76.512.184.254.2]The correlation between infections and Diabetic Ketoacidosis is presented below.
Table 19: Association between Infections and Diabetic Ketoacidosis (DKA)
DKA present No DKA
Infections present 5 51
No Infections 9 47
Infections and Severity of diabetic ketoacidosis:
[image:74.612.70.530.193.587.2]Of the 14 children admitted with diabetic ketoacidosis during the study period, five had infections.
Table 20: Infections and Severity of diabetic ketoacidosis
Infections
Yes No
N %
n % n % p-value
Severity
Mild 9 64.3 3 33.3 6 66.7 Moderate 5 35.7 2 40.0 3 60.0 1.00*
Shock
Yes 8 57.1 3 37.5 5 62.5 No 6 42.9 2 33.3 4 66.7 1.00*
Insulin infusion
duration (Hours) 20 ± 18 24 ± 24 17 ± 15 0.61+
Subcutaneous
insulin duration
(Hours) 68 ± 43 46 ± 36 80 ± 43 0.19+
Days of hospital
stay 6 ± 4 4 ± 2 7 ± 5 0.44+
* Chi-square test + Mann-Whitney U test
SEVERITY OF DKA
Venous pH Clinical
Normal 7.35 – 7.45 No change Mild DKA 7.25 – 7.35 Fatigued
Moderate DKA 7.15 – 7.25 Kussmaul’s respiration, sleepy, arousable Severe < 7.15 Kussmaul or depressed
respiration, depressed sensorium to coma.
Other factors like shock, duration of insulin infusion, duration of subcutaneous insulin administered and duration of hospitalisation was compared between the children with infections and diabetic ketoacidosis and children with diabetic ketoacidosis only.
8. SUMMARY OF THE STUDY
At the end of a one-year follow up period, it is seen that the number of infections are more common in the diabetic children than in the normal children. Though the actual number of infected children in both the group is not very different between the groups, it is seen that the number of times a diabetic child got infected is more than the number of times a normal child got infected during the same one year follow up period, i.e.; whenever a diabetic child got infected it had a tendency to have recurrent infections which was not so in the case of normal children. It is also seen that infections of the skin and soft tissue and urinary tract are more common in the diabetic children than in the normal children whereas respiratory tract infections are more common in the normal children. While three children in the diabetic group were hospitalized for infections, in addition to five children with infections and diabetic ketoacidosis, none of the normal children were hospitalized for infections. None of the diabetic children in the study suffered from Hepatitis B infection or tuberculosis.
incidence of skin infections is affected by the glycemic status, with poor glycemic control predisposing to more infections of the skin and soft tissue. However, incidence of urinary tract infections and respiratory tract infections is not affected by the glycemic status.
9. DISCUSSION
Comparison with Other Studies and Literature Review:
Most of the studies on infections in diabetes mellitus have been done only in adults with either Type 2 or Type 1 diabetes. There is paucity of data on infections in diabetes mellitus in children. Review of the literature shows that, people with diabetes mellitus have incompletely defined abnormalities of the cell mediated immunity, phagocytic function, hyperglycemia and diminished vascularisation all of which leads to an increased incidence and frequency of infections in these patients1. In fact, the World health Organisation lists diabetes as a secondary immunodeficiency disease13. There is also evidence to support the concept that hyperglycemia per se or the metabolic abnormality in diabetes is sufficient to explain the impaired immune response in patients responding to infections15. It is also true that infections in diabetic patients may worsen the glycemic control12, 79. So, poor glycemic control may impair the immune response and cause infections, which in turn may worsen the glycemic status, and, hence, these children with poor glycemic control have recurrent infections.
furunculosis, erysipelas, and carbuncle. In our study also diabetic patients had more number of skin and soft tissue and urinary tract infections. Bacterial infections of the skin were more common than fungal. However impetigo was the commonest bacterial infection in our study among the diabetic population. Abscesses and nail infections were more commonly seen in the diabetic population. Staphylococcus aureus was the commonest organism isolated. There is no clear evidence for increased incidence of Staphylococcal infections in diabetics, though the risk of increased nasal61 and skin colonization39 by the same is noted, especially for those on insulin therapy.
As seen in the study by Alteras et al.69, Candida was more common in the diabetics than non –diabetics (11.11%Vs 0%) whereas their study showed a difference of 31% Vs 5%. Dermatophytosis was not more common in the diabetics than in the non diabetics (3.33% Vs 8.75%) as observed in the study by Alteras as well.
hospital. Our study also shows that, Klebsiella is the next most common pathogen causing urinary tract infection in diabetics. One child admitted with Diabetic ketoacidosis grew Pseudomonas aeruginosa, which again could be explained by the hospitalisation.
increased. Fraser et al.46 only found an association with chronic Hepatitis C virus infection and not chronic Hepatitis B virus infection.
A number of in vitro studies done by Moutschen et al.15 and Gin H et al.21have proved the impaired immune response in poorly controlled diabetes. Our study has established that poor glycemic control does lead on to infections in diabetic children, especially of the skin and soft tissue.
10. CONCLUSIONS
Infections occur more frequently in diabetic patients and with more severity.
Diabetic children are more prone to infections of the skin and urinary tract when compared to normal children.
None of the diabetic children in our study suffered from tuberculosis.
None of the diabetic children in our study had Hepatitis B Virus infection.
Poor glycemic control predisposes to infections in diabetic children, especially of the skin and soft tissues.
There is no correlation between infections and diabetic ketoacidosis or its severity in children with diabetes.
Maintaining good glycemic control could prevent infections in children with
diabetes mellitus. Hence, there is an urgent need to create awareness in children
Data Entry Form for Registration of Group 1:
S.no
Name Age Sex Address
Date of Registration
Family history of Diabetes mellitus: Diabetic age as on registration
Previous hospitalisations: Y /N
Diagnosis: Other medical illnesses :
Clinical Examination:
Height (cm): Weight (kg): BMI:
Baseline Investigations:
Total count: Differential count : Peripheral smear : Urine : Albumin Sugar Deposits
Urine C/S :
Mantoux Chest X ray: HBsAg :
Data Entry Form for Registration of Group 2:
S.No Name Age Sex Address
Date of Registration
Family history of Diabetes mellitus: Medical History:
Clinical Examination: Height (cm):
Follow up Visits – Data entry form for Groups 1 and 2:
Date: S.no: Visit number : Height (cm): Weight(kg) : BMI :
Complaints: Nil / Fever/ Cough/ breathlessness/diarrhea/vomiting/ Skin infection/ dysuria/ itching over genitals/injection site injection/ ear discharge/convulsions/ altered sensorium/ others.
Duration of illness:
Examination: HR RR BP Temp CVS RS P/A CNS
ENT SKIN
Severity of illness: treated as op/ip Investigations:
TC DC PS Blood Glucose (Group 1):
Urine R/E:
Urine Culture and sensitivity:
Non Enteric Culture and sensitivity: Body Fluids Culture and sensitivity:
Proforma for children with Diabetic Ketoacidosis:
Complaints: Duration :
Examination: HR RR BP Temp CVS RS P/A CNS SKIN ENT
Focus of Sepsis:
Features of DKA:
1.Dehydration : Y/N 2.Acidotic features: Y/N 3. Shock : Y/N
Predisposing Factors :Infections/ Poor compliance/ none
Investigations:
TC DC PS
B.Sugar S.electrolytes B.urea S.creatinine pH :
Urine R/E
Urine Culture and sensitivity:
Non Enteric Culture and sensitivity:
Duration of insulin infusion (hrs): Duration of subcutaneous insulin(hrs): Duration of Hospitalisation(days): Final diagnosis:
BIBLIOGRAPHY
1. Alvin C.Powers.Diabetes Mellitus .In: Harrison’s principles of Internal Medicine, 16th edition, Kasper, Braunwald, Fauci and Hauser et al. (eds), McGraw Hill Medical Publishing Division, 2005; 2152-80.
2. Michael J Haller MD, Mark A Atkinson Ph.D, Desmond Schatz MD. Type 1 Diabetes Mellitus: Etiology, Presentation and Management. Pediatr Clin N Am 52 (2005) 1553 – 78.
3. Ramin Alemzadeh and David T.Wyatt.Diabetes Mellitus in Children .In: Nelson Textbook of Pediatrics, 17th edition, Behrman, Kleigman and Johnson (eds), Thomson press (India) Ltd, 2004; 1947-72.
4. P.Tong and Clive S.Cockram. Economics of Care: South and East Asia. In :International Textbook of Diabetes Mellitus, 3rd edition, R.A.Defronzo, E.Ferrannini, H.Keen and P.Zimmet (eds), John Wiley and Sons, Ltd.ISBN:0-471-48655-8,2004;Vol 2; 1855-1859
5. Shobana R,Rama Rao P,Lavanya A,Williams R,Padma C,Vijay V,Ramachandran A. Costs incurred by families having type I diabetes in a developing country-a study from Southern India. Diabetes Res Clin Pract 2002; 55:45-8
6. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin dependent diabetes mellitus. New Engl J Med 1993;329:977 – 86.
7. Robbins SL, Tucker AW Jr. The cause of death in diabetes: a report of 307 autopsied cases. N Engl J Med 1944;231:865 – 8.
8. Seymour A, Phear D. The causes of death in diabetes mellitus. A study of diabetic mortality in the Royal Adelaide Hospital from 1956 to 1960. Med J Aust 1963;1:890 – 4.
10. Sasaki A, Horiuchi N, Hasegawa K, Uehara M. Mortality and causes of death in Type 2 diabetic patients: a long-term follow-up study in Osaka District, Japan. Diabetes Res Clin Prac 1989;7:33 – 40.
11. Kessler II. Mortality experience of diabetic patients: a twenty-six year follow-up study. Am J Med 1971;51:715 – 24.
12. Nirmal Joshi and Monica Mahajan. Infections and Diabetes. In: Textbook of Diabetes, 3rd edition, John C.Pickup, Gareth Williams (eds), Blackwell Science, 2003;Volume 1; 40.1-40.16
13. World Health Organization. Immunodeficiency: Report of a Scientific Group. Technical Report Series 630. Geneva: World Health Organization, 1978.
14. Paolo Pozzilli and R.D.G Leslie.Infections, Immunity, and diabetes .In :International Textbook of Diabetes Mellitus, 3rd edition, R.A.Defronzo, E.Ferrannini, H.Keen and P.Zimmet (eds), John Wiley and Sons, Ltd.ISBN:0-471-48655-8,2004;Vol 2; 1729-39
15. Moutschen MP, Scheen AJ, Lefebvre PJ. Impaired immune responses in diabetes mellitus: analysis of the factors involved. Relevance to the increased susceptibility of diabetic patients to specific infections. Diabete Metab 1992;18: 187-201.
16. Wilson RM, Reeves WG. Neutrophil function in diabetes. In Nattrass M (ed). Recent Advances in Type 2 Diabetes. London: Churchill Livingstone 1986; pp 127 – 38.
17. Walenberger J, Lange J, Kranz A. Vascular endothelial growth factor-A-induced chemotaxis of Monocytes is attenuated in patients with diabetes mellitus: a potential predictor for the individual capacity to develop collaterals. Circulation July 11, 2000;102:185 – 90.
18. Simmons D, Lelong LNG, Bomford J. Abnormal myoinositol influx in human leukocytes in diabetes but not specifically in diabetic neuropathy. Diabetes 1992;41:760 – 65.
19. Wykretowicz A, Wierusz-Wysocka B. Infection and diabetes: role of polymorphonuclear neutrophils. In Pozzilli P, Signore A (eds). Diabetes Prevention and Therapy, Vol. 6. Chichester, UK: Wiley, 1992;p 27