Vol.57,No. 1 JOURNALOFVIROLOGY, Jan. 1986, p.50-64
0022-538X/86/010050-15$02.00/0
Copyright©D1986, AmericanSociety forMicrobiology
Simian Virus
40
Origin
DNA-Binding Domain
on
Large
T
Antigen
EVA PAUCHA,t* DANIEL KALDERON,t ROBERT W.HARVEY,§ ANDALAN E. SMITH§
Biochemistry Division, National InstituteforMedicalResearch, MillHill, London NW7JAA, United Kingdom Received 28May1985/Accepted3 September1985
Fifty variant forms of simian virus 40 (SV40) large T antigen bearing point, multiple point, deletion, or termination mutations within aregion of the proteinthought to be involved inDNA binding were tested for theirability to bindtoSV40 originDNA. A number of the mutantlargeTspecies including some with point mutations were unable tobind,whereas many were wild type in this activity. The clustering of the mutations that are defective inorigin DNA binding both reported here and by others suggests aDNA-bindingdomainon large T maps between residues 139 and approximately 220, with a particularly sensitive sequence between amino acids 147 and 166. The resultsindicate that the domain is involvedinbindingtobothsite I and site II onSV40 DNA, but it remains unclear whether it is responsible for binding to cellular DNA. Since all the mutantsretain theability to transform Rat-1 cells, we conclude that the ability of large T to bind to SV40 origin DNA is not aprerequisite for its transforming activity.
Simian virus40(SV40) largeTantigen,theproductofthe
viralAgene, is requiredfor replicationofthevirusas well as
forthetransformation ofnonpermissive cells in culture(59).
Anumber ofbiochemical activities havebeen shown to be intrinsic to the proteinincluding an ATPase activity(4, 58)
and theabilitytoform complexes withthehost phosphopro-teinp53(24, 26). Large T has also been showntobind with
high
affinitytoviral(8, 10,31, 55-57)andcellular(2, 11, 41) DNA sequences. The role of the DNA-binding activity inboth replicative and transforming functions of large T has
been widely investigated. From analysis of a number of mutants isolated in several laboratories, it appears that the
bindingoflarge TtoSV40origin DNAisnecessary (30, 32,
50, 54) thoughnotsufficient (5,29, 43)forreplication,but not
essential for efficient transformation ofcells in culture (29, 43, 54).
In mapping the domain of large T involved in DNA binding, two types ofactivity
have
been measured in dif-ferent studies:theabilitytobindtocellular double-stranded (ds) DNA-cellulose (2,11, 41)and theabilitytobindtoSV40 originDNA sequences (8, 10, 31, 55-57). Itis notyet clearwhether these assays measure the activity of one or more
domains. The variantsused to map theDNA-binding domain include: (i) fragments of large T produced by in vitro translation of unspliced SV40cRNA (42); (ii) various trun-catedformsoflargeTpresent in differentSV40-transformed celllines(3, 46);(iii) various chimericproteinscodedforby adeno-SV40 hybrid viruses containing defined portions of
large T (33, 41, 57); (iv) defective large T species isolated frompermissiveornonpermissive transformedcells(12, 28,
29, 43, 49, 54);(v)pseudorevertant forms oflargeTthat are
ableto replicate froma defective origin sequence (30, 50); (vi)proteolyticfragments ofwild-type large T (34); and(vii) large T variants encoded by constructed SV40 A gene deletion mutants(5, 39). Almost all of the data are consistent insuggestingthatamino acids between 83(correspondingto
* Correspondingauthor.
tPresent address: Dana-Farber Cancer Institute, Boston, MA 02115.
tPresent address: Department of Biochemistry, University of California, Berkeley, CA94720.
§Presentaddress: IntegratedGenetics, Framingham, MA 01701.
the splice site in the large T mRNA) andabout 270 constitute part, if not all of aDNA-bindingdomain.
Wehave previously reported theisolation of over 50large
T variants, most of which carry mutations in the DNA coding for amino acids 106 to 158 oflargeT(19). Almostall of the mutants retain theabilitytotransform Rat-1 cells,but most are reduced or defective in theirability to replicate. Some of the mutations affect residues that are normally
phosphorylated in vivo, and others are located within a sequence that isimportantinlocalizing largeT tothe nucleus (17, 18, 25). Some of the mutants isolated had a
super-transforming activityinthatthey produced transformedfoci ingreater number and morerapidlythan
wild-type
largeT. A further seriesofmutantshave been producedby introducing termination codons at different positions throughout the large T coding region (18; B. Roberts, unpublished data). Here we report the DNA-binding properties of the large Tspeciespresent in cells transformed bythedifferent largeT
variantswehaveisolated. The datashow clearlythatpoint, multiple point, deletion, and termination mutations within the target area used here canabolish the ability tobind to
SV40 origin DNA sequences, as measured by an immuno-precipitation assay. This, together with data from other
laboratories,allows us to definepreciselythoseaminoacids thatconstitute the amino-terminalportionof aDNA-binding
domain onlargeT.The data alsoconfirm thatSV40largeT does notrequire the ability tobind to SV40originDNA to
transform established cells. It remains to be seen whether the ability to bind to cellular DNA plays a part in this process.
MATERIALSANDMETHODS
Cells. Allcellsweremaintained inDulbeccomodifiedEagle
medium
supplemented
with10%fetal calf serum. The rat cells (RE52) transformed by microinjected Taq-BamHI fragmentof SV40large T, called70K,werethegiftofA. Graessman.
SVA31E7 cells, SV40-transformed mouse cells, have been
described previously (13, 38). The generation of cell lines transformedbyeachof the mutant DNAs has been described indetailpreviously(19). Thewild-typeSV40-transformedrat line used in these experiments was generated as described (19) by transfection with the wild-type SV40-containing plasmid pPVU-0. Cell lines transformedby the termination
50
on November 10, 2019 by guest
http://jvi.asm.org/
mutants
H23, dH,
andHHpa
andby
the deletion mutants RL75-RL88 and RL12-S24were alsogenerated
by
transfec-tion of Rat-1 cells with
plasmid
DNAsby
theprocedure
previously
described(19).
The construction ofthe termina-tion mutants isgiven
in Kalderon et al.(18).
The deletion mutants were createdby
ligating
BamHI-EcoRI-cut DNAfrom the
appropriate pairs
oflinkerinsertionmutants(19).
Labeling
and extractionofcells. Theprocedures
forlabel-ing
and extraction of cells were carried outessentially
asdescribed
previously
(38, 51, 52).
Cells were labeled withmethionine for 5 h
by replacing
the medium on confluentmonolayers
of cellsgrowing
on50-or90-mmdishes(Nunc,
Roskilde, Denmark)
withgrowth
medium minus methioninesupplemented
with 1%complete
medium(containing
10% fetal calfserum)
and 200,uCi
of[35S]methionine
(specific
activity,
>1,000
Ci/mmol;
AmershamInternational)
perml.32P04
labeling
was for2 h ingrowth
medium minusphos-phate containing
250,uCi
of32p,
(Amersham
International)
per ml. After
labeling,
the cells were rinsed twice withice-cold Tris-buffered saline and then
lysed
by
the additionof1
ml/90-mm
dish or0.5 ml/50-mm dishofcell extraction buffercontaining
50mM Tris(pH 8.0),
120 mMNaCl,
and0.5% Nonidet P-40
(NP-40).
Thesamples
were spun at10,000
rpm in anEppendorf microcentrifuge
for 10 min at4°C,
and the supernatants were useddirectly
or stored at-700C.
Immunoprecipitation
of cell extracts.Samples
oflysates
containing
3 x 106trichloroaceticacid-precipiptable
35Scpm(generally corresponding
to 40 to 100 ,ul ofextract)
weremixed with0.5 mlof buffer
containing
20 mM Tris(pH 7.0),
100 mM
NaCl,
2 mMdithiothreitol,
1mM EDTA and 0.5% NP-40. Portions(10 ,ul)
of anti-SV40tumorcellserum wereadded,
and the mixture was incubated for 60 min at 18 to20°C.
A 10%suspension
ofwashedStaphylococcus
aureusbacteria
(50 ,lI)
wasadded,
andincubation continued for 10 min. The bacterialpellet
was washed twice in a buffercontaining
20 mMTris(pH 8.0),
2 mMdithiothreitol,
1 mMEDTA,
0.5%NP-40,
and 0.5 MNaCl. Afterathirdwash in the same buffercontaining
0.1 MNaCl,
the boundproteins
wereeluted with
gel-loading
buffer(0.0625
MTris[pH 6.8],
2% sodiumdodecyl sulfate,
10%glycerol,
0.1 Mdithiothrei-tol,
0.01%bromophenol blue). Sample
preparation
andpoly-acrylamide gel electrophoresis
weredone asdescribedpre-viously
(51, 52).
Gelscontaining
[35S]methionine-labeled
samples
were soaked for1 h in 10 volumes of 1 M sodiumsalicylate
beforebeing
dried.Autoradiography
wasfor1to4days
at-700C
onFuji
Rx film with an Ilford fast tungstateintensifying
screen.SV40
origin
binding.
Four DNAs were used in theseexperiments. pSV328
(a
gift
from G. C.Grosveld)
contains thelarger
BamHI-EcoRIfragment
ofSV40 inserted between the BamHI and EcoRI sites ofpBR322.
RL18 and034 areboth derivatives of
pPVU-0 (16, 19),
aplasmid
thatcontains the BamHI to PvuIIearly-region fragment
of SV40(SVS
strain)
between theBamHIandPvuII sites inpBR328.
RL18 has an EcoRI linker(CGGAATTCCG)
inserted betweenpositions
5194 and5186,
while 034 contains the linker inplace
ofSV40 sequences betweenpositions
5215and 4739. These DNAs weredigested
with the restriction enzymeBstNI,
and in the case of RL18 also with EcoRI. Therestricted DNAs were treated with calfintestinal
phospha-tase
(Boehringer
MannheimBiochemicals, Indianapolis,
Ind.),
and thenend-labeled with[-y-32PIATP
(specific
activ-ity,
5 to7,000 Ci/mmol;
AmershamInternational)
and T4polynucleotide
kinaseby
the methodof Maniatis etal. (27).The
specific activity
of DNA obtained wasgenerally
be-tween 1 x
107
and 3 x107
cpm/,ug. The DNAbinding
procedure, essentially that of McKay (31), has been de-scribed in detail previously (38). In these experiments,samples of cell extracts containing equivalent amounts of
35S-labeledlarge T, as judged fromexperiments suchasthat shown inFig. 1, generally 40 to 100 ,ul,weremade upto 100 ,ul with extraction buffer and mixed with 0.4 ml ofbinding
buffer(20 mM Tris [pH 7.0], 1 mMEDTA, 2.5 mM dithio-threitol, 125 ,ug of bovine serum albumin, 0.05% NP-40) and 10 ng of the appropriate labeled DNA. After incubation at 18°C for 40 to 60 min, 5 p.l of a mixture of the monoclonal antibodies pAb419 andpAb413 was added. Incubationwas continued for a further 40 to 60min,and 25 ,u1of washed S. aureus bacteria was added. After an additional 5 to 10-min
incubation, the pellets were washed three times in binding
bufferwithout bovine serumalbumincontaining 0.2 MNaCl.
The DNA was eluted in 15 p.1 ofgel loadingbuffer (10mM Tris [pH 7.5], 10 mM EDTA, 2% sodium dodecyl
sulfate)
and loaded directly onto 2% agarose gels in Tris-acetate buffer (27). After electrophoresis, the gels were fixed in 2 volumes of ethanol before drying. Autoradiography was
generallyfor 1 to 2 days.
Binding to ds calfthymus DNA immobilized on cellulose. Confluentmonolayers of cells on 50-mm dishes(Nunc)were labeled with
32p,
for2 h as described above.Themonolayerswerewashed withTris-saline and lysed in 200 ,ul of buffer A (10 mM HEPES[N-2-hydroxyethylpiperazine-N'-2-ethane-sulfonic acid], 1 mM MgCI2, 0.5% NP-40, 0.3 mg of
phenylmethylsulfonyl fluoride per ml) containing 0.45 M NaCl. Thelysate was removed andimmediately diluted by
the addition of2 volumes of buffer without salt. ThepH was thenadjusted to6.5by the addition of 0.01 M HCI, and the extractwas mixedwith 0.5ml ofpacked native calfthymus DNA-cellulose (P-L Biochemicals, Inc., Milwaukee, Wis.)
whichhad beenequilibratedin bufferA atpH 6.5 with 0.15 M NaCl. The mixture was keptat 4°Cfor 1 h on a rotary
mixer. The DNA-cellulose was then pelleted by centrifuga-tion for 1 minat4°C in an Eppendorf microcentrifuge, and thesupernatant was removed. The DNA-cellulose pelletwas washed three times withbuffer A atpH6.5 with 0.15 M NaCl andthen incubated for 1 h at 4°Cwith0.5 ml ofbuffer A at pH 8.5 with 0.15 M NaCl to elute bound protein. After three washes with this buffer, a second elution step was then carriedoutwith buffer AatpH8.5 with 1.0 MNaCl. Both of the eluted fractions and 20-,ul samples of starting material andof the unbound fractionwereadjustedtopH 8.5 and 0.5 MNaClbefore
overnight
incubation with10 p.1 of anti-SV40tumor cell serum. Immunoprecipitateswerethen collected, washed, andanalyzed as described above.
RESULTS
As part ofourefforts to assign biochemical functions to domains of large T, we examined many of the large T variants we have isolated (19) for their ability to bind to SV40
origin
DNA. The mutants used fall into two groups. The larger group consists of those variants carrying pointmutations or small deletions in the DNA coding for amino acids 106 to 158 of large T. The sequence of the SV40 variants encoded by most of the mutants used has been
reportedin detail (19)and issummarized in Table 1. The second group contains the so-called termination mu-tants which encode truncated large T protein (17, 19;
Roberts, unpublished data). Three of these have been used in the studiesreportedhere:H23,dH(previouslyreferred to asAHindlIl [18]),andHHpa, which encodeamino-terminal
fragments oflarge T consistingof 147, 272, and 362 amino
on November 10, 2019 by guest
http://jvi.asm.org/
52 PAUCHA ET AL.
TABLE 1. DNA-binding activity of mutantSV40 large T proteina Sequencealteration
Mutant 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 Ser Glu Glu Met Pro Ser Ser Asp Asp Glu Ala Thr Ala Asp Ser Gln His Ser Thr Pro Pro Lys Lys Lys Arg Lys Val
C8 Phe
K79(Kl)cd Lys
C37c,d Lys
C22d Lys Lys
K7d Lys Asn
pPVU_1d Tyr
W7 D19 D20 D29 T23-L7 X46 X12e sif S17-S29e S17-S36e W34 W38 U33 W67 W75 L27-S26 S1-S32 Dlll D119 D1209 U249 B9c Bllg U199 U20 W1289 W134 RL239 D889
D899
D90 D94 D96c D104C D98
pPVU-2 DeletesGlu-Gln-Trp-Trp-Asn (92-96) RL148 Tyr-Ser (179-180) - Ser-Glu-Phe-Arg B18cd Ser 189 -. Asn
RL75 Ser-Tyr-Asn-His-Asn (189-193)-- Asi
RL115 Phe-Leu-Ile-Cys(220-223)-* Arg-Asr H23 Terminatesat147
dH Terminates at 272 HHpa Terminates at362
70K Transformed bymicroinjected Taq-Ba
Tyr Phe Phe
Ile Arg Asn Ser Ala
Ser
Thr Thr
Met Thr
Arg Asn Ser Glu Arg Asn Ser Glu
;n-Gly-Ile-Pro n-Ser-Gly
rmfragment
acid residues, respectively. As
virtually
all ofthe large T variants that weisolatedgaverise,albeit to differentextents, to foci of Rat-1 cells overgrowing a monolayer ofnormalcells, the transformed cell lines were used as a source of
largeTforthe DNAbinding studies. The
biological
proper-ties ofthecell lines transformedbytheselargeTfragments
will be reported elsewhere. We also tested the large T
fragment produced in the so-called 70K cell line that was
isolated after needle microinjection into rat cells of the smaller Taq-BamHIfragment of SV40 whichlacks the first
exon of large T
corresponding
to amino acids 1 to 82(13).
To establish the relative levels of
large
Tpresent in the different cell lines, extracts were made from cells afterlabeling
with[35Slmethionine
for 5 h. Thislabeling
periodwaschosen because it should be sufficienttolabel the cells to areasonablyhigh
specific
activity
atsomething
approach-ing steady state. When equal numbers of trichloroaceticacid-precipitable
countsof[35S]methionine-labeled
material wereimmunoprecipitatedwithanti-T serum, the amountof J. VIROL.on November 10, 2019 by guest
http://jvi.asm.org/
TABLE 1-Continued
SV40 DNA-133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 binding Glu Asp Pro Lys Asp Phe Pro Ser Glu Leu Leu Ser Phe Leu Ser His Ala Val Phe Ser Asn Arg Thr Leu Ala Cys activityb
++ ++ ++ ++ ++ ++ ++ ++ ++ ++ ++
++
++ ++ ++
Leu Arg Asn Ser Val
Lys Lys
Ala Glu Phe Arg
Asn Asn
Asn Asn Asn
Tyr Val Asn
Thr Val
Met Thr Met
Ile
Met Pro Glu Phe Arg
Thr
Asn Thr
Tyr Asn
Asn Lys
Asn Lys
Phe
++ ++ ++
++
++ +
Tr+
++
(+)
(+)
++ ++
Asn Thr
t+
(+)
+
++
a The alterationspredictedin the aminoacidsequenceof themutantlarge Tproteinsareindicated. Thesuperscripts refertothefollowing properties of the
mutantplasmidsasdemonstratedby Kalderon and Smith (19).c,Mutantplasmidscanreplicate in CV1 cellsaswellaswild-typeSV40,bothasplasmids andas
re-constructed virus.d, Plasmidsaredefective in transformation. Fewer fociareproduced permicrogram of DNA withalonger latentperiodthanwithwild-type DNA.e,Mutantlarge T is detected only in the cytoplasm by immunofluorescent staining.f, Mutant large T is found in both the cytoplasm and the nucleus of
trans-formedcells. g, Mutant DNA producesmore focipermicrogram with ashorter latent period than does wild-type DNA. This phenotype has been called supertransforming(19).
b The ability of themutantlarge TstobindSV40origin DNA has been expressedas:++,binding equivalenttowild type;+,decreasedabilitytobind;(+)weak
binding detected withverysmall site I-containing DNA fragments; -,nodetectablebinding underanyconditionswithanyDNA tested. Leu
Lys Asn
Asn
on November 10, 2019 by guest
http://jvi.asm.org/
54 PAUCHA ET AL.
labeledlargeTdetected wasreasonably consistentbetween the different extracts, but some cells clearly contained
significantly
morethanothers(Fig. 1). Each of the cell lines was developed from a randomly picked dense focus after transformation by eachofthe mutant DNAs underequiva-lentconditions. Differencesin theamountoflargeTbetween
individual cell lines might therefore reflect their nonclonal nature.Inaddition,thestability ofthemutantproteinsorthe extent to which the mutant proteins are expressed might
varybetween thedifferenttransformed lines. Further
exper-iments would be required to distinguish between these
possibilities
for individual mutants. It isstriking,
however, that all ofthe largeTvariants thatcontain mutationswithinthe sequence 127-Lys-Lys-Lys-Arg-Lys-Val-132 are
over-produced6to20-foldrelativetowild type. We havealready shown that thesemutantlarge Tsare located
entirely
(X12, S17-S29, S17-S36) or partly (dl) in the cytoplasm oftrans-formed or
microinjected
cells (17, 18). Itispossible,
there-fore,
that the observedoverproduction might
be caused bythe
inability
ofcytoplasmic large T to autoregulate itsownproduction. Lack ofautoregulationmight explain whysome
of the other large T
species
appear to be present in largeamounts.
The large T fragments immunoprecipitated from Rat-1 cells transformedby thetermination mutants H23,dH,and
HHpa are also shown in Fig. 1. The apparent molecular
weights ofthe 147, 272, and 362 amino acid fragments are
14,000, 30,000, and45,000, respectively. The largeT found
in 70K cells is shown in Fig. 1. Since these rat cells lack
coding
sequencescorrespondingtothefirstexonoflargeT, the large T variant expressed cannot include any of theresidues 1 through82. Itisthoughtthattranslationbeginsat the first in-frame methionine ofthe large T gene, Met-109
(13). Thepredicted molecular weight ofthe resulting protein
fragment
is about 70,000; however, the mutant large Tmigrates
with an apparent molecularweight of84,000 (Fig. 1).Although
large T species are known to migrateanoma-lously
onsodiumdodecyl sulfate-polyacrylamide gels,some doubt is cast ontheexact natureof 70KlargeTas aresultof the difference between the predicted and the observedmolecular
weights.
Forthe
experiments
described here we assumed that thetotal amountoflargeT present in the different transformed cells was
proportional
to that detected aftera 5-hlabeling
period. Samples
ofextracts of the different cell linescon-taining equivalent
amountsoflargeT wereincubated withamixture of
32P-end-labeled
fragments of SV40 DNA which had beenproduced by cleavage with the restriction enzyme BstNI.Digestion
with this enzyme produces among others an SV40fragment
of 311 base pairs (bp)which contains allthree ofthe sitestowhich SV40largeTis knowntobind(5,
8,
9, 35, 55-57). After incubation for 60 min, any large T bound tothe SV40DNA was recovered byimmunoprecipi-tation with amixture ofthe monoclonal antibodies PAb413 and PAb419 which recognize determinants localized at the
carboxyl
and theaminotermini, respectively,oflargeT(15).The labeled DNA was then fractionated by agarose gel
electrophoresis. Figure
2 shows the results of the so-calledMcKay
assay (31),using
extracts from the cells shown inFig. 1. Someof themutantscoimmunoprecipitatedwild-type levels of the 311-bp BstNI fragment, whereas others had reduced levelsofbindingand somegave none. Addition of moreextract from cells which were negative forbindingin the
experiment
in Fig. 2 still failed to give any signal,emphasizing
that thelargeTspeciespresentin theseextracts wereunabletorecognizeSV40originDNAin this assay. Forsuch mutants, DNA-binding activity was consistently absent in all independently derived transformed cell lines exam-ined.
These data, together with the predicted amino acid se-quencesof the different mutant large Ts, are summarized in Table 1. These data show that the mutations which affect the ability of large T to bind toSV40 origin-containing DNAare tightly clustered. Small deletions or point mutations up to and including amino acid 137 have little effect on theability of the mutatedlarge T to bind to SV40 origin sequences. This includes the region around the Lys-128 mutations which
produces cytoplasmic large Ts (17, 25) and all of the phos-phorylation sites mapped at the amino terminus of large T (Ser-106, Ser-111, Ser-112, Ser-123, and Thr-124 [48, 60]). Mutation ofPro-139, however, or deletions encompassing this residue result inlargeTwhich is defective in SV40 origin
binding. Likewise,
mutation of Ser-147 -- Asn(D119)
ofAla-149-- Val (U24) andof
Ser-152+Arg-154
-*Asn+Lys
(U19) all abolish the ability of large Ttobind to SV40 origin DNA. A number of multiple and deletion mutants encom-passing these residueswerealsonegative for DNA binding. Othermutants in the region between residues 140 and 160 hadaseverelyreducedability tobind toDNA.The cluster-ing of all of these mutations suggests that they all lie within afunctional domain whoseamino-terminal end is defined by Pro-139.
There are fewmutants in ourcollection that map down-streamof residue 158;consequently, mapping of the extent of the domain is difficult. However, two mutants in the downstream sequences (RL148 and RL75) were reduced in theirorigin-binding activity, and the mutant RL115 (which has alteredamino acids 220 to223)wasunable to bind origin DNAatall.This maymeanthat thedomainextends at least asfarasamino acid 223.
The dataobtained with the other mutants testedis
consis-tentwiththisinterpretation.The 70Klarge T,whichlacks at leastamino acids 1to82,exhibitedwild-type levels of origin
binding (Fig. 2), indicating that the domain lies within the secondexonoflargeT.The147-amino acidfragment oflarge T coded for by the termination mutant H23 failed to bind DNAin this assay(datanotshown).dHlarge T, which is 272 amino acids long, and therefore includes the sequences
makinguptheregion defined above,didbind to origin DNA,
though weakly. HHpa largeT bound DNA better, perhaps
suggestingthat theconformation ofthe362-aminoacid-long fragment more closely resembles that ofwild-type large T than do the shorter forms. Taken together, these data supportthenotionthataregion involved inbindingto SV40
originDNAbegins at Pro-139 andextendsatleastasfaras amino acid 223 of large T. Within this domain, a region
between residue 147 and 154 appears to be particularly
sensitiveto mutation.
Ability of the mutants to bind to the SV40 site II origin region. The EcoRII Gfragmentof SV40 DNAcontainsthree sites to which large T is known to bind. Site II spans the
originofSV40DNAreplication.Site I is locatedontheearly
side of the origin,and site III ison thelateside. Bindingof
large T to site I is believed to block transcription of SV40
earlymRNAresultinginautoregulation of largeTsynthesis
(1, 9, 44). Binding to site II ispresumably essential for the initiation ofreplicationof viral DNA (8, 9, 50, 55, 56), while the consequencesof siteIIIbindingare not yetunderstood. De Lucia et al. (8) have established that each sitecontains
severalcopiesof theconsensuspentanucleotide 5'-GAGGC-3' which are protected from methylation by dimethylsulfate when large T is bound. The sequence of the SV40 origin-J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
OnN 1-r
-C½ CNJr~ r~J rN<WWCOCY
Cm CO j- ciW.- v-X q< n U
NCJ.
> OO O 0) X)'0CD C.q rN P.NNa.. N CYC3 CYCN t t- - -CL2Q zC>)]C X X Xx lo CO ora~~~0oo00~~~~-xcx~*,,,w'w w
gO
N rZ
-IzI WN
* - S - Large-T
.a
44
=p53
a0
Small-t
-aw < in
J- 'f-NM
4
7
- v- - T- C-4 04 0)
3: a O N:
_r Cm <: m m:D
C: n
in ci c-i cm
cD
0;
a) o_r _r _r CM
N CY CN N
vNCo C c
_ r-- oc0o
Do
31
,.QCi
6 t, Q o 0(
ao
ai)
a cXkC]QC
cuJ N N
Thu (0%r
N Ch_ - T-U) Cl) Cl) 00
t0
.,
NNI
I 1-i- XCi COo IONte NN'-_J
¢ -j -iwCcO
Large-T- * W.*S*4.0 " 0 *o
s.0
**h *
p53
-Small-t
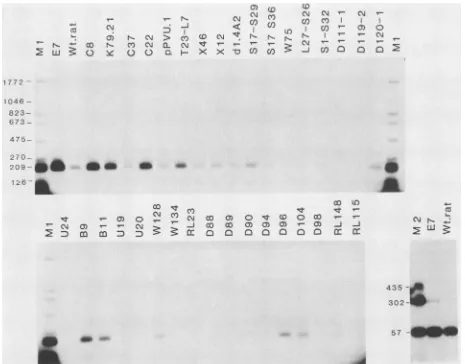
FIG. 1. ImmunoprecipitationofmutantcellextractswithSV40 anti-'1 serum. Kat-IcellstransformedbyeachofthemutantDNAswere
labeledfor 5 h with 100,Ciof[35S]methionineperml,andcellextractswereprepared. EqualnumbersofTCA-precipitable [35S]methionine
countsperminute of eachextractwereimmunoprecipitatedwithhamsteranti-SV40tumorcellserum.ThepositionsoflargeT, smallt,and p53areindicated. WT, wild-typeSV40-transformedratcellextract;E7,extractof theSV40-transforitiedmousecellline SVA31E7.Notethat
mousep53 migrates morerapidlyin thesegelsthandoesratp53in theadjacent70Ktrack. Thename of eachmutant is givenabovethe appropriatetrack. Thepredictedaminoacidsequenceof the largeTencoded bythemutantsis shown in Table1.Thelettersaand bfollowing thenameindicatetwoseparately preparedextractsof thesamecellline,e.g., K79.21aandK79.21b. Differentnumbersfollowingthename
indicateextractspreparedfrom celllines derived fromindependent fociproducedby transformation withthesameDNA,e.g., D20.1 and D20.2. Intwocases,D20.1 andS17-S36.1,nolargeT could bedetecteduponimmunoprecipitation ofthe cellextracts. Furtherexperiments
werecarriedoutwithcell lines derived from differentfoci (D20.2 and S17-S36.2)which did contain large T. spanning regions I and II is shown in Fig. 3 (top) with the
recognition pentanucleotide boxed. Measurements of the relativeaffinity of largeTbindingtothedifferent sitesat4°C have ledtotheconclusion that the protein bindsmosttightly to the two perfect pentanucleotides in site I, then to the
sequencesinregionII,andlastlytoregionIII(8).Itislikely thatbindingoflargeTtothe 311-bpBstNI fragmentinthe immunoprecipitationassayusedherelargelyreflectsbinding to the highest-affinity site I (32). In an attempt to assess whetherbindingtositeIand siteIIwasaffectedinparallelin
.0 CM CM C *
a 0) cO N
a3
.(. IC eLarge-T
-. ,
p53
-CU4
, N_
eacc ii
.0
040.16.00
*0
AMIJAL 000
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.612.56.553.80.569.2]56 PAUCHA ET AL.
co m b- C4 N > a) o o:, cn N0)A s lq*O CY CO
co N r 004 n
Y Y N C\ < U)CO
1772_404030404_- r '-CM <
CON
NO~r-~C'4
N N4oc)
04Nr
-'~~w~oe~~eoor
a~~~~OoQQHx>c>CV00~6
CY1772~~~Lj
1046-
823-
592-
444-
311- 249-
200-4111_a4-0_00 C_ a*b Am
cdd
CO)
04 \ _ _ 4 CO 04
'-
'-v-C/)COl~
co 0 <0 04 40 0F~~~~~~~~~~~~T C\3 1< im C\ I\ C\j CX)o (,0
Vi
Ln I CO'- 0)OI
?-N (V, (QNNN T- - \ C r- S-0 ) - - C
M
lL
3i
2
3
J
cn)
0c
a D D CD Go co 0 0 D:D
3:
2
-o
eo
--04CNJC\j N C\j~~~~~~~0
£6Cj
N\
Nj C) It L
04004000C)c) 0 t(D O 'r- a
CX
COC3M
0 o O oD
OC0 0
m :-C5
O 0
Nl N cc~
.4
:Ipk
e_ _
_ _
823
-6 7 3
,n. -592
,-n -5 5 2
:g -486
444
-31 1
-- 249
126
VP
CY) 00 0
m..
I.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
the different mutants, we carried out immunoprecipitation assayswith a numberof differentDNAfragments.
Initially, we separated site I and site II sequences by digesting the SV40 HindlIl C fragment with Dde. Site I was present as a 57-bp fragment, and site II was included in a
fragment of 302 bp (Fig. 3, bottom). The small site
I-containing Dde-HindIII C fragment was preferentially rec-ognized by wild-type large T, and recovery of the 302-bp site Ifragment was poor (Fig. 4). This may have been areflection of the lower affinity ofTantigenforsiteIIbut couldequally
have been due to the position of the Dde site which is
immediately adjacent to the large T-binding sequences on
site II (shownin Fig. 3, bottom) at the extreme end ofthe
302-bp fragment. Therefore, two mutant plasmid DNAs were used, RL18 and 034, in which two or all three of the
siteIpentanucleotides weredeleted. The DNA sequenceof
theoriginregionofthese two mutantsis illustrated in Fig. 3,
bottom. The mutant DNAs were digested with BstNI or BstNIandEcoRI, end labeled,and usedforDNAbindingas
before. Resultsobtained withRL18 areshown inFig.4, and someof themutantstested with 034areshown inFig.5.All
ofthe resultsobtained followedthe patternobserved in Fig. 2. Those mutantswhich bound the 311-bp wild-type BstNI
fragmentalsoimmunoprecipitated theorigin-containing
frag-ments of bothmutantDNAs; those mutantswhich failed to
bindto thewild-type origin fragmentalsofailedtorecognize the altered origins. In addition to the 858-bp SV40
origin-containing fragment bound bythedifferentmutantsinFig.5, the 1,772-bp plasmid DNA fragment was precipitated in some cases. Since that band was not detected in previous experiments using the same extracts under the same condi-tions, webelieve this reflects nonspecific binding whichcan
sometimes occurin immunoprecipitation assays and which
has been seen by several other authors (32, 43).
Neverthe-less, these results suggest that the ability of large T to
recognize siteII sequences is lost inparallel withtheability
to bind to site I. This observation must be tested more rigorously by using precise measurements of DNA binding such as dimethylsulfate protection assays. However, our
initialresults withalarge number ofmutantsimplythat the
functional domain involved in binding to site I sequences
also plays a role in recognizing sequences at the origin of replication.
Binding to cellular DNA. In addition to binding to se-quences at theviralreplicationorigin,SV40largeTisknown
tobind tightlytocellularDNA sequences(2, 10, 11, 41). It
hasnot yetbeenestablishedwhether one or twofunctional domains are responsible for these activities. From
experi-mentsusingaseries of truncatedlargeTmolecules, Chaudry
et al. (3) tentatively mapped a region on large T between aminoacids 109 and 272that was sufficient forbinding to ds cellular DNA. Thisregion includesthe sequenceswhichwe haveshown here areinvolvedinbinding specificallyto SV40
originDNA. In afurther attempt to determine whether these sequencesplayarolein bindingto cellular DNA, we tested anumberofmutants carrying alterationswithinthedomain
fortheirabilitytobind tods calf thymusDNAimmobilized on cellulose.
Ofthe mutants we have constructed, we chose to examine the
point
mutantsU24(Ala-149
-) Val)and U19(Ser-152
-Asnand
Arg-154
->Lys),
both of which had lost theability
to bind to the SV40origin,
and Bl1(Ala-149
-- Thr andVal-150 -*
Met),
which could bindorigin
butnotreplicate.
To probe the region on the carboxyl side of these point
mutants, we used the smalldeletion mutants RL148 (Tyr-179 and Ser-180 -*
Ser-Glu-Phe-Arg)
and RL115(Phe-220-Cys-223 -*
Arg-Asn-Ser-Gly)
sincewehavefewpoint
mutantsin that area. RL148 bound origin DNA weakly in an origin immunoprecipitationassay, while RL115showednodetect-able binding. Because we anticipated that the so-called
nonspecific binding to ds DNA might be less sensitive to
point mutation than was binding to origin-specific se-quences, we also examined mutants carrying larger
dele-tions. This ensured that the protein domain under scrutiny
was tested in a severely altered form. The mutant RL75-RL88 has four amino acids (Arg-Asn-Ser-Asp) inserted in placeof thesequencebetweenamino acids 188and 212. The other mutant, RL12-S24, has Gly-Ile-Pro replacing amino
acids 230 to 251. Both of these mutants are capable of producing dense foci on Rat-1 cells, and DNA binding experimentslike those shown inFig. 1and 2establishedthat both mutants are defective in origin binding. We also used the large T fragment of 272 amino acids coded for by the
termination mutant dH.
Cells transformed by each ofthe mutants were labeled with
32p,
and cell extracts wereprepared. The extracts were mixed with calf thymus ds DNA-cellulose at pH 6.5, and bound proteins were eluted atpH 8.5, first with a low-salt buffer and then in buffer containing 1 MNaCl. Samples ofthe starting material, of the unbound fraction, and of the
entiresample of bothlow-salt andhigh-salt eluateswerethen immunoprecipitated as described. Some representative re-sults are shown in Fig. 6. A fraction of the population of
large Tofeach mutant tested wascapableof binding tightly
to calf thymus DNA-cellulose asjudged by elution under
conditionsofhigh pHandhighsaltconcentration(tracks 4).
Our results with the mutant dH confirm that the
amino-terminal272aminoacids oflargeT aresufficientforbinding
tocellular DNA. However, these results showthatthere is nocorrelation betweentheabilitytobindwithhighaffinityto
SV40
origin
DNAand the abilityto bind to cellular DNA. When considered together with theobservation
that large deletions within the putative origin-binding domainappar-ently donotaffecttheability ofthemutant
protein
tobindtocellular DNA, these results might imply that there is a second site on large T that is capable offunctioning inde-pendently inbindingto cellular DNA.
DISCUSSION
SV40 origin region DNA-binding domain. Earlier data
indicatingthat aDNA-binding domainonSV40large T lies
FIG. 2. Immunoprecipitationof mutant large T bound toSV40 origin-containing DNA. Samples of each cell extract containing equivalent amounts ofI'S-labeledlarge T (Fig. 1) wereincubated with 10 ng of _P-labeled BstNI-digested DNA from the plasmidpSV328, which contains the entireearly region of SV40. LargeT wasimmunoprecipitated withamixtureof the monoclonal antibodies pAb419 and pAb413 which recognize amino- and carboxyl-terminal determinants, respectively,on large T (15). Theimmunoprecipitates wereseparated on 2% agarosegels. TracksM:0.5 ng ofBstNI-digested pSV328DNA.The sizes ofthedifferentfragmentsaregiven inbasepairs. E7, extractof SVA31E7cells; WT,extractofwild-type SV40-transformed ratcells. The names of the different mutants are given above theappropriate tracks.70Ka,70Kcell extractimmunoprecipitatedwith pAb413;70Kb,70Kcell extractimmunoprecipitatedwithpAb419; Rat-1, extractof untransformed Rat-1 cells. The tracksshownonthe bottom right wereanalyzed in a separate gel which was electrophoresed for a longer time, toachieve better separation of the markerfragments.
on November 10, 2019 by guest
http://jvi.asm.org/
58 PAUCHA ET AL.
Site
ESite
I
4 3 2 1 3 2
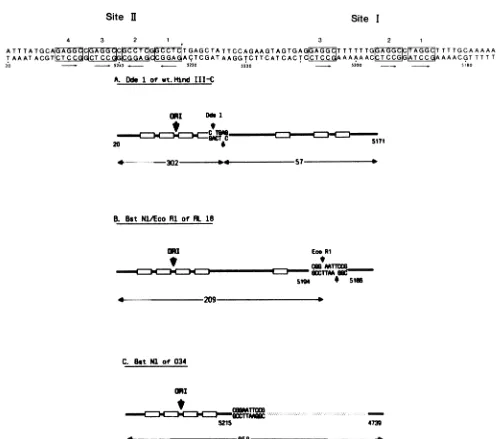
ATTTATGCAAGGqCI AGAGCCTCCTCPCCCTCTGAGCTATTCCAGAAGTAGTGAGAGG
TTTTTTGGAGGC;rkTGGqTTTTGCAAAAA
T AAATACGTICTCC
CiGGILCrG
GAGlqCGGAdAC.TCG
ATAAGGTCTTC AT CAACTr
CCTCCG|AAAAAAC¢CTCCGIGATCCGAAAACGTTT T T20 5243 55230 5220 5200 5180
A. Dde 1 of
wt.Kind
111-CORI Dd 1
C T
71OCTC
1 " _ 8 1720 S7
-302 57
B. Bst Nl/Eco Rl of RL 18
Eco RI
510 4 5168 S104 4 Sias
C. Bet Ni of 034
t
.----CZZHZ::
c
3:i
I-C21
GCTTAAR9C
" '"5215 4739
FIG. 3. (Top) Sequence of SV40 DNA about the origin ofreplication. The nucleotides are numbered according to Tooze (59). The consensuspentanucleotide recognition sequence whichDeLuciaetal.(8) have showntobeprotectedfrommethylation bythebindingof SV40 large T are boxed and numbered as described by those authors. Arrows beneath the boxes indicate the 5' -+ 3' polarity of the
pentanucleotides. (Bottom) Structure of theorigin-containingDNAfragmentsproduced bydigestionofwild-typeandmutantSV40DNAs with different enzymes. The sequence between nucleotides 20 and 5171, shown in the toppanel,ispresentedhere inadiagrammaticform with theboxesrepresenting the recognition pentanucleotide. Theposition oftheoriginofreplicationis indicated. (A) Fragmentsproduced by digestion of thepurified wild-type HindIII CfragmentwithDdeI. (B) Fragmentsproduced bydigestionof RL18 DNA with both BstNI and EcoRI. RL18 hasan EcoRIlinker(CGGAATTCCG) inserted between positions5194and 5186ofwild-typeSV40.(C) Fragmentsproduced by digestion of 034 withBstNI.Mutant034 hastheSV40 sequences betweenpositions5215and 4739deleted andreplacedwith theEcoRI linker.
between amino acids 83 and 250 was summarized in the
Introduction. The datapresentedhereallow amoreprecise
map to be deduced. These data, together with relevant
informationfrom otherlaboratories,aresummarizedin Fig. 7. The most amino-terminal of the point mutations we examined that is negative in the DNA binding assay is Pro-139-- Leu (W75). Deletion of the
surrounding region
also produces SV40 origin DNA-binding-negative large T
(L27-S26). Mutationsatresidues 147
(Ser--
Asn;D119),149(Ala-- Val; U24),and 152
plus
154(Ser-*Asn,Arg--Lys;
U19) also abolish origin DNA binding as do a number ofmultiple point and deletion mutations in the same
region
(Dlll,
W134, RL23,D89,D98;
listedinTable 1). Several ofthepointmutations in this
region
arereduced intheirbinding
activity relative to wild type (W67, D120, Bl, U20,D88,
D90, D94). It is striking that mutation of Ala-149 to Thr
(D120) retains some binding activity, whereas mutation to Val(U24)doesnot.Evenmorenoticeably, conversiontothe
adjacent
residue Val-150-+Met(B9)generatesavirusthat is able to replicate and has a wild-type SV40origin
DNA-binding phenotype.
The closeclustering of mutations witha common defect
J. VIROL.
ani
t
----.C:Dmc::30cz:oc::?
on November 10, 2019 by guest
http://jvi.asm.org/
[image:9.612.64.563.61.500.2]L a) N- N >
C0
CD N 'tW2 0 OO a QX XvN
0C4) 0
Co
CD)
U) Lot N N N
U)r 1 5N
4 CM
r--CY)
C/) V- C5) 0
I r- Ir- 04
r W r
C 0 Q
270 -1
209-S
b
b
126-0O
It rf0) C
i10 0o) ,- 1- Cj
2 cnt_D
aM
1t0)
CY)04%
00 L)
0 O~qt tOO 0
00 00C)0) 0) cJ m
000
ECD
D 00) 112z a [image:10.612.75.540.71.435.2]dB_
FIG. 4. Binding of large Tto SV40origin DNA containingadeleted site I.(Upper panel and lower left panel) Samples of different cell
extractscontaining equivalentamountsof"S-labeled large Twereincubated with 10ngof RL18 DNA which had been digested with EcoRI and Bst NI andthen end labeled with32P.Large T boundtoDNAwasimmunoprecipitated and analyzedasdescribed in thetext.Tracks Ml, 0.5 ngofEcoRl-BstNI-digested RL18 DNA. The sizes of the different fragmentsaregiven in base pairs. (Lower right panel) Extractswere
incubated with 2.5ngofpurified HindIII-C which had been digested with DdeI before labeling.
EllJ XC 'IT
M w ~- 3'
v- 0) C\ CM
M D N cu
1772-1046_
858- _
673-
475-270.
249-
126-FIG. 5. Binding oflarge T to SV40origin DNA lacking site
Samples of the different cellextractswereincubated with 10ngof
BstNI-cut, end-labeled 034 DNA. Track M, 0.5ngof startingDNA.
The sizesof the differentfragmentsaregiven in base pairs.
strongly suggests that they lie within a functional domain. This is madeevenmorelikely by findings fromtwoseparate studies. By recovering the integrated SV40 DNA from transformed permissive monkey cells and sequencing the early regions,the defects thatpreventthe expected produc-tive infection of these cellsby the virus have been identified (12). In thecaseof the C6-2mutation,the defectappearsto be inSV40 origin binding(43),andthelesionmapstoresidue 153. Similarly, the SV40 insert coding for the large T in human SV80 cells hasbeen isolated and found to be repli-cation defective. Again,theproteinappearstobe reduced in itsSV40 origin DNA-binding activity (14). The lesion in this case maps to amino acid 147 (W. Gish and M. Botchan, personal communication). Using a totally different
ap-proach, Nathans and colleagues (50) have isolated pseudorevertant (sr)forms ofSV40 largeT that are ableto replicateSV40viruscontainingadefective origin of replica-tion. Presumably, the pseudorevertant large T species are altered inthose amino acid residues intimately involved in the sequence-specific interaction with origin DNA. sr-3 maps to amino acid 157, and sr-2 mapsto amino acid 166 (30). We believe, therefore, thatthe amino-terminal end of the origin DNA-binding domain on large T can now be 177 2
-1 046-823
-673
-
475-0
a
4-cri 1-:
C,v p.- 4-2 Uj .:
435 302
on November 10, 2019 by guest
http://jvi.asm.org/
60 PAUCHA ET AL.
Wt.rat A
I I
1 2 3 4
Wt.rat
f-1 2 3 4
U
1 9
[ I
1 23 4
I.I.
..
**
_F**.__~~~~
.1iI-:.1;li,~~ ~ ~ ~ ~ ~ ~4..
0
0-.,.0
_-RL148
RL75-RL88
RL1
15
RL 1 2-S24
I I
12 3 4
I n
[image:11.612.71.554.75.519.2]1 2 3 4
FIG. 6. Binding ofmutantlargeTtocalfthymusDNA-cellulose.Samples (500 ,ul)of 32P-labeledlysatesofdifferentmutantcelllineswere
mixed withcalfthymus DNA-cellulose, washed, and elutedasdescribedin thetext. Allsampleswereimmunoprecipitated with anti-SV40
Tserumbefore analysisonSDS-containing15%acrylamide gels. Extracts ofwild-type SV40-transformedratcells werealso analyzedon
underivatized cellulose as acontrol (WTratA). Lanes: 1, 20-,ulsampleof starting material; 2, 20,ul ofunboundsupernatantfraction; 3, material elutedatpH8.5 with0.15MNaCl; 4,materialelutedatpH 8.5 with 1.0 MNaCl.Thepositionsof migration of large T and p53are
indicated.
localizedtoaregion beginningatPro-139 withaparticularly importantsequencebetween amino acids 147and 166.
Wedo notknow how far the putative domain extendsto
the carboxy-terminal side becausewehavenotmade many
mutantsmappingtothis region. However, themutantRL115 whichaffects residues 220to223is origin-binding defective, and two other SV40 point mutants recovered from trans-formed permissiveor nonpermissive cells that mapto resi-due 203 (T22) (29) and 214 (SVR 9D) (54) also lack origin DNA-binding activity. Since domains mappedon procary-oticDNA-binding proteins areusually of the order of 70to 90 residues (37), these mutations may lie at the extreme carboxyl endof the samedomain.
The dataobtained with the otherSV40 largeTvariants(70 K, H23, dH) reported here are also consistent with this location. Thus, with the exception ofone published report claiming that a proteolytic fragment oflarge Tcomprising onlyresidues 1 to 130 hasorigin DNA-binding activity (34), almost all other data are consistent with the site identified here between residues 139 and 223beingamajorcomponent in defining origin-specific DNAbinding.
Comparison of the SV40 large T amino acid sequence between residues 135 and 225 withtheregionbetweenamino acids 289 and 379 of large T of the related papovavirus, polyomavirus, is shown in Fig. 7. The underlined amino acids are conserved between the two viruses. It is striking
U24
1 2
3
4
Large-T
p53
-B1 1
1 2 3 4
A.W
1 2 3 4
.4 -#
Large-T
p53
-1 2 3 4
dH
1 2 3 4
0.
a 4
J.VIROL.
A& 41i it
IV
on November 10, 2019 by guest
http://jvi.asm.org/
SV40 ORIGIN DNA-BINDING DOMAIN
N Y
>1
a _'
-,
II
I>l
a
I-a
o,
O 0
XZ
a o ,
I
,,-z
I ca:
I'I
. .,
0i
a ILI
IU.,
aIILL
a
oZ
a ~0
Z
,-U.
a 0>~
a -:I
!
~~~~>1
oILl
* b~~~~~~~-I
Zi
I,-\ ~~~~ZI
\
<~~~
~~~~~~IL \ Ow1. a..
(a
Z
wi
0
a
I-x
I..
Z
U.
Z c,
1
-J
0
@a
°
a,
co , )-. 9 0 aIIo0 N g
rw. > N 0 m = N> N .Ji-i
3: O03 3000 I-0 -i
VOL.57, 1986
a
61
0
2
C
I-0
CO)
qI
0
0
on November 10, 2019 by guest
http://jvi.asm.org/
62 PAUCHA ET AL.
that all themutantsdefectiveinorigin bindingshownin Fig.
7 map to residues shared with polyomavirus large T. The
high degree of conservationof amino acids suggests that the
region is essentialtoeach virusand hasasimilarfunction. It
hasrecently beenshown (40)thatpolyomavirus largeTcan
bindto thesame pentanucleotide recognized bySV40large
T.
DNA-binding domains in procaryotic proteins have been characterized in some detail (37). Forexample, they
com-prise66, 73,and 92residues, respectively,for theCro, CAP,
and X repressor proteins. Although the domain described here could be of approximately this size our preliminary
searcheshavefailedtofindsequencehomologybetween the
SV40 amino acid sequences and those of the 20-residue helix-bend-helix motif characteristic oftheprocaryotic
pro-teins.
Site I, site II, and dsDNA binding domains. The data
reported here do not allow us to distinguish whether the
domain has differentspecificities for bindingsites IandIIin
the SV40 origin. As far as it is possible to tell, the site II
binding can be detected in such an immunoprecipitation
assayby usingDNAwhichlacks site I sequences appearsto
parallelthesiteIbinding results, suggestingthatthedomain
hasactivityforboth sites. Furthermore,thepseudorevertant largeT antigensarepresumably effective inorigin, i.e., site
II, binding since they are capable of initiating viral DNA
replication and yet they map to the site I domain defined
here. Thus, itseems likelythattheregion betweenresidues
139and 223 recognizes bothsites I and II in the SV40origin region, but this conclusion must be tested more rigorously before being regarded asestablished.
It remains to be seen whether other regions on large T
formanotherDNA-binding domain. Onereport suggeststhat a second domain witha specificity fornon-SV40 DNA lies between residues245and 325 (42). The results reported here are consistent with that hypothesis since mutations within the SV40 origin binding region, including some large
dele-tions,
donot seem toaffecttheability ofthemutantproteinstobindtodscellular DNA. These resultsmustbeinterpreted
with some caution, however, because ofthesevere
limita-tions oftheDNA-cellulosebindingassayitself.In ourhands, largeTbindsefficientlyto underivatized cellulose (compare
Fig. 6, panelWTratA, tracks 1and 2) although apparently with low affinity as the bulk ofthe protein can be eluted under low-salt conditions (Fig. 6, WT rat A, track 3).
Furthermore, we can only recover 10 to 20% of the
32p_
labeled protein bound to calfthymus DNA-cellulose in theelution steps. Any conclusions reached on DNA binding
may, therefore, apply to only a small fraction of the entire
protein population. The existence of a separate domain
capable of binding independently to cellular DNA thus
remainsan open question.
SV40origin bindingandreplication. Very few(5of50)of themutantsdescribed in thisstudyare capableof viralDNA
replication. All othersare severely defective ordeficient in thatfunction.Thosemutantswhichcanreplicate (K79, C37, B9, and D96/D104)bind efficiently to SV40 origin DNA as
expected. The only exception to this rule is mutant B18,
which canreplicate but shows weak DNA bindingin these assays. B18transforms cells verypoorly, so the possibility existsthat thelargeTfound in the B18-transformed line used here was modified in some way, perhaps by a second site mutationorbygene rearrangement. Itis easytorationalize the inability to replicate of those mutants whose origin
binding
activity is severely impaired. Similarly, the replica-tiondefect in thosemutants aroundLys-128 which producea cytoplasmic large T could perhaps be explained by an inability to accumulate functional large T in the nucleus. This idea is supported by the apparent failure of these mutants toautoregulate the levels of large T synthesized. It is more difficult to understand why the remaining mutants fail to replicate, even though they appear to bind well to SV40origin DNA, site II aswellas site I, asjudged by the
immunoprecipitationassaysreported here. Perhaps virtually anymutation in thisregion of large T,even apoint mutation 40 to 50 residues removed from theDNA-binding domain, disrupts the structure sufficiently to affect the intimate association reported toexistbetween the protein and SV40
site II DNA (6). Such mutations might prevent replication
but still allow an interaction measureable by the McKay assay.
SV40origin bindingandtransformation. Allof the mutants described herewereable to transform Rat-1 cells regardless of their ability to bind to SV40 origin DNA. In fact, some mutantswhich cannot bind DNAproduced foci more rapidly and in greater numbers than wild-type virus. Kalderonand Smith (19)suggested thatthis supertransformingphenotype
might be caused by an overproduction of large T since all mutants showing this property displayed brighter than nor-mallarge T fluorescence. Although not consistently reflected in the amountoflarge T immunoprecipitated from individual celllines,amodestoverproductionof mostmutants
display-ingthe supertransforming phenotype(Bli, U19, U20, U24,
W128, RL23,D88, D89)isseenhere. More significantly, all
ofthesupertransforming mutants showa discernibledefect inSV40origin DNA binding, with the mutants U19 and U24
displaying the most extreme phenotype in both respects. Furthermore,mutantsmapping to the same region (B9, D96, D104) that are able to bind to SV40 origin DNA and to
replicate do not discerniblyoverproduce or transform with an efficiency greater than wild type. Thus, the hypothesis
that the observed supertransforming activity ofsomeofthe mutants can beexplained in terms of an overproduction of
protein is consistent with the deficiencies observed in this
study in bindingto site I, the siteof transcriptional control by largeT.
The work described here supports the conclusion that
largeTdoesnotneedtobe able tointeractwithSV40origin
DNA to transform established Rat-1 cells (29, 43, 54).
However, any suggestion that transformation by large T does not involve an interaction with cellular DNA must be
tempered by the finding that all of the mutants retain the
ability to bind to cellular ds DNA-cellulose. Schelleret al. (49) havealreadydescribedasimilar phenotypefortheC6-2 mutant. If transformation of established cell lines by SV40 does not involve binding to DNA, it might imply that this aspectof transformationisacytoplasmic eventcatalyzed by
the membrane-associated forms of large T detected by a number of workers (7, 20, 21, 47 [and references therein];
53). This would be consistent with the emerging view that
transformation, asopposed toimmortalization, iscatalyzed bycytoplasmicevents(22, 23, 36,45). It would alsoexplain
why severalof ourmutantlargeTspeciesthat appearunable to localize to the nucleus of cells still retain the ability to transform established cells (17).
Domain structureof largeT. Frompreviousstudiesonthe
biological properties of the many mutants we have con-structed and from the data reported in this paper, it has become apparent that the linear aminoacid sequence oflarge T can be divided into discrete blocks each of which is associated with adistinct functionor property.
Allof thephosphorylationsites in theamino-terminalhalf J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
oflarge T are clustered between amino acids 106 and 124: Ser-106, Ser-111, Ser-112, Ser-123, and Thr-124 (48). Our studies have shown that mutants carrying alterations in amino acids 106 to 114 generally produced foci on Rat-1 cells moreslowly and in reduced numbers than did wild-type large T (19). These mutants, therefore, define a domain which mustplay a rolein the transformation process. We do not yet
knowwhethermutationswithin theputativetransformation domain alter the phosphorylation patterns of large T or whether some otherfunction is responsible for their reduced transforming ability. The next domain includes the sequence
125-Pro-Pro-Lys-Lys-Lys-Arg-Lys-Val-132 which we showed constitutes a nuclear location signal. Mutations
within this region result in large Ts which are wholly or partially located in the cytoplasm (17, 18, 25). Thesemutants retain the ability to transform Rat-1 cells to continuous
growth. Finally, in this publication, we defined a
DNA-binding region which begins at Pro-139 and which may extend as far asCys-223. Whether each of these functional
domains corresponds to a structural domain remains to be determined. Nevertheless,the existence of mutationswithin each ofthe different functional domainsfacilitates the sys-tematicanalysisoflargeTfunctions and the rolethey playin itsbiological activities, particularly in transformation.
ACKNOWLEDGMENTS
WethankA.Graessmann forthegifts of the70Kcellline,Carol Prives for information and adviceoncalfthymusDNAbinding,and ourcolleaguesatMill Hill and elsewhere forhelpfuldiscussions.We arealsogratefultoLydiaPearsonforhelp with the tables andtoAnn Desai fortyping themanuscript.
LITERATURECITED
1. Alwine, J. C.,S. I.Reed,andG. R. Stark. 1977. Characteriza-tionof the autoregulation of simian virus40 gene A. J. Virol. 24:22-27.
2. Carroll, R. B., L. Hager, and R. Dulbecco. 1974. Simian virus40 T antigen binds to DNA. Proc. Natl. Acad. Sci. USA 71:3754-3757.
3. Chaudry, F.,R. Harvey,and A. E. Smith. 1982. The structure
and biochemical functions of four SV40 truncated large T antigens. J. Virol. 44:54-66.
4. Clark, R.,D. P.Lane,and R.Tjian.1981.Theuseof monoclo-nal antibodies as probes of simian virus 40 Tantigen ATPase activity.J. Biol. Chem. 256:11854-11858.
5. Clark, R., K. Peden, J. M. Pipas, D. Nathans,and R. Tjian. 1983. Biochemical activity of T-antigen proteins encoded by simian virus 40 A gene deletion mutants. Mol. Cell. Biol. 3:220-228.
6. Cohen,G.L.,P. W.Wright,A.DeLucia,B. A. Lewton,M. E. Anderson, and P. Tegtmeyer. 1984. Criticalspatial requirement withintheorigin of simian virus 40 DNAreplication. J. Virol. 51:91-96.
7. Covey, L.,Y.Choi,and C. Prives. 1984. Associationof simian virus 40 T antigen with the nuclear matrix of infected and transformedmonkey cells. Mol. Cell. Biol. 4:1384-1392. 8. DeLucia,A.L.,B.A.Lewton,R.Tjian, andP.Tegtmeyer. 1983.
Topography of simian virus 40 A protein-DNA complexes: arrangement ofpentanucleotideinteractionsitesattheoriginof replication. J. Virol. 46:143-150.
9. DiMaio, D., and D. Nathans. 1982.Regulatorymutantsof simian virus40:effect ofmutations at a Tantigen bindingsite on DNA replication and expression of viral genes. J. Mol. Biol. 156:531-548.
10. Fanning, E., K.-H. Westphal,D. Brauer,and D.Corlin. 1982 Subclasses of simian virus 40 large T antigen: differential binding of two subclasses of T antigen from productively infectedcells to viral and cellular DNA. EMBO J. 1:1023-1028. 11. Gidoni, D.,A. Scheller, B. Barnet, R.Hantzopoulos, M.Oren,
and C. Prives. 1982. Different forms ofsimian virus 40 large tumor antigen varying in their affinities for DNA. J. Virol. 42:456-466.
12. Gluzman, Y.,and B.Ahrens.1982. SV40earlymutantsthatare
defective for viral DNA synthesis butcompetent for transfor-mation of cultured ratand simian cells. Virology 123:78-92. 13. Graessmann, M., and A. Graessmann. 1982. Simian virus 40
cRNA is processed into functional mRNA in microinjected monkeycells.EMBOJ. 1:1081-1088.
14. Gruss, C., E.Baumann, and R. Knippers. 1984. DNA binding properties of a mutant T antigen from the simian virus 40-transformed human cell line SV80.J. Virol. 50:943-946. 15. Harlow, E.,L. V.Crawford,D. C.Pim,and N. M. Williamson.
1981. Monoclonalantibodiesspecific for simian virus40tumor
antigens. J. Virol. 39:861-869.
16. Kalderon, D., B. A.Oostra,B.K. Ely,and A. E.Smith. 1982. Deletion-loop mutagenesis:anovelmethod for the construction ofpointmutationsusingdeletionmutants. Nucleic Acids Res. 10:5161-5171.
17. Kalderon, D.,W.D.Richardson,A.Markham,and A. E.Smith. 1984.Sequence requirementsfor nuclear location ofSV40large T. Nature(London)311:33-38.
18. Kalderon, D., B. L. Roberts, W. D. Richardson, and A. E. Smith. 1984. A short amino acid sequence able to specify nuclearlocation. Cell39:499-509.
19. Kalderon, D.,and A. E. Smith.1984. In vitromutagenesisofa
putative DNA binding domain on SV40 large T. Virology 139:109-137.
20. Klockmann, U.,and W.Deppert. 1983.Acylated simianvirus 40 large T antigen: a new subclass associated with a detergent-resistant lamina of the plasma membrane. EMBO J. 2: 1151-1157.
21. Klockmann, U., M. Staufenbiel, and W. Deppert. 1984. Mem-braneinteractionsofsimian virus 40largeTantigen:influence ofprotein sequencesandfatty acidacylation. Mol. Cell. Biol. 4:1542-1550.
22. Land, H., L. Parada, and R. A. Weinberg. 1983. Cellular oncogenesandmultistep carcinogenesis.Science 222:771-778. 23. Land, H.,L.F.Parada,and R. A.Weinberg.1983.Tumorigenic
conversion ofprimary embryofibroblastsrequiresatleasttwo
cooperatingoncogenes. Nature (London)304:596-602. 24. Lane,D.P.,and L. V.Crawford. 1979.LargeTantigenisbound
to ahostprotein in SV40 transformed cells. Nature(London) 278:262-263.
25. Lanford,R.E.,andJ. S.Butel.1984.Construction and charac-terization ofanSV40mutantdefective in nucleartransportofT antigen. Cell 37:801-813.
26. Linzer, D., and A. Levine. 1979. Characterization of a 54K cellularSV40tumorantigenpresentin SV40 transformed cells anduninfectedembryonalcarcinoma cells. Cell 17:43-52. 27. Maniatis, T.,E. F.Fritsch,andJ. Sambrook. 1982. Molecular
cloning,alaboratorymanual. ColdSpringHarborLaboratory, Cold Spring Harbor, N.Y.
28. Manos,M.M.,and Y.Gluzman. 1984. Simian virus40largeT antigen pointmutantsthataredefectiveinviralDNAreplication but competent in oncogenic transformation. Mol. Cell. Biol. 4:1125-1133.
29. Manos,M.M.,and Y.Gluzman.1985.Geneticandbiochemical analysis of transformation-competent, replication-defective simian virus40largeTantigenmutants.J. Virol.53:120-127. 30. Margoiskee,P.F.,and D. Nathans.1984.Simian virus40mutant
Tantigens withrelaxedspecificityforthenucleotidesequence
atthe viralDNAoriginofreplication. J.Virol. 49:386-393. 31. McKay, R. D. G. 1981. Binding of SV40 T antigen related
proteintoDNA. J.Mol. Biol. 145:471-488.
32. McKay,R.D.G.,and D.DiMaio.1981. Bindingofan SV40T antigenrelatedproteintothe DNAofSV40regulatorymutants.
Nature(London)289:810-813.
33. Montenarh, M.,W.Deppert,and R.Henning.1982.Mappingof
a DNA-binding domain of simian virus 40 T antigen using non-defective adenovirus-2-simian virus 40 hybrid viruses. FEBS Lett. 142:129-132.
34. Morrison, B., M. Kress,G. Khoury, and G. Jay. 1983. SV40
on November 10, 2019 by guest
http://jvi.asm.org/
64 PAUCHA ET AL.
tumor antigen: isolation of the origin specific DNA binding domain. J. Virol.47:106-114.
35. Myers,R. R., andR. Tjian. 1980. Construction and analysis of simian virus 40origins defective in tumor antigen binding and DNAreplication. Proc. Natl. Acad. Sci. USA 77:6491-6495. 36. Newbold, R. F., andR. W.Overell. 1983. Fibroblastimmortality
is a prerequisite for transformation by EJ c-HA-ras oncogene. Nature(London)304:648-651.
37. Pabo, C. O., and R. T. Sauer. 1984. Protein DNA recognition. Annu. Rev.Biochem. 53:293-321.
38. Paucha, E., R. W. Harvey, andA.E. Smith. 1984.Antibodies to synthetic peptides thatimmunoprecipitate someforms of SV40 large T antigen. J. Virol. 51:670-682.
39. Pipas, J. M.,K.W. C. Peden,and D.Nathans. 1983. Mutational analysis of simian virus 40 Tantigen: isolation and character-ization of mutants with deletions in the T-antigen gene. Mol. Cell. Biol. 3:204-213.
40. Pomerantz,B.J.,and J. Hassel. 1984.Polyomavirus and simian virus 40 large Tantigens bind to common DNA sequences. J. Virol.49:925-937.
41. Prives, C., B. Barnet, A. Scheller, G. Khoury, and J. Gilbert. 1982. Discrete regions of simian virus 40large T antigenare required for nonspecific and viral origin-specific DNA binding. J.Virol. 43:73-82.
42. Prives,C., Y. Beck, and H. Shure. 1980. DNAbinding proper-ties ofsimian virus 40 Tantigen synthesized in vivoand invitro. J. Virol. 33:689-696.
43. Prives,C.,L. Covey,E.Scheller,and Y.Gluzman. 1983.DNA binding properties of simian virusTantigenmutantsdefective in viral DNA replication. Mol. Cell. Biol.3:1958-1966.
44. Rio, D.,A.Robbins,R.Myers, and R. Tjian.1980.Regulation of simian virus 40early transcription in vitro by purified tumor antigen.Proc. Nati. Acad. Sci. USA 77:5706-5710.
45. Ruley, H.E.1983.Adenovirus early region 1A enables viral and cellulartransforming genes to transform primary cells in cul-ture.Nature (London)304:602-606.
46. Rundell, K., J.K.Collins,P.Tegtmeyer,H.Ozer,C.J. Lai,and D. Nathans.1977.Identification of simianvirus 40 Aprotein. J. Virol. 21:636-646.
47. Santos, M., and J. S.Butel. 1984.Antigenicstructureof simian virus 40 large tumor antigen and association with cellular protein p53 on the surfaces of simian virus 40-infected and
-transformed cells. J.Virol.51:376-383.
48. Scheidtmann,K.H.,B.Echle,andG. Walter.1982. SV40large Tantigenisphosphorylated atmultiple sites clustered in two
separateregions. J. Virol. 44:116-133.
49. Scheller, G. E., L. Covey, B. Barnet,and C. Prives. 1982. A small subclass of SV40Tantigen binds to the viral origin of replication. Cell 29:375-383.
50. Shortle,D. R.,R. F.Margolskee,and D.Nathans. 1979. Muta-tionalanalysis of the simian virus 40 replicon:pseudorevertants ofmutantswithadefectivereplication origin. Proc. Natl. Acad. Sci. USA 76:6128-6131.
51. Smith, A. E., R. Smith, andE. Paucha. 1978. Extraction and fingerprint analysis of simian virus 40 large T and small t
antigens.J. Virol. 28:140-153.
52. Smith,A.E.,R.Smith,and E.Paicha. 1979. Characterization of differenttumorantigens present in cells transformed by simian virus40. Cell 18:335-346.
53. Staufenbiel, M., and W. Deppert. 1983. Different structural systems of thenucleusaretargets forSV40 largeTantigen. Cell 33:173-181.
54. Stringer, J. R. 1982.Mutantof simianvirus 40large T antigen that is defective for viral DNA synthesis but competent for transformation of culturedratcells. J. Virol. 42:854-864. 55. Tegtmeyer, P.,B. A.Lewton, A. L. D)e Lucia,V.G.Wilson, and
K.Ryder.1983. Topography of simian virus 40 Aprotein-DNA complexes: arrangement of protein bound to the origin of replication. J.Virol.46:151-161.
56. Tenen, D.G.,T.S. Taylor,L.L. Haines,M. K.Bradley,R.G. Martin, andD. M.Livingston. 1983. Binding of simian virus 40 large T antigen from virus-infected monkey cells towild-type and mutant viral replication origins. J. Mol. Biol. 168:791-808.
57. Tjian, R. 1978. The binding site on SV40 DNA for a T antigen-related protein. Cell 13:165-179.
58. Tjian, R.,and R. Robbins. 1979. Enzymatic activity associated with purified simian virus 40 T antigen-related protein. Proc. Natl. Acad. Sci. USA76:610-615.
59. Tooze, J. 1980.DNAtumorviruses, 2nd ed., part 2. ColdSpring HarborLaboratory, Cold SpringHarbor, N.Y.
60. VanRoy, F., L.Fransen, and W. Fiers. 1983. Metabolic turn-overofphosphorylation sitesinsimianvirus 40largeTantigen. J.Virol. 45:442-446.
J. VIROL.