JOURNALOFVIROLOGY, June 1991,p. 3151-3160 0022-538X/91/063151-10$02.00/0
CopyrightX 1991,American Society forMicrobiology
Immediate-Early
Gene
Expression
Is
Sufficient for Induction
of
Natural Killer
Cell-Mediated
Lysis of Herpes
Simplex
Virus
Type
1-Infected Fibroblasts
PATRICIAFITZGERALD BOCARSLY,l* DONNA M. HOWELL,' LISAPETTERA,1 SHAHRAM
TEHRANI,1
AND CARLOS LOPEZ2
Departmentof Laboratory Medicine andPathology, University of Medicine andDentistryofNewJersey-NewJersey MedicalSchool, Newark, NewJersey
07103-2757,1
and VirologyResearch, LillyCorporate Center,Indianapolis, Indiana 462852 Received 27July1990/Accepted 7March 1991
Herpes simplex virustype1(HSV-1)-infectedhumanfibroblast (HSV-FS)targetsaresusceptibletolysis by
naturalkiller(NK)cells,whereasuninfected FSareresistanttolysis.Studieswereundertakentodetermine the mechanismof this preferentialsusceptibility.HSV-FSwerenotintrinsically less stable than FS,asdetermined
bya
5"Cr
releaseassayunder hypotonic shock in the presence ofratgranule cytolysin and by sensitivity to anti-human leukocyte antigen classIantibody plus complement. Single-cell assays inagarose demonstrated that although similar numbers oflarge granular lymphocytes bound to the HSV-FS and FS targets, theconjugateswith HSV-FSwerelysed atamuchhigher frequencythanthose withFS. Theseresultssuggested
thatboth targetsareboundbytheNKcellsbut only theHSV-FS wereable totrigger lysis. The requirement foractive virusexpressionwasdemonstratedbyfailure of emetine-treatedHSV-FStargetsortargetsinfected
withUV-inactivatedHSVtobelysed by NK effectors. Toevaluatethe roleof viral glycoproteins in conferring susceptibilitytolysis, Fabwereprepared fromHSV-l-seropositivesera;these Fabwereunabletoblocklysis
of theHSV-FS. Furthermore, incubationinphosphonoacetic acidfailedtoreduceNK(HSV-FS)activitydespite sharp reductions in viral glycoprotein synthesis. Finally, targets infected with tsLB2 at the nonpermissive
temperature were lysedaswell as orbetter than targets infected with wild-type virus, indicating that HSV immediate-early geneproduct expressionissufficient for conferringsusceptibility tolysis. We conclude that
expression of nonstructural viral proteins or virally induced cellular gene products early in the course of
infectionrather thanstructuralglycoproteinsisrequired forNKlysis ofHSV-FStargets.
Naturalkiller(NK) cellsarecapable of lysingavariety of virus-infected target cells as well as certain tumor targets and normal cells. Because they are relatively nonspecific, NK cells are available immediately as a first-line defense against an invading viral pathogen and are postulated to serve tolimit theextent ofvirusdissemination priortothe
more specific immune responses. In vitro studies with hu-man effector cells have indicated that NK cells can effi-cientlyreduce theextentofreplicationofcertaincytopathic viruses, includingherpes simplex virustype 1(HSV-1), and
adoptive transfer studies with a murine system have indi-cated a role for NK cells against a number ofcytopathic viruses(10).
Thenature of thetargetantigens recognized byNKcells on virus-infectedtargets has remained controversial. Roles forboth the majorstructural glycoproteinG and nonstruc-tural matrix protein M havebeen implicated in the lysis of targets infected with vesicular stomatitis virus (VSV) (32, 37). Bishopetal. (3, 5, 6) reportedthat HSV glycoproteins aretherelevant structuresrecognizedon infectedfibroblast
orWISHepithelialcell targets.However,Borysiewiczetal.
(7)andLopez-Guerreroetal.(31)havefailedtodemonstrate
arole for viralglycoproteins in thelysisofcytomegalovirus (CMV)- or HSV-infected targets, respectively, and have instead implicated a role for early viral genes or a cellular
gene product. Similarly, in a bovine herpesvirus (BHV) system, Cook and Splitter (11) have suggested that viral
*Correspondingauthor.
glycoproteins are not essential for the lysis of targets in-fected with BHV.
Theissueof thetarget structure present oninfectedcells
is potentially complicated by several factors: the nature of
thetarget cells to be infected, the infecting virus itself,the recentfindingsof therequirementfor HLA-DR+ accessory cells for thelysisofmanyinfected targets(2, 18, 33),and the statusof the effectorcells(i.e.,interferon[IFN]preactivated or not). For example, the CD16+ NK cells and the
HLA-DR'
accessorycells,whicharebothrequiredfor thelysisofHSV-infected fibroblasts(HSV-FS),maypotentially require
different interactions with the virus-infected targets. The studiespresentedinthisarticlewereundertakentoevaluate the requirements for development ofsusceptibility ofNK targets to lysis by human peripheral blood NK cells. We haveevaluated thestatus ofcellularstabilityof the infected targetscomparedwiththatof the uninfectedtargets andthe relative target cell-binding efficiency of the effectors and have examined the role of viralgeneexpressioninconferring susceptibility of the target cells to lysis. In contrast tothe conclusions ofBishopetal. (3, 5, 6),wefindnoevidencefor arequirementfor viralglycoprotein synthesisforthelysisof HSV-FS.
MATERIALSANDMETHODS
Viruses. HSV-1 strain 2931 was originally isolated by C.
Lopez and is grown and assayed on Vero monkey kidney
cells. The temperature-sensitive mutant ofHSV-1, tsLB2
(24),wasobtained fromP. Schaffer andwaspropagatedand
3151
Vol.65, No. 6
on November 10, 2019 by guest
http://jvi.asm.org/
titered at the
permissive
temperature of33°C.
VSV wasoriginally
obtained from N. Ponzio of the NewJersey
Medical School and was
propagated
and titered on Verocells. All virus
preparations
were stored at-70°C
andthawed
immediately prior
to use.Cell lines. Human foreskin fibroblast
(FS)
cells wereprepared
by enzymatic digest
of foreskins from full-terminfants. These cells were grown in Dulbecco's modified
Eagle's
medium(DME;
HazeltonLaboratories, Lenexa,
Kans.)
supplemented
with 10% heat-inactivated fetal calfserum
(FCS;
Hyclone Labs, Logan, Utah),
penicillin,
strep-tomycin,
andL-glutamine
in a 5%Co2 atmosphere
in ahumidified incubator. These cells were
passaged
twiceweekly
and are used up to passage 20 to 22. GM-2767fibroblasts,
which are trisomic for chromosome21,
were obtained from the Human Mutant Genetic CellRepository
(Camden,
N.J.)
andpassaged
likeFS cells. K562 cellswere grown insuspension
culture in RPMI 1640 mediumsupple-mented with 10%
FCS,
penicillin, streptomycin,
L-glu-tamine,
and HEPES(N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic
acid)
buffer. All cell lines were screenedroutinely
formycoplasmas
withMycotrim
Culture Kits(Hanna
Biologicals, Alameda, Calif.)
and werenegative
when used in assays.
Preparation
of effector cells. Mononuclear effector cells wereseparated
from freshheparinized peripheral
blood obtained after informed consent fromhealthy
donorsby
centrifugation
onLymphoprep
gradients (Accurate
Chemi-cal
Co., Westbury, N.Y.).
Mononuclear cellswere washed and enumerated with ahemacytometer
or with a Coulter modelZBI electronicparticle
counter.NK cell assays. NK cell assays were
performed
as de-scribedpreviously (15). Briefly, newly
confluentflasks of FS cells wereeither infected with HSV-1 at themultiplicity
of infection(MOI)
statedforeachexperiment
for 3 hormock infected.Simultaneously,
the cellswerelabeledwith150,uCi
of 51Cr
(as
Na51CrO4;
NewEngland Nuclear, Boston,
Mass.).
The targetswerethentrypsinized
andwashedthor-oughly.
Nonadherent K562 cells were spun down andla-beled with 51Crinavolumeof300 ,ul ofmediumfor1to3 h. AtotalofS x
103
targetsin 0.1 ml ofRPMI-10% FCSweredispensed
into each well of flat-bottomed microtiter plates(Costar, Cambridge,
Mass.)
whichalready
containedef-fector cells
plated
intriplicate
in 0.1 ml toyield
effector/target cell
(E:T)
ratios of100:1 to 12:1.Assay
mixes wereincubated at
37°C
for 14 h in a 5%CO2
atmosphere in anincubator,
after which 0.1 ml of the supernatant wasre-movedfrom each well and the
radioactivity
wasdetermined withaPackardGammaCounter.51Cr
releasewascalculatedasfollows: %
51Cr
release =[(experimental
cpm -sponta-neous
cpm)/(total
cpm - spontaneouscpm)]
x 100; wherespontaneouscpmis theamountof51Cr released
by
targetsin the presenceof medium alone and total cpm isthe amountof51Cr
releasedby
targets incubated in the presence of 5% TritonX-100detergent. Spontaneous
releasedidnotexceed30% forHSV-FS and FS targetsor25%for K562 targets. The data
generated
from each NKtest wereanalyzedwith the VonKrogh equation
(35)andareexpressedasestimated kill atan E:T ratio of 50:1.IFNassays. Atthetermination of NKcytolyticassays,an additional35
RI
ofsupernatantwascollectedfromeach 50:1 E:T wellfor the determination of antiviral activity.Super-natantswerestored frozen at-20°C priortoquantitationof IFN
activity
with GM2767 indicator cells and VSV as thechallenge
virus incytopathic
effectreduction assays. Each IFN assay mix contained cell controls, virus controls, andthe internationalleukocyteIFN(IFN-ot)reference standard G-023-901-527, obtained from the National Institute of Al-lergy and Infectious Diseases.
Preparation of Fab fractions fromseropositive and
seroneg-ative donors. Fab fractions were prepared from
Sandoglob-ulin(Sandoz, East Hanover,N.J.) and fromserumobtained fromanHSV-1-seropositive and aseronegative donor. The serological status of the donors was determined by the ability of theirserumsamplestoinduceantibody-dependent
cellular cytotoxicity (ADCC) against HSV-FS and FS
tar-gets.Briefly, 20to35 ml ofserum wascollected from each of the donors. The sera were centrifuged and then passed
through a Millex GU filter. Saturated ammonium sulfatewas
added to 40%drop by drop withconstantstirring in thecold;
the mixture was centrifuged, the supernatant wasdecanted, and the pellet was resuspended in PBS anddialyzed
exten-sively against PBS. A DEAE column was prepared and equilibrated with 0.02 M sodium phosphate buffer, pH 8.0. The saturated ammonium sulfate precipitate was dialyzed
overnight in the sodium phosphate startingbuffer, the
mate-rial was passed through the column, and the first 9 ml of eluant was collected and pooled. The material was made isotonic with lOx saline, and protein concentration was
determined by OD280 measurement with a Beckman spec-trophotometer.
For preparation of Fab fractions, 0.5 ml of immobilized papain (Pierce Chemical Co., Rockford, Ill.) was added to 10 mgof immunoglobulin G (IgG) in 1.0 ml of freshly made 20 mMNaH2PH4 buffer containing 20 mM cysteine-HCl and 10
mMsodium EDTA, pH 6.2. The reaction mix was incubated for 5 h at 37°C with rocking, and then 3.0 ml of 1.0 mM Tris-HCl (pH 7.5) wasadded.The mixture was centrifuged, and the supernatant was applied to a 5-ml immobilized protein A (Pierce Chemical Co.) (22) column equilibrated with 10 nM Tris-HCl, pH 7.5. The column was washed with 15 mlof 10 nM Trisbuffer, and the eluate containing Fab was collected. Proteinconcentrations were determined with the BCA assay (Pierce Chemical Co.). The presence of Fab but notFcin the eluatefraction was confirmed by radial immu-nodiffusion (27) with goat anti-human IgG Fab and Fc reagents (Cappel Laboratories, Westchester, Pa.). The
in-tegrity ofthe Fab regions within the Fab preparations was confirmed by indirect immunofluorescence against
HSV-infected fibroblasts. The HSV-FS targets were incubated with the DEAE IgG preparation or the Fab fractions for 30 min at 4°C, followed by incubation for 45 min in a 1:20
dilution of fluorescein isothiocyanate-conjugated goat
anti-mouse IgG (Cappel Laboratories), and were visualized un-derafluorescencemicroscope.
Incubation of targetcells incytolysin. Ratgranulecytolysin
preparedfroma rat LGL tumor cell line was obtained from
Craig Reynolds, National Institutes of Health (25). The granules were stored frozen at -70°C until immediately before use and were thawed and diluted in
Ca2'-
andMg2+-free
PBS togiveaconcentrationof 20U/ml.Twofold dilutions of thegranulepreparationwere made in theCa2+-and
Mg2'-free
PBS, and 100 ,ul of cytolysin were added to 100RI
of51Cr-labeled targets (at105 cells per ml,diluted inRPMI-1%bovine serumalbumin) in round-bottomed plates inreplicates of six. The mixtures were incubated for various timesat37°C and centrifuged at 4°C, and 100 [L of
superna-tant washarvestedfordetermination of 51Cr release. Exposureoftargetstohypotonic shock. Forexperiments to
measure the sensitivity of targets to hypotonic shock, 5 x
10351Cr-labeledtargetcells (50
RI
perwell) were incubated in six replicates in the presence of an additional 150RI
ofon November 10, 2019 by guest
http://jvi.asm.org/
NK CELL-MEDIATED LYSIS OF HSV-1-INFECTED TARGETS 3153
fluid ranging from 0 to 62% distilled, deionized water (ddH2O), with the remaining volume made up in medium. The cells were then incubated for 14 h at 37°C, after which thesupernatants were harvested and the percent51Cr release in each group was determined by dividing the mean cpm in each group by the mean cpm of a total release obtained by incubating the target cells in 5% Triton X-100 detergent.
Anti-HLA class I plus complement treatment of target cells. FS targets were infected for either 3 or 14 h or leftuninfected and labeled with 51Cr. Ten thousand targets were plated into replicate wells of round-bottomed microtiter plates and incubated for 30min at 37°C with various dilutions of rabbit anti-human leukocyte antigen (HLA) class I framework antibody (New England Nuclear, Cambridge, Mass.). Baby rabbit complement (1:4) was then added to each well at the concentrations described, and the plates werefurther incu-bated at 37°C for 1 h, after which the microtiter plates were centrifuged at 1,000 rpm for 10 min, and the top 100 ,ul of each well was harvested for determination of51Cr release. 51Cr release was calculated as for NK assays.
Single-cell assays in agarose.Peripheral blood mononuclear cells obtained from Lymphoprepgradients were allowed to adhere to nylon wool columns for 30 min and eluted with warm RPMI-10% FCS. The nylon wool-nonadherent frac-tion was separated on seven-step discontinuous Percoll gradients as described previously (15), and fractions 2 and 3, which wereenriched for large granular lymphocytes and NK activity, were collected. The large granular lymphocyte-enriched fraction was depleted of CD3 (anti-Leu-4)-positive T cells by panning (15). Target cells were prepared at an MOI of 5:1 or were mockinfected as described above. For targetbinding assays (23), targets and effectors were resus-pended at 2 x 106/ml in RPMI-10% FCS and 0.1 ml of effectorcells was incubated with 0.2 ml of target cells at 30 or 4°C for 5 min; the tubes were then centrifuged at 200 x g for 5 min, the residualmedium was decanted, and the cells were gentlyresuspended three or four times with a capillary pipette in a volume of 50
RIu
ofRPMI-10% FCS. To each tube was then added 0.1 ml of molten agarose (1.3% Sea Plaque Agarose; FMC Corp., Rockland, Maine)inRPMI-10% FCS at 39°C, and the solution was gently mixed and spread over microscope slides. After allowing the solution to harden for 2 to 3 min, the slides were placed in a staining dish with RPMI-10% FCS and incubated for 6 h at 37°C in a5%CO2incubator. At the end of the incubation, the cells were immersed in a trypan blue-PBS solution for 5 min, after which the slides were washed threetimes, 10 min per wash, in a PBS-0.3% Formalin solution. Duplicate slides were prepared for each group. The slides were viewed with a phase-contrast microscope by an individual blind to the experimental conditions, and the percentageofeffector cells adhering to targets was determined bycounting 200 effector cells on each slide. The proportion of lysed targets was
determined by counting the number of targets inconjugates which stained with trypan blue minus the percentage of targets spontaneously staining with trypan blue on slides
incubated without effector cells. Target-effector conjugates were easily discriminated from target-target and effector-effector interactions because the target cells were much larger than the effectors.
Polyacrylamide gel electrophoresis (PAGE). Target cells in
75-cml
flaskswereinfected with HSV-1 orHSVmutantsfor 3 h in the presence or absence of antiviral drugs or mock infected and then incubatedovernightin the presenceof 100 to 300 ,uCi of[35S]methionine
or[3H]glucosamine
(NewEngland Nuclear, Cambridge, Mass.). The cells were then
gentlytrypsinizedandincubatedinlysing buffer(0.05 M Tris
base,
0.6 M KCI, 0.5% TritonX [pH 7.5]) for10minonice. The lysed cells were clarified by centrifugation (32,000 rpm for 90 min). In some experiments, the supernatants wereimmunoprecipitated overnightat4°Cwith 100,llof the virus preparation and 0.1 mg of primary antibody, followed by incubation for 2 h on ice with protein A-Sepharose. The
precipitateswerewashed three times in RIPA buffer (0.05 M
Tris-HCl, 1 mMEDTA, 0.15 MNaCl, 0.25%bovine serum albumin [pH 8.0]) and incubated insamplebufferovernight.
The samples were then heated at 100°C for 5 min,
centri-fuged briefly to remove the Sepharose beads, and run on
PAGE withan8.5%resolving gel and3%acrylamide stack-ing gel cross-linked with N,N-diallyltartardiamide (Aldrich Chemical Co., Milwaukee, Wis.) in an amount correspond-ing to4%of the weight of acrylamide. Each liter ofrunning buffer contained 3 gof Tris base, 14.4 g of glycine, and 1 g of SDS.
Treatment of cells with metabolic inhibitors and antiviral drugs. Theacid formofphosphonoacetic acid(PAA; Sigma
Chemical Co., St. Louis, Mo.), which is an inhibitor of HSV-encoded DNA polymerase (26), was used ata concen-tration of300 ,ug/ml and was present throughout the course
of infection and NK assays.
Emetine-HCl(Sigma Chemical Co.), which is an irrevers-ible inhibitor of protein synthesis, was usedat a
concentra-tion 0.1 mM to treat thetarget cells priorto theaddition of virus. Following treatment with emetine for 1 h, cultures were rinsed extensively with PBS and then infected with HSV-1 as described above. Following trypsinization of FS and HSV-FS target cells or the pelleting of K562 cells, the targets receivedfour washes in 15 ml of medium to eliminate anycontaminating emetine.
UV-inactivated virus.UV-inactivated HSV-1 was prepared byirradiating HSV-1 with a UV sterilizing lamp (254 nm) at
a distance of 10 cm to yield 1,340 i±W/cm2 for the times shownin the experiments. The efficacy of the treatmentwas confirmed by titration of the UV-irradiated virus on Vero cell monolayers.
RESULTS
HSV-FS targets are not instrisically less stable than unin-fected targets. In an early study, Brooks et al. (9) demon-strated a correlation between the osmotic stability of a series of rat tumor targets and their susceptibility to lysis by rat splenic NK cells. Onepossible explanation forthe increased susceptibility of HSV-FS over uninfected FS targets was that the infected targets were unable to repair membrane damage due to shutoff of host macromolecular synthesis by
thevirus. Totestthishypothesis, we subjected infected and
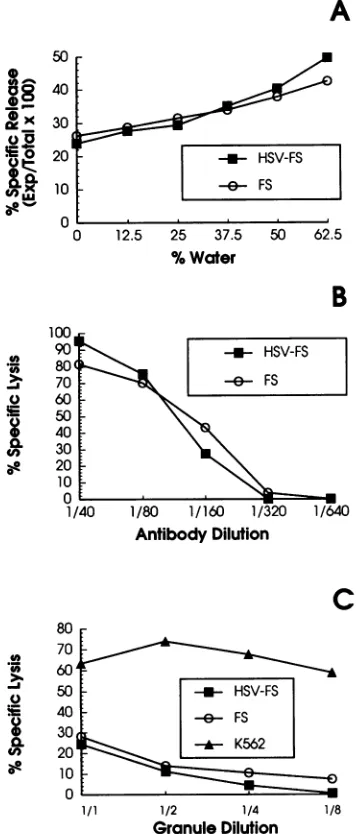
uninfected targets tohypotonicshock, rat granule cytolysin, and treatment with anti-HLA class I antibody plus comple-ment. Figure 1A shows the results of a representative experiment in which 51Cr-labeled HSV-FS and FS targets were incubated in the presence of increasing amounts of
distilled water for 14 h (the time of our typical NK assay), after which the supernatants were harvested for determina-tion of51Cr release. The infected targets were found not to
be more sensitive to hypotonic lysis than the uninfected targets. In parallel studies with the same targets, infected targets werepreferentially lysed by effector cells in14-h NK assays (data not shown).
Next we tested thesensitivity of both FS and HSV-FS to
lysis by anti-HLA class I polyclonal antibody and comple-ment in 1-h 51Cr release studies. For these studies,
51Cr-VOL. 65,1991
on November 10, 2019 by guest
http://jvi.asm.org/
3154 FITZGERALD-BOCARSLY ET AL.
A
-i- HSV-FS
-- FS
0 12.5 25 37.5
% Water
[image:4.612.92.270.77.494.2]50 62.5
TABLE 1. Targetbinding and single-cellassays inagarosea
Target %Conjugates %of conjugates lysedb
cells
4°C
300C4°C
300CFS 10.8 ± 2.3 17.5 + 3.2 3.3 ± 0.8 2.0 ± 0.5
HSV-FS 11.8± 3.0 17.7± 0.7 10.7 ± 3.2 8.7 ± 2.8
aLight-density cellsfromPercoll gradients were depleted ofT cells by panning and used in single-cell target-binding assays in which the
target-effectorconjugateswereformedat4 or300C.Conjugatelysiswasdetermined
after 6 hofincubation,with 200cells countedperslide.Data arethemeansof
threeexperiments. Statisticalsignificancewasdetermined by Student'sttest;
P < 0.01 for percent lysed (FS versus HSV-FS); P > 0.1 for percent
conjugates(FSversusHSV-FS).
bPercentageoflysedtargetswithintarget-effector conjugates.
B
._ 100
90
80 70 60
50
40 30 20 10
0
1/i
-in- HSV-FS -e&- FS
.-I
1/80 1/160 1/320 1/640
AntibodyDilution
j
.~~
-m HSV-FS
. O~~-& FS
|
~~~~K562
I 8070
.r 60A
50
40
s 40
9
30i20
10k 110
0
1/1 1/2 1/4
Granule Dilution 1/8
FIG. 1. Comparison of intrinsic stability of FS and HSV-FS
targets. (A) HSV-FS and FS targets were incubated in various concentrations ofddH2O for 14 h, after which releaseof51Crinto
the supematants was determined. (B) Susceptibility of HSV-FS (infected overnight) and FS targets tolysis with anti-HLA class I
andcomplement. Targets were incubated for30 minat 37°C with variousconcentrations of anti-HLA class I framework followed by incubation in baby rabbit complement for 1 h. (C) Sensitivity of HSV-FS and FS to treatment with rat granule cytolysin was determinedby incubatingtargetsfor 1 hinCa2+- and Mg2+-free PBS
inthepresenceof dilutions ofcytolysin. Dataforeach experiment
shownarerepresentative ofatleast three similar experiments.
labeledtargets wereincubated with anti-HLA antiserum for 30 min prior to the addition of baby rabbit complement. Figure 1B shows the results ofarepresentative experiment
demonstrating that HSV-FS are not more susceptible to
complement-mediated lysis than arethe FStargets. HSV-FS and FS targets were also tested for their sensi-tivity to lysis by cytolysin, the lytic material purified from
the granules of a rat large granular lymphocytic leukemia (25). A representative experiment in which FS, HSV-FS, and K562 targets were incubated in cytolysin for 1 h is
shown inFig. 1C. FSdemonstrated aslightbutconsistently greatersusceptibilitytolysis bycytolysinthandid HSV-FS.
Compared with K562, both the infected and uninfected FS showed relatively low susceptibility to cytolysin (Fig. 1C)
and required longer incubation times in the presence of
cytolysin tobe lysed (datanot shown).
Together, the data presented in Fig. 1 indicate that
al-thoughthe HSV-FStargetsareproductivelyinfected witha lytic virus andultimately go on to produce infectious viral progeny(16, 30), theirpreferential lysis byhuman NK cells
withinourassayconditionscannotbe explained byagreater
intrinsic instability than exists in their uninfected counter-parts.
We alsoquestionedwhethertrypsinization of targets had anyeffect on thedifferential susceptibility of the targets to lysis since we observed that in wells to which trypsinized uninfectedtargetswereadded,these targets readheredtothe microtiter plates, whereas trypsinized HSV-FS readhered less well to the microtiter wells during the course of the
assay. We therefore compared the lysis of targets which
wereinfectedin75-cm2 flasksfollowedbytrypsinizationand
plating into microtiter plates with lysis of targets when uninfected cells were platedin microtiter wells, allowed to reattachovernight, and theninfected and 51Cr-labeled in the
wells. Each of these infected populations were equally susceptible to lysis, whereas both the recently trypsinized
andovernight-readhered, uninfected targetswereinsensitive toNKlysis (datanotshown.)
Similar numbers of effector cells bind to infected and uninfected FS targets. We havepreviouslydemonstrated that uninfected targetsare efficient coldcompetitorsfor thelysis
of HSV-FS (15). It was therefore of interest to determine whether therewasasimilar leveloftargetbindingof effector cells to FS and HSV-FS. In these experiments, partially
enrichedlargegranular lymphocytepreparationswereused, and the binding of the effectors to the targets and lysis of targets were monitored in single-cell assays. Therewas no significant difference in the binding of the effectors to the HSV-FS and the FS targets(Table 1). Incontrast, ahigher proportion ofthe HSV-FS than ofthe FS targets in conju-gateswerefoundto belysedat6 h.
Active viralexpression is required for making target cells
susceptible to lysis. To determine whether passive viral
expressionwassufficienttomake thetargetcellssusceptible to lysis or whether active viral expression was required, fibroblasttargetswereinfected with eitherHSV-1orHSV-1 whichhad beenUVinactivated for variousamountsof time
(UV-HSV)and thentested for theabilityof the targetstobe
lysed byNKcells(Table 2). Additionally,titersof virus after UV irradiation were determined; by 5 and 10 minof
irradi-08
X
Qx
_L
-50
40
30
I
20
10
0
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.612.317.560.89.143.2]NK CELL-MEDIATED LYSIS OF HSV-1-INFECTED TARGETS 3155
TABLE 2. NK activity against targets infected with UV-inactivatedHSV-la
UVexposure HSV titer HSV-FS
(min) (PFU/ml) % Lysis (SD) IFN-a(IU/ml)
0 5.0 x 106 20.0 (3.6) 300
1 1.0 X 103 23.0 (3.0) 300
5 <10 11.0 (4.0) 100
10 <10 6.4(3.0)b 300
a HSV-1wasirradiated witha UV lamp at 1,380
p.W/cm2
forthe times listed andusedtoinfect FS.Values are percentlysisat an E:T ratio of 50:1 followingVonKroghanalysis for sixseparate blood donors. IFNgenerationin response
to HSV-FS and virus titers following irradiation are shown for a
repre-sentativeexperiment.
bp = 0.05 versus lysis of uninfected FS (Student's t test); lysis of
uninfected FSwas3.2%+ 1.5%, andtheyinduced<3 IU of IFN-a perml.
ation, infectious virus was no longer observed. Targets infected with UV-HSV were not lysed by the effector cells; thiseffect was directly related to the dose of UV, and NK susceptibility was reduced almost to the level of uninfected cellkilling after irradiation for10 min. In contrast, infection
oftargets with UV-inactivated virus failed to decrease the production of IFN-a by the effector cell population which was induced by those targets (Table 2). The inability of UV-inactivated virus to confer NK susceptibility to FS targets was observed at awide range of MOIs. Even at an
MOI of20:1,UV-inactivatedvirus failed to make the targets
susceptibletolysis (data not shown).
Further evidence for the requirement of active viral
expression for making infected targets susceptible to lysis
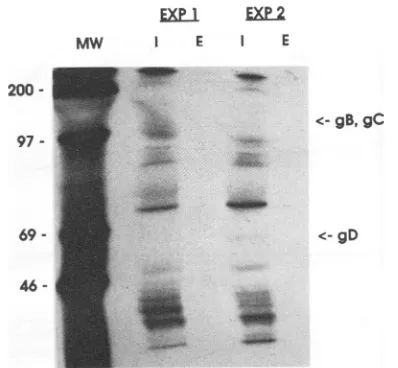
was obtained by treating FS targets with the irreversible proteininhibitoremetine priortoinfection with HSV-1. FS targetcellswereincubatedwith 0.1 mM emetine for1h, and the cultures were washed extensively and infected. As a
control, K562 tumor targets were incubated in emetine for thesameperiod of time. At the end of infection and labeling with 51Cr, the targets were thoroughly washed and usedin 14-h NK assays. The efficacy of emetine treatment was confirmed by PAGE; protein synthesis was completely
turned off in the infected targetsin the presenceof emetine (Fig. 2). Inhibition ofprotein synthesis by emetine in the K562 and HSV-FS targets was further confirmed by mea-surement of[3H]leucine incorporation into these targets. A 1-htreatmentofthe targets withemetine resulted ina94.6%
reductionin theincorporation of[3H]leucine in the emetine-treated versusuntreated K562 targets anda100%reduction in the emetine-treated versus untreated HSV-FS targets. Treatment of the FS targets with emetinepriortoinfection resulted ina dramatic reduction in NKactivity against the infected FS (Table 3). In contrast, lysis of K562 cells was
unaffected by thistreatment.
Roleof viral glycoproteinsinmaking HSV-infected targets susceptible to lysis. Bishop et al. (3, 5, 6)have suggested a rolefor viralglycoproteins astargetstructures for NKlysis
of HSV-infected endothelial cells. They described lower
lysis oftargets infected with viralmutants which underpro-ducedglycoprotein C(gC) orgBandwere
partially
ableto block NK lysis by the addition of Fab fragments directedagainst viral glycoproteins. To evaluate the role of viral
glycoproteinsinmakingviral targetssusceptibletolysis,we
used Fab fragments of serum IgG from
HSV-seropositive
and seronegative donors and PAA treatment of infected cultures toprevent viral
glycoprotein expression.
Fab frac-tions were prepared by papain digestion ofa commercialEXPI EXP2
E I E
<-gB,gC
<-gD
46
-FIG. 2. SDS-PAGE analysis of emetine-treated, HSV-infected targets. SDS-PAGE analysis of two experiments with 0.1 mM emetine treatment of HSV-FS targets is presented. Targets were treatedwith emetine for 1h, washed,and then infected with HSV-1
at anMOI of 1. Cultures werelabeled with[35S]methionine, lysed, and immunoprecipitated with a polyclonal anti-HSV antiserum.
Lanes: MW, 14C-labeled molecularmassstandards(shownin kilo-daltons);I,culturesinfected in the absence ofemetine; E,cultures infectedin the presence of emetine.
pooled
human serumgammaglobulin
(Sandoglobulin)
orfroman
HSV-1-seropositive
and aseronegative
donor. The presenceof Fab butnotFcfractionswasconfirmedby
radial immunodiffusion. Intactrecognition
of viralproteins by
the Fabprepared
fromtheseropositive
butnottheseronegative
donorwas confirmed
by
indirect immunofluorescence withHSV-FS;
intactSandoglobulin
andtheimmunoglobulin
pre-pared
from theseropositive
donor and Fabfragments
pre-pared
from theseimmunoglobulins
gave strongsurface im-munofluorescencestaining
of HSV-FS but not FS targets, whereas neither theimmunoglobulin
nor the Fab from theseronegative
donor gavepositive
fluorescence. The intactIgG
fractionfrom both theSandoglobulin
and theseroposi-tive donor but not the
seronegative
donor wascapable
ofinducing
ADCCagainst
HSV-infected targets.However,
TABLE 3. Effect of emetineonNKactivitya
%Lysis
Expt Donor HSV-FS K562 +
HSV-FS +
emetine
K562 emetine1 1 33.8 3.7 62.8 66.2
2 33.2 3.9 72.0 73.9
2 1 20.7 7.7 58.5 68.7
2 31.1 7.0 52.7 55.9
Mean(SEM) 29.7(3.1) 5.6(1.0)b 61.5(4.1) 66.2(3.8)c
aTargetcellswereinfectedin the presenceofemetine(0.1mM)for1 hand
then washedextensivelybefore beinginfectedorusedinthe assay. Lysis
valuesareforanE:Tratioof 50:1 for fourseparatemononuclear cell donors
(two in eachexperiment). Protein synthesiswasmonitoredby [3Hlleucine
uptake. Emetine treatment of K562 cells and HSV-FS decreased protein synthesis by96.4and100%,respectively. Failure ofemetine-treatedFSto replicateHSVwasalsoconfirmedbyPAGE(Fig.2).
bSignificantly different from lysis ofHSV-FSin theabsence of emetine (P=0.0003).
cNotsignificantlydifferentfrom
lysis
of K562 in the absenceof emetine.VOL.65, 1991
mw I
IRP-111,
A.
".,
". joibk,b
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.612.343.542.79.263.2] [image:5.612.61.299.102.173.2]70 60
,50
-a40
20
10
0
80 70 60
~.5
dE40
a
20
10 n
MW U I +PAA
A
- ii1'
L + Fab
200-97
-69
-MIl'l 500 166 55.5
(IgGorFab) (jg/mi)
18.5 6.2
B -in- IgG Seroneg.
-/- Fab Seroneg.
-/- IgG Seropos.
-e/- Fab Seropos.
iI
X
it
I 500 100 20 4
(IgGorFab) (jg/ml)
FIG. 3. Fab prepared from HSV-seropositive serafail toblock NK(HSV-FS) activity. IgG and Fabwereprepared from Sandoglo-bulin(A) and from HSV seropositiveandseronegativedonors(B). The IgG and Fab were added at the concentrations shown to HSV-FStargetsand effectorsatanE:Tratio of 50:1. Percent 51Cr
releasewasdetermined in 14-hassays.(A) Dataarerepresentative of four similar experiments. (B) Data are representative of two experiments. Lysis of uninfected FSwas -5%.
when the Fab fractions wereaddedtotheNKassays, they neither suppressed nor augmented the response at any dilution (Fig. 3). Thus, we have failed to reproduce the
resultsof Bishopetal. (6)inoursystem.
PAA is a specific inhibitor of the virally encoded DNA
polymerase (26). Inthe absence of viral DNAreplication, it has been demonstrated thatlittleorno HSV-1gamma gene
products are expressed by infected cells. We therefore infected FS cellsandperformedNKassaysin thepresence of PAA(300,ug/ml). SDS-PAGE results confirmed that viral
glycoprotein bandsweresharplyreduced in the PAA-treated HSV-infected fibroblasts (Fig. 4). NK activity against the HSV-FS targets was not reduced and in some cases was augmented bythetreatmentwith PAA(Table 4).Thelysisof K562 cells and uninfected FS was also not affected by treatmentwith PAA (datanot shown). Furthermore, treat-mentwith PAAhadnosignificant effectonspontaneous51Cr
release valuesfor anyof thetargets.
Role ofHSV-1 immediate-early geneexpression in confer-ring targetsusceptibility tolysis. Convincing evidence fora roleforearlyviralgeneexpressioninconferring susceptibil-ity ofvirus-infected targets tolysis by both NK and cyto-toxic T cells has been developed recently (8, 12, 31). To evaluate the role of immediate-early gene expression in HSV-1-infected targets, we used tsLB2, a temperature-sensitive HSVmutantwhichatthenonpermissive
tempera-<-gB,gC
. c-<-gD
46
-30
-FIG. 4. SDS-PAGEanalysis ofPAA-treated, HSV-infected
tar-gets. Targets were infected with HSV-1 at an MOI of 1 in the continuous presence of PAA (300 ,ug/ml) in low-glucose DME medium.The targetswerelabeledovernight with[3H]glucosamine,
lysed, immunoprecipitated withapolyclonalanti-HSV antiserum,
run onSDS-PAGEgels, anddevelopedbyautoradiography.Lanes: MW, 14C-labeledmolecularmassstandards(showninkilodaltons); U, uninfectedFS:I,FS;infectedintheabsence ofPAA;+PAA,FS infected in thepresenceofPAA.
ture(39°C)failstomake betaand gamma geneproductsand overproducesthealphagene
product
ICP4(13,14).At33°C,however, viralreplication proceeds normallyand infectious progenyareproduced. Targetswereinfectedat33 and39°C
with either tsLB2orwild-type2931virus,and the NK assays were carried out at these temperatures as well. At the
permissivetemperature,bothsetsof targetswerekilledtoan equivalentextentbythe NKeffectors.Atthe
nonpermissive
temperature, however, targets infected with tsLB2 were killedas well as or even more efficiently than the FS cells infected with the wild-type virus (Table 5). The failure of tsLB2 to produce normal levels of structuralglycoproteins
[image:6.612.53.302.72.378.2]and
overproduction
of ICP4 at39°C
were confirmed by SDS-PAGE(Fig.
5).TABLE 4. Effectof PAAonlysis of HSV-FSa
% Lysis
Donor MOI 0.2:1 MOI 1.0:1
-PAA +PAA -PAA +PAA
1 35.6 34.7 56.7 51.6
2 60.7 50.8 74.9 67.9
3 35.4 50.8 34.8 37.8
4 33.9 37.0 28.1 33.8
5 25.9 35.0 27.8 34.2
Mean(SEM) 38.3(5.9) 41.7(3.8) 44.5 (9.3) 45.1(6.6)
a PAA (300 ,ug/ml) was present throughout infection and NK assays.
HSV-FStargets wereprepared at an MOIof0.2:1or 1:1. Datarepresent
estimatedlysisatan E:Tratio of 50:1followingvonKroghanalysis.There
was nostatistical difference(P>0.05,Student's ttest)with orwithoutPAA
ateach MOI.Lysisof uninfected FSwasalways.5%.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.612.341.534.76.286.2] [image:6.612.323.561.572.685.2]NK CELL-MEDIATED LYSIS OF HSV-1-INFECTED TARGETS 3157
TABLE 5. NKactivityagainstFStargetsinfectedwithtsLB2a
Target cells
TTemp
% Lysis Virus titer(') Donor 1 Donor 2 (F/l
2931 33 18.3 21.6 1.5 x 105
39 15.6 19.8 1.2 x 105
tsLB2 33 21.1 24.7 1.2 x 105
39 31.1 33.7 0.2 x 102
Uninfected 33 7.8 6.6 NAb
39 4.6 4.8 NA
aFS targets were infected with tsLB2 or HSV-1 strain 2931 (MOI, 1:1) at 33 or39°C, and the entireexperiment was carriedout atthese temperatures.NK
activity and virus replication in spontaneous release wells were measured after 14 h of incubation. NK data are expressed for an E:T ratio of 50:1 following von Krogh analysis.Data arefromoneexperiment representative of six similar experiments.
bNA, notapplicable.
Effect of 20-h preinfection of targets on susceptibility of HSV-FS to NK lysis. The majority of the NK studies of
Bishop et al. (3-6) wereperformed with targets which had
been infectedovernight with HSV-1 before being used inNK assays. In contrast, our studies havebeen performed with 3-h-infected targets followed by 14-h NK assays. We have now directly compared 3- and 20-h-infected targets for susceptibility to lysis in 14-h NK assays (Table 6). The
viability of the targetspostinfection was found to beidentical forthe 3- and20-h-infectedtargets
(>95%),
asdeterminedbytrypan blue exclusion. Moreover, spontaneous release val-uesfromthe 3- and20-h-infectedtargets were
indistinguish-able at the endof14h NK assays(datanotshown).When we
compared NK lysis ofthe 3-h and 20-h targets, however,
there was asignificant decrease in NK susceptibility ofthe
20-h-infected targets; for some donors, the 20-h-infected
targets were lysed toextremely low levels, whereas some
donorsshowed lesser degrees ofsuppression. Thedecreased sensitivity of the 20-h-infected targets occurred despite equivalent induction ofIFN
production
andan evengreatersurface immunofluorescence for HSV
glycoproteins
thanUnif 2931 hLB2
MW 330 390 330 390 330 390
200- <-ICP4
4 w " <-gB,gC
95.
-H
V
2931
at
<- gD55-
"
''R'
FIG. 5. SDS-PAGE analysis of targets infected with tsLB2 or HSV 2931 atpermissiveand nonpermissive temperatures. Targets
wereuninfected(Uninf)orinfected with HSV-1 2931 or tsLB2 at an
MOI of1 at33or39°C, labeled with[35 ]methioninein methionine-free medium, lysed, and run without immunoprecipitation on an
SDS-PAGEgel. Sizes are shown in kilodaltons.
TABLE 6. Extendedinfection of targets decreasessensitivityof HSV-FStolysisa
3-h-infected targets 20-h-infectedtargets
Donor IFN IFN
%Lysis generated %Lysis generated
(IU/ml) (IU/ml)
1 30.2 300 13.1 200
2 42.0 300 23.2 200
3 18.4 1,000 3.9 1,000
4 16.2 3,000 5.0 3,000
5 26.6 700 17.6 700
6 15.1 1,000 6.8 1,000
7 26.6 1,000 21.0 1,000
8 26.0 1,000 16.8 1,000
9 41.4 10,000 21.1 10,000
10 28.2 300 17.9 300
11 22.9 300 6.6 300
12 19.3 300 20.4 300
x(SD) 26.1(8.8) 780 14.5(7.1)b 729
aFS targetswereinfected with HSV-1 for3or20hat anMOI of1andthen
usedin 14-h NK assays. Data represent estimatedlysisat anE:Tratio of 50:1
followingvonKroghanalysisand IFNgeneratedatanE:Tratio of 50:1 for12
different donors. Arithmetic mean NK activity and geometric mean IFN productionareshown in the lastline. Uninfected FSlysiswas '10%for all donors.
bSignificantlydifferent fromlysisof 3-h-infectedtargetsasdeterminedby
Student'sttest(P<0.05).
observed for the 3-h-infected targets when stained with
anti-HSV antiserum.
HSV-seropositive
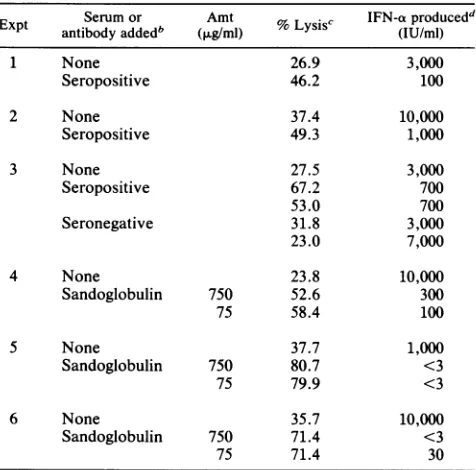
serum induces ADCC but blocks induc-tionofIFN.SerumfromHSV-1-seropositive
orseronegative
individuals orreconstituted
Sandoglobulin
immuneglobulin
were added to 14-h NK cellsagainst
HSV-FS targets.Sandoglobulin
andserumfromseropositive
butnotseroneg-ative donors
augmented
lysis
of the HSV-FS targets viaADCC. Incontrast, IFN
production
in the presence oftheHSV-immunesera was
depressed,
whereasHSV-seronega-tiveserumhadnoeffectonIFN
production (Table 7). Thus,
antibodiesagainst
viralproteins expressed
on the cellsur-face blocked the
ability
ofthe infected cells toprovide
aninductive
signal
totheIFN-producing
cells.DISCUSSION
In this
study,
we have examined the mechanism of thepreferential
lysis
of HSV-infected fibroblasts over theiruninfected counterparts. Since HSV-1
efficiently
turnsoff
hostcellmacromolecular
synthesis (28),
itwaspossible
that the increasedlysis
ofthe infected targets could be duetoaninherent
fragility
ofthese targets and aninability
torepair
membranedamage.
Using
three different measurements ofsensitivity
tolysis,
wehavefailedtodemonstrateincreasedsensitivity
ofthe HSV-FS:they
showedsimilarsensitivity
tohypotonic
shockandto treatmentwithanti-HLAclass Iplus
complement
and were not moresusceptible
to ratgranule
cytolysin,
thelytic
material from a ratlarge
granular
lym-phocyte
tumorline(25)
(Fig. 1).
Thesensitivity
tolysis
by
cytolysin
of both HSV-FS and FS targets was farlower,
however,
than thatofK562,
afinding
which is inagreement
with the report of the relative
insensitivity
offibroblasts to the action ofcytolysin.
We also observed nosignificant
difference in the percentageof effector cells which boundto HSV-FS andFS targets, whereas the HSV-FS
targets
werepreferentially
lysed
(Table 1).
These resultssupport
ourprevious
observationsthatuninfected FStargetsareefficientVOL.65, 1991
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.612.62.301.489.670.2]TABLE 7. Suppression ofIFNproductionbyHSV-immune but notnonimmuneserum7
Expt Serum or Amt %Lysis' IFN-aproducedd
antibodyaddedb (>tg/ml) s (IU/ml)
1 None 26.9 3,000
Seropositive 46.2 100
2 None 37.4 10,000
Seropositive 49.3 1,000
3 None 27.5 3,000
Seropositive 67.2 700
53.0 700
Seronegative 31.8 3,000
23.0 7,000
4 None 23.8 10,000
Sandoglobulin 750 52.6 300
75 58.4 100
S None 37.7 1,000
Sandoglobulin 750 80.7 <3
75 79.9 <3
6 None 35.7 10,000
Sandoglobulin 750 71.4 <3
75 71.4 30
a Serum(5 ,ul) fromHSV-immune(seropositive)ornonimmune (seronega-tive) donorswasaddedtoNK(HSV-FS)assays at thebeginning ofculture.
Sandoglobulinwasaddedto theassaysat afinalconcentration of 750 or 75
jig/ml.Lysis of uninfected FSwas -5%.
bInexperiment 3,serafromtwoeachseropositiveandseronegative donors
weretested.
c NK or ADCC activity at an E:T ratio of 50:1 following von Krogh
analysis.
dIFNproductionat anE:Tratioof 50:1.
coldcompetitorsfor thelysisof HSV-FS targets(15)and the resultsof Borysiewicz etal.,who demonstratedcompetition between CMV-infected and uninfected fibroblasts(7,8).Our
competition and targetbinding results support the concept thatthe initial recognition structureis presenton the
unin-fected targets, whereas the infected targets are selectively triggered to preferentially lyse theinfected targets. In con-trast to ourtarget-binding results, Borysiewiczet al. (7, 8)
have reported a significantly greater (approximately
50%)
bindingofeffector cells to human CMV-infected fibroblasts thantouninfected fibroblasts. These latterresults, however,
were obtained with IFN-pretreated effectors, which may have influenced thetarget-binding profile.
The role ofactiveexpression of virus inmakingthe targets
susceptible to lysis was evaluated by using UV-irradiated virus toinfect targets. Our results indicate that adsorption of the UV-damaged virus to the FS targets was insufficient to make the target cells susceptible to lysis. In contrast, the
UV-inactivated virus induced levels of IFN-a similar to thoseinduced by nonirradiated virus. It has previously been suggested that lysis ofvirus-infected targets occurs
nonspe-cificallyandis due to promiscuous killing by NK cells which have been activated by the IFN induced by the viral infec-tion (38, 39). However, the observainfec-tion that high levels of IFN wereinduced by UV-treatedvirus-infected targets but thatthese targets were notlysedindicates that IFN induc-tion alone is insufficient to lead to
lysis
of the fibroblast targets (Table 2). Rather, active expression of the viral genome,perhaps leadingtotheinductionofatriggerstruc-tureontheinfectedtargets, is alsorequired. That
preferen-tiallysis of virus-infected targets is not due solelyto
IFN-induced NK cytotoxicity is further supported by the
observationsthatIFN-pretreatedeffectors stillpreferentially
lyse virus-infected over uninfected targets (4, 17) and that
inclusion ofanti-IFN antiserum in NK assays failstoreduce
lysis ofthevirus-infectedtargetsdespite neutralizing detect-able supernatant IFN(2, 17).
The
suppression
ofNK(HSV-FS) but notNK(K562)ac-tivity by emetine (Fig. 2, Table 3) further indicates that active viral expression is required to make the virally
in-fected targets susceptible to lysis. Protein synthesis
inhibi-tion
by
emetine,
as measuredby
[3H]leucine uptake, wasfound tobe similarfor the HSV-FS and K562 targets, ruling out a differential effect of the emetine on the two targets. These results confirm and extend those ofBishop etal.
(3),
who demonstrated that 18-h treatment of HSV-infected targets with emetine also diminished NK susceptibility.
Theseinvestigators, however,failedtoreportcontrol exper-iments with anoninfected NK target cell such as K562. This is an important control, sinceblockade of protein synthesis
in targetsforsuch an extendedperiod prior to the NK assay would also be expected to affect cellular proteinturnover.
Althoughboth the seropositive antiserum and
Sandoglob-ulin(but not the seronegative serum) induced ADCC of the HSV-FS targets, the Fabfailed to block cytotoxicitydespite theirretainingtheability torecognize virus-infected targets. This failure to inhibit lysis is in contrast to the partial inhibition observed by Bishop et al. (6) with Fab prepared
from a seropositive donor oramonoclonal antibody to gBor
gC.
Further evidence for alack of requirement for structural glycoproteins comes from our data demonstrating intact NK(HSV-FS) activity in the presence of PAA (Table 4) and with the temperature-sensitive mutant tsLB2 (Table 5) de-spite the reduced production of viral glycoproteins. These results are similar to those indicating a lack ofrequirement for structural glycoproteins for the lysis of CMV-infected human fibroblasts (8) or BHV-infected fibroblasts (11). The data we haveobtained with the tsLB2 mutant at the nonper-missive temperature further indicate that expression of the immediate-early genes of HSV is sufficient to make the targets susceptible to lysis. In fact, the tsLB2-infected targets were killed better at the nonpermissive temperature than the targets infected with wild-type virus despite the failure of the tsLB2 virus to replicate or express structural glycoproteins (Table 5, Fig. 5). These results are consistent with the observation that expression of immediate-early gene products is sufficient to induce lysis of CMV-infected FS targets (8).
Our findings of a lack of requirement for structural glyco-proteins of HSV-infected targets for conferringsusceptibility to NK lysis contradict those of Bishop et al. (3, 5, 6). One difference in our assay systems is that they routinely used targets that were infected overnight, whereas we used acutely infected targets. However, when we directly com-pared 3- and 20-h-infected targets in 14-h NK assays, we found that the 20-h-infected targets demonstrated signifi-cantly less susceptibility to NK lysis despite strong induc-tion of IFN by these targets and high levels of cell surface viral glycoproteins (Table 6). These results again argue for NKrecognition of a relatively early event in viral replication (whether it be a cellular or viral gene product) which is at least partially downregulated following 20 h of infection. Studies are under way to more fully characterize the bio-chemical differences between the 3- and 20-h-infected tar-gets.
on November 10, 2019 by guest
http://jvi.asm.org/
NK CELL-MEDIATED LYSIS OF HSV-1-INFECTED TARGETS 3159
Although we have demonstrated that expression of the
HSV genome is required to make the infected targets sus-ceptible to lysis, the nature of the target signal and/or
triggeringmolecules remains unclear. It is possible that the immediate-early gene products are the actual target struc-tures recognized by NK cells. Peptide sequences of
imme-diate-earlygeneproductsofanumber of viruses are known to be capable of inducing cytolytic T cells. These peptide sequences are thought to associate with major
histocompat-ibility
complex (MHC) antigens inside the cell and to be transported to the cell surface. It is possible that somesimilar mechanism might occur for the recognition of
in-fected cells by NK cells; however, NK cells are not MHC restricted, nor would they be expected to have the same degree of fine specificity as cytotoxic T cells. Alternatively, expressionof immediate-early gene products of HSV, some
of which are known to affect cellular gene expression (13, 20), may be altering a cellular gene product (either upregu-lating, downreguupregu-lating, or modifying it) that may influence targetsusceptibility.
NK lysis was found to correlate with increased total and recycling transferrin receptor expression by CMV-infected targets by Borysiewicz et al. (7); however, these authors concluded that the NK target structure was not the transfer-rin receptor itself, since they were unable to inhibit lysis with either iron-saturated transferrin or affinity-purified transfer-rin receptor. Lopez-Guerrero et al. (31) have also implicated host cell transferrin receptor in the killing process of both tumor and virus-infected targets, yet were unable to block lysis of the HSV-infected targets with certain anti-transferrin receptor monoclonal antibodies which were able to block lysis of the tumor targets. These authors have also demon-strated that inhibition of N-linked glycosylation of HSV viral glycoproteins leads to decreased sensitivity of the targets to lysis, yet, like us, were unable to correlate sensitivity with levels of surface expression of viral glycoproteins B, C, and D
(31).
The conflicting data in the literature regarding the relative role of viral structural proteins, immediate-early genes, and host factors in making HSV- or CMV-infected targets sus-ceptible to lysis may reflect different requirements for acti-vation of the
CD16+
effector cells and HLA-DR+ accessory cells, both of which are required for lysis of HSV-FS and CMV-FS (2, 18, 33). Our data with UV-inactivated virus indicates that input viral glycoproteins themselves are suffi-cient to induce IFN production by theHLA-DR+
accessory cells but not NK susceptibility of the targets. Similarly, blocking of viral glycoproteins with antibody led to a dra-matic suppression of IFN production (Table 7). We predict that Fab against viral glycoproteins would also decrease IFN production, but we have not yet performed these experi-ments. In one report, blocking of gD with monoclonal antibody was found to result in a decrease in IFN production (3). In the case of tsLB2-infected targets or assays per-formed in the presence of PAA, we observed levels of induced IFN similar to those obtained with wild-type virus (data not shown), again indicating that input virus is suffi-cient to stimulate IFN production. Thus, we propose that stimulation of accessory cell activity may be dependent upon viral glycoprotein expression, whereas the NK cells them-selves require recognition of the infected target cell, for which de novo immediate-early gene expression, either directly or indirectly, is required. Studies are under way to evaluate the relative roles of specific immediate-early gene products of HSV in making targets susceptible to lysis, utilizing newly available HSV deletion mutants and vectorscontaining specific HSV-1 genes. In addition, the role of
cellular proteins which are up- or downregulated by HSV
(e.g., heatshockproteins, which areupregulated,andMHC
antigens, which aredownregulated) in HSV-infectedcellsis being evaluated.
ACKNOWLEDGMENTS
Wethank JoanMack fortyping themanuscript, Michael Feldman for preparingthefigures,andMonicaMendelsohnandShelleyCurl for technicalassistance.
This study wassupportedUSPHSgrantCA42093fromthe NCI.
REFERENCES
1. Abb, J., H.Abb,and F. Deinhardt. 1983. Phenotypeofhuman alpha-interferon producingleucocytesidentifiedby monoclonal antibodies. Clin. Exp. Immunol.52:179-184.
2. Bandyopadhyay,S., B.Perussia,G.Trinchieri,D.S.Miller,and S. Starr. 1986. Requirement forHLA-DR+ accessory cells in natural killing ofcytomegalovirus-infected fibroblasts. J. Exp. Med. 164:180-195.
3. Bishop,G. A., J.Glorioso,and S. A. Schwartz. 1983. Relation-shipbetween expression ofherpes simplex virusglycoproteins andsusceptibility oftargetcellstohumannatural killeractivity.
J. Exp. Med. 157:1544-1561.
4. Bishop,G. A., J. C. Glorioso,and S. A. Schwartz. 1983. Roleof interferon in human natural killer activity against target cells infected withHSV-1. J. Immunol. 131:1849-1853.
5. Bishop, G. A., G. Kumel, S. A. Schwartz, andJ. C. Glorioso. 1986.Specificityof humankiller cellsinlimitingdilution culture fordeterminantsofherpessimplex virustype 1glycoproteins. J. Virol. 57:294-300.
6. Bishop, G. A., S. D.Marlin,S. A. Schwartz,andJ. C.Glorioso. 1984. Human natural killer cell recognition ofherpes simplex
virus type 1 glycoproteins: specificity analysis with the use of monoclonal antibodies and antigenic variants. J. Immunol. 133:2206-2214.
7. Borysiewicz, L. K., S. Graham, and J. G. P. Sissons. 1986. Human natural killer cell lysis of virus-infected cells. Relation-ship toexpression of thetransferrinreceptor. Eur. J. Immunol. 16:405-411.
8. Borysiewicz, L. K., B. Rodgers, S. Morris, S. Graham, and J. G. P. Sissons. 1985.Lysisofhumancytomegalovirus-infected fibroblasts by naturalkiller cells: demonstration ofan interfer-on-independent component requiring expression ofearly viral proteins and characterization of effector cells. J. Immunol. 134:2695-2701.
9. Brooks, G. C., E. A. Wayner, P. J. Webb, J. D. Gray, S. Kenwick,and R. W. Baldwin. 1981. Thespecificityofratnatural killer cells and cytotoxic macrophages on solid tumor derived target cells and selected variants. J. Immunol. 127:2477-2483. 10. Bukowski,J. F., J. F. Warner, G. Dennert, and R. M. Welsh.
1985. Adoptive transfer studies demonstrating the antiviral effect ofnatural killer cells in vivo. J. Exp. Med. 161:40-52. 11. Cook, C. G., and G. A. Splitter. 1989. Characterization of
bovine mononuclear cell populations with natural
cytolytic
activity against bovineherpesvirus 1-infected cells. Cell. Immu-nol. 120:240-249.12. Cook,J. L., D.May,A.Lewis,andT.Walker. 1987.Adenovirus ElA gene induction ofsusceptibility to lysis by natural killer cells and activated macrophages in infected rodent cells. J. Virol. 61:3510.
13. Estridge, J. K., L. M. Kemp, N. B. La Thangue, B. S. Mann, A. S.Tyms, and D. S.Latchman.1989. Theherpessimplexvirus type 1immediate-early protein ICP27isobligatelyrequired for the accumulation of a cellular protein during viral infection. Virology 168:67-72.
14. Feldman, M., and P. Fitzgerald-Bocarsly. 1990.
Sequential
en-richment and immunocytochemical visualization ofhuman in-terferon-a producing cells. J. InterferonRes. 10:435-446. 15. Fitzgerald,P.A.,R.Evans,D.Kirkpatrick,andC.Lopez.1983.Heterogeneity ofhumanNKcells: comparisonof effectorsthat lyse HSV-1-infected fibroblasts and K562
erythroleukemia
tar-VOL.65, 1991
on November 10, 2019 by guest
http://jvi.asm.org/
gets. J.Immunol. 130:1663-1667.
16. Fitzgerald, P. A., M. Mendelsohn, and C. Lopez. 1985. Human natural killer cells limitreplication of herpes simplex virus type 1invitro.J. Immunol. 134:2666-2672.
17. Fitzgerald, P. A., P. von Wussow, and C. Lopez. 1982. Roleof interferon in natural kill of HSV-1 infected fibroblasts. J. Immunol. 129:819-824.
18. Fitzgerald-Bocarsly,P., M.Feldman, S. Curl, J.Schnell,and T. Denny. 1989. Positively selected Leu-lla (CD-16+) cells require the presence of accessory cells or factors for the lysis of HSV-infectedfibroblasts but notHSV-infected Raji. J. Immu-nol. 143:1318-1326.
19. Fitzgerald-Bocarsly, P., M. Feldman,M. Mendelsohn, S. Curl, andC. Lopez. 1988. Humanmononuclear cellswhichproduce interferon-alpha during NK (HSV-FS) assays are HLA-DR positive cells distinct fromcytolytic natural killer effectors. J. LeukocyteBiol.43:323-334.
20. Gelman, I.H., and S.Silverstein. 1985. Identificationof imme-diateearlygenes from herpes simplex virus that transactivate thevirusthymidine kinase gene. Proc. Natl. Acad. Sci. USA 82:5265-5269.
21. Gobi,A. E., K. Funa, andG. V. Alm. 1988.Different induction patterns ofmRNA for IFN-a and
-P
in human mononuclear leukocytes after in vitrochallenge with herpes simplex virus-infected fibroblasts and Sendai virus. J. Immunol. 140:3605-3609.22. Goding, J. W. 1978. Use of staphylococcal protein as an immunological reagent. J.Immunol. Methods 20:241-253. 23. Grimm, E., and B. Bonavida. 1979. Mechanism of cell-mediated
cytotoxicityatthesingle cell level. I. EstimationofcytotoxicT lymphocyte frequency and relative efficiency. J. Immunol. 123:2861-2869.
24. Halliburton, I. W., R. E. Randall, R. A. Killington, and D. H. Watson. 1977.Some properties of recombinantsbetween type 1 andtype2herpes simplex viruses.J. Gen.Virol. 36:471-484. 25. Henkart, P. A., P. J. Millard, C. W. Reynolds, and M. P.
Henkart. 1984. Cytolytic activity of purified cytoplasmic gran-ules fromcytotoxic rat large granular lymphocyte tumors. J. Exp. Med. 160:75-93.
26. Honess, R. W., and D. H. Watson. 1977. Herpes simplex virus resistance and sensitivity to phosphonoacetic acid. J. Virol. 21:584-600.
27. Johnson, M. J. 1986. Immunoprecipitationin gels,p. 15-24. In H. Rose, H.Friedman,andJ. L. Fahey (ed.), Manual of clinical
laboratory immunology. American Society for Microbiology, Washington, D.C.
28. Kwong, A. D., J. A. Kruper, and N. Frenkel. 1988. Herpes simplexvirusvirion host shutoff function. J. Virol. 62:912-921. 29. Lebon, P. 1985. Inhibition ofherpes simplex virus type 1-in-duced interferon synthesis by monoclonal antibodies against viral glycoprotein D and by lysosomotropic drugs. J. Gen. Virol. 66:2781-2786.
30. Leibson, P. J., M. Hunter-Laszlo, and A. R. Hayward. 1986. Inhibition of herpes simplex virus type 1 replication in fibroblast cultures byhuman blood mononuclear cells. J. Virol. 57:976-982.
31. Lopez-Guerrero, J. A., B. Alarcon, and M. Fresno. 1988. Mech-anism ofrecognition ofherpes simplex virus type 1-infected cellsbynaturalkiller cells. J. Gen. Virol. 69:2859-2868. 32. Moller, J. R., B. Rager-Zisman, P.-C. Quan,A. Schattner,D.
Panush, J. K.Rose,and B. R. Bloom. 1985. Natural killer cell recognitionof target cellsexpressingdifferentantigensof vesic-ular stomatitis virus. Proc. Natl. Acad.Sci.USA82:2456-2459. 33. Oh, S. H., S. Bandyopadhyay, D. S. Miller, and S. E. Starr. 1987. Cooperation between CD16 (Leu-11b)+ NK cells and HLA-DR+cells innaturalkilling of herpes virus-infected fibro-blasts.J. Immunol. 139:2799-2802.
34. Perussia, B., V. Fanning, and G. Trinchieri. 1985. Aleukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferonproduction inresponse toviruses. Nat.Immun. Cell GrowthRegul. 4:120-137.
35. Pross, H. F., M. G. Baines, P. Rubin, P. Shragge, and M. S. Patterson. 1981. Spontaneoushumanlymphocyte-mediated cy-totoxicity against tumor target cells. IX. The quantitation of natural killer cell activity. J.Clin. Immunol. 1:51-63.
36. Ronnblom, L.,and G. V. Alm. 1982. Limiting dilutionanalysis of humanperipheral blood mononuclear leukocytes thatreactto human amnioncells and protectthese against viral challenge. Eur. J. Immunol. 12:437-441.
37. Schattner, A., and B. Rager-Zisman. 1986. Lysis by natural killer cells requires viral replication and glycoprotein expres-sion. Immunol.Lett. 13:261-268.
38. Trinchieri, G. 1989.Biology ofnaturalkillercells, p. 187-303. In F. Dixon (ed.), Advances in immunology. Academic Press, Inc.,SanDiego, Calif.
39. Welsh, R. M. 1986. Regulation of virus infections by natural killer cells. Areview. Nat. Immun.CellGrowthRegul. 5:169-199.