0022-538X/85/040199-08$02.00/0
Copyright© 1985, American Society forMicrobiology
Monoclonal Antibodies Specific for African Swine Fever Virus
Proteins
ANTONIO SANZ,t BLANCA GARCIA-BARRENO,t MARIA LUISA NOGAL, ELADIO VINUELA,* AND LUIS ENJUANES
Centro deBiologia Molecular (CSIC-UAM), Universidad Aut6noma, Canto Blanco, Madrid-34, Spain
Received 6September 1984/Accepted 19 December 1984
We haveobtained 60stablehybridomas which produced immunoglobulinsthatrecognized12proteins from African swine fever virus particles and African swine fever virus-infected cells. Most of the monoclonal antibodies were specific for the threemajorstructural proteins p150, p72, and p12. The specificityofsome
monoclonal antibodies for the structuralproteins p150andp37and the nonstructural proteins p220and p60
indicatedthat proteins p150 andp220areantigenicallyrelatedtoproteins p37 and p60.The associationofsome viral antigenstospecific subcellularcomponents wasdetermined by immunofluorescence andanalysisof the bindingofmonoclonalantibodies to infected cells. A hostprotein (p24)seemedtobeassociatedwith the virus particles.
African swine fever (ASF) isadisease of swine produced byanicosahedral, cytoplasmicdeoxyvirus, sensitivetolipid
solvents, which containsadouble-stranded DNA with about 170kilobase pairs and more than 30 structural proteins(22; E. Vinluela, Curr. Top. Microbiol. Immunol., in press).
Some ASF virusisolatesproduce anacute diseasewitha
mortality close to 100% in pigs, but chronic diseases pro-duced byattenuated virusisolates arebecoming more com-mon in natural infections (9). The infected host cannot
eliminatethevirus; this maybe relatedtothesusceptibility ofporcine macrophages(15) andtothe lack of induction of
neutralizing antibodies, although virus-precipitating,
comple-ment-fixing, and hemadsorption-inhibiting antibodies are present in the seraof infected animals (5, 14).
Theavailability ofASFvirus-specificmonoclonal antibod-ies(mAbs)willbe useful in theidentificationof viralproteins and the determination of their distribution in both virus
particles and virus-infected cells. By using the hybridoma technology developed by Kohler and Milstein (11), we have
obtained a collection of241 hybridomas from mice immu-nized with eitherASFvirusparticles orASFvirus-infected
cells. Inthispaperwegive thecharacterization of60mAbs that has allowed us to determinethe distributionof several
viral proteins in the infected cells, to show that two struc-turalproteins areantigenicallyrelatedto twovirus-induced, non-structural proteins, and to ascribe a cellular origin to onevirus structural protein.
MATERIALS ANDMETHODS
Animals, BALB/c mice, 8 to 10 weeks old, were used for
immunization. They were a gift from R. A. Fox, Frederick
CancerResearchCenter, Frederick, Md.
Cells. Porcine alveolar macrophages were obtained by
broncho-alveolarlavage aspreviously described (2). Mono-cytes from porcine peripheral blood were isolated as
indi-*Corresponding author.
tPresent address: INGENASA, S.A., Hermanos Garcia Nobl-ejasno.41,Madrid-17, Spain.
tPermanent address:CentroNacional deInmunologia, Virologia
yBacteriologfa Sanitarias, Majadahonda, Madrid, Spain.
cated by Enjuanes et al. (6). Vero cells (CCL81) were obtainedfrom theAmerican Type CultureCollection, Rock-ville, Md.
Viruses. Two ASFviruses were used in this study. ASF virus BA71-5 was isolated from the spleenofaninfectedpig
and cloned bylimiting dilution in porcine macrophages (3).
ASF virus BA71-V was a Vero cell-adapted clone derived from the same stock from which ASF virus BA71-5 was
isolated (6).
ASFviruswaspartially purified fromtheculturemedium of virus-infected cells, asdescribed elsewhere (20). Highly purifiedASFvirus, producedin Verocells,wasobtainedas
described byA. L. Carrascosa, M. del Val, J. F. Santardn,
and E. Vinluela (unpublished results). Sucrose gradient-pu-rified Moloney leukemia virus (MoLV) and influenza virus
were provided by J. N. Ihle (Frederick Cancer Research
Center, Frederick, Md.) and C. Lopez(Centro Nacionalde
Microbiologfa, Virologia eInmunologia Sanitarias, Madrid, Spain), respectively.
Antisera and other immunological reagents. Rabbit
anti-mouse immunoglobulins (heavy and light chain specific)
were purchased from Cappel Laboratories, Cochranville, Pa., and'25I-labeledgoatanti-mouse immunoglobulinswere
purchased from New England Nuclear Corp., Dreieida,
Federal Republic ofGermany. To determine the isotypeof the immunoglobulins produced by the hybridoma clones,
goatorrabbit antiseraspecific forthedifferentisotypeswere
purchased fromResearch Products International Corp.,Elk
Grove Village, Md. Mouse immunoglobulin chains were
purchased fromLittonBionetics, Kensington,Md. The mAb
6D.2, specific for protein p30 ofMoLV, was provided by
J. C. Lee, Frederick Cancer Research Center, Frederick,
Md. The culture medium from P3-X63/Ag8 myeloma cells (11)was usedas acontrol.
lIybridoma
production and selection. To obtainhybrid-omas secretingASF virus-specific mAbs, fusions were car-ried out with the mouse myeloma X63/Ag 8.653 (10) (pro-videdby L. Cicurel, Wistar Institute, Pa.) and spleen cells from BALB/c mice immunized by intraperitoneal injection
of the antigen as described previously (19). Table 1 shows the different antigens used for mouse immunization. The
199
on November 10, 2019 by guest
http://jvi.asm.org/
200 SANZ ET AL.
TABLE 1. Antigens used for mouse immunization andhybridoma selection
Fusion Antigenfor:
no. Immunization Selection
1, 2, 14 Partially purified ASF virus (BA71-5) ASF virus (BA71-V)-infected Vero cell extracts 7, 17 Partially purified ASF virus (BA71-V) ASF virus (BA71-V)-infected Vero cell extracts 18 ASF virus (BA71-5)-infected macrophages Partially purified ASF virus (BA71-5)
19 ASF virus (BA71-V)-infected Vero cells ASF virus (BA71-V)-infected Vero cellextracts 20 ASF virus (BA71-V)-infected Vero cells Partially purified ASF virus (BA71-V)
hybridizations were carried out as described by Nowinski et al. (17). To increase the plating efficiency in the cloning steps, 106 mouse thymocytes and 3 x 106 peritoneal macro-phages were added per well. With this procedure, 80 to 100% of the wells contained viable hybrids.
Thehybridomas and the mAbswerenamed in the follow-ing way: the first number indicates the fusion number, the firstletter indicates the microtest plate of that fusion, and the
second letterand numberindicate the position in the plate of the well with the selected hybridoma.
Radioimmunoassay. A 1-pLg sample of ASF virus protein or 4 ,ug of protein from an extract of ASF virus-infected
cells, in 50
pul
of phosphate-buffered saline (PBS), wasadsorbedtothe well ofpolyvinyldisposable U plates (Dyna-tech Laboratories, Inc., Alexandria, Va.) by overnight incu-bation at 4°C. The subsequent steps of the assay were
carriedout as described byNowinski et al. (17).
Immunoprecipitation and binding analysis. The specificity ofthe mAbs was determined by two procedures,
immuno-precipitation and binding of antigen to mAb-coated plates
(immunoadsorption). The antigens used in both procedures (virus particles or uninfected or ASF virus-infected Vero
cells) were labeledfor 24h with 20 ,uCi of
[35S]methionine
per ml (1,300 Ci/mol) in Dulbecco modified Eagle mediumwith the methionine concentration reduced 10-fold. The ASF virus particles and the uninfected or ASF virus-infected cells were treated for 1 h at 37°C with 2%
Nonidet
P-40(NP-40)-4Murea-0.2%mercaptoethanolin the presence of 0.1 mM L-1-tosylamide-2-phenylethylchloromethyl ketone, 0.1 mM
N-o-p-tosyl-L-lysinechloromethyl
ketone, and1 mM phenylmethylsulfonyl fluoride. Insoluble material was re-movedby centrifugation at 140,000x gfor 2 h at 4°C. Before the test, the extracts werediluted in 10 or more volumes ofPBS. Portions (100 ,ul) of
[35S]methionine-labeled
antigens(about105cpmofASF virus or 5 x 106cpmof extracts from
uninfectedorASFvirus-infected cells)wereincubated
over-nightat4°C with
400-pul
portions of hybridoma supernatants or1/20 dilutionsofascitic fluidorantiserum. Then 20 ,u ofa10% suspensionof Staphylococcus aureus (Cowan I strain;
Pansorbin; Calbiochem-Behring, San Diego, Calif.) coated with rabbit
serikm
anti-murine immunoglobulins was added, and the incubation was continued for 4 h. The immunecomplexwascollectedbycentrifugation, washed four times with0.5% NP-40 in PBS, and solubilized by treatment with
2% sodium dodecyl sulfate-0.7 M 2-mercaptoethanol-1 M
glycerol-0.02% bromophenol blue-0.04 M Tris-hydrochlo-ride (pH 6.3) (dissociation buffer). After removal of the bacteria by centrifugation, a sample of the supernatantwas subjected to electrophoresis in a 7 to 20% acrylamide
gradient(12). The gels were preparedfor autoradiographyby
using sodiumsalicylate (4), dried,andexposed with RP-X1 films (Mafe, Madrid, Spain) at -70°C.
The mAb specificity was also determined by the proce-dure ofMeleroand Gonzalez-Rodriguez(16).
Determination of mAbisotypes. The class and subclass of themAbs weredetermined by immunodiffusion with antise-ra specific for mouse heavy and light chains (18). Protein
precipitation lineswere stained with 0.1% amido black. The presence ofp.chains or more than onelight chaintype was
confirmed by polyacrylamide electrophoresisof [35S]methio-nine-labeledimmunoglobulins immunoprecipitatedwith rab-bit anti-mouse immunoglobulin-coated S. aureus cells, as described above.
Binding ofmAbs touninfectedorASF virus-infectedVero cells. Vero cells at adensity of about 8 x 104 cells percm2, in microtest plates (MIT; Becton Dickinson Labware, Ox-nard,Calif.),weremockinfected or infected with ASFvirus at amultiplicity ofinfection of 5 PFU percell. At14 to24h afterinfection, the cellswereeither unfixed or fixed with 1%
formaldehyde in PBS for 20 min at room temperatureand,
afterwashing, incubated for 1 h at room temperature with
hybridoma culture supernatants, ascites fluid, or immune mouseserum,thelasttwobeingdiluted100-foldwith0.05%
bovineserumalbumin inPBS. Themonolayerswerewashed three times with PBS before theaddition of2.5 x
105
cpmof125I-labeled
goatimmunoglobulins anti-mouse immunoglobu-lins (specific activity, 5 ,uCi/pug; New England NuclearCorp., Boston, Mass.) in 0.05% bovine serum albumin in PBS. Afterincubating the cells for 45 min at 37°C followed bywashingthem three times withPBS,200pJof2MNaOH
was added to each well, and the radioactivity was deter-mined in gamma counter (Wallace model 80000; LKB,
Sweden).
Immunofluorescence. Vero cells at adensity ofabout 2 x
104 cells per cm in microslide culture chambers (Miles Scientific, Naperville, Ill.) were mock infected or infected withASF virus at a multiplicity of infection of 0.3 PFU per cell. At 24 h afterinfection, thecellswereeither unfixed or fixed with methanol-acetone (1:1) for 10 min at -20°C and washed three times with PBS and once with 0.25% bovine serumalbumin in PBSfor 10 minatroomtemperature. The cells were reacted with hybridomasupernatants containing
the indicated mAbs. After three additional washings with
PBS,the cells were covered witha 1/20 dilution of fluores-ceinatedgoatanti-mouse
immunoglobulins
(Cappel Labora-tories) in 0.25% bovineserum albumin in PBS for45minat room temperature, washed three times with PBS, and mounted on glycerol-PBS (9:1). The cells were examined underaZeiss Photomicroscope IIequippedwith anepifluo-rescencedeviceIIIRS.
RESULTS
Production and general properties of the mAbs. The first
screening forASF virus-specific mAbs was carried out by using an indirect radioimmunoassay in multiwell plastic
plates. The wells containing antibody showing a positive
reaction with extracts from ASF virus-infected cells were detectedbyautoradiography (Fig.1A,A', B, B').At10 to15 J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
ASF VIRUS-SPECIFIC MONOCLONAL ANTIBODIES 201
;
0
.
*
.
AI
0
.
C
0 *
9
B~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
l
*-
B
||
..
D
I~~~~~9
.
.
11 yE;s
S
8*~~~~~~~~~*
*0
00~~~~~~~~~
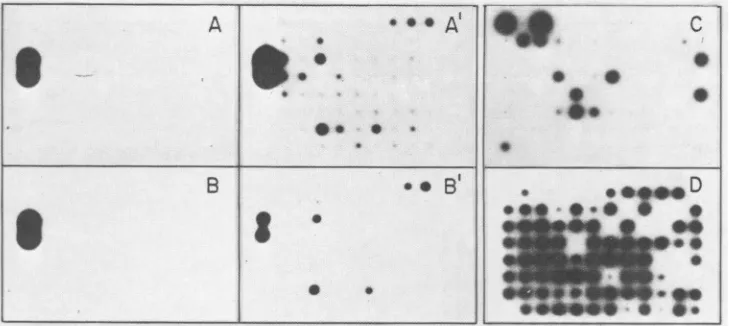
FIG. 1. Selection of anti-ASF virus immunoglobulins by radioimmunoassay. A 4->xg sample of protein antigen concentrated by centrifugation from culture medium (A, A') orcellextracts (B, B') from uninfected (A, B) orASF virus-infected (A', B') Vero cellswas
adsorbed toeach well of Microtest II plates. Each well received hybridoma culture supernatant, goatanti-mouse serum, and 125I-labeled protein A (3 x 107cpm/lLg; 5 x 104cpm perwell). After autoradiography, the hybridomas showingaspecific, positive reaction with ASF
virus-infected cell extractswere cloned and testedagainst partially purified ASF virus particles (C). The positive clones werecloned and
testedonce more asbefore (D). The well in theupperleft side in A, A', B, and B' contained 1jigof MoLVprotein and anti-p30 specific mAb 6D.2.
days after the fusion, when the positive clones contained about 5 x 103 cells, the hybridswerecloned,and 8to10days laterthe culture mediumwas assayed for reactivity against
partially purified ASF virus (Fig. 1C). After one to three
morecloning steps, mostof the cloned cellswerestable and
produced mAbs which reacted with partially purified ASF virus (Fig. 1D).
From eight independent fusions (Table 1), 241 stable hybridomas were obtained. The protein specificity was
de-termined in90 hybridomas, and from these, 60 hybridomas
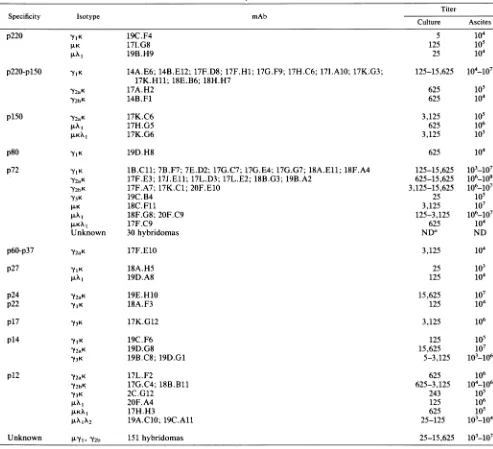
were characterized in more detail. Table 2 shows the
spec-ificity, isotype, and titer range of the mAbs produced by thosehybridomas. Themost frequent isotypewas
immuno-globulin Gl (IgGl; 24 mAbs), followed by IgM, IgG2a, IgG2b, andIgG3, present in 13, 12, 6, and5 mAbs, respec-tively. The13IgM immunoglobulins showed X1, K, KX1, and X1X2chains5, 3, 3,and 2times, respectively.The mAb titers
in culture supernatants and ascitic fluids ranged from 5 to 15,625andfrom
103
to 108, respectively.To assess the specificity of the mAbs and test whether they were reacting with ASF virus ornonviralcomponents present in the virus preparation, representative mAbs of each specificity were tested againsteither partially purified ASF virus (BA71-5), produced in swine macrophages, or
partiallyorhighly purifiedASFvirus(BA71-V), producedin Vero cells. All the mAbs reacted with both highly and partially purified ASF virus but not with the serologically unrelated influenza virusor MoLV(Table 3). Thestrongest binding was obtained with the mAbs specific for proteins
p220, p150, p72, p37,p24, p17, p14, and p12 from ASF virus producedinVero cells. The mAbsagainst proteins p80, p27, and p24 from ASF virus produced in swine macrophages showed littlebindingtothecorresponding proteins. By using
an improved binding assay, partially purified ASF virus produced in swine macrophages bound at least 10 times
more19E.H10than did the control mAb(B. Garcia-Barreno, A. Sanz, E. Vifluela, and L. Enjuanes, manuscriptin prep-aration).
Proteinsrecognized bythe mAbs. NinetymAbsbound, by immunoprecipitation or immunoadsorption, proteins which were identified by polyacrylamide electrophoresis.
Im-munoadsorption was more efficient than immunoprecipita-tion for protein selection, as several mAbs that could not precipitate the corresponding protein could bind enough material for determination oftheprotein specificity.
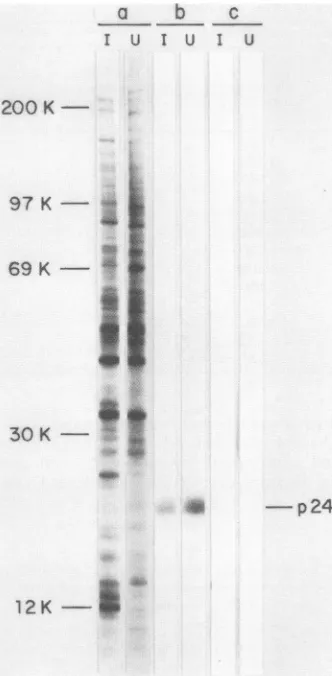
Figure2shows theproteinsselectedby some mAbs from
partially purified [35S]methionine-labeled ASF virus, disso-ciated withNP-40-urea-mercaptoethanol,and Table 2shows the mAbs thatrecognized eachprotein. Thelargestnumber of mAbs of knownspecificitywerethosespecificforprotein p72 (52 mAbs), followed by p150 (16 mAbs), p12 (8 mAbs), p14 (4 mAbs), and p27 (2 mAbs). Only one mAb was obtained for each of the other five proteins. Ingeneral, the majorviralproteins (p150, p72,andp12)gaverisetoastrong band afterelectrophoresis. Incontrast,the mAbsspecificfor proteins p14, p17, p37, and especially p80 produced weak bands. Protein p24, which will be shown later to be a cell protein, gave a broad diffuse band that suggested some
heterogeneity.
Figure3 shows the proteinsselectedbysome mAbs from ASF virus-infectedcellsorASF virusparticles,and Table 2 shows the mAbs whichrecognizedeach of theseproteins. A
setof three mAbs whichrecognized protein p220inextracts from infected cells did not recognize any protein in viral particles (Fig. 3b). Anothersetof 13 mAbsrecognized both proteins p220andp150in infected cells and protein p150 in virus particles (Fig. 3c). A third set of three mAbs recog-nizedprotein p150in virusparticles and recognizednone in virus-infected cells (Fig. 3d). Finally, mAb 17F.E10 recog-nized protein p60in extracts from ASF virus-infected cells andprotein p37in virusparticles. These results suggestthat thenonstructuralproteins p220andp60are precursorsof the structural proteins p150and p37, respectively, orthat they sharea similar epitope.
The mAb 19E.H10 recognized protein p24, which is presentinpurifiedASFvirusparticles (Table 3)aswellasin uninfected and ASF virus-infected Vero cells (Fig. 4). A similar protein recognized by the mAb 19E.H10 has been found in uninfected and ASF virus-infected swine
macro-phages (datanotshown).
Bindingof mAbstouninfected and ASFvirus-infectedVero cells.(i) Bindingassays. Table 4shows thebinding of mAbs
A
S
VOL.54, 1985
. *0
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.612.124.490.69.232.2]202 SANZ ET AL.
TABLE 2. Properties of mAbs
Titer
Specificity Isotype mAb Culture Ascites
p220 'ylK 19C.F4 5 104
JK
p220-plSO 'YIK Y2aK Y2bK p150 Y2aK J.KX1 17I.G8 19B.H9
14A.E6; 14B.E12; 17F.D8; 17F.H1; 17G.F9; 17H.C6;17I.A10; 17K.G3; 17K.Hll; 18E.B6; 18H.H7
17A.H2 14B.F1 17K.C6 17H.G5 17K.G6 125 105 25 104 125-15,625 104-107 625 105 625 104 3,125 625 3,125
S05
106 105 625 1041B.Cll; 7B.F7; 7E.D2; 17G.C7; 17G.E4; 17G.G7; 18A.Ell; 18F.A4 17F.E3; 17J.Ell; 17L.D3; 17L.E2;18B.G3; 19B.A2
17F.A7; 17K.C1;20F.E1O 19C.B4 18C. Fl1 18F.G8; 20F.C9 17F.C9 30hybridomas 125-15,625 625-15,625 3,125-15,625 25 3,125 125-3,125 625 NDa 3,125 103-107 106_108 106_107 105 107 106_107
l61o7
104 ND 104 25 10l 125 104 15,625 107 125 104 3,125 106 19C.F6 19D.G8 19B.C8; 19D.Gl 125 15,625 5-3,125 105 107 103_106 p12 Y2aK Y2bK Y3KLKA
1KFAKX1
17L.F2 17G.C4; 18B.Bll 2C.G12 20F.A4 17H.H3 19A.C1O; 19C.AllWY1Y Y2b 151 hybridomas 25-15,625 101-107
representative of each of the specificities shown in Table2to uninfected or ASF virus-infected Vero cells fixed with 1% formaldehyde. The mAbs specific for proteins p220, p72, p24, p14, p12, and an unidentified component bound to infected, fixed cellsto agreater extent thandid the control mAb,butonly the anti-p12 mAb and,toalesserextent,the anti-p72 mAb bound specificallytoASF-virusinfected cells. These results indicate that mAbs17K.G3, 19E.H10, 19D.G8, and 19B.G7, specific for proteins p220, p24, p14, and an
unidentifiedcomponent,respectively, reacted with cell com-ponents. They alsosuggest that proteins p24, p14, and p12 werepresentin the cell membrane of the infected cells(see below). Similar results were obtained with unfixed cells
(datanot shown).
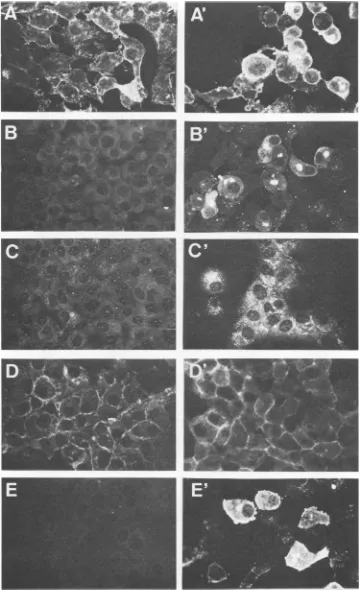
(ii) Imnmunofluorescence patterns. Figure 5 shows some
characteristic immunofluorescence patterns for uninfected
and ASF virus-infected Vero cells in thepresence ofsome mAbs. Four different fluorescence patterns were observed: (i) fluorescence limitedtodiscretecytoplasmicareas,which corresponded tothelocalization ofviralfactories(1, 7);this pattern was obtained with the p72-specific mAbs with the strongest intensity (Fig.SB and B'); (ii)fluorescence
cover-ing cytoplasmicareasmoreextensivethan inpatternianda
weak fluorescence in the nuclei of both uninfected and virus-infected cells; this pattern was characteristic of the
p27-specificmAbs(Fig. SC and C'); (iii) fluorescencelimited to the cytoplasmic membrane from both uninfected and virus-infected cells; this pattern was shown by the
p24-specific mAb(Fig. SDand D');and(iv) diffuse and intense cytoplasmic fluorescence covering all the cytoplasm and, probably, the cell membrane (Table 4); this pattern was characteristic of allthe p12-specificmAbs (Fig.SE andE').
p80 p72 19D.H8 Y1K 'YIK 'Y2aK Y2bK 'Y3K .LK .LKXI Unknown p60-p37 p27 'Y2aK 17F.E1O 18A.H5 19D.A8 p24 p22 p17 'Y2aK
yIK
Y3K p14 19E.H1O 18A.F3 17K.G12 yIK 'Y2aK Y3K Unknown
aND,Not done.
625 625-3,125 243 125 625 25-125 106 104_106 105 106 i05 103-104 J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
TABLE 3. Binding of mAbstoASF virus, influenza virus, and MoLV Binding (cpm per well)of:
Highly purified Partially purified ASF virus from:
Specificity mAb ASF virus from Influenza MoLV(1 Fg
Vero
cells
(05Veocelo(m.
Pigmacro- virus(1~tgVerocells(0.5 Verocells (2
)Lg
phages(2tig perwell) perwell)pLgperwell)
prwl)per
well)P220-p1S0 14B.E12 9,436 7,798 3,220 395 568
p220-p1S0 17F.D8 9,001 8,165 3,221 417 507
p80 19D.H8 2,845 3,448 703 394 515
p72 1B.C11 14,881 17,006 7,394 322 518
p37 17F.E10 7,218 6,540 2,658 387 588
p27 18A.H5 2,047 4,915 1,061 411 713
p24 19E.H10 10,445 10,650 1,200 288 993
p22 18A.F3 2,500 2,624 4,100 341 522
p17 17K.G12 8,395 15,500 2,600 333 546
p14 19D.G8 5,438 6,374 3,031 427 546
p12 17G.C4 5,057 9,800 3,500 480 740
p30 (MoLV) 6D.2 1,077 2,160 732 392 9,738
Control P3-X63/Ag8 956 1,277 782 394 770
Table5 summarizes the reactivity of representativemAbs ofknown specificity and that of mAb 19B.G7 of unknown specificity with uninfected and ASF virus-infected cells. Of the 12mAbs studied, 10 stained the infected cells. The p20-and p14-specific mAbs did not bind to either acetone- or
200K a b c
d
e
f
g h ij
4.
97 K
-69
K-30K
-S
k
-p150
mt
formaldehyde-fixed infected cells. The mAbs 19E.H1O (spe-cific for protein p24) and 19B.G7 (which reacted with an unidentified component) bound to the cell and nucleus membrane, respectively, of both uninfected and virus-in-fected cells.
a b c
d
ef
c v c v c v c v c v c v
200K -- -
p220
-p 150
-p80
97K-
P72_
-p37 69K-i
-p27
30K-1
- p24 *
me
- p60
-
p37
-
p22
12
K-N
Q-- p14
_m -p12
FIG. 2. Polyacrylamide gel electrophoresis of proteins from ASF virus particles recognized by mAbs. Partially purified ASF virus particles labeled with [35S]methionine were dissociated with
NP-40-urea-mercaptoethanol, andafterselectionwithmAbstheywere
subjected to7 to20% polyacrylamide electrophoresis in the pres-ence ofsodium dodecyl sulfate before(a) orafter immunoprecipi-tation withmAbs17F.D8(b), 19D.H8 (c), 17L.D3 (d), 17F.E10 (e), 18A.H5 (f), 19E.H10 (g), 18A.F3 (h), 19D.G8 (i), 18B.B11 (j), and culture medium from P3-X66/Ag8 myeloma cells (k). After elec-trophoresis, thegel wasdried andsubjectedtoautoradiography.
12K-FIG. 3. Polyacrylamide gel electrophoresis of proteinsfrom ASF virus particles and virus-infected cells selected by mAbs. ASF virus-infected cells (C) or virus particles (V) labeled with [35S]methionineweredissociated with NP-40-urea-mercaptoethan-ol and subjected to 7 to 20% polyacrylamide gel electrophoresis, before(a)orafterimmunoprecipitationwith the mAbs 19B.H9(b), 17L.A10 (c), 17K.C6 (d), 17F.E10 (e), and culture medium from P3-X66/Ag8 myeloma cells (f). Afterelectrophoresis, the gel was dried andsubjectedtoautoradiography.
am
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.612.60.300.343.625.2] [image:5.612.317.560.346.634.2]204 SANZ ET AL.
a b C
I u1 u I 1
200
K-97 K-
-69 K --- I
i..
I
30 K
K-* -p24
12K-FIG. 4. Polyacrylamide gel electrophoresis of proteinsfrom unin-fected (U) and ASF virus-infected (I) cells selected with mAb 19E.H10. Mock-infected and ASFvirus-infected cells labeledwith [35S]methioineweredissociated withNP-40-urea-mercaptoethanol
andsubjectedto7to20%polyacrylamide gel electrophoresis inthe presenceofsodiumdodecylsulfate, before (a)orafter
immunopre-cipitation with mAb 19E.H10 (b) or culture medium from P3-X66/Ag8 myeloma cells(c).Afterelectrophoresis,the gelwasdried
andsubjectedtoautoradiography. DISCUSSION
In thispaperwedescribe the isolation of 241 hybridomas
obtained after immunization of mice with either partially purified ASF virus or virus-infected porcine macrophages.
We obtained 90 hybridomas which produced mAbs that recognized specific proteins; 60 of these were stable. In
total, 12 proteins wererecognized by the mAbs: p220, p150, p80,p72, p60, p37, p27, p24, p22, p17, p14, and p12 (Fig. 2 and3).
Most of the mAbs produced by the stable hydridomas
were specific for three major structural proteins, p72 (22
mAbs), p150 (16 mAbs), and p12 (8 mAbs). All the mAbs studiedin thiswork,except thosespecific for proteins p24,
an unidentified component, and perhaps p14, seemed tobe ASFvirusspecific because they reacted with highly purified ASF virus but not with the serologically unrelated MoLV and influenza virus (Table 3). Furthermore, none of those mAbs reacted with componentfrom uninfected Verocells, exceptthose indicated above(Tables4and5).
The mAbsspecific for the structural protein p150 and the virus-induced, nonstructural proteinp220could beclassified
into three groups: (i) those which recognized only protein p220in infected cells (Fig. 3b); (ii) those which recognized proteins p220 and p150 in infected cells and protein p150 in virus particles (Fig. 3c); and (iii) those which recognized only protein p150 in virus particles (Fig. 3d). These results suggest that protein p220 is a precursor of the structural protein p150 and thatthe lattercontainsatleastoneepitope,
which would appear in the latest stepsofvirus maturation andthereforewouldnotbepresentin theprotein p150 in the infected cells.
The virus-induced nonstructural protein p60 is antigenic-ally related to the structural protein p37, since the mAb 17F.E10precipitated p60 from extracts from infected cells and precipitated p37 from virus particles. Although ASF virus-infected cells contain protein p37, as revealed by gel
electrophoresis ofextracts prepared with sodium dodecyl sulfate, we could not achieve the immunoprecipitation of that protein fromextracts prepared with NP-40-urea. Two possibilities that couldaccountforthat resultarethateither p37 isnot solubilized or thatp60 is cleavedto produce p37 onlyatthelateststepof virus maturation, when the virion is releasedfrom the cell.
Anexceptiontothe virusspecificity of the mAbs studied in thiswork is that of the mAb 19E.H10, specific for protein p24, which is a host cell protein as shown by the binding
assays (Table 4) and immunofluorescence studies (Fig. 5a;
Table5). Furthermore, 19E.H10immunoprecipitateda pro-tein with the same electrophoretic mobility from both
unin-fected and virus-inunin-fected cells. Protein p24 was present in the membranes of both uninfected and virus-infected cells (Fig. 5) andwasincorporated in the virusparticles, probably
during the budding process, through the cell membrane (1, 7), since highly purified ASF virus particlescanbe
neutral-ized about 100-fold by 19E.H10 (L. Enjuanes, B. Garcia-Barreno, A. Sanz, M. L. Nogal, and E. Vifiuelda, unpub-lished results). Thus, ASF virus incorporatesatleastahost
protein (p24) into the virion, like some other enveloped viruses (8, 13, 21). Whether ASF virus infections can be
limited byan immune responsedirected against the specific
host surface antigen, as in the case of vesicular stomatitis virus (8), isan open question which deserves further study.
The binding studies shown in Table 4 suggest that protein p14 is also a host cell membrane component or a viral
protein thatcross-reactswithahostprotein(Tables 4 and 5).
Also, from binding(Table 4) and immunofluorescence (Table 5) studies, an unidentified component, recognized by the mAb 19B.G7, seemstobelocalized in both thecell and the nucleus membrane from uninfected and virus-infected cells.
TABLE 4. Binding of mAbstoformaldehyde-fixedVerocells Binding(cpm perwell)in:
Specificity mAb Uninfected Infected
cells cells
p220-p150 17K.G3 2,495 2,975
p220-p1S0 17A.H2 1,334 1,465
p80 19D.H8 1,456 1,239
p72 19B.A2 1,879 3,452
p37 17F.E10 1,697 2,610
p27 19D.A8 2,184 1,944
p24 19E.H10 7,757 8,254
p22 18A.F3 1,691 1,841
p17 17K.G12 1,305 1,564
p14 19D.G8 6,399 6,835
p12 18D.B11 1,637 6,969
Unknown 19B.G7 6,261 6,908
Control P3-X63/Ag8 1,710 1,403
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.612.99.265.68.406.2] [image:6.612.319.559.561.715.2]FIG. 5. Immunofluorescencepatternsof selected mAbsreactingwithuninfected(A, B,C,D,E)andASFvirus-infected Vero cells(A', B',C', D', E'). A, A',Mouseanti-ASFvirus serum; B,B', mAb 19B.A2(p72);C, C',mAb 19D.A8 (p27);D, D',mAb 19E.H10(p24); E, E',mAb 18B.B11(p12).
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.612.126.489.84.674.2]206 SANZ ET AL.
TABLE 5. Reactivity of mAbs with cell components from uninfected and ASF-virusinfected Vero cells
Cellcompo- Binding of mAb specific for proteinform':
nent Infected cells Uninfectedcells
Cellmem- p24(19E.H10; ++) p24 (19E.H10; ++) brane p220-p1S0 (17K.G3; +) p220-p1S0(17K.G3; +)
p14(19D.G8)* p14(19D.G8)* Unknown (19B.G7)* Unknown (19B.G7)* p12(18B.B11)*
Cytoplasm p12 (18B.B11; ++) p220-p1S0(17A.H2; +) Virus fac- p72 (19B.A2; +++)
tory p220-p1S0(17A.H2; ++); (17K.G3; +)
p80(19D.H8; +) p60-p37(17F.E10; +) p17(17K.G2; +) Perinuclear p27 (19D.A8; + +)
cyto-plasm
Nucleus Unknown(19B.G7; +) Unknown (19B.G7; +)
mem-brane
Nucleus p220-p1S0(17A.H2; +)
p27(19D.A8; +) p27 (19D.A8; +)
aThedatawere obtained from fluorescenceassays(Fig. 5), except those
indicatedwith an asterisk, which were obtained from the bindingexperiments
shownin Table4. The numberof+ signs indicates the relativefluorescence
intensity.
ACKNOWLEDGMENTS
We are grateful to J. Palacin and J. G. Miguet for excellent technical assistance.
This investigation has beenaided by grantsfrom theComisi6n Asesoraparael DesarrollodelaInvestigaci6nCientificayTdcnica toIngenasa and the Fondo deInvestigaciones Sanitarias.
LITERATURECITED
1. Breese, S. S., and C. J. De Boer. 1966. Electron microscope observations of ASFV in tissue culture cells. Virology 28:420-428.
2. Carrascosa, A. L., J. F. Santarin, and E. Viduela. 1982. Pro-duction and titration of African swine fever virus in porcine alveolarmacrophages. J. Virol. Methods3:303-310.
3. Casal, I.,L.Enjuanes,and E.Vinuela. 1984.Porcineleucocyte cellular subsets sensitivetoAfrican swinefever virusinvitro.J. Virol. 52:37-46.
4. Chamberlin, J.P.1979.Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate.Anal.Biochem. 98:132-135.
5. DeBoer, C. J.,I. C. Pan, and W. R. Hess. 1972.Immunologyof Africanswinefever. J. Am. Vet. Med. Assoc. 160:528-532. 6. Enjuanes,L., A. L.Carrascosa, M. A. Moreno, and E. Vinuela.
1976. Titration of African swine fever virus. J. Gen. Virol. 32:471-477.
7. Haag, J., A. Lucas, B. Larenaudie, F. Ruiz Gonzalvo, and R. Carnero. 1966. Pesteporcine africaine. Recherches sur la taille etlamorphologie duvirus. Rec. Med. Vet. 142:801-808. 8. Hecht, T. T., and W. E. Paul. 1982. Limitation of VSV infection
by the host's response to VSV-associated cellularantigens.J. Immunol. 129:1736-1741.
9. Hess, W. R. 1981. African swine fever: areassessment. Adv. Vet.Sci. Comp. Med. 25:39-69.
10. Kearny, J. F., A. Radbruck, B. Liesegang, and K. Rajewsky. 1979.Anew mousemyeloma line whichhas lost immunoglobu-linexpression but permits the constructionofantibody secreting hybrid cell lines. J.Immunol. 123:1548-1550.
11. Kohler, G., and C. Milstein.1975.Continuous culturesoffused cellssecreting antibody of predefined specificity.Nature (Lon-don) 256:495-497.
12. Laemmli, U. K.1970.Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680-685.
13. Lodish, H. F., and M. Porter. 1980. Specific ihcorporation of host cellsurfaceproteins into budding vesicular stomatitis virus particles. Cell 19:161-169.
14. Malmquist, W. A.1963.Serologic and immunologic studies with ASFV.Am. J. Vet. Res. 24:450-459.
15. Malmquist, W. A., and D. Hay. 1960. Hemadsorption and cytophatic effectproduced by African swine fever virus in swine bone marrow and breffy coat cultures. Am. J. Vet. Res. 21:104-108.
16. Melero,J. A.,andJ.Gonzalez Rodriguez. 1984.Preparationof monoclonal antibodiesagainstglycoprotein Illa ofhuman plate-lets: their effect on platelet aggregation. Eur. J. Biochem. 141:421-427.
17. Nowinski, R.C., M. E. Lostrom, M. R. Tam, M. R. Stone, and W. N.Burnette.1979. Theisolation ofhybrid cell lines produc-ing monoclonal antibodies against the p15 (E) protein of murine leukemia viruses. Virology 93:111-126.
18. Ouchterlony,O., and L.A.Nilsson. 1978.Immunodiffusion and immunoelectrophoresis, p. 19.1-19.44. In D.M. Weir (ed.), Handbook of experimental immunology. Blackwell Scientific Publications, Ltd., Oxford.
19. Stahli, C.,T.Staehelin, V. Miggiano, J.Schmidt,and P.Hazing. 1980.High frequencies of antigenspecifichybridomas:
depend-enceonimmunizationparametersandprediction of spleen cell analysis. J. Immunol. Methods 32:297-304.
20. Tabares, E., F. R.Gonzalvo, M. A. Marcotegui, and A. Ordas. 1977. Growth, purificationand fractionation ofAfrican swine fever virus. Comm. Eur. Communities Eur. Rep. 5904EN:507-531.
21. Wagner, R. R. 1975.Reproduction ofrhabdoviruses,p. 1-93. In H. Fraenkel-Conrat and R. R. Wagner (ed.), Comprehensive virology, vol.4. Plenum Publishing Corp.,New York. 22. Wardley, R.C., C. de M.Andrade,D. H.Black, F. L. de Castro
Portugal, L. Enjuanes, W. R. Hess, C. Mebus, A. Ordais, D. Rutili, J.Sanchez-Vizcaino,J. D.Vigario,P.J.Wilkinson, J.F. Moura-Nunes,andG. Thomson. 1983.African swine fevervirus. Arch.Virol. 76:73-90.
J. VIROL.