Copyright01974 AmericanSociety for Microbiology Printed in U.S.A.
Encephalomyocarditis Virus Infection of Mouse Plasmacytoma
Cells
I.
Inhibition
of
Cellular
Protein
Synthesis
CHARLES LAWRENCE ANDROBERT E. THACH
DepartmentofBiological Chemistry, Division of Biology and Biomedical Sciences, Washington University, SaintLouis, Missouri 63110
Received forpublication29April 1974
Mouse plasmacytoma
ascitestumorcells (MOPC
460)wereefficiently
infectedwith
encephalomyocarditis
virus.Inhibition
ofhostprotein synthesis
wasevidentafter 2 h and complete by 4 h
postinfection.
The mechanism by which virus infection results in inhibitionof host
cell
proteinsynthesis
wasstudied in vitro.Cell-free
protein-synthesizing
systems,prepared
fromuninfected and infected
cells, were found tobe equally active with respect totheirabilities
totranslate cellular and viral mRNAs. Theplasmacytoma cell-free
systemwasalso
showntobe insensitivetotheadditionof
double-stranded
viral RNA. Hostcellular
mRNAwas isolated from uninfected and
infected
cells. No difference in theamountorsizedistribution of the mRNAwas
detected. However, the
mRNA from infectedcells was translated only 46 to 49% as actively as that from
uninfected
cells.mRNA isolated from cells in which initiation of
protein
synthesiswasinhibitedwith
pactamycin
wassimilarlyinactivated. Simultaneous addition
ofviral RNAand cellular mRNA to the
plasmacytoma
cell-free
systemresulted
in acomplete suppression
of the translation of thecellular
message,whereas viral
RNA wastranslated normally.
The infection
ofanimal cells
with
picor-naviruses
results
inaninhibition of host protein
and RNA
synthesis (2). We have investigated
the
virus-induced inhibition of cellular
proteinsynthesis
inencephalomyocarditis (EMC)
vi-rus-infected plasmacytoma
ascitestumor
cells.
To
study
this
problem
invitro,
wehave isolated
acellular
mRNA from
plasmacytoma
tumorsand
analyzed
the
ability
of
acell-free system
made from
infected cells
totranslate this
mes-sage.
We have also
isolated this mRNA from
infected
cells
and studied
itstranslation
innormal cell
extracts.Finally,
wehave
at-tempted
to reconstruct invitro
the conditions
found inthe infectedcytoplasm by
simultane-ously translating viral and cellular mRNAs.
MATERIALS AND METHODS
Plasmacytoma cell line. An MOPC 460 ascites tumor(obtainedfromR.Graff)wasseriallypassaged by intraperitoneal injectionsof 0.2ml of ascitesfluid at10-day intervals withBALB/cmice(alsoobtained from R. Graff). One mouse produced 3 to 5 ml of asciticfluid containingapproximately2 x
10'
to3 x10'
tumorcells.EMCvirus.EMCvirusstocksweregrowninKrebs II ascitescells (initialcells andvirusweregenerously providedto usbyA.T. H.Burness).Theprocedures
forgrowth(25) and
purification
(4)ofEMCvirushavebeenextensively describedbyotherinvestigators and have beenadoptedinthislaboratory.
The virus stock used in these experiments was obtainedbylow-multiplicitypassage inKrebs ascites cells and had a titer of 5.1 x 105 hemagglutination units(HAU) perml.
Infection of MOPC 460 ascites cells with EMC virus. Asciticfluid, 9to14daysaftertheinjectionof thetumorcells,wasdiluted with10volumes ofsterile ice-cold phosphate-buffered saline (PBS) without calcium ormagnesium (Grand IslandBiological Co., GrandIsland, N.Y.),and thetumorcellswerewashed by repeated centrifugation at 200 x guntil free of contaminating erythrocytes. The cells were
resus-pendedinLeibowitz L-15medium
(Schwartz/Mann)
to a concentration of4 x 106 or 5 x 106cells per ml. Infections wereinitiatedby addinganequalvolumeof 60% L-15 medium (0.6 volume L-15, 0.4 volume of water)containing the indicatedamountofvirusstock togiveafinal concentration of80%L-15medium and 2 x106 or2.5x 106cellsperml. Theviruswasallowed to attach at room temperature for 0.5 h in an Erlenmeyer flask withacapacity of5 to 10timesthat of thevolume of the culture. Afterviral attachment, the flaskwasplacedin a 37Cwaterbath and the cells were kept in suspension by gentle swirling. "Hours postinfection" usedinthetextreferstothe time after theshift-upto37C. In the indicatedexperiments, the unattached viruswasremoved at 1.5hpostinfection by centrifugationofthecells and resuspensioninfresh 100%L-15 mediumtogive theoriginal cell concentra-598on November 10, 2019 by guest
http://jvi.asm.org/
ENCEPHALOMYOCARDITIS VIRUS INFECTION. I. tion.All culturescontained50U ofpenicillinperml,
50
Ag
ofstreptomycin perml, and 2% heat-inactivated horse serum (Grand Island Biological Co., Grand Island, N.Y.). In all experiments, an uninfected control was used and treatedby the same procedures as the infected culture, except for the addition of virus.Inpreliminary experiments,it wasdetermined that cells were maximally infected with 0.5 x 10-2HAU/ cell. At this andhigher virus inputs, 15 to 20% of the cellswereresistant toinfection asdeterminedbythe absence of cytopathic effect. In these experiments, a 4- to 20-fold excess of infecting virus was used to initiate infection.
Hemagglutination. EMC virus can agglutinate erythrocytes and the amount of hemagglutinating activity isproportional tothe concentration of infec-tious virus (25). Portions ofa culture to be assayed were withdrawnand frozen at-20C, and the hemag-glutinating activity of a 50-uliter sample was deter-mined as described by Martin et al. (25). Human erythrocytes were used for the assay, as they gave higher and more reproducible titers than commer-cially prepared sheeperythrocytes.
Rate of protein synthesis in uninfected and infected cells. Cellswere infectedat aconcentration of 2.5 x 106 cells per ml and 2.55 x 10-2 HAU/cell. Unattached viruswasremovedat1.5hpostinfection. At various times,0.2-ml portions werewithdrawn and washed two timesbycentrifugation inice-coldPBS. The cells were resuspended in 0.5 ml of Earle bal-anced salt solution (Grand Island Biological Co., GrandIsland, N.Y.) containing0.1MCiofamixture of 15 '4C-labeledL-aminoacids(NewEnglandNuclear, Boston, Mass.) and incubated for 10 min at 37C. Ice-cold PBS was added (3 ml), and the cells were immediately filtered through a glass-fiber filter (Whatman GF/C) and washed with 5% trichloroace-ticacid, followed by washes of95%ethanol andethyl ether. The filters were dried, and radioactivity was determined intoluene scintillationfluidin aPackard Tri-Carbliquid scintillationcounter(Packard Instru-mentCo., Inc., DownersGrove, Ill.).
Synthesis of viral protein duringinfection. For polyacrylamide gel electrophoretic analysis of the proteins synthesized during infection, cells were in-fectedataconcentration of 2.5 x 106cells per ml and 2.04x 10-2HAU/cell.Unattached viruswasremoved at 1.5 h postinfection. At various times 0.2-ml por-tionswereremoved andwashed two timesby centrifu-gation inPBS. The cells were suspended in 0.5 ml of Earle balanced salt solution containing 5
ACi
of[35S]methionine (170 Ci/mmol, Amersham/Searle) and incubated for 5 min at37C. Cellswere precipi-tatedby the addition of 0.5 ml of10%trichloroacetic acid. Theprecipitate was centrifuged at 860 x g and washedtwotimeswith acetone. Residualacetone was removed by warming the precipitate at 37 C. The dried precipitatewasdissolvedin50ylitersofsample buffer for electrophoresis and analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electropho-resis, asdescribed below.
Purification of a cellular mRNA. Solid MOPC 460 tumors grown inBALB/c mice (generously pro-vided for us by E. Simms) were collected in 2.5
volumes of ice-cold buffer containing 0.05 M Tris-hydrochloride (pH 7.4), 0.025 MKCl, 0.005 M MgCl2, 0.007 M f,-mercaptoethanol, 0.8 M sucrose, and 0.5 mg of heparin per ml (SigmaChemical Co., St. Louis, Mo.). The tumors were dispersed in a Waringblender at low speed for 1 min and at highspeed for 0.5 min at 4C. The dispersed tumor was homogenized by five strokes in a motor-driven Teflon-glass homogenizer, and the homogenate wascentrifuged at 15,000 x g for 20 min. The supernatant was mixed with an equal volume of 0.2 M Tris-hydrochloride (pH 9.0), 0.2M NaCl, 0.01 M EDTA, and2% SDS, and this mixture was extracted twice at room temperature with an equal volume ofwater-saturated phenol-chloroform-isoamyl alcohol (50:50: 1). RNA was precipitated fromthe aqueous phasebythe addition of 0.1 volume of 20%potassiumacetate(pH 5.5) and 2.5 volumes of 95% ethanol, and stored at -20C. A polyadenylic acid [poly(A) ]-containing fraction was obtained by chromatography on oligodeoxythymidylic acid (oli-go-dT)cellulose as describedbySwanetal.(39). The proportion ofthe total RNA which boundtocellulose was about 1.1%. The poly(A)-containing fraction elutedatlow saltwasprecipitatedby the addition of 0.1 volume of 4 M NaCl and 2.5 volumes of 95% ethanol, collected bycentrifugation, and dissolved in 1%SDS.Approximately 540absorbancyunitsat260 nm(A260)of RNA werelayeredonfive17-ml5 to30% (wt/wt) sucrose gradients containing 0.01 M Tris-hydrochloride (pH 7.5), 0.1 M NaCl,0.001 MEDTA, and1%SDS.Centrifugation,at20C,wasfor 18.5hat 26,000 rpm ina Spinco SW27rotor. The 10Sregion was pooled, precipitated with ethanol, and recen-trifuged on two SDS-sucrose gradients as described above, except thatcentrifugation wasfor 21 h. The indicated fractions were pooled and the RNA was precipitated as above, dissolved in water, and re-precipitatedwithethanolto removeanyresidualSDS. The RNAwasfinallydissolved in sterilewaterfor use in cell-freeproteinsynthesisand storedat -20C.
EMC viralRNA, rabbitglobinmRNA, and EMC replicative form (RF). EMC viral RNA was prepared from virus grown inKrebs II ascitestumor cellsbythemethod of Boime and Aviv (4). The RNA extracted from purifiedviruswaspurified furtherby SDS-sucrose gradient sedimentation, as described above for the preparation of cellular mRNA except that centrifugation was performedfor 12h. The 35S RNA species was pooled and prepared for use in cell-freeproteinsynthesis, as described above.
The rabbit globin mRNA used was the poly(A)-containing fraction of RNA fromrabbitreticulocytes (prepared byP.Yau).
EMC RF(prepared byD. Dobbertin)waspurified fromKreb's cells infected for5h withEMC virus by a method described previously (S. S. Thach, D. Dob-bertin, C. Lawrence,F.Golini, and R. E. Thach, Proc. Nat. Acad. Sci.U.S.A., inpress).
Preparation of cytoplasmic extracts for cell-free protein synthesis. Uninfected or infected plas-macytoma cells were washed two times by centrifuga-tion in anice-cold solutionof0.035 M Tris-hydrochlo-ride (pH 7.5) and 0.14 M NaCl. The cells were resuspendedin 2 volumes ofDouncebuffer (0.01M Tris-hydrochloride, [pH 7.5],0.01MKCl,and0.0015
VOL.14, 1974 599
on November 10, 2019 by guest
http://jvi.asm.org/
M magnesium acetate) and homogenized with 25 strokes in a tight-fitting Dounce homogenizer. The homogenate was adjusted to 0.03 M Tris-hydrochlo-ride (pH 7.5), 0.12 M KCl, 0.005 M magnesium acetate, and 0.007 M,-mercaptoethanolbythe addi-tionof 0.1 volume of a concentratedsolution, and then centrifugedat30,000 x gfor 10 min. The supernatant wasapportioned and stored in liquidN2.
Preincubated Krebs ascites cell cytoplasmic ex-tract was prepared by the procedure of Boime and Aviv (4).
Cell-free proteinsynthesis.Reaction mixtures for cell-free protein synthesis contained 0.03 M Tris-hydrochloride (pH 7.5), KCl as indicated below, 0.0035 M magnesium acetate, 0.007 M ,B-mercapto-ethanol,10-3MATP, 10-4MGTP,6x 10-4M CTP, 0.01M creatinephosphate,0.16 mg ofcreatinekinase perml (Boehringer-Mannheim), 19aminoacids (me-thionine omitted; 4 x 10-5 M each), 3 1Ci of [35S]methionine (195 Ci/mmol, Amersham/Searle), mRNA as indicated, and 0.5 A260 of cytoplasmic
extractinafinalvolume of50
lAiters.
Translation of cellular and globin mRNAwas performedat85 mM KCl and viral RNAwastranslatedat115 mM KCl. When both cellular and viral messageswere present simultaneously, the reaction mixture contained 104 mMKCI.Efficienttranslation of each message alone occurs at this intermediate concentration. Incuba-tions were performedat 30C for 2.5 hduringwhich incorporation intomessenger-dependentpolypeptides islinear in the plasmacytoma cell-free system. Addi-tion of tRNAwas notrequiredforactivity of either the plasmacytomaorKrebs cell-free systems.Analysis of the cell-free reaction products by SDS-polyacrylamide gel electrophoresis. SDS-polyacrylamide gel electrophoresis wasperformed by themethod of Laemmli (22). Proteinsynthesis reac-tionswerestopped bythe addition of 100glitersof 1% SDS. Proteinwasprecipitated bytheaddition of2ml ofacetone and collected by centrifugation. The ace-tone wasremovedby aspirationandthe dried
precipi-tate was dissolved in 40
lAiters
of sample buffer containing 5% 2-mercaptoethanol and heated for 2 min at 90 to 95C.Twenty-five-iAliter
samples were layered on a slab gel consisting ofa4% acrylamide stacking gel and a 7.5 to 20% acrylamide linear gradient separating gel (5). Electrophoresis was per-formedat100Vfor6h.Thegelwasfixed inasolution of 25% ethanol plus 10% aceticacid followedby
7% aceticacid,and the fixedgelwasdried undervacuum ontoWhatmanno. 1chromatographypaper. Autora-diographywasperformedwith KodakRP/R54
X-ray film for 1 to3 days. Radioactivity invarious bands wasdetermined by cuttingoutthe desired regionof thegelandincubatingitfor2hat70C with1.0ml of 30%NH4OHplus15%H202. The elutedradioactivitywasquantitatedin aPackardTri-Carbliquid scintil-lationcounterusingBrays scintillation solution. Ra-dioactivitywasdetermined byscanningtheexposed X-ray film in a Joyce-Loebl double-beam scanning microdensitometer(seeFig. 10).Thenetdensityina
messenger-dependent band was determined by sub-tracting thedensityoftheappropriate minus-messen-ger control. The data presented in Fig. 10 are the relative densities calculated by dividing the net
density by the maximal density in the experiment. Isolation of mRNA from untreated, infected, or pactamycin-treated cells. Untreated, infected, or pactamycin-treated cells were collected by centrifuga-tion and washed with ice-cold PBS. The cells were suspendedin 2volumes of Dounce buffer and homoge-nized with25strokes of a tight-fitting Dounce homog-enizer. The homogenate was adjusted to 0.03 M Tris-hydrochloride (pH 7.5), 0.12 MKCl, and 0.005 M magnesium acetate and was centrifuged at 15,000 x g for 10 min. Apoly(A)-containing RNA fraction was obtained by SDS-phenol extraction and oligo-dT cellulose chromatography, as described above for the purification ofcellular mRNA. This RNA was layered on a4.2-ml5 to 30%(wt/wt)sucrosegradient contain-ing 0.01 M Tris-hydrochloride (pH 7.5), 0.1 M KCl, and 0.001 M EDTA. Centrifugation was performed at 55,000 rpm for4h at 2C inaSpinco SW56 rotor.
RESULTS
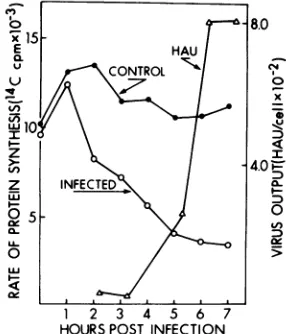
Effect of
infection
oncellular protein
syn-thesis
and demonstration of viral protein
synthesis.
The rateof
protein
synthesis during
infection
ofMOPC
460plasmacytoma
cells byEMC virus
wasestimated by pulse-labeling
portions
ofuninfected and
infected cultureswith
a mixture of 15"4C-labeled
L-aminoacids,and
by determining the
incorporation of labelinto
acid-precipitable radioactivity.
A decline inthe
rate of proteinsynthesis began
between 1 and 2 h postinfection, and this inhibition con-tinued until about 5 h postinfection (Fig. 1). After 5 h of infection, no further inhibition occurred and aresidual
level ofproteinsynthe-o
~~~~~~~~~~8.0
7
15
-E HAU
HOURSPCONTROL
) ~~~~~0
wi10
z
Z
[image:3.493.292.435.423.590.2]INFECTED-0
FIG.1.ate of protein synthesis after )
0 >:
uJ
HOURS POST INFECTION
FIG. 1. Rate of protein synthesis after infection and time courseof virusproduction. Portionsofan
uninfectedandaninfectedculturewerepulse-labeled with 14C-labeled L-amino acidsatvarious timesafter infectionandassayed for incorporatedradioactivity. Unattached viruswasremovedat1.5h
postinfection,
and portions of the infected culture were taken atvarious timesfordeterminationofhemagglutination activity.
on November 10, 2019 by guest
http://jvi.asm.org/
ENCEPHALOMYOCARDITISVIRUS INFECTION. I.
sis
remained.
Some
of
this residual
proteinsynthesis
probably
occurred in thepopulation
ofcells which remained
resistant toinfection.
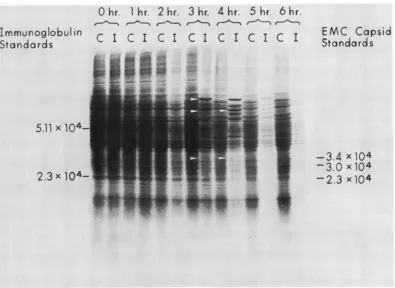
To
further analyze the
proteinssynthesized
during
infection,cells
werepulse-labeled
with
[35SImethionine
atvarious times
postinfection,
and
the total cellular protein
wasanalyzed by
polyacrylamide gel electrophoresis. As
isevi-dent
inFig.
2,the
viral
inhibition of
protein
synthesis
wasdue
to acoordinate inhibition in
the synthesis of all the
cellular proteins. At
3and
particularly 4 hpostinfection,
alarge
amountof
the
label
wasincorporated
into
apopulation of
polypeptides
notpreviously
pres-ent.
Three
ofthese
newpolypeptides
(indicated
by
arrows)have
molecular
weights
ofapproxi-mately
105, 8.2 x 104,and
3.7 x 104,which
correspond closely
tothe
molecular
weights ofthe three primary translation products
ofthe
viral
messageobserved
by Butterworth
et al.(6)
in
HeLa
cells infected with
EMC. Much
ofthe
protein
synthesis observed
at 3and
4h
postin-fection
(Fig. 2),
therefore,
appeared
tobe virus
specified,
and the inhibition of cellular protein
synthesis
wasactually
complete by
4h
postin-fection.
Viral RNA
synthesis
during
infection.
Viral
RNAsynthesis
ininfected cells
wasmo-nitered by measuring the
actinomycin
D-resist-ant
accumulation of
[3H]uridine
intoacid-precipitable radioactivity. Viral RNA
wasmax-imally
synthesized between
3and
4.5h
postin-fection,
but
newly synthesized
viral RNA could
be
detected
asearly
as 2h
postinfection.
Syn-thesis
ofviral
double-stranded RNA
(dsRNA)
was
barely
detectable
atthat
time(unpublished
data).
Isolation
of
acellular
mRNA. To
quanti-tatively
study the
ability
of
acell-free
protein-synthesizing
system totranslate cellular
mRNA,
a
relatively
pure messenger was necessary.We
initially attempted
topurify
the mRNA for
im-munoglobulin light chain from
MOPC
460
cells;
however,
itbecame
apparentthat another
cellu-lar mRNA
could be
moreeasily obtained
inhigher
purityby
a verysimple purification
scheme.
Cytoplasmic
RNA
wasOhr.
1
hr. 2hr. 3hr. 4 hr.
5
hr. 6hr.
_ r-H1 rt
Immunoglobulin
c
I
C I C I
C I
c I C I C I
Standards
prepared by
SDS-EMC
Capsid
Standards
5.11
x104-_*
-3.4
x104
2
,33
x104-
33.0x 1044
3.0.
x104
FIG. 2. Polyacrylamidegelelectrophoresis of proteins synthesized during infection. Portions of control (C) and infected (I) cultures were pulse-labeled with [35S]methionine at theindicated times after infection and prepared for electrophoresis. The figurepresented is an autoradiograph of the gel described. Unlabeled marker proteinswerealsoruninthegel, and the position of their migration and molecular weight are indicated (right sideof gel: EMC capsid polypeptides; left side of gel: purified myeloma protein from MOPC 460 tumors). The arrowsindicatepresumptive viral polypeptides synthesized in infected cells.
601
VOL.14, 1974I,
I
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.493.44.439.331.619.2]phenol extraction
ofapost-nuclear
supernatantfrom
solid MOPC
460 tumors,and
apoly(A)-containing fraction
wasobtained
by oligo-dT
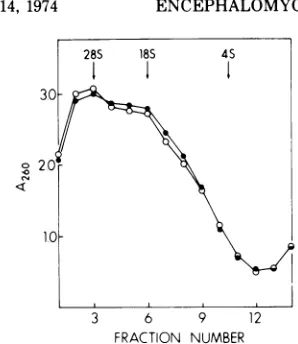
cellulose chromatography. This fraction
wassedimented
in anSDS-sucrose
gradient (Fig.3A). Various fractions from this gradient
weretested
in acell-free protein-synthesizing
sys-tem,
and
the products
wereanalyzed by
SDS-polyacrylamide gel electrophoresis. Whereas
most
fractions
stimulated incorporation
ofra-dioactive
amino acids into a number ofpolypep-tides,
RNA
fromthe
10S
region of
the gradient
stimulated
incorporationinto only
onemajor
polypeptide.
This RNA
waspurified further
by
sedimentation
in anadditional
SDS-sucrose
gradient (Fig. 3B). The translation
of thismRNA
fraction in acell-free
systemfrom
MOPC
460ascites
cells
isdemonstrated
inFig.
4b. The
polypeptide
synthesized
has
amolecu-lar weight
ofapproximately
20,000,and
co-elec-trophoreses with
aprotein
found in the
plas-macytoma
cells. The
apparentidentity of this
cellular protein with the
polypeptide
synthe-sized
inresponse tothe
10S mRNA
in vitro wasindicated
by two-dimensional mapping
ofthe
methionine-containing
trypticpeptides
(data
not
shown). We have also demonstrated that
synthesis
ofthis protein
invivo is sensitive
tocycloheximide
and
insensitive tochloram-phenicol, confirming that synthesis
ofthis
pro-tein occurs on
cytoplasmic ribosomes. Thus, the
10S
mRNA isolated
fromMOPC
460 tumorsappears to
be
avalid
representativecellular
mRNA
and
is anappropriate standard for
studies
onthe
ability
of
aprotein-synthesizing
system from
infected cells
totranslate cellular
messenger.Activity of
acrude
cell-free
protein-syn-thesizing
systemfrom infected cells. The
abil-ity of
the
protein-synthesizing machinery from
infected cells
totranslate cellular mRNA
wasanalyzed
by studying the cell-free translation of
cellular 10S mRNA
incrude
cytoplasmic
ex-tracts.
The
extracts wereprepared by
homoge-nization of
uninfected
orinfected cells
inhypo-tonic buffer followed
by centrifugation
at30,000xg.
Preincubation and
dialysis
orchromatogra-phy
onSephadex
G-25
wasavoided
toeliminate
thepossibility
ofinactivating
orremoving
aninhibitor which
might
beinvolved
inshut-off
ofprotein
synthesis.
Cellular
10SmRNA,
rabbit
globin mRNA,
andEMC
viral
RNAwereincubatedwith crude
extracts of
uninfected
andinfected
cells in the presence of[35S]methionine,
and the
reactionproducts
wereanalyzed
by
SDS-polyacrylamide
gel
electrophoresis.
Anautoradiograph
ofthis
experiment
isshown
inFig.
4. Noqualitative
difference
couldbe observed
inthe translation
A B -30
28S 18S 4S
1002
10
<~~~~~~~~~~2
5.50 -10
10 20 10 20
[image:5.493.268.463.61.169.2]FRACTION NUMBER
FIG. 3. Purification of cellular 10S mRNA. (A) Poly(A)-containing RNA fraction isolated from MOPC 460 tumors wassedimented inaSDS-sucrose gradient. The position of rRNAspeciesin aparallel gradient is indicated. The 10S region (indicated by bracket inA)waspooledandrecentrifugedinanother SDS-sucrosegradient which is shown in B. The 10S region (indicated by bracket inB) from this second gradient waspooledandprocessed foruseincell-free proteinsynthesis.
of
10S mRNA and
globin mRNA in uninfected
or
infected
extracts.In
uninfected
extracts,viral
RNA
stimulated incorporation into
alarge
polypeptide with
amolecular weight of about
110,000
and
anumber
ofsmaller
polypeptides
(Fig.
4d). The
products
aresimilar
tothe EMC
RNA-dependent cell-free products reported by
other investigators (5, 20),
and
areidentical
tothe products obtained
by
translation
ofthe viral
RNA
inKrebs cell
extracts(unpublished data).
In
infected
extracts,however, viral RNA
stimu-lated incorporation into
adifferent
setof
poly-peptides
(Fig. 4h).
The
largest
ofthese has
amolecular
weight
ofabout
100,000and
co-elec-trophoreses with the EMC
capsid
precursorfound in
vivo ininfected cells
(unpublished
data).
Preliminary
evidence
suggested
that
this
new setofpolypeptides
wasproduced
asaresult
of a
post-translational
endoproteolytic activity
present
in
extractsof
infected cells.
The
messenger-dependent
polypeptides
in
Fig.
4 wereanalyzed quantitatively
by cutting
them
outofthe
gel,
solubilizing
the
protein,
and
measuring the
radioactivity
inthe bands
di-rectly.
The results
(Table
1) show
nosignificant
quantitative difference
inthe translation
ofthese
messengers inuninfected
orinfected
ex-tracts.
The
response of uninfectedand infected
ex-tracts tovarying
concentrations of10S
mRNA isdemonstrated
inFig.
5.Again
there is noapparent restriction of the translation of cellu-lar
mRNA
in infected extracts.Lack
of
sensitivity
ofplasmacytoma
cell-free protein
synthesis
toEMC
RF.Since
dsRNA
hasbeen
implicated
in theviral-induced
shut-off
of proteinsynthesis,
weat-tempted
to demonstrate an effect ofEMC RF
on in vitro
protein
synthesis
intheMOPC
460on November 10, 2019 by guest
http://jvi.asm.org/
ENCEPHALOMYOCARDITIS VIRUS INFECTION. I.
Molecu
lar
Weight:
m
11.0
x104
10.0
X104
_i.
-
2.0
x104
41
-
1.55
x
104
ab
c
d
e
f
g
h
FIG. 4. Translation of various mRNAs inuninfectedandinfected cell-freesystems.MOPC460ascites cells wereinfectedwithEMC virus (5 x 10-2 HAU/cell)orincubated withoutvirusfor4h.The cellswereharvested andcytoplasmicextractswereprepared.mRNAs (asindicated) wereincubated inreactions containing either uninfected or infected cytoplasmic extract and [35S]methionine. The reaction products were analyzed by SDS-polyacrylamide gel electrophoresis and autoradiography. (a-d) Uninfected extracts; (e-h) infected
extracts.Additions:(aande)noRNAadded; (b andf)0.037A26o 10SmRNAadded; (c andg) 0.052A260rabbit globin mRNA added; (dandh) 0.05
A260
,EMC
RNAadded.cell-free
system.Figure
6comparesthe
responseof
cell-free
systems fromMOPC
460cells and
from
Krebs
IIascites
cells
toEMC RF. The
translation
ofcellular mRNA
inthe
plas-macytoma system is not
significantly
affectedby EMC RF,
whereastranslation
in theKrebs
system can be inhibited up to
75%.
Theinsensi-tivity
ofthe
plasmacytoma
system toEMC
RFis
apparently
not due to degradation ofthe
dsRNA
sinceincubation
ofradioactive
EMC
RFwith
plasmacytoma
extracts does notrender
theradioactivity
acid soluble(unpublished data),
ashas
been
reported
forKrebs cell
extracts(34).The
plasmacytoma
cell-free system is alsoin-sensitive to
polyinosinic acid: polycytodylic
acid
inconcentrations
ranging
from10-'
to 100,ug/ml
(unpublished
data).
Activity
of cellular mRNA from infected
cells.
Poly(A)-containing
RNA was isolatedfrom
uninfected
and infected cells after 4 h ofincubation.
The yield ofpoly(A)-containing
RNA was
1.8%Y and 2.0%
ofthe
total RNA from uninfected and infectedcells,
respectively.
The RNA was analyzed by sucrose gradient sedi-mentation, and Fig. 7 demonstrates that there was noapparent difference in the size distribu-tion of the RNA isolated fromuninfected
or infectedcells.
This is consistent with a recent603
VOL. 14, 197446%0. ..;.. .4imp,
40
4M&
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.493.97.383.75.444.2]TABLE 1. Translation ofcellular10S mRNA and EMC RNA in cell-free systems from uninfected
and infected cells
35S counts/min eluted fromgela mRNA Regionofgeleluted
(mol wt) Unin- Infected fected cells
cellsb
-mRNA 20,000 762 446
+ 10SmRNA 20,000 4,626 3,826
-mRNA 110,000 867
+EMCRNA 110,000 7,695
-mRNA 100,000(doublet) 1,303 +EMC RNA 100,000(doublet) 16,007 -mRNA Totalgel 25,983 19,888
+EMC RNA Totalgel 85,065 77,159
aThe indicated regions of thegels shown in Fig. 4
were cut out and the radioactivity was eluted and countedasdescribed in Materials and Methods.
Where cell-free extractwasfrom.
6000
0
4000-0
¢ 2000
,4,
.02 .04 .06
A260UNITS
RNA ADDEDFIG. 5. Translationofcellular10S mRNA in
unin-fected and infected cell-free systems. Cellular 10S mRNAwasincubated withcytoplasmicextractsfrom uninfectedorinfectedcells and[35S]methionine. The reaction productswereanalyzed by SDS-polyacryla-mide gel electrophoresis and autoradiography. The 20,000-molecular weight region of each gel was cut out,andtheradioactivitywaseluted and counted(see Fig. 4). Symbols: radioactivity stimulated by 10S mRNA (circles), or rRNA (squares), in uninfected
extract (solid symbols) or infected extract (open symbols).
study
of thepoly(A)-containing
RNA isolatedfrom
mengovirus-infected
L cells (8).The
1OS
region of thegradient
wasassayed
in acell-freeprotein-synthesizing
systemtodeter-mine
the activity
ofthe cellularlOS
mRNA. Asshownin
Fig. 8A,
the10S
message from infectedcells
was57%
asactive asthat fromuninfectedcells.
Since
15 to 20% ofthe cells
in ourinfected
cultures
wereresistant toinfection, themessen-ger
from
the population that
wasinfected
mayhave been only
46 to 49% asactive as that fromuninfected cells. The loss of
messenger activitywas not
restricted
tothe lOS
mRNA
sinceall
messenger activity inthe poly(A)-containing
fraction from
infected
cells
wassimilarly
re-duced (data
notshown).
The decrease
of messengeractivity
described
above
mayhave accounted, in
part, forthe
observed decline
inthe
rateofproteinsynthesis
in
infected cells.
However, adistinction
mustbe
made
as towhether
messenger inactivationoccurred directly
as a resultof a specificviral-induced
function, or whether it occurredsecond-arily
as aresult
of aninhibition
ofhost
mRNAtranslation.
Inthe latter
case,the untranslated
host mRNA would be subjected
to normalcellular
inactivationand
degradation
processes,thus accounting for the
observed decrease
inspecific
translation
activity.To
distinguish between these
twopossibili-ties,
weisolated poly(A)-containing
RNA fromcells incubated
in the presence of 10-7 Mpactamycin.
Proteinsynthesis
under
thesecon-ditions
wasinhibited
80%.Since low
concentra-tions
ofthis drug inhibit initiation
of protein800(
0-LI)
600(
400(
200(
30,000
20,000
0
to
10,000
2x10-72x1O-62x1f2WX142x10-3
A260UNITSEMC RF ADDED
FIG. 6. Sensitivity of plasmacytoma and Krebs cell-free protein synthesis to EMC RF (dsRNA). Varying amountsof EMC RF and 0.034A26, of IOS mRNA were added to reactions containing either Krebs or uninfected plasmacytoma cellcytoplasmic
extractsand [35S]methionine. Thereactionproducts
wereanalyzed by SDS-polyacrvlamide gel
electropho-resis and autoradiography. The 20,000-molecular weight region of each gel was cut out, and the radioacitivy was eluted and counted. A
minus-mes-senger background of 1,484 and 12,764 counts/min
was subtracted from the Krebs and plasmacytoma 20,000-molecular weight region, respectively. Sym-bols: *, radioactivity stimulated by 1OS mRNA in
Krebsextracts;0,plasmacytomaextracts.
I
0
D
I
X
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.493.87.232.299.475.2] [image:7.493.268.456.374.531.2]VOL.1,1974ENCEPHALOMYOCARDITISVIRUS INFECTION. I.60
2.0
1.0-~
3 6 9 12 FRACTION NUMBER
FIG. 7. Sucrose gradient analysis of
poly(A)-con-tamning RNA from uninfected and infected cells. MOPC 460 ascites cellswereinfectedwithEMC virus
(10X 10-2HAU/cell)orincubated withoutvirusfor4 h. Thecellswereharvested andapoly(A)-containing
RNAfraction waspreparedand analyzed bysucrose
gradient centrifugation. Theposition ofrRNA
stan-dardsinaparallel gradientis indicated.S~ymbols:0,
poly(A)-containing RNA from uninfected cells; 0,
infectedcells.
synthesis (7), we can studythe effects of
block-ing initiationon mRNAactivityindependentof virus infection. The yieldofpoly(A)-containing
RNA was 1.9 and 1.5% from untreated and
pactamycin-treated cells, respectively, and
su-crose gradient analysis revealed no detectable differences in the size distribution of the RNA
(data not shown). However, Fig. 8B
demon-strates that an inactivation ofthe mRNA took
placeinpactamycin-treatedcells similartothat which took place during viral infection.
It is interesting that under two different conditions where initiation ofprotein synthesis
was inhibited, virus infection and pactamycin
treatment, a functional inactivation of mRNA occurred in the absence of detectable physical
alteration of the mRNA. Although the
signifi-cance of this observation in terms of mRNA
metabolism is not understood, it suggests that suchafunctional inactivationmaybea
prelimi-nary event in mRNA degradation.
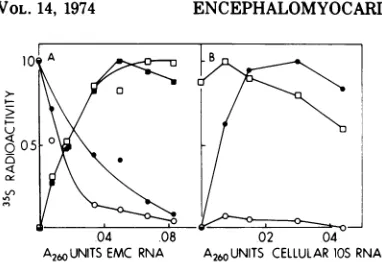
Simultaneous cell-free translation of viral RNA andcellular RNA. Translation of
exoge-nously added cellular mRNA in an infected
cytoplasmic extract is not an accurate
recon-struction of the infected cell cytoplasm, since much of the endogenous microsomal cellular and viral messenger is removed by
centrifuga-tion at 30,000 x g. Furthermore, the extensive dilution ofthe cytoplasm, which takes place in
preparing the cell-free system, lowers the
con-centrationofbothprotein synthesisfactors and
endogenous mRNA so that any effect of, for
example,
the
presenceof
viral RNA
onthe
translation
ofcellular mRNA
maybe
elimi-nated.
Therefore,
the
effect ofthe
presence ofviralRNA on
the translation
ofcellular
lOS
mRNAwas
studied
by adding
both mRNAs
insaturat-ing
amounts toacytoplasmic
extract andana-lyzing
theproducts by
SDS-polyacrylamide gel
electrophoresis.
Thetranslation
ofcellular
10S
mRNA
wassuppressed by
the
presence ofviral
RNA
whereastranslation
ofthe latter
wasunaffected
(Fig.
9). This
phenomenon
wasin-vestigated
morequantitatively by varying
viral
RNA
concentration while
holding
cellular
mRNA concentration
constant(Fig.
IOA),
orby
varying
cellular mRNA concentration and
hold-ing
viral RNA
constant(Fig.
lOB).
The reaction
products
wereanalyzed by
SDS-polyacrylamide
gel electrophoresis,
and the
radioactivity
inthe
cellular 105
mRNA-dependent polypeptide
and
in a viral
RNA-dependent polypeptide
was IA10OO00 r
0
LO
5,000[
0 01 0203 01 02
A260 UNITS RNA ADDED
FIG. 8. Translation of 10S mRNA isolated from uninfected, infected, and pactamycin-treated cells. (A) Varying amounts of fraction 9from Fig. 5 were
added to a reaction containingpreincubated Krebs cell cytoplasmic extract and [35S]methionine. The
reaction products wereanalyzed by
SDS-polyacryla-mide gel
electrophoresis
andautoradiography.
The20,000-molecular weight region was cut outand the
radioactivity
eluted andcounted.Symbols:@,
radio-activity stimulated by uninfected 105 mRNA; 0, infected lOS mRNA; A, rRNA. (B) Plasmacytoma ascitescells wereincubatedata concentrationof3 x106cells per mlfor2.5hat37C in mediumcontaining
noadditionsor1O-7Mpactamycin. Poly(A)-contain-ingRNAwasisolatedfromthesecells andanalyzedas
inFig. 7. The mRNAsedimentingat105wasassayed
foractivityasdescribedin A.Symbols:
0b,
radioactiv-itystimulatedby 10SmRNAfromuntreatedcells;0, pactamycin-treated cells; A,rRNA.605
VOL.
14,
1974on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.493.59.208.56.228.2] [image:8.493.243.438.300.493.2]p
4.,Molecular
Weight:
-a
W
C'.t.
4,07
q
_' '
-_b'.
-
10.0
x
104
1.
-
2.0
x
104
a
b
c
d
FIG. 9. Simultaneous translation of EMC viral RNA and cellular 10S mRNA. Varying amounts of mRNAs wereadded toareactioncontaining infected MOPC 460 cytoplasmic extract (see Fig. 4) and [35S]methionine. The reaction products were analyzed by SDS-polyacrylamide gel electrophoresis and autoradiography. Additions: (a) no RNA added; (b) 0.03A260 10S mRNA; (c) 0.05
A2,,0
EMC RNA; (d) 0.03A26010SmRNA plus 0.05A260EMC RNA.determined.
The presence ofviral RNA could
completely
suppress thetranslation
ofcellular
mRNA,
whereasthe
presence ofcellular
mRNAhad minimal effect
on viral RNAtranslation
(Fig.
10). Fig.1OA indicates
that viralRNA
wassomewhat more effective in
inhibiting
the trans-lation of cellular mRNA in infected extracts than inuninfected
extracts. This wasprobably
due to the presence of someendogenous
viral message in theseextracts. The moststraightfor-ward
interpretation of these experiments is that viral RNA has ahigh
affinity for acellular
factor necessary for thetranslation
ofcellular
mRNA,
possibly
an initiation factor.In
other
experiments, wedemonstrated
thatviral RNA will suppress the
translation
of cellular mRNAspecies
other than10S
and ofrabbit
globin mRNA, suggesting that thedomi-nance ofviral RNA is
general
with respect tocellular
mRNAs.Interestingly,
thecellular
10S
606
J.VIROL.on November 10, 2019 by guest
http://jvi.asm.org/
[image:9.493.122.406.73.504.2]ENCEPHALOMYOCARDITISVIRUSINFECTION.I.
04 .08
A265UNITS EMC RNA
02 04
A260UNITS CELLULAR lOS RNA
percell cytoplasm. We have estimatedthat the total number of viral RNA molecules synthe-sized inan infected plasmacytoma cellis about
105 and the total number ofpoly(A)-containing cellular mRNA moleculespercell isabout 1.5 x
105. These values indicate that the mRNA concentrations used in vitro are within the
physiological range and, therefore, that our
experimental results are likely to reflect the translation of viral andcellular mRNA in vivo.
FIG. 10. Simultaneous translation of EMC viral RNA and cellular IOS mRNA. Reactions contained varying amounts of RNA, uninfected or infected
plasmacytoma cytoplasmicextract,and [35S]methio-nine. Reactions were analyzed by
SDS-polyacryla-mide gel electrophoresis and autoradiography. The radioactivity ina viral RNA-dependent polypeptide
(indicated byarrowinFig. 9)and in the1OS
mRNA-dependent polypeptide was determinedas described
inMaterials and Methods.Symbols: (A)0,reactions
contained infected cytoplasmic extract plus viral RNA; E, 0, viral RNA and 0.03
A2,0
1OS RNA: , contained uninfected cytoplasmic extract plus viral RNA and0.03A2,0
1OSmRNA; radioactivity incorpo-rated into viralprotein, (squares); and cellular 20,000-molecular weight protein, (circles). (B) 0,reactionscontained infected cytoplasmic extractpluscellular mRNA; 0, 0, cellular mRNA and 0.05 A260 EMC
RNA; radioactivity incorporated into viralprotein, (squares); and cellular 20,000-molecular weight
pro-tein, (circles). The relative radioactivity of1.0for the cellular 20,000-molecular weight polypeptide andfor the viralpolypeptide correspondstoabout2,000and 4,000 counts/min, respectively. The background
ra-dioactivity which is subtractedfrom this data is less than500counts/min.
mRNA will suppress the translation of globin
mRNA when both messages are present in a
cell-free reaction (unpublished data), indicat-ing that
10S
mRNA is neither an abnormallyweak message norseriously inactivated byour
purification scheme.
Tocompareourin vitroresultsto eventsthat takeplace in vivo,wehavemadecalculations of
the concentrations of viral andcellular mRNA molecules present in our reaction mixtures as
well as in the cell cytoplasm in vivo. The
concentrationof viral RNAnecessarytoachieve maximal invitro proteinsynthesis and maximal inhibition of cellular mRNA translation is about 3 x 10-8M.The concentration of cellular
10S
mRNAnecessarytoachieve maximaltrans-lation in vitro is
about
1.5 x 10-7 M. We haveestimated that thevolume offree cytoplasm in
an MOPC 460 cell is approximately 6 x 10-13
liters. The above in vitro concentrations are
therefore equivalent to 104 molecules of viral RNA and 5 x 104 molecules ofcellular mRNA
DISCUSSION
One feature common to many types of viru-lent viruses is their abilitytoinhibit the synthe-sis of host cellproteins duringinfection. Viruses for which this property has been demonstrated include picorna (2), Sindbis (36), Newcastle disease (16), vesicular stomatitis (41), adeno (14), herpes (3),andvacciniaviruses(27).Since viral proteins must be synthesized in cells infected with these viruses under conditions where synthesis of cellular proteins is
sup-pressed, this phenomenon offers the opportu-nity to study a unique type of translational
control. EMC virus was chosen for this study because the replication of picornaviruses has been describedinsomedetail(2), because EMC
virus canbe grownand purified in large
quan-tities (4), and because the RNA extracted from purified virions is an active messenger in
cell-free protein-synthesizing systems (5, 20). The MOPC 460 mouse plasmacytoma cell linewas
chosen as a suitable host cell because the cells canbegrownefficiently in mice in either ascites orsolid tumor form. The former are useful for
studying viral infections in vivo and for the preparation of active cell-free systems for in vitro studies of protein orRNA synthesis. The
latter is convenient starting material for large-scale purification of subcellular components such as mRNA andRNApolymerases.
Inhibition of host protein synthesis during picornavirus infection is characterized by a
gradual disaggregation of the polysomes synthe-sizing cellular proteins (11, 30, 37). However, the rate of polypeptide elongation on the
re-maining polysomes is unchanged during disag-gregation (37), and cellular mRNA appears to
be unaltered physically during infection (8, 21, 42), suggesting that inhibition of protein
syn-thesistakesplaceatthe level ofinitiation. (The activity of this cellular mRNAwasnotanalyzed directly, however.) At high multiplicities of infection, host shut-off is induced in the
pres-ence of drugs which prevent viral replication such asguanidine and penicillamine (1, 17, 26, 31),and inthepresenceofamino acid analogues 104
05
A [B
0
VOL.14, 1974
607
on November 10, 2019 by guest
http://jvi.asm.org/
[image:10.493.41.232.57.188.2]such
asfluorophenylalanine and azetidine
(9, 17). Use of thelatter
drugs results in bothsynthesis
ofnonfunctional viral
proteins and aninhibition
ofviral replication.
This wouldsug-gest
that
a component ofthe
infectingvirion,
if present in highenough concentration,
can causethe inhibition of host
proteinsynthesis.
An
alternative model
for theshut-off
of hostprotein
synthesis
hasrecently been
proposed,namely
that
viral dsRNA (RF,
formed as aby-product
ofreplication) specifically inhibits
translation of host mRNAs (12). dsRNA
is apotent
inhibitor
ofinitiation
ofprotein synthe-sis inreticulocyte cell-free
systems (12)and
asomewhat less
potentinhibitor
inother cell-free
systems
(34). Furthermore, dsRNA
is cytotoxicand
caninhibit
proteinsynthesis
in vivo whenadded exogenously
to tissueculture cells
(10).It
has
been
proposed that dsRNA inhibits
proteinsynthesis by binding
to an initiationfactor (19)or
by stimulating the production
of aninhibitor
(23). However,
certain evidence
suggeststhat
the
notion ofdsRNA involvement
inviral
in-duced host
shut-off
mustbe viewed with
cau-tion:
(i) inhibition by dsRNA
in vitro isnonse-lective with respect
tothe translation
of viraland
cellular mRNAs (34);
(ii) asmentioned
above, host shut-off
can occur athigh
multiplic-ities of
infection
inthe absence
ofreplication,
and
presumably replication is
necessary fordsRNA
synthesis; (iii) the lack
of sensitivity ofthe
plasmacytoma cell-free
system toEMC RF
(Fig. 6)
suggeststhat
somecell
types canbe
resistant
tothe
effects ofdsRNA
and
yetdemonstrate
apronounced
inhibition
ofprotein
synthesis
when
infected
by
picornavirus (this
has also
been shown
tobe
true forchicken
embryo fibroblasts which
areshut
offby
infec-tion with
Sindbis
virusbut whose
cell-free
pro-tein
synthesis
isinsensitive to dsRNA (35); (iv)it
has
recently been demonstrated that EMC
viral RNA
isreplicated through
single-stranded
structures (28;
Thach
etal.,
inpress)
implying
that the
amountoftruly
dsRNA
early
ininfec-tion may
be
verysmall.
Forthese
reasons, weare
inclined
toseek
an alternativeexplanation
for
the shut-off
phenomenon.
It is apparent that
protein
synthesis
during
EMC
infection ofMOPC
460cells
is character-izedby
two distinctphenomena: (i)
ageneral
inhibition
ofprotein
synthesis;
and
(ii)
aswitch-over
to thesynthesis
of viralprotein
(Fig. 1 and
2).
However,
ourstudy
of theactivity
of thecrude cell-free
protein-synthesiz-ing system from infected cells indicates neither a
general
debility
inprotein
synthetic
capacity
nor a
specific
restrictionagainst
translation ofcellular mRNA
(Fig. 4and
5,Table
1).This
result
has anumber
ofimplications.
First, it isevident that the total
cellular
protein-synthe-sizing
machinery,
including ribosomes, tRNA,
initiation
and elongation
factors,
andamino-acyl-tRNA
synthetases,
isunaffected by
infec-tion. Moreover,
it seemslikely that
nospecific
inhibitor
oftranslation
orviral-induced
mecha-nism
for inactivating
cellular
mRNA is presentin
the
cell-free
system.Apparently, the
mech-anisms
which
causethe
overall decline
inpro-tein
synthetic activity and which
causeswitch-over from
host
toviral
proteinsynthesis
havebeen lost
ormasked
inthe
cell-free
reactionmixtures.
One important
factorwhich
maycontribute
to
the overall decline
in proteinsynthesis,
especially
verylate
in infection,could be
adepletion
ofthe
cellular
energypools (ATP,
GTP, etc.).
Infact, the
rateof
polypeptide
elongation
onvirus-specific
polysomes has been
shown
todecrease late
ininfection
(38), thus
supporting this
concept.An
alternative
explanation
forthe
general
inhibition
ofprotein
synthesis is that the
ribo-somal
transit time(per
codon)
onviral RNA
might be
intrinsically longer than that of the
average
cellular mRNA.
Since viral RNA
re-places
most ofthe cellular mRNA
inpolysomes
during
infection, the result would be
alowering
of
the total
rate ofprotein
synthesis. The
rate ofelongation
onpoliovirus and EMC-specific
polysomes
in vivohas been
estimated
tobe
205to250 amino
acids
per min(33). In
contrast,the
in vivo rates ofelongation
ofalpha- and
beta-hemoglobin
chains
(molecular
weight
15,500)
are 600
and
400 aminoacids
per min,respec-tively (18); ovalbumin
(molecular weight
42,000)
and
conalbumin (molecular weight
76,000)
are 320and
224 aminoacids
per min,respectively (in chicken oviducts
at henbody
temperature) (29); and
collagen
precursor(molecular weight 115,000)
is 209 amino acidsper min
(40).
Inaddition, the
average rate ofelongation
inChinese
hamster ovarycells
(weight average molecular weight 85,000) is 420 aminoacids
per min (13). Thus, different mRNAs canbe translated
withdifferent
elonga-tion rates in the samecell
typeor tissueand,
ingeneral, long
mRNAs aretranslated
at lowerspecific
rates than shorter ones. This suggeststhatthe
replacement
ofcellular
mRNAby
viralRNA on
polysomes
could itself account formuch
of thegeneral
decline inprotein
syn-thetic activityduring infection.
The switch-over to
synthesis
ofviralprotein
during
infection isprobably
caused
by
the608
on November 10, 2019 by guest
http://jvi.asm.org/
ENCEPHALOMYOCARDITIS VIRUSINFECTION. I.
ability
of
viral RNA
toout-compete
cellular
mRNA for
acomponent necessary for
the
initia-tion of protein
synthesis
(Fig. 10).
This property
of viral RNA
isalso
likely
tobe
indirectly
responsible
forthe low
specific
activity
ofcellu-lar mRNA
isolated
during infection.
Thus,
asthe
amount ofviral RNA
increasesduring
infection,
the
rate ofinitiation on cellular
mRNA
will
decline,
possibly
resulting in
agreater
availability of cellular mRNA
tothe
normal
cellular
processes ofmRNA inactivation
and
degradation.
Our results
areconsistent
with
much
ofthe
published literature
dealing
with host
shut-off.
At
high
multiplicities
ofinfection, sufficient
viral RNA may
be present
inthe cell
toinduce
the decrease
incellular protein
synthesis
eveninthe
absence
ofreplication
(17). The acceleration
of
viral induced shut-off of
protein
synthesis
caused
by actinomycin D (24) may be due
toitseffect
directly
orindirectly
oninitiation of
pro-tein
synthesis (15, 32). When the
rateof
initia-tion is
nonspecifically
decreased by an agent
such
asactinomycin D, the
ability
ofviral RNA
to
exclude cellular RNA
fromthe protein
syn-thesis
machinery
maybe
greatly enhanced.
The
ability
toutilize
cellular protein
synthe-sis
machinery
moreefficiently
than
cellular
mRNA may
be
ageneral property
ofthe
mRNA
of
the virulent
viruseswhich suppress cellular
protein
synthesis. This would be
alogical way to
insure that
amaximal
amount ofviral
specified
protein will
be synthesized
from aminimal
amount of
mRNA. The
general inhibition
ofprotein
synthesis
incells
infected with such
virulent
virusesmay
be
only
asecondary and
relatively unimportant consequence
ofthe
effi-cient
translation
ofviral RNA.
ACKNOWLEDGMENTS
Wewould liketothankDarrell Dobbertin forhis expert technicalassistance; Ernie Simms.Ralph Graff,DickLynch,
A.Burness, andIrvBoimeforhelpfuldiscussions andadvice; and MiltSchlesingerforprovidinguswithpactamycin.
This workwassupported by Public Health Service grant CA-13008 from the NationalCancer Institute and by Health Services Advancement Award (to Washington University) 5-504-FR-06115 fromtheDivision ofResearch Facilitiesand Resources.
C. L. isa Medical Scientist traineesupported by Public HealthServices grant GM-02016 from the National Institute ofGeneral Medical Sciences.
LITERATURE CITED
1. Bablanian, R., H. J. Eggers, and I. Tamm. 1965. Studies onthe mechanismofpoliovirus-induced cell damage I. The relation betweenpoliovirus-induced metabolic and morphological alterations in cultured cells. Virology 26:100-113.
2. Baltimore, D. 1969. The replication ofpicornaviruses, p. 101-176. In H. B. Levy (ed.), The biochemistry of viruses.Marcel Dekker, Inc., New York.
3. Ben-Porat,T., T. Rakusanova, and A. S. Kaplan. 1971. Early functions of the genome of herpes virus. II. Inhibition of the formation of cell-specific polysomes. Virology46:890-899.
4. Boime, I., and H. Aviv. 1973.Cell-freesynthesisofviral proteins in the Krebs II ascites tumor system, p. 187-215. In A. Laskin andJ. Last (ed.), Methods in molecular biology, vol. 4. Marcel Dekker, Inc., New York.
5. Boime, I., H. Aviv, and P. Leder. 1971. Protein synthesis directed by encephalomyocarditis virus RNAII.Thein vitro synthesis ofhigh molecular weight proteins and elements of the viral capsid. Biochem. Biophvs. Res. Commun.45:788-795.
6. Butterworth, B. E., L. Hall, C. M. Stoltzfus, and R. R. Rueckert. 1971.Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells.Proc. Nat. Acad. Sci. U.S.A. 68:3083-3087.
7. Butterworth, B. E., and R. R. Rueckert. 1972. Gene order ofencephalomyocarditis virusasdeterminedby studies with pactamycin. J. Virol. 9:823-828.
8. Colby, D. S., V. Finnerty, andJ. Lucas-Lenard. 1974. Fateof mRNA of L-cells infected with mengovirus. J. Virol.13:858-869.
9. Collins, F. D., and W. K. Roberts. 1972. Mechanism of mengo virus-induced cell injury in L cells: use of inhibitors of protein synthesis to dissociate virus-specificevents.J. Virol. 10:969-978.
10. Cordell-Stewart, B., and M. W. Taylor. 1973. Effect of viral double-stranded RNA on protein synthesis in intactcells. J. Virol. 11:232-237.
11. Dalgarno, L., R. A. Cox, and E. M. Martin. 1967. Polyribosomes innormal Krebs2 ascites tumorcells andincells infected withencephalomyocarditis virus. Biochim.Biophys. Acta 138:316-328.
12. Ehrenfeld,E., andT.Hunt. 1971. Double-strandedpolio virus RNA inhibits initiation of protein synthesis by reticulocyte lysates. Proc. Nat. Acad. Sci. U.S.A. 68: 1075-1078.
13. Fan, H., and S. Penman. 1970. Regulation of protein synthesisin mammalian cells II.Inhibition ofprotein synthesis at the levelof initiation duringmitosis. J. Mol. Biol.50:655-670.
14. Ginsberg, H. S. 1969. Biochemistry ofadenovirus infec-tion, p.329-359. InH. B.Levy(ed.). The biochemistry ofviruses.Marcel Dekker, Inc., New York.
15. Goldstein. E. S., and S. Penman. 1973. Regulation of proteinsynthesis in mammalian cells V. Further stud-iesonthe effectofactinomycinDontranslation control inHeLa cells. J. Mol. Biol. 80:243-254.
16. Hightower, L. E., and M.A.Bratt.1974.Protein synthe-sis inNewcastle disease virus-infected chickenembryo cells.J. Virol. 13:788-800.
17. Holland,J.J. 1964.Inhibitionofhost cell macromolecu-larsynthesis by high multiplicitiesofpoliovirus under conditions preventing virus synthesis. J. Mol. Biol. 8:574-581.
18. Hunt, T., T. Hunter. and A. Munro. 1969. Control of haemoglobin synthesis: rateoftranslation of the mes-senger RNA for the a and d chains. J. Mol. Biol. 43:123-133.
19. Kaempfer, R., and J. Kaufman. 1973. Inhibition of cellular protein synthesis by double-stranded RNA: inactivation of aninitiation factor. Proc. Nat. Acad. Sci.U.S.A. 70:1222-1226.
20. Kerr, I. M., R. E. Brown, and D. R. Tovell. 1972. Characterization of the polvpeptides formed in re-sponsetoencephalomvocarditis virus ribonucleic acid
609
VOL. 14, 1974
on November 10, 2019 by guest
http://jvi.asm.org/
inacell-freesystemfrommouseascitestumorcells. J. Virol. 10:73-81.
21. Koschel, K. 1974. Poliovirus infection and poly(A) se-quences of cytoplasmic cellular RNA. J. Virol. 13:1061-1066.
22. Laemmli, U. K. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature (London) 227:680-685.
23. Legon, S.,A.Brayley, T. Hunt, and R. J. Jackson. 1974. The effectofcyclicAMP and relatedcompoundsonthe control ot protein synthesis in reticulocyte lysates. Biochem.Biophys.Res. Commun. 56:745-752. 24. Leibowitz, R., and S. Penman. 1971. Regulation of
protein synthesis in HeLa cells III. Inhibitionduring poliovirusinfection. J. Virol.8:661-668.
25. Martin,E. M., J.Malec, S. Sved, and T. S. Work.1961. Studies on protein and nucleic acid metabolism in
virus-intectedmammalian cells. I.
Encephalomyocar-ditis virusinKrebsIImouse-ascites-tumor cells.
Bio-chem. J.80:585-597.
26. Merryman, P.,I. A.Jaffee,and E. Ehrenfeld. 1974. Effect of D-penicillamine on poliovirus replication in HeLa cells. J.Virol. 13:881-887.
27. Moss, B. 1968. Inhibition of HeLa cellprotein synthesis
2.by thevaccinia virion. J.Virol.2:1028-1037.
28. Oberg, B., and L. Philipson. 1971.Replicativestructures ofpoliovirus RNA in vivo.J. Mol. Biol.58:725-737. 29. Palmiter, R.D. 1972.Regulationofprotein synthesis in
chick oviductII.Modulation ofpolypeptide elongation
ratesand initiationratesbyestrogenandprogesterone.
J.Biol. Chem.247:6770-6780.
30. Penman, S., K. Scherrer, Y. Becker, and J. E. Darnell. 1963. Polyribosomesinnormal andpoliovirusinfected HeLa cells and theirrelationship tomessengerRNA. Proc. Nat. Acad. Sci.U.S.A. 49:654-662.
31. Penman, S., and D. Summers. 1965.Effectsonhost cell
metabolism followingsynchronousinfection with polio-virus.Virology 27:614-620.
32. Reichman, M., and S. Penman. 1973. Stimulation of
polypeptide initiation in vitro after protein synthesis
inhibition invivoinHeLacells. Proc. Nat. Acad. Sci. U.S.A. 70:2678-2682.
33. Rekosh, D. 1972. Gene order ofthe poliovirus capsid proteins. J. Virol.9:479-487.
34. Robertson, H. D., and M. B. Mathews. 1973. Double-strandedRNAas aninhibitor ofproteinsynthesisand as a substrate for anuclease in extracts ofKrebs II ascitescells. Proc. Nat. Acad. Sci. U.S.A.70:225-229. 35. Shenk, T. E., and V. Stellar. 1972. Effect of
double-stranded RNAs from Sindbis virus-infected cells on
rabbitreticulocyte and chick embryo cell-freeprotein synthesis. Biochim. Biophys. Acta287:501-513. 36. Strauss, J. H., B. W. Burge, andJ. E. Darnell. 1969.
Sindbis virus infection of chick and hamster cells: synthesis of virus specific proteins. Virology 37:367-376.
37. Summers,D. F.,andJ.V.Maizel. 1967.Disaggregation of HeLa cellpolysomes after infectionwith poliovirus. Virology31:550-552.
38. Summers, D. F., J. V. Maizel, and J. E. Darnell. 1967.
The decreaseinsizeand syntheticactivity ofpoliovirus polysomes late in the infectious cycle. Virology 31:427-435.
39. Swan, D.,H.Aviv.,andP.Leder.1972. Purificationand
propertiesofabiologicallyactivemessengerRNA fora
myeloma light chain. Proc. Nat. Acad. Sci. U.S.A.69: 1967-1971.
40. Vuust, J., and K. A. Piez. 1972. A kinetic study of collagen biosynthesis. J. Biol. Chem. 247:856-862. 41. Wertz, G. W., and J. S. Younger. 1972. Inhibition of
protein synthesis in L cells infected with vesicular stomatitis virus.J. Virol. 9:85-89'
42. Willems, M., andS. Penman. 1966.The mechanism of host cell protein synthesis inhibition by poliovirus. Virology30:355-367.