Copyright © 2004, American Society for Microbiology. All Rights Reserved.
Increased Incidence of Squamous Cell Carcinomas in
Mastomys
natalensis
Papillomavirus E6 Transgenic Mice during
Two-Stage Skin Carcinogenesis
Iris Helfrich,* Min Chen,† Rainer Schmidt, Gerhard Fu¨rstenberger,
Annette Kopp-Schneider, David Trick, Hermann-Josef Gro¨ne,
Harald zur Hausen, and Frank Ro¨sl
Deutsches Krebsforschungszentrum, D-69120 Heidelberg, Germany Received 7 August 2003/Accepted 23 December 2003
Papillomaviruses cause certain forms of human cancers, most notably carcinomas of the uterine cervix. In contrast to the well-established involvement of papillomavirus infection in the etiology of cervical carcinomas and in carcinomas of a rare hereditary condition, epidermodysplasia verruciformis, a causative role for cu-taneous human papillomavirus types in the development of nonmelanoma skin cancer has not been proven. In order to better understand the functions of individual genes of cutaneous papillomavirus types, we generated
transgenic mice carrying oncogene E6 of theMastomys natalensispapillomavirus (MnPV), which causes
kerato-acanthomas of the skin in its natural host. In the present study, we demonstrate that under conditions of experimental two-stage skin carcinogenesis, fast-paced squamous cell carcinomas develop in nearly 100% of
MnPV E6 transgenic mice in comparison to 10% in their nontransgenic littermates (log rank test;P< 0.0001).
Therefore, we conclude that the MnPV E6 transgene favors the malignant progression of chemically induced
tumors. Whereas an activating H-ras mutation is a consistent feature in benign and malignant tumors in
wild-type mice, the majority of papillomas and keratoacanthomas and all squamous cell carcinomas obtained
in MnPV E6 transgenic mice contain nonmutatedrasalleles. These results indicate that the development of
squamous cell carcinomas in MnPV E6 transgenic mice does not depend on an activated H-rasoncogene.
Papillomaviruses are widely known to play a causative role in the development of tumors in humans. Certain genotypes cause mostly benign epidermal tumors in humans and animals. Another subgroup of genotypes, the so-called “high-risk” ano-genital human papillomavirus (HPV) types, are causally in-volved in the development of malignant tumors, such as car-cinoma of the cervix (17, 47, 49). The carcinogenic potential was first detected in 1935, when Rous and Bernard showed that inoculation into domestic rabbits of the filterable extracts from warts of cottontail rabbits was able to induce skin carcinomas (37). A direct involvement of cutaneous HPV types in the development of squamous cell carcinomas, one of the most frequent malignancies in humans of Caucasian origin, is sus-pected but still not proven (7, 34). The earliest hints of an involvement of specific HPV types in human skin cancer orig-inated from studies of patients suffering from the very rare hereditary disease epidermodysplasia verruciformis (EV) (32, 21). Only a limited number of HPV types, commonly referred to as EV-HPV types, such as HPV5, -8, and -14, are frequently detected in squamous cell carcinomas that develop in⬃30% of cases within multiple flat warts in sun-exposed areas of the skin in such patients (25). More recent data suggested a role for EV-related papillomaviruses in the origin of skin cancer in immunosuppressed patients (39) and those with renal
trans-plants (28). Thus, specific high-risk cutaneous HPV types com-parable to carcinogenic anogenital HPV types have not been identified (48, 49).
The tumorigenic potential of some cutaneous animal papil-lomaviruses has been demonstrated (for a review, see refer-ence 10), including that ofMastomys natalensispapillomavirus (MnPV) (29), a rodent papillomavirus known to cause kera-toacanthomas in its natural host,M. natalensis(1). Reinacher et al. (36) described spontaneous keratoacanthomas inM. na-talensisand observed MnPV particles in keratinized cells of the stratum granulosum and the stratum corneum. Infection of young M. natalensis with MnPV particles resulted in benign skin tumors in 40% of the infected animals (36). During their life spans, the animals remained persistently infected, and the number of viral genomes in their skins steadily increased. At the age of 1 year, skin tumors developed spontaneously (1).
In the case of high-risk anogenital HPV types, the E6 and E7 genes cooperate in the transformation of human cells and induce carcinomas in transgenic mice (2, 3, 15, 16, 19, 24). In order to assess the role of the E6 protein of a cutaneous papillomavirus type in vivo, we generated transgenic mice ex-pressing the MnPV E6 gene in the skin under the control of the humancytokeratin-14promoter. MnPV E6 transgenic mice showed an increased incidence of squamous cell carcinomas when the classical mouse skin two-stage carcinogenesis proto-col (6, 13) was applied. These results demonstrated a cooper-ating function of the MnPV E6 protein with chemical carcin-ogens.
The ras family oncogenes have been detected with high frequency in both human (26) and animal (4, 31) cancers. The
* Corresponding author. Mailing address: Forschungsschwerpunkt Angewandte Tumorvirologie, Abteilung Virale Transformationsmech-anismen, Deutsches Krebsforschungszentrum, Im Neuenheimer Feld 280, D-69120 Heidelberg, Germany. Phone: 496221/424919. Fax: 496221/424902. E-mail: i.helfrich@dkfz.de.
† Present address: Texas Heart Institute, Houston, TX 77303.
4797
on November 8, 2019 by guest
http://jvi.asm.org/
mouse model system for skin tumorigenesis has been invalu-able in dissecting tumor development into distinct stages, i.e., initiation, promotion, and progression (17, 46). Molecular analyses of the stages of chemical skin carcinogenesis have indicated that the H-rasgene is a major target for a mutation event that takes place at the time of initiation (4, 5). Mutant H-rasalleles are found in a high proportion of premalignant tumors, indicating that the H-rasmutation is an early event (4). Furthermore, the specific mutations observed depend on the nature of the carcinogen used as an initiator (5, 35), suggesting a direct interaction between the carcinogen and the H-ras
gene. Treatment with chemical carcinogens, specifically with 7,12-dimethylbenz[a]anthracene (DMBA), mainly resulted in mutations and the frequent accumulation of tumor cells har-boring a nucleotide transversion at position 2 of H-rascodon 61 (35). Also, mutations in K-rashave been reported after the use of DMBA–12-O-tetradecanoylphorbol-13-acetate (TPA) during a classical two-stage mouse carcinogenesis in H-rasnull mutant mice (22). Therefore, we examined the mutation rates of H-rasand K-rasoncogenes for potential codon 12, 13, and 61 mutations in tumors obtained from MnPV E6 transgenic mice and nontransgenic littermates exposed to the two-stage skin carcinogenesis protocol. In both mouse lines, no muta-tions were found for K-rasin codons 12, 13, and 61. Tumors obtained from nontransgenic animals revealed H-ras muta-tions at position 2 in codon 61 in 70%, in contrast to 24%, of MnPV E6 transgenic mice. Most notably, no mutated H-ras
gene was found in any malignant tumors expressing high levels of MnPV E6. The same was true for a cell line established from a squamous cell carcinoma of the MnPV E6 transgenic-mouse line after chemical carcinogenesis.
MATERIALS AND METHODS
Generation of transgenic mice.Mice were purchased from Charles River Wiga (Sulzfeld, Germany). We generated hemizygous MnPV E6 transgenic mice in an inbred FVB/N genetic background by injection of vector-free DNA into a pro-nucleus of fertilized oocytes. Transgenic animals were identified by PCR (30 cycles of 94°C for 60 s, 55°C for 60 s, and 72°C for 120 s) with DNAs obtained from tail biopsies (44) using primers ATV11 and ATV12, specific for thelacZ
reporter gene, together with primers Mgp1 and Mgp2 for the endogenous myo-genin gene as a control. The MnPV E6 transgene was assembled in pBluescript KS (Stratagene, La Jolla, Calif.) by inserting a 2-kb XhoI fragment carrying the promoter of humancytokeratin-14and a 650-bp BamHI-EcoRI fragment con-taining the rabbit-globin intron from plasmid pHR2 (44) (a generous gift of S. Werner, ETH Zu¨rich, Zu¨rich, Switzerland), a 440-bp HindIII fragment with the complete open reading frame of the MnPV E6 gene obtained by PCR from a MnPV genomic construct (42) (kindly provided by E. Amtmann, Deutsches Krebsforschungszentrum, Heidelberg, Germany), a 3.9-kb SpeI-XbaI fragment from plasmid pIRESlacZ containing the encephalomyocarditis virus internal ribosome entry site (IRES) element (provided by G. Sczakiel, University of Lu¨beck, Lu¨beck, Germany), and the lacZgene including a simian virus 40 polyadenylation signal from plasmid BGZ40 (45). For injection, the 7-kb MnPV E6 transgene construct was excised from the vector by cutting with the restriction endonucleases SalI and NotI.
Analysis of transgene expression.Transgene expression from the bicistronic MnPV E6 transgene was assessed by visualizing-galactosidase reporter enzyme activities with X-Gal (5-bromo-4-chloro-3-indolyl--D-galactopyranoside) on whole embryos or on 5- to 10-m-thick cryostat sections of adult mouse skin or skin tumor tissue (20). Expression of the E6 transgene was monitored by reverse transcriptase (RT) PCR performed on total RNA from primary keratinocyte cultures isolated from 2- to 3-day-old mice. Briefly, the skin of each animal was floated on 0.25% trypsin in phosphate-buffered saline (PBS) at 4°C overnight. The epidermis was peeled off, minced, and stirred for 25 min in Keratinocyte SFM (Life Technologies, Karlsruhe, Germany) supplemented with 0.05 mM CaCl2. After filtration of the cell suspension through nylon nets, the cells were
pelleted for 15 min at 1,200⫻g, washed with PBS, and seeded onto fibronectin-and collagen-coated plates in Keratinocyte SFM with 0.05 mM CaCl2, 50g of amphotericin B (Fungizone)/ml, and 20g of gentamicin/ml. The cells were maintained at 37°C in a humidified atmosphere with 5% CO2. Dead and differ-entiated cells were washed off with PBS the next day. Total RNA was prepared from primary keratinocytes or fromM. natalensisskin tumor tissue using Trizol (Life Technologies) according to the manufacturer’s instructions. Samples were treated with DNase I and reverse transcribed with SuperScript II (Life Technol-ogies) using the primer ATV175 or T22. After the removal of RNA by RNase H (Roche, Mannheim, Germany), PCRs for E6 were performed withTaq polymer-ase and ATV176 and ATV177 primers for 30 cycles (94°C for 30 s, 50°C for 45 s, and 72°C for 60 s). Control reactions for-actin employed PCRs with primers ATV49 and ATV50 for 27 cycles (94°C for 30 s, 55°C for 45 s, and 72°C for 60 s). Control PCRs were routinely carried out using samples without prior reverse transcription. The PCR products were visualized on ethidium bromide-stained agarose gels.
Two-stage mouse skin carcinogenesis.Induction of tumor development was performed on 6-week-old female MnPV E6 transgenic mice of the strongly E6-expressing line HP22-42 and their nontransgenic littermates. The dorsal skins of the mice were shaved 1 week prior to topical application of a single dose of the initiator DMBA (0.1mol dissolved in 100l of acetone) or acetone only (18). Starting 1 week later, the dorsal skins were treated twice weekly with the tumor promoter TPA (5 nmol in 100l of acetone) or acetone alone for 20 weeks. Sixty MnPV E6 transgenic and 56 nontransgenic mice were treated with DMBA-TPA. Controls receiving acetone or acetone-TPA consisted of 10 MnPV E6 transgenic animals and 10 nontransgenic littermates per treatment. DMBA-acetone con-trols had 37 animals per group. The tumor incidence and number were recorded individually for each mouse once per week. All of the animals were observed until week 36. Mice that developed tumors with a diameter of⬎1 cm were sacrificed. Tumors were scored by morphological appearance as papillomas or keratoacanthomas/squamous cell carcinomas at the time of collection. Papillo-mas and all other tumors were reexamined histologically on hematoxylin-eosin-stained paraffin sections for detailed diagnoses. A part of each tumor tissue was frozen in liquid nitrogen for further analyses.
Analysis of tumor data.The average number of papillomas per animal was calculated by dividing the total papilloma count by the total number of surviving animals. The papilloma incidence was monitored over time as the percentage of papilloma-bearing animals in a group. The distribution functions describing carcinoma and keratoacanthoma incidences were based on individual animal observations and were determined by using the Kaplan-Meier estimator. Differ-ences in carcinoma and keratoacanthoma occurrence times between MnPV E6 transgenic and wild-type mice were evaluated using the log rank test (13).
Detection of H-rasmutation at position 2 in codon 61.The presence of the activating A-to-T transversion of the second nucleotide of codon 61 of the H-ras
coding region generates a diagnostic XbaI restriction enzyme recognition site, which was identified through PCR-based RNA and DNA analyses after isolation from tumor tissue. Following RNA isolation using Trizol (Life Technologies), the samples were treated with DNase I and reverse transcribed with SuperScript II using primer ATV192. After treatment with RNase H, PCRs for H-raswere performed withTaqpolymerase and ATV190 and ATV192 primers for 35 cycles (94°C for 30 s, 65°C for 30 s, and 72°C for 30 s). Control reactions for-actin using primers ATV49 and ATV50 and reactions without prior reverse transcrip-tion were made to exclude amplificatranscrip-tion of genomic DNA. For DNA isolatranscrip-tion, the tumors were digested with 0.5 mg of proteinase K/ml in lysis buffer (100 mM NaCl, 10 mM Tris [pH 7.5], 1 mM EDTA, 1% sodium dodecyl sulfate) at 55°C overnight. After phenol-chloroform extraction and ethanol precipitation, the resulting DNA was subjected to 30 cycles of PCR (94°C for 30 s, 60°C for 30 s, and 72°C for 30 s) to amplify the respective H-rasfragment using ATV190 and ATV192 primers. PCR products from RNA and DNA were gel purified and digested with XbaI. The resulting fragments were separated by electrophoresis on 5% MetaPhor agarose gels (Biozym, Hessisch Oldendorf, Germany).
Sequencing analysis of Ras gene mutation.Isolated RNAs from papillomas, keratoacanthomas, and squamous cell carcinomas of MnPV E6 transgenic mice and wild-type controls were used as templates for RT-PCR. PCRs for H-rasusing ATV193 and ATV194 primers for 35 cycles (94°C for 30 s, 64°C for 30 s, and 72°C for 30 s) and K-rasusing ATV195 and ATV196 primers for 35 cycles (94°C for 30 s, 62°C for 30 s, and 72°C for 30 s) were performed. PCR products from RNA were gel purified using a Qiaex II gel extraction kit (Qiagen). Nucleotide sequences containing codons 12, 13, and 61 of the H-rasor K-rasgene were determined by dye terminator cycle sequencing (Applied Biosystems) using the primers listed below.
Southern blot analysis.RNAs isolated from papillomas, keratoacanthomas, and squamous cell carcinomas of MnPV E6 transgenic mice were used as
on November 8, 2019 by guest
http://jvi.asm.org/
plates for RT-PCR. PCRs for MnPV E6 and-actin were performed using primers ATV176, ATV177, ATV49, and ATV50 in one reaction for 29 cycles (94°C for 30 s, 50°C for 45 s, and 72°C for 60s) and separated on 1.5% agarose gels in the presence of ethidium bromide. The amplified PCR products were transferred to GeneScreen Plus membranes (DuPont NEN). For hybridization, 2.5 ng of transgenic MnPV E6 construct and 2.5 ng of linearized pHFA1 using BamHI were labeled with [32P]dCTP by random priming using the HexaLabel Plus DNA-labeling kit (MBI Fermentas). The filters were washed in 2⫻SSC (1⫻ SSC is 0.15 M NaCl plus 0.015 M sodium citrate) including 0.1% sodium dodecyl sulfate at 68°C and exposed by phosphorimager (Amersham Pharmacia Biotech). The counts were quantified using ImageQuant (Amersham Pharmacia Biotech).
PCR primers.The DNAs were synthesized in an Applied Biosystems synthe-sizer using phosphoramitide chemistry and further purified by high-performance liquid chromatography.
The following primers were used: ATV11 (5⬘-GCGACTTCCAGTTCAACA TC-3⬘), ATV12 (5⬘-GATGAGTTTGGACAAACCAC-3⬘), ATV49 (5⬘-ACCCA CACTGTGCCCATCTACGA-3⬘), ATV50 (5⬘CTTGCTGATCCACATCTGC T-GGA-3⬘), ATV175 (5⬘-AGACGGCAATATGGTG-3⬘), ATV176 (5⬘TGCAC ATTCTGCTCGAGGTT-3⬘),; ATV177 (5⬘ -CCCTCTTTCTGCACACTCTA-3⬘), ATV190 (5⬘-CTGGAGGCGTGGGAAAGAGTG-3⬘), ATV192 (5⬘-CTGT ACTGATGGATGTCCTCGAAG-3⬘), ATV193 (5⬘GCAGCCGCTGTAAAAG CTAT-3⬘), ATV194 (5⬘-GTCCTCGAAGGACTTGGTGT-3⬘), ATV195 (5⬘GG CCTGCTGAA-AATGACTGAG-3⬘), ATV196 (5⬘-CTAACTCCTGAGCCTGT TTCGTG-3⬘), Mgp1 (5⬘-CCAAGTTGGGTCAAAAGCC-3⬘), and Mgp2 (5⬘-C TCTCTGCTTTAAGGAGTCAG-3⬘).
RESULTS AND DISCUSSION
Phenotype of K14MnPV E6 transgenic mice. In order to
analyze the role of a cutaneous papillomavirus type in skin tumor formation, we generated transgenic mouse lines carry-ing the MnPV E6 gene under the control of the human cyto-keratin-14promoter. This directs transgene expression to the basal layer of the skin (Fig. 1A) (43). The mRNA transcribed from the transgene was designed as a bicistronic message. The MnPV E6 gene constituted the first open reading frame, and thelacZreporter constituted the second open reading frame, preceded by an encephalomyocarditis virus IRES element for efficient translation of thelacZreporter. This permits easy and fast detection of transgene expression by monitoring -galac-tosidase activities in transgenic animals. Initial analysis of MnPV E6 transgenic mouse lines hemizygous for the trans-gene and derived from different founders was performed by whole-mount staining of 15.5-day-old embryos with the chro-mogenic -galactosidase substrate X-Gal. This revealed the presence of -galactosidase in developing hair follicles and epidermis, a pattern expected for the activity of the cytokera-tin-14promoter at this stage (Fig. 1B and C) (9). Variations in the strength of transgene expression in 15.5-day-old embryos among eight expressing MnPV E6 transgenic lines corre-sponded to-galactosidase reporter activities observed in cry-ostat sections of the respective adult skins (Fig. 1B to E).
-Galactosidase activities were detected throughout the epi-dermal layers of adult skin in the strongly expressing lines, reflecting the production of large amounts of this enzyme and its stability throughout differentiation processes of the mouse epidermis (Fig. 1D).-Galactosidase activity in skin sections of weakly expressing MnPV E6 transgenic lines was typically patchy and confined to the basal layer of the epidermis (Fig. 1E). Expression of the MnPV E6 gene was analyzed by RT-PCR. Reproducibly, the RT-PCR signals for E6 were strongest in those lines with the highest-galactosidase activities. This points to equal MnPV E6 expression levels and-galactosidase
reporter activities, as expected from the translation of the bicistronic E6 IRES-lacZtranscript (Fig. 1F).
Mice from different MnPV E6 transgenic lines displayed no transgene-dependent phenotype and did not develop sponta-neous tumors by the age of 2.5 years. The strong E6-expressing lines HP22-42, HP21-2, and HP21-13 were used for further analyses. For a complete phenotyping of the MnPV E6 trans-genic animals, hematoxylin and eosin-stained paraffin sections from the skin of different regions of the body (back, tail, and ear) and also from the tongue, salivary glands, esophagus, thyroid gland, heart, liver, lung, spleen, small intestine, thymus, adrenal glands, and pancreas of 10-day- and 3-, 6-, 12-, 24-, and 36-month-old MnPV E6 transgenic mice and wild-type con-trols were prepared (three animals per time point). We found that MnPV E6 transgenic mice were histologically indistin-guishable from their nontransgenic littermates. They showed no increase in proliferation, as was also demonstrated by im-munohistochemical staining using Ki-67 antibody (data not
shown). Thus, the MnPV E6 transgene expression per se did not perturb normal skin differentiation and tissue homeostasis. This is in contrast to results obtained with HPV16 E6 trans-genic mice (41), where two out of five transtrans-genic lines showed a consistent thickening of the epidermis due to hyperprolifera-tion of the basal keratinocytes. A small percentage of these HPV16 E6 transgenic mice developed spontaneous, mostly malignant skin tumors by the age of 1 year (41). The differ-ences between HPV16 E6 and transgenic MnPV E6 lines may be indicative of distinct biological properties of MnPV E6 as “skin-type” papillomavirus in comparison to anogenital high-risk-type HPV16 E6.
MnPV E6 increases the yield of squamous cell carcinomas after treatment with chemical carcinogens and primarily
en-hances malignant progression. High-risk papillomavirus E6
protein cooperates with a second viral oncoprotein, E7, during tumor formation and immortalization or transformation of primary human cells (16, 30, 40). The expression of the E6 protein of HPV16 alone was found to affect malignant tumor progression in chemically induced skin tumors (40). We chose to test MnPV E6 transgenic mice of the strongly positive line HP22-42 in a two-stage mouse skin carcinogenesis assay using DMBA as an initiator and TPA as a tumor promoter. This treatment results in the formation of papillomas, keratoacan-thomas, and squamous cell carcinomas almost exclusively as a consequence of the clonal selection and expansion by TPA of keratinocytes carrying a DMBA-induced mutation in the H-ras
gene (8, 35).
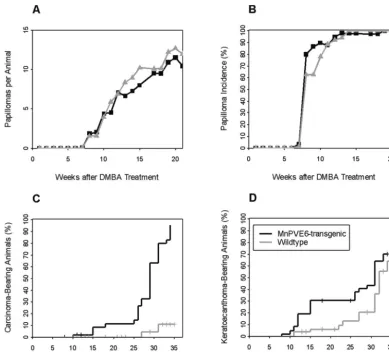
In MnPV E6 transgenic mice and wild-type littermates, treatment with DMBA and TPA predominantly produced pap-illomas. Less frequent tumor types were diagnosed as squa-mous cell carcinomas and keratoacanthomas (Fig. 2). The in-cidence of papillomas and the papilloma number per surviving animal were monitored weekly over a period of 36 weeks after DMBA initiation. The mean papilloma number per surviving animal was associated with large variability (variation coeffi-cient, 80 to 120% in both groups). No significant differences were found between the numbers and incidences of papillomas for transgenic and wild-type mice (Fig. 3A and B). MnPV E6 transgenic mice, however, developed 27 squamous cell carci-nomas, while only 2 squamous cell carcinomas grew in the nontransgenic animals. Most squamous cell carcinomas
on November 8, 2019 by guest
http://jvi.asm.org/
peared after cessation of TPA treatment between weeks 21 and 36. By using the Kaplan-Meier estimator, taking into account animals that had to be sacrificed prior to the end of the ob-servation time due to large carcinoma size, the percentages of animals with carcinomas were calculated at the end of the treatment to exceed 90% among MnPV E6 transgenic mice while reaching⬃10% among the nontransgenic control mice (Fig. 3C). The difference in carcinoma incidence evaluated over the whole study period was highly significant (log rank test;P⬍0.0001).
A statistically significant difference (P⫽0.02) was also ob-served for the incidence of keratoacanthomas: MnPV E6 transgenic mice developed keratoacanthomas earlier than their nontransgenic littermates (Fig. 3D). No tumors devel-oped in groups of mice treated with acetone only, DMBA-acetone, or acetone-TPA. This result suggests that the MnPV E6 transgene acts neither as an initiator nor as a tumor pro-moter of mouse skin carcinogenesis.
In a second MnPV E6 transgenic mouse line, HP21-2, we obtained essentially the same results by using the same
proto-FIG. 1. Transgene expression in MnPV E6 transgenic mice. (A) Schematic representation of the transgene construct. K14, promoter of the humancytokeratin-14gene;-glo, 5⬘untranslated region of the rabbit-globin gene with intron; E6, coding region of the MnPV E6 gene; IRES, IRES element of encephalomyocarditis virus; LacZ,Escherichia coli lacZgene encoding the reporter enzyme-galactosidase; pA, simian virus 40 polyadenylation signal. (B and C) Reporter gene expression monitored by-galactosidase activities in 15-day old transgenic mouse embryos (lateral views). Transgene expression in embryos from the lines with the strongest expression, such as HP22-42 (B), was seen in the epidermis and developing hair follicles all over the body, whereas in embryos of weakly expressing lines, such as HP22-12 (C), transgene expression was restricted to the epidermis of the extremities, face, ears, and prominent early-developing hair follicles, such as the whiskers. (D and E) In the skin of adult transgenic mice, expression of the transgene was confined to the epidermis and hair follicles. The scale bar in panel E corresponds to 50m (D and E) and 0.9 mm (B and C). Nontransgenic animals showed no-galactosidase activities on day 15 of development or in adult skin (not shown). (F) E6 expression in primary keratinocytes from MnPV E6 transgenic lines analyzed by RT-PCR. Lanes: wt, wild-type control; Mn tumor, positive control with RNA from MnPV-infected tumor tissue fromM. natalensis; HP22-42, HP21-2, HP21-13, HP22-3, HP22-21, HP22-12, HP21-23, and HP21-24, RT-PCR products with RNAs from different MnPV E6 transgenic lines. Top, PCR products for the amplified MnPV E6 gene; bottom, amplified fragments of the-actin control. No PCR products were obtained with samples without RT.
on November 8, 2019 by guest
http://jvi.asm.org/
FIG. 2. Histological analysis of skin tumors from MnPV E6 transgenic mice developed during chemical carcinogenesis. All tumors were paraffin embedded, cut into 2-m-thick sections, and stained with hematoxylin-eosin. (A) Papilloma developed during week 12 of chemical-carcinogenesis treatment. The papillomas showed hyperplasia of the squamous cell layer (left arrow), a typical hyperkeratinized layer (middle arrow), and an intact basal membrane (lower arrow) (magnification,⫻50). (B) Squamous cell carcinoma developed during week 10 of treatment (overview). The arrow shows the infiltration of the carcinoma into the underlying dermis (magnification,⫻25). (C) Enlargement of boxed portion of panel B showing typical carcinoma-like areas, including cells with enhanced mitosis (upper arrow). Infiltrations from carcinoma into the muscle layer are clearly visible (middle and lower arrow). (D) Keratoacanthoma developed during week 12 of treatment (overview) (magnification,⫻25) showing a horn-filled crater and clear demarcation of the muscle layer (lower arrow). (E) Enlargement of boxed area in panel D showing dysplastic epithelia (upper arrow), keratin pearls (middle arrow), and inflammation areas (lower arrow), typical characteristics of keratoacanthomas (magnification, ⫻25).
on November 8, 2019 by guest
http://jvi.asm.org/
col (data not shown). Thus, our data point to an involvement of the MnPV E6 protein in the malignant progression of chem-ically induced skin neoplasias (41).
Given that the MnPV E6 protein is involved in malignant expression, we reasoned that the expression level of the trans-gene may be increased in squamous cell carcinomas carrying normalrasalleles. To scrutinize this assumption, RNAs from benign and malignant tumors of MnPV E6 transgenic mice were isolated for RT-PCR using specific primers for MnPV E6 and-actin (Fig. 4B). As depicted in Fig. 4C, all squamous cell carcinomas lacking an H-ras mutation expressed at least a threefold-higher steady-state level of E6 (as calculated by phosphorimager analysis) than benign tumors, using-actin as an internal reference. In addition, we also examined a cell line established from a DMBA-TPA-induced squamous cell carci-noma of the MnPV E6 transgenic line HP22-42 for the rele-vant mutation of H-ras(Fig. 4, HP22-42 scc cell line). In agree-ment with the tumor data, these cells also expressed high levels of MnPV E6 and contained nonmutatedrasalleles.
The molecular mechanism by which E6 enhances malignant progression remains unknown. The ability of E6 proteins from high-risk anogenital HPV types to interfere with p53-regulated pathways and to cause genomic instability may be involved in this process. It has indeed been reported that the absence of p53 enhances the malignant progression of DMBA-TPA-in-duced tumors (23). Moreover, high-risk HPV E6 protein is known to neutralize p53 by binding to p53 and the ubiquitin ligase E6-AP, thereby recruiting the latter to p53 and trigger-ing its proteasome-mediated degradation (38). Interaction with E6-AP and an efficient degradation of p53 has not yet been shown for E6 proteins of cutaneous HPV types (12). Further studies are required to test whether MnPV E6 can induce p53 degradation or whether it interferes with p53-reg-ulated processes.
H-rasgene is not activated in chemically induced skin
tu-mors with high expression of MnPV E6. The majority of
[image:6.603.97.487.68.424.2]tu-mors generated according to the two-stage carcinogenesis pro-tocol are known to harbor DMBA-induced activating ras FIG. 3. Time course of tumor formation in mice treated initially with 100 nmol of DMBA, followed by treatment with 5 nmol of TPA as described in the text. The number of tumors was examined weekly to the end of the observation time. MnPV E6 transgenic mice (solid lines) developed similar average numbers of papillomas per mouse (A), with time courses (B) similar to those of nontransgenic (wild-type) controls (shaded lines) during the 21 weeks of initiation and promotion. After the end of tumor promotion, the average number of papillomas decreased in both groups due to spontaneous regression of papillomas (not shown). MnPV E6 transgenic mice showed a highly significant increase in the percentage of carcinoma-bearing animals compared to wild-type controls throughout the time of the experiment (C), as well as an earlier appearance of keratoacanthomas (D). No tumors were found in mice with mock treatments.
on November 8, 2019 by guest
http://jvi.asm.org/
mutations (14, 22, 33, 35). The most frequent mutation re-sulted in a CAA-to-CTA transversion of codon 61 in the H-ras
gene (11). In addition, an activated H-raswas found to coop-erate with HPV18 E6 in malignant cell transformation in tissue culture experiments (1). To investigate whether an activating mutation of H-rasis involved in tumor development in MnPV E6 transgenic animals, we PCR amplified the relevant part of the H-rasgene after RNA and DNA isolation and digested the product with XbaI, allowing the detection of activating muta-tions at position 2 in codon 61 by virtue of an XbaI restriction fragment length polymorphism (24). Seventy-one tumors, in-cluding papillomas, keratoacanthomas, and squamous cell car-cinomas, derived from MnPV E6 transgenic mice and 45 tu-mors from control mice were screened for mutations by this method. We found mutations of H-rasat the second position of codon 61 in 24% of MnPV E6 transgenic tumors, in contrast to 70% in tumors obtained from nontransgenic mice. Figure 5A shows a panel of tumors received from nontransgenic and MnPV E6 transgenic mice (papillomas, keratoacanthomas, and squamous cell carcinomas), revealing in some tumors a mutated H-ras allele in a heterozygous state. To determine which allele was transcribed, RT-PCR was performed. The ratio of the transcription efficiencies of the two H-rasalleles corresponds to that obtained in DNA analysis (compare Fig. 5A and B).
Additionally, we evaluated the tumors for the presence of other activating mutations of the H-rasand K-rasgenes, espe-cially codons 12, 13, and 61, by PCR amplification and direct sequencing of PCR products (Table 1). In all tumors of MnPV E6 transgenic mice and wild-type controls, no mutations were found in codons 12, 13, and 61 of K-ras. Also, no mutation in codons 12 and 13 of H-raswas detectable, in accordance with previous results using DMBA (22, 35). Thirteen of 34 E6 transgenic papillomas contained an A-to-T transversion at the
[image:7.603.59.265.68.193.2]second position of codon 61, in comparison to 24 out of 34 in the nontransgenic littermates, where single CAA-to-CTT and CAA-to-wAG transversions were also found (w indicates that both nucleotides A and T were detected at the same position). A lower mutation rate was also recognized for transgenic keratoacanthomas. Four of 14 MnPV E6 transgenic kerato-acanthomas harbor the A-to-T mutation in the middle base of
[image:7.603.307.536.70.182.2]FIG. 4. H-rasactivation in comparison to MnPV E6 expression level. Tumors induced during two-stage carcinogenic treatment were tested for H-rasactivation. All MnPV E6 transgenic squamous cell carcinomas containing high levels of E6 had no mutations in H-ras. (A) Selection of PCR products for amplified H-rasgene in squamous cell carcinomas (scc), keratoacanthomas (kerato.), and papillomas (pap.) derived from MnPV E6 transgenic (tg) mice. The HP22-42 scc cell line was established from a squamous cell carcinoma developed during tumorigenic treatment. No mutations in H-raswere detected in malignant MnPV E6 transgenic tumors or the HP22-42 cell line. (B) For quantification of the MnPV E6 expression level, PCR was performed using specific MnPV E6 and-actin primers for reference. (C) Hybridization of signals (shown in panel B) using Southern blot-ting technique followed by phosphorimager quantification.
FIG. 5. Activation of H-ras in skin tumors developed in line HP22-42 of MnPV E6 transgenic mice during chemical carcinogenesis. The presence of the activating A-to-T transversion of the second nu-cleotide of codon 61 of the H-rascoding region generates a diagnostic XbaI restriction enzyme recognition site. Tumors were analyzed using restriction fragment length polymorphism. The following cell lines were used for control: SN161, derived from squamous cell carcinoma (a gift from A. Balmain, University of California—San Francisco); MK2 immortalized BALB mouse keratinocytes (a generous gift from S. Aaronson, National Institutes of Health); and 3P2, derived from a squamous cell carcinoma induced in NMRI mice by chemical carcino-genesis using DMBA-aplysiatoxin (R. Schmidt, unpublished data). (A) PCR products for amplified H-rasgene in tumors (papillomas [pap.], keratoacanthomas [kerato.], and squamous cell carcinomas [scc]) derived from wild-type (wt) controls and MnPV E6 transgenic (tg) mice and control cell lines. (B) RT-PCR products for H-rasgene amplified after RNA isolation in the same tumors and cell lines as for panel A.
TABLE 1. rasgene mutations in skin tumors
Genotype Histologya gene andMutant
codon Mutation
b No. of mutanttumors/total
no. tested
Wild-type Papilloma 0/34
H-ras13 0/34
H-ras61 CAA3CwA 24/34 H-ras61 CAA3CTT 1/34 H-ras61 CAA3wAG 1/34 Keratoacanthoma H-ras12 0/9
H-ras13 0/9
H-ras61 CAA3CwA 4/9
SCC H-ras12 0/2
H-ras13 0/2
H-ras61 CAA3CwA 1/2 E6 transgenic Papilloma H-ras12 0/34
H-ras13 0/34
H-ras61 CAA3CwA 13/34 Keratoacanthoma H-ras12 0/14
H-ras13 0/14
H-ras61 CAA3CwA 4/14
SCC H-ras12 0/23
H-ras13 0/23
H-ras61 CAA3CwA 0/23
aSCC, squamous cell carcinoma.
bw indicates that both nucleotides, A and T, were detected at the same
position. No mutations were found in codons 12, 13, and 61 of K-ras.
on November 8, 2019 by guest
http://jvi.asm.org/
[image:7.603.300.541.484.699.2]codon 61, in contrast to 4 of 9 tumors in the wild-type controls. Of particular interest is the mutation incidence in malignant tumors. One of the two carcinomas obtained in wild-type mice harbored an A-to-T transversion at position 2 of codon 61. In contrast, no mutations were detectable in 23 tested squamous cell carcinomas of the MnPV E6 transgenic mouse line.
The majority of papillomas and keratoacanthomas and all squamous cell carcinomas in MnPV E6 transgenic animals carry normalrasalleles. Nevertheless, their formation critically depended on initiation by DMBA, which induces an activating
rasmutation in wild-type skin. This may indicate that in MnPV E6 transgenic mice, the development ofras-mutated papillo-mas is impaired in the presence of the transgene protein. Also, keratinocytes initiated by DMBA-induced genetic alterations, other thanrasmutations, appear to be preferentially selected and expanded to papillomas with normalrasalleles, which in addition exhibit an accelerated transgene-dependent malig-nant progression. The wild-type papillomas show the known low progression rate. This observation may point to an addi-tional function of the MnPV E6 protein in tumor promotion of DMBA-initiated keratinocytes with normalrasalleles.
ACKNOWLEDGMENTS
We thank I. Moll for help with histological preparations, K. Wayss for tumor material from M. natalensis, A. Balmain (University of California—San Francisco) for providing SN161 cells, S. Aaronson (National Institutes of Health, Bethesda, Md.) for MK2 cells, A. Hun-ziker for sequencing the tumor samples, H. Poepperl for generation of the MnPV E6 transgenic mice, and B. Goedecke and G. Schulze for excellent animal care. M. Chen was supervised by H. Poepperl.
This work was supported by the Bundesministerium fu¨r Gesundheit, Bonn, Germany.
REFERENCES
1. Amtmann, E. and K. Wayss.1987. The Mastomys natalensis papillomavirus, p. 187–198.InN. P. Salzmann and P. M. Howley (ed.), The Papovaviridae, vol. 2. Plenum Press, New York, N.Y.
2. Arbeit, J. M., K. Mu¨nger, P. M. Howley, and D. Hanahan.1994. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 trans-genic mice. J. Virol.68:4358–4368.
3. Baker, C. C., W. C. Phelps, V. Lindgren, M. J. Braun, M. A. Gonda, and P. M. Howley.1987. Structural and transcriptional analysis of human papil-lomavirus type 16 sequences in cervical carcinoma cell lines. J. Virol.61:
962–971.
4. Balmain, A., M. Ramsden, G. T. Bowden, and J. Smith.1984. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin pap-illomas. Nature307:658–760.
5. Balmain, A., and K. Brown.1988. Oncogene activation in chemical carcino-genesis. Adv. Cancer. Res.51:147–182.
6. Boutwell, K.1964. Some biological effects of skin carcinogenesis. Prog. Exp. Tumor Res.4:207–250.
7. Boxman, I. L., L. H. Mulder, F. Noya, V. de Waard, S. Gibbs, T. Broker, F. ten Kate, L. Chow, and J. ter Schegget.2001. Transduction of the E6 and E7 gene of epidermodysplasia-verruciformis-associated human papillomavi-ruses alters human keratinocyte growth and differentiation in organotypic cultures. J. Investig. Dermatol.117:1397–1404.
8. Brown, K., M. Quintanilla, M. Ramsden, I. B. Kerr, S. Young, and A. Balmain.1986. v-rasgenes from Harvey and BALB murine sarcoma viruses can act as initiators of two-stage mouse skin carcinogenesis. Cell46:447–456. 9. Byrne, C., M. Tainsky, and E. Fuchs.1994. Programming gene expression in
developing epidermis. Development120:2369–2383.
10. Campo, M. S.1997. Bovine papillomavirus and cancer. Vet. J.154:175–188. 11. Chakravarti, D., J. C. Pelling, E. L. Cavalieri, and E. G. Rogan.1995. Relating aromatic hydrocarbon-induced DNA adducts and c-H-ras muta-tions in mouse skin papillomas: the role of apurinic sites. Proc. Natl. Acad. Sci. USA92:10422–10426.
12. Elbel, M., S. Carl, S. Spaderna, and T. Iftner.1997. A comparative analysis of the interactions of the E6 proteins from cutaneous and genital papillo-maviruses with p53 and E6AP in correlation to their transforming potential. Virology239:132–149.
13. Fu¨rstenberger, G., and A. Kopp-Schneider.1995. Malignant progression of papillomas induced by initiation-promotion protocol in NMRI mouse skin. Carcinogenesis16:61–69.
14. Greenhalgh, D. A., X. J. Wang, J. A. Rothnagel, J. N. Eckhardt, M. I. Quintanilla, J. L. Barber, D. S. Bundman, M. A. Longley, R. Schlegel, and D. R. Roop.1994. Transgenic mice expressing targeted HPV-18 E6 and E7 oncogenes in the epidermis develop verrucous lesions and spontaneous, Ha-ras activated papillomas. Cell Growth Differ.5:667–675.
15. Griep, A. E., R. Herber, S. Jeon, J. K. Lohse, R. R. Dubielzig, and P. F. Lambert.1993. Tumorigenicity by human papillomavirus type 16 E6 and E7 in transgenic mice correlates with alterations in epithelial cell growth and differentiation. J. Virol.67:1373–1384.
16. Hawley-Nelson, P., K. H. Vousden, N. L. Hubbert, D. R. Lowy, and J. T. Schiller.1989. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J.8:3905–3910.
17. Hecker, E., N. E. Fusenig, W. Kunz, F. Marks, and H. W. Thielmann.1982. Carcinogenesis: a comprehensive survey. Raven Press, New York, N.Y. 18. Hennings, H., A. B. Glick, D. T. Lowry, L. S. Krsmanovic, L. M. Sly, and
S. H. Yuspa.1993. FVB/N mice: an inbred strain sensitive to the chemical induction of squamous cell carcinomas in the skin. Carcinogenesis14:2352– 2358.
19. Herber, R., A. Liem, H. Pitot, and P. F. Lambert.1996. Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J. Virol.70:1873–1881.
20. Hogan, B., R. Beddington, F. Constantini, and L. Lacy (ed.).1994. Manip-ulating the mouse embryo: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
21. Jablonska, S., S. Majewski, S. Obalek, and G. Orth.1997. Cutaneous warts. Clin. Dermatol.15:309–419.
22. Kazuihiro, I., K. Nakamura, K. Nakao, S. Shimizu, H. Harada, T. Ichise, J. Miyoshi, Y. Gondo, T. Ishikawa, A. Aiba, and M. Katsuki.2000. Target deletion of the H-ras gene decreases tumor formation in mouse skin carci-nogenesis. Oncogene19:2951–2956.
23. Kemp, C. J., L. A. Donehower, A. Bradley, and A. Balmain.1993. Reduction of p53 gene dosage does not increase initiation or promotion but enhances malignant progression of chemically induced skin tumors. Cell74:813–822. 24. Lambert, P. F., H. Pan, H. C. Pitot, A. Liem, M. Jackson, and A. E. Griep.
1993. Epidermal cancer associated with expression of human papillomavirus type 16 E6 and E7 oncogenes in the skin of transgenic mice. Proc. Natl. Acad. Sci. USA90:5583–5587.
25. Majewski, S., and S. Jablonska.2002. Do epidermodysplasia verruciformis human papillomaviruses contribute to malignant and benign epidermal pro-liferations? Arch. Dermatol.138:649–754. (Erratum,138:1104.)
26. Malumbres, M., and M. Barbacid.2003. RAS oncogenes: the first 30 years. Nat. Rev. Cancer.3:459–465.
27. Mantovani, F., and L. Banks.2001. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene20:7874–7887. 28. Meyer, T., R. Arndt, I. Nindl, C. Ulrich, E. Christophers, and E. Stockfleth.
2003. Association of human papillomavirus infections with cutaneous tumors in immunosuppressed patients. Transpl. Int.16:146–153.
29. Mu¨ller, H., and L. Gissmann.1978. Mastomys natalensis papilloma virus (MnPV), the causative agent of epithelial proliferations: characterization of the virus particle. J. Gen. Virol.41:315–323.
30. Mu¨nger, K., W. C. Phelps, V. Bubb, P. M. Howley, and R. Schlegel.1989. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J. Virol.
63:4417–4421.
31. Nelson, M. A., B. W. Futscher, T. Kinsella, J. Wymer, and G. T. Bowden.
1992. Detection of mutant Ha-ras genes in chemically initiated mouse skin epidermis before the development of benign tumors. Proc. Natl. Acad. Sci. USA89:6398–6402. (Erratum,90:781, 1993.)
32. Orth, G., M. Favre, and F. Breitbund.1980. Epidermodysplasia verrucifor-mis: a model for the role of papillomaviruses in human cancer, p. 259–282. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
33. Pellison, I., Y. Chardonnet, S. Euvrard, and D. Schmitt.1993. Low incidence of c-Ha-ras gene mutations in benign and malignant cutaneous lesions from transplant recipients. Int. J. Cancer55:915–920.
34. Pfister, H.2003. Human papillomavirus and skin cancer. J. Natl. Cancer Inst. Monogr.31:52–56.
35. Quintanilla, M., K. Brown, M. Ramsden, and A. Balmain.1986. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogen-esis. Nature322:78–80.
36. Reinacher, M., H. Muller, W. Thiel, and R. L. Rudolph.1978. Localization of papillomavirus and virus-specific antigens in the skin of tumor-bearing Mastomys natalensis (GRA Giessen). Med. Microbiol. Immunol. (Berlin)
165:93–99.
37. Rous, P., and J. W. Beard.1935. The progression to carcinoma of virus-induced rabbit papillomas (Shope). J. Exp. Med.62:523–548.
38. Scheffner, M., J. M. Huibregtse, R. D. Vierstra, and P. M. Howley.1993. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell75:495–505.
39. Shamanin, V., H. zur Hausen, D. Lavergne, C. M. Proby, I. M. Leigh, C.
on November 8, 2019 by guest
http://jvi.asm.org/
Neumann, H. Hamm, M. Goos, U. F. Haustein, E. G. Jung, et al.1996. Human papillomavirus infections in nonmelanoma skin cancers from renal transplant recipients and nonimmunosuppressed patients. J. Natl. Cancer. Inst.88:802–811.
40. Song, S., A. Liem, J. A. Miller, and P. F. Lambert.2000. Human papilloma-virus types 16 E6 and E7 contribute differently to carcinogenesis. Virology
267:141–150.
41. Song, S., H. C. Pitot, and P. F. Lambert.1999. The human papillomavirus type 16 E6 gene alone is sufficient to induce carcinomas in transgenic ani-mals. J. Virol.73:5887–5893,
42. Tan, C. H., R. Tachezy, M. Van Ranst, S. Y. Chan, H. U. Bernard, and R. D. Burk.1994. The Mastomys natalensis papillomavirus: nucleotide sequence, genome organization, and phylogenetic relationship of a rodent papilloma-virus involved in tumorigenesis of cutaneous epithelia. Virology198:534–541. 43. Vassar, R., M. Rosenberg, S. Ross, A. Tyner, and E. Fuchs.1989.
Tissue-specific and differentiation-Tissue-specific expression of a human K14 keratin gene in transgenic mice. Proc. Natl. Acad. Sci. USA86:1563–1567.
44. Wankell, M., B. Munz, G. Hu¨bner, W. Hans, E. Wolf, A. Goppelt, and S. Werner.2001. Impaired wound healing in transgenic mice overexpressing the activin antagonist follistatin in the epidermis. EMBO J.20:5361–5372. 45. Yee, S. P., and P. W. Rigby.1993. The regulation of myogenin gene
expres-sion during the embryonic development of the mouse. Genes Dev.7:1277– 1289.
46. Yuspa, S. H. 1994. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis—33rd G. H. A. Clowes Memo-rial Award Lecture. Cancer Res.54:1178–1189.
47. zur Hausen, H.1996. Papillomavirus infections–a major cause of human cancers. Biochim. Biophys. Acta1288:F55–F78.
48. zur Hausen, H.2000. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J. Natl. Cancer Inst.92:690– 698.
49. zur Hausen, H.2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer2:342–350.
![FIG. 5. Activation of H-ras[pap.], keratoacanthomas [kerato.], and squamous cell carcinomas[scc]) derived from wild-type (wt) controls and MnPV E6 transgenic(tg) mice and control cell lines](https://thumb-us.123doks.com/thumbv2/123dok_us/226200.57162/7.603.300.541.484.699/activation-keratoacanthomas-squamous-carcinomas-derived-controls-transgenic-control.webp)