Comparative study of the effects of intravenous palonosetron versus
ondansetron and dexamethasone for prevention of postoperative
nausea and vomiting (PONV) after laparoscopic cholecystectomy: A
prospective randomized control study.
A dissertation submitted to The Tamil Nadu Dr. MGR Medical University
in part fulfillment of the degree MD ANAESTHESIA
By
Dr. Karen Lynn Lee
Christian Medical College and Hospital – Vellore
Tamil Nadu – 632004
CERTIFICATE
This is to certify that the dissertation titled “Comparative study of the effects of
intravenous palonosetron versus ondansetron and dexamethasone for prevention of
postoperative nausea and vomiting (PONV) after laparoscopic cholecystectomy: A
prospective randomized control study” is the bonafide work of Dr. Karen Lynn Lee
in partial fulfillment of the requirements for the M.D Anaesthesia (final)
examinations of The Tamil Nadu Dr. M.G.R medical university to be conducted in
April 2017.
Signature:
Dr. Anna B. Pulimood, Principal,
Christian Medical College, Vellore
Dr. Sajan Phillip George, Head of Department, Anaesthesia,
Christian Medical College, Vellore
Dr. Raj Sahajanandan, Guide,
Department of Anaesthesia, Christian Medical College, Vellore
DECLARATION
I hereby declare that this dissertation titled “Comparative study of the effects of intravenous palonosetron versus ondansetron and dexamethasone for prevention of postoperative nausea and vomiting (PONV) after laparoscopic cholecystectomy: A prospective randomized control study” was prepared by me in partial fulfillment of the regulations for the award of the degree of M.D Anaesthesia of The Tamil Nadu Dr. M.G.R medical university, Chennai. This has not formed the basis for the award of any degree to me before and I have not submitted this to any other university previously.
Vellore Dr. Karen Lynn Lee
ACKNOWLEDGEMENTS
I acknowledge God, for all guidance, mercies and support.
Dr. Raj Sahajanadan for all that he has taught me and for mentoring me. Dr. Sajan Philip George for being supportive throughout the course.
I acknowledge all my teachers, for making this study and this course a reality. I also thank my family for being a constant source of support and encouragement.
Finally and most importantly, I would like to express my gratitude to all the patients for their participation.
TURNITIN REPORT
CONTENTS
INTRODUCTION ... 7
AIMS AND OBJECTIVES ... 10
REVIEW OF LITERATURE ... 13
DEFINITION ... 14
CAUSES OF NAUSEA AND VOMITING ... 21
POSTOPERATIVE NAUSEA AND VOMITING ... 25
MANAGEMENT OF POSTOPERATIVE NAUSEA AND VOMITING ... 32
METHODS ... 51
RESULTS ... 59
DISCUSSION ... 81
LIMITATIONS ... 85
CONCLUSION ... 86
BIBLIOGRAPHY ... 88
ANNEXURES ... 94
Annexure – 1 : Pro forma ... 94
Annexure – 2: Patient Information Sheet ... 96
Annexure – 3: Consent Form ... 98
Annexure – 4: Ethics committee approval ... 99
INTRODUCTION
INTRODUCTION
A wide variety of prophylactic antiemetic regimens have been used for the prevention of PONV. Many of the traditional antiemetics produce undesirable side effects and have limited efficacy. Therefore, the search for more ideal compounds has continued.
Ondansetron is considered as the “gold standard” of treatment when compared with the other antiemetics. The combination of ondansetron with dexamethasone has been found to be highly effective in the reduction of PONV.(5)(6) However, ondansetron has to be administered thrice daily when used alone and the addition of dexamethasone may be deleterious in diabetics as this may hamper optimum glycemic control. Palonosetron, a newer 5-hydroxytryptamine 3 (5-HT3) receptor antagonist that has recently been introduced and has a longer half-life and a better safety profile when compared to the older generation of 5-HT3 receptor antagonists such as ondansetron.(7) There is limited literature comparing the efficacy of palonosetron with ondansetron and dexamethasone, especially when comparing laparoscopic operations.
Aim of the study
Primary objectives
To compare the efficacy of a single dose palonosetron vs. a combination of ondansetron and dexamethasone for the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy.
Secondary Objective
1. To study the requirement for rescue anti-emetic medications
DEFINITION
Vomiting is defined as the expulsion of contents of the gastrointestinal tract through the oral cavity. This forceful expulsion of gastrointestinal contents is the end product of contraction of the upper gastrointestinal musculature and synchronous contraction of the muscles of the thoracoabdominal wall. This must be differentiated from regurgitation, which is generally used to define a condition where the passage of stomach contents into the mouth is in a passive manner. The term retching can be described as the presence of only the forceful muscular events present in vomiting without the actual expulsion of gastric contents. Nausea is described as a painless unpleasant feeling that vomiting is imminent(8)(9). This is a subjective sensation and the patient that feels nauseated may not actually retch or vomit. Vomiting may actually ease the sensation of nausea. This is differentiated from dyspepsia, which presents as epigastric burning pain, bloating or discomfort. Nausea may be present along with dyspepsia however they are two distinct events(10).
The complex mechanisms present in nausea include the CNS, the endocrine system, the autonomic nervous system, psychological states, and gastric dysrhythmias.
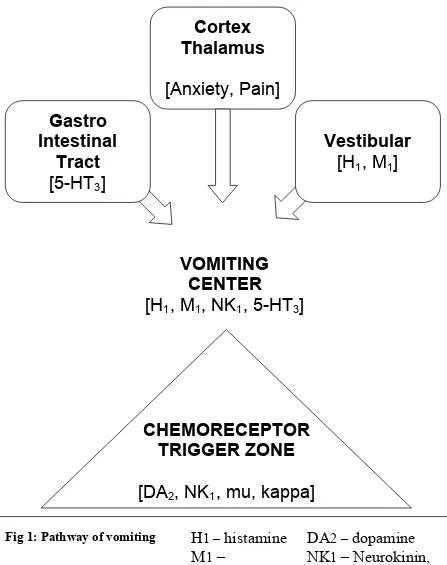
The medulla oblongata houses the vomiting center, which is comprised of the nucleus tractus solitaries and the reticular formation. Activation of this center causes motor efferents to descend to the upper gastro intestinal tract within cranial nerves V, VII, IX, X and XII, to the lower gastro intestinal tract via the vagal and sympathetic nerves and to the diaphragm and abdominal muscles via the spinal nerves.
DA
2 –dopamine
NK
1– Neurokinin,
substance P
mu/kappa – Opioids
Cortex
Thalamus
[Anxiety, Pain]
Gastro
Intestinal
Tract
[5-HT
3]
Vestibular
[H
1, M
1]
VOMITING
CENTER
[H
1, M
1, NK
1, 5-HT
3]
CHEMORECEPTOR
TRIGGER ZONE
[DA
2, NK
1, mu, kappa]
H
1 –histamine
M
1–
acetylcholine
5-HT
3 – [image:16.612.80.527.42.607.2]serotonin
The Central Nervous system
Although the neurocircuitry in the process of vomiting has been better illustrated, the central mechanism that results in nausea is yet to be clearly explained(12). Autonomic responses such as sweating or salivation may be seen during nausea or vomiting. These are brought about by the medulla oblongata. The chemoreceptor trigger zone (CTRZ) is found between the floor of the IV ventricle and the medulla oblongata. This receptor, unlike other centers in the brain is not shielded by the blood-brain barrier. This implies that due to lack of the surrounding glial cells, emetic irritants directly permeate the endothelium of its capillaries irrespective of the size of the molecule or its lipid solubility. They then relay information to the nucleus tractus solitarius. Afferent fibers from the gastro intestinal tract are carried by the vagus nerve. They detect the gastric contents as well as the gastric tone, which is also projected to the nucleus tractus solitarius. From the nucleus tractus solitarius the neurons carry impulses to a central pattern generator that coordinates the many actions that result in vomiting(13).
The Autonomic Nervous system
The physiological changes seen before vomiting such as pallor, tachycardia, increased blood pressure, cutaneous vasoconstriction, sweating and decreased gastrointestinal motility are directed by the autonomic nervous system. Afferent signals arise from vagal inputs that are a result of chemical or mechanical stimuli(16). Many studies now show that an increased perception of nausea is seen with increased sympathetic and decreased parasympathetic modulation(17)(18). This explains the presence of the symptoms mentioned earlier. Therefore autonomic outflows as well as the CNS network that controls it may in fact determine the overall intensity of nausea that is perceived. Understanding these in detail could have therapeutic importance.
The Endocrine System
mediator in the functioning of the foregut. It stimulates the inhibitory motor nerves that are present in the dorsal motor nucleus of the vagal nerve and causes delayed gastric emptying along with nausea(22).
Gastric dysrhythmias
PATHOGENESIS OF NAUSEA – in summary
Cerebral cortex and limbic system – providing
cognitive and emotional input
Cerebellar and Vestibular signals from motion induced nausea
Area prostema recognizes the presence of emetic agents in blood
Nucleus Tractus Solitarius receives afferent
information from various parts of the body including the Vagus
Increase in Vasopressin level Autonomic nervous system response
Gastric
Dysrhythmias
Nausea
Vagus mediated
CENTRAL
CAUSES OF NAUSEA AND VOMITING
MEDICATIONS AND TOXIC ETIOLOGIES(24)
Cancer chemotherapy Antibiotics/antivirals
Mild -fluorouracil, vinblastine, tamoxifen Erythromycin Moderate - etoposide, methotrexate, cytarabine Tetracycline Severe - cisplatinum, dacarbazine Sulfonamides
Analgesics Antituberculous drugs
Aspirin Acyclovir
Nonsteroidal antiinflammatory drugs Gastrointestinal medications
Antigout drugs Sulfasalazine
Cardiovascular medications Azathioprine
Digoxin CNS active drugs
Antihypertensives Narcotics
Antiarrhythmics Antiparkinsonian drugs
Calcium channel antagonists Anticonvulsants
Beta blockers Antiasthmatics
Diuretics Theophylline
Hormonal preparations/therapies Radiation therapy
Oral anti diabetics Ethanol abuse
INFECTIOUS CAUSES
Gastroenteritis Nongastrointestinal infections
Viral Otitis media
Bacterial
DISORDERS OF THE GUT AND PERITONEUM
Mechanical obstruction Organic gastrointestinal disorders
Gastric outlet obstruction Pancreatic adenocarcinoma
Small bowel obstruction Inflammatory intraperitoneal disease
Functional gastrointestinal disorders Peptic ulcer disease
Gastroparesis Cholecystitis
Chronic intestinal pseudo-obstruction Pancreatitis
Nonulcer dyspepsia Hepatitis
Irritable bowel syndrome Crohn disease
Mesenteric ischemia
CNS CAUSES
Migraine Psychiatric disease
Increased intracranial pressure Psychogenic vomiting
Malignancy Anxiety disorders
Hemorrhage Depression
Infarction Pain
Abscess Anorexia nervosa
Meningitis Bulimia nervosa
Congenital malformation Labyrinthine disorders
Hydrocephalus Motion sickness
Pseudotumor cerebri Labyrinthitis
Seizure disorders Tumors
Demyelinating disorders Ménière disease
ENDOCRINOLOGIC AND METABOLIC CAUSES
Pregnancy
Other endocrine and metabolic
Uremia
Diabetic ketoacidosis Hyperparathyroidism Hypoparathyroidism Hyperthyroidism Addison's disease
Acute intermittent porphyria
MISCELLANEOUS CAUSES
Postoperative nausea and vomiting Cyclic vomiting syndrome
Cardiac disease
Myocardial infarction
Heart failure
POSTOPERATIVE NAUSEA AND VOMITING
Nausea and vomiting are one of the most common complaints that follow surgery under general anaesthesia. It has been described as “the big little problem” that complicates postoperative recovery(25). 18 months after the introduction of chloroform to anaesthesia, Sir John Snow in 1848 was the first to extensively describe the phenomenon of PONV(1). Older inhalational anaesthetics such as chloroform and ether were used before the 1960s; the incidence of postoperative vomiting at that time was as high as 60%(26). Advances in the field of anaesthesia with better techniques as well as the development of newer anti-emetics and shorter acting anaesthetic drugs has decreased the overall incidence of PONV in the first 24hours to around 30%(27)(28). However high risk patients have an incidence of up to 80% following surgery(29). Therefore nausea and vomiting that follows surgery is known to be multifactorial as it includes anaesthetic factors, surgical factors as well as individual patient risk factors(30)(31).
postoperative care unit, cause an increase unanticipated hospital admission in out-patients and also significantly increase overall costs of health care(3)(34).
There are numerous anti-emetic drugs that are available in the market today that have been proven to be safe. However there is no drug that is available that does not have side effects. These side effects can be mild, for example headache may occur with ondansetron or can even be severe, for example QT prolongation that is also seen with ondansetron. Therefore it is important to identify those individuals who are at risk of developing nausea and vomiting after surgery and reduce it with appropriate anti-emetic prophylaxis without the unnecessary risk of side effects of medications(29).
The etiology of postoperative nausea and vomiting is often multifactorial. These factors can be classified under the following -
1. Patient factors
Patient Factors
Gender:
The female gender has been consistently noted as the strongest risk factor for developing postoperative nausea and vomiting. Women in the reproductive age group are three time more likely to suffer from PONV than men thereby suggesting the involvement of hormones(28)(2).
Smoking Status:
Non-smokers have double the risk of developing PONV. Although the protective mechanism is unknown, it has been suggested that cytochrome P450 enzyme is induced by the polycyclic aromatic hydrocarbons found in cigarette smoke. This increases the metabolism of the emetogenic anaesthetic agents(35).
History of PONV or motion sickness:
Patients who suffer from motion sickness, past history of nausea or vomiting following surgery or both are 2-3 times more likely to develop PONV(36).
Age:
Delayed gastric emptying:
Those patients with diabetes mellitus, pregnancy, hypothyroidism, intra abdominal pathologies such as pyloric stenosis, and increased intra cranial tension that have delay in gastric emptying are also at the risk of developing PONV.
Obesity:
Although several studies have suggested that an increased BMI may predispose the patient to develop nausea and vomiting after surgery, a systematic review done in 2001 showed no correlation.(37)
Anesthesia Factors
Inhalational agents:
Opioid use:
The use of opioids in the intraoperative and the postoperative period also increases the risk of developing postoperative nausea and vomiting, in a dose dependent manner. Opioids reduce the muscle tone and the peristaltic activity, causing delayed gastric emptying and inducing gastric distension. This triggers the vomiting reflex.
Duration of Anesthesia:
The duration of surgery and thereby anaesthesia can also predict the risk of developing nausea and vomiting in the postoperative period. The duration of exposure to emetogenic stimuli such as intraoperative opioids and volatile anaesthetics is also increased with prolonged duration of surgery.
Neostigmine:
Due to insufficient evidence it is not possible to conclude whether the dose of neostigmine causes an increase in the risk of nausea and vomiting following surgery.
Method of Anesthesia:
Surgical factors
Postoperative Factors
Early intake of food, postoperative pain, early ambulation and dizziness are said to be associated with increased incidence of nausea and vomiting.
Although various factors seem to determine the incidence of developing post operative nausea and vomiting, the data available is sometimes conflicting or of no clinical relevance. As per the consensus guidelines from 2014, the risk factors were classified based on the level of evidence as well as the clinical implication. (4)
RISK FACTORS FOR PONV – IN ADULTS
EVIDENCE RISKFACTORS
POSITIVE OVERALL
Female sex (B1)
History of PONV or motion sickness (B1) Nonsmoking (B1)
Younger age (B1)
General versus regional anesthesia (A1)
Use of volatile anesthetics and nitrous oxide (A1) Postoperative opioids (A1)
Duration of anesthesia (B1)
Type of surgery (cholecystectomy, laparoscopic, gynecological) (B1)
CONFLICTING
ASA physical status& BMI (B1) Menstrual cycle (B1)
Level of anesthetist’s experience (B1) Muscle relaxant antagonists (A2)
DISPROVEN OR OF LIMITED RELEVANCE
Anxiety (B1)
MANAGEMENT OF POSTOPERATIVE NAUSEA AND VOMITING
The goal of prophylaxis is to decrease the incidence of nausea and vomiting that follows surgery and thereby reduce the distress caused as well as the cost of health care. There are several guidelines that have been published on the management of postoperative nausea and vomiting. As per the most recent guidelines published in 2014, by Gan et al, the first step in management is to identify the patient’s risk of developing PONV. As discussed earlier, various risk factors are said to influence the incidence of developing PONV. However not all play a significant role as the data available is sometimes conflicting or of limited clinical relevance. In adults, the patient specific risk factors that were identified include a younger age, the female sex, and a past history of PONV or motion sickness and non-smoking status.
As seen with all drugs that are available, antiemetics also carry some risk of adverse effects that may occur. Therefore it is important to objectively assess the individual’s risk of developing postoperative nausea or vomiting and thereby decide on the need for antiemetic prophylaxis. Therefore it is essential to objectively assess the baseline risk for developing PONV by using a validated risk score. Although there are certain risk factors for PONV that have strong evidence, no single predictor is used to clinically decide on the need for antiemetic prophylaxis.(44) There are 2 risk scores that are commonly used in adults, the Apfel score and Koivuranta score.(45)
on the implications of vomiting in certain clinical scenarios such as oesophageal or gastric surgeries, increased intracranial pressures or in patients after facial surgeries with wired jaws.
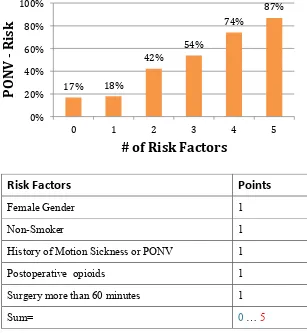
[image:33.612.160.467.331.664.2]Koivuranta et al. developed a scoring system that consisted of 5 predictors of PONV – Female sex, non-smoking status, history of motion sickness or PONV in the past, the use of opioids postoperatively and the duration of surgery more than 60 minutes. It is an additive score and the incidence of postoperative nausea and vomiting is 17%, 18%, 42%, 54%, 74% and 87% if 0, 1, 2, 3, 4 or 5 risk factors are present (28).
Fig. 2: Koivuranta score
Risk Factors Points
Female Gender 1 Non-Smoker 1 History of Motion Sickness or PONV 1 Postoperative opioids 1 Surgery more than 60 minutes 1 Sum= 0 … 5
17% 18%
42%
54%
74%
87%
0% 20% 40% 60% 80% 100%
0 1 2 3 4 5
P
O
N
V
-‐
R
isk
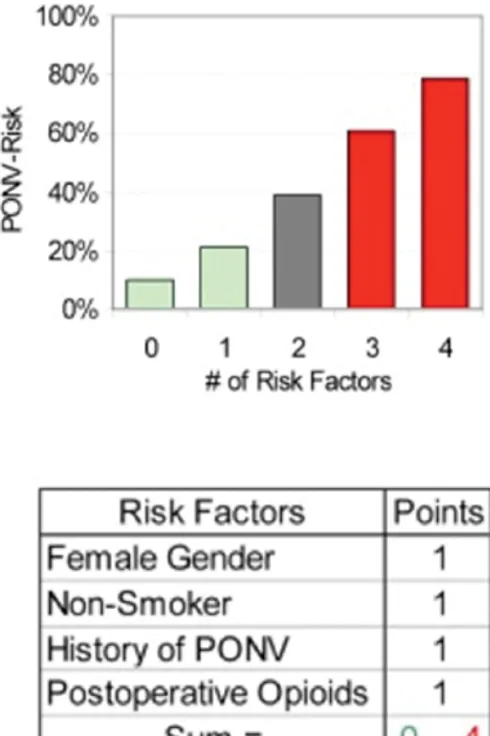
Subsequently Apfel et al developed a simplified scoring system that reduced the number risk factors in the scoring system from five to four, based on the data from Koivuranta et al. as well as their own previous data. As per the Apfel simplified score, 4 risk factors were taken into consideration for the prediction of PONV. Nonsmoking status, the female sex, history of motion sickness/ PONV and the use of postoperative opioids are included in this score. The presence of 0, 1, 2, 3 or 4 risk factors implies that the risk of PONV is 10%, 20%, 40%, 60% or 80% respectively.
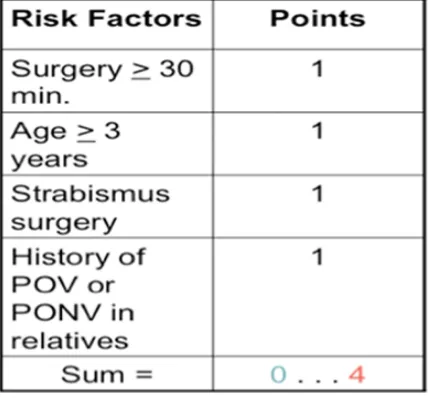
In children, postoperative vomiting (POV) can be predicted using a simple score described by Eberhart et al., which identifies 4 risk factors of developing POV – age of the child more than 3 years; duration of surgery more than 30 minutes; history of vomiting after surgery in the patient, sibling or parent; and strabismus surgery. The risk of postoperative vomiting was 9%, 10%, 30%, 55% and 70% with a risk score of 0, 1, 2, 3 or 4.
As per the 2014 guidelines, patients were categorized as “low”, “medium” or “high” risk based on their Apfel scores of 0 – 1, 2 – 3 and 4 respectively. Depending on which category the patient belonged to, antiemetic prophylaxis as well as anaesthetic management guidelines were suggested to prevent the occurrence of PONV.
It is also important to minimize the baseline risk factors for developing PONV. Strategies that were suggested include the preference of regional anaesthesia over general anaesthesia. The incidence of developing PONV after regional anaesthesia was almost 9 times less than after general anaesthesia. When it was necessary to give general anaesthesia, induction and maintenance of anaesthesia with propofol decreased the incidence of nausea and vomiting in the early postoperative period. According to the IMPACT study, which evaluated 6 strategies to reduce postoperative nausea and vomiting, it was found that total intravenous anaesthesia (TIVA) with propofol reduced the risk of PONV by 25% (46). Reducing the dose of intraoperative as well as postoperative opioids by using alternative methods of pain relief decrease the risk of PONV as well as hyperalgesia (27)(47) . Another simple strategy suggested was adequate hydration, which has also shown to decrease PONV (48).
5-‐Hydroxytryptamine (5-‐HT3) Receptor Antagonists
Radioligand binding studies have demonstrated a high density of 5HT3 receptors in areas known to be involved in the emetic reflex, including vagal afferent terminals in gastric mucosa, the brainstem dorsovagal nucleus, the nucleus tractus solitarius and the area postrema. It is possible that anesthetic agents may stimulate neurons within the area postrema and activate the vomiting reflex via 5 HT mediated pathway. Anesthetics can disrupt mucosal enterochromaffin cells and induce the release of paracrine transmitters, including 5HT, resulting in afferent vagal firing and this initiate the vomiting reflex. Gastrointestinal distension caused by diffusion of nitrous oxide, laparotomy and manipulation of gastro intestinal tract, can activate vagal afferents via mucosal 5HT release.
5HT3 antagonists belong to the class of antiemetics that act by blocking the 5HT3 receptor. The pathway involved in emesis consists of several sites, at which the 5HT3 receptors are present, such as the vagal afferents, the nucleus tractus solitarius and also the area postrema. These drugs prevent the binding of serotonin to the 5HT3 receptor and thereby suppress nausea and vomiting. Based on their chemical structure they may be classified further into 3 classes. (51)
Fig.5: Chemical structure of 5HT3 antagonists
Carbazole derivatives:
Ondansetron belongs to this class of 5HT3 receptor antagonist. It is one of the most researched amongst its class of drugs and is often considered as the “gold standard” of antiemetics. Ondansetron is a selective antagonist at the 5HT3 receptor and is the first drug in the class of 5HT3 antagonist to be introduced for the clinical management of vomiting associated with chemotherapy and radiotherapy. Ondansetron has been in
Name Structure
Ondansetron
Granisetron
Dolasetron
Ramosetron
clinical development for the prevention and treatment of PONV since 1988. The initial literature recommended dose is 0.1 to 0.15 mg/kg and effective blood levels are attained 30-60 minutes after administration. The elimination half-life of ondansetron is
approximately 3.5-4 hours in adults. Due to its relatively short half-life it may be relevant to administer the drug intraoperatively towards the end of the surgical procedure,
especially those surgeries of more than 2 hours duration. Large multicenter studies have demonstrated that intravenously administered ondansetron 4 mg is effective in preventing and treating established PONV. This is confirmed in the 2014 consensus guidelines in the management of PONV by Gan etal The recommended intravenous dose of ondansetron is 4mg, usually at the end of surgery(4).
Farhat etal compared 4 mg ondansetron with 10 mg metoclopramide for prevention of PONV after laparoscopic cholecystectomy and found that prophylactic ondansetron was more effective with fewer side effects. Hemley compared ondansetron with droperidol and metoclopramide for laparoscopic cholecystectomy under propofol TIVA and found that PONV in the ondansetron group was significantly less in the 1 -4 hours, but similar in the rest of the 24 hours.
The adverse effects that are seen with the use of ondansetron include headache, diarrhea or constipation, dry mouth, and even QT prolongation.
Indole derivatives:
Indazole derivatives
Granisetron was found be as effective in the treatment of nausea and vomiting when compared to the other first generation 5HT3 receptor blockers. The dose needed was 0.3 to 3mg given intravenously. When compared to 8mg of dexamethasone, a 3mg dose of IV granisetron was found to be equally effective but when used as a combination it was found to be better than using each drug alone.(53)
Ramosetron has a higher affinity for the 5HT3 receptors than the older drugs in this class. The antiemetic property of this drug is present for more than 2 days. At a dose of 0.3mg IV, it is effective in preventing nausea and vomiting in patients that are on patient controlled analgesia (PCA) with fentanyl. It is available only in certain South East Asian countries. (54)
5HT3 receptor antagonists are usually well tolerated. Minor side effects of these medications include mild headaches seen in 15 to 20% of patient population or constipation that occurs in 5 – 10% of the population. Electrocardiogram interval changes, QT prolongation is seen in the first – generation 5HT3 receptor antagonists.
Corticosteroids
In 1980 methyl prednisolone was first reported to be an effective antiemetic in chemotherapy patients. Since 1981, dexamethasone has been reported to be more effective than metoclopramide in controlling chemotherapy induced nausea and vomiting and was preferred by the patients’ treated. The antiemetic effect of dexamethasone was reported to be equal or better than the 5HT3 receptor antagonists such as ondansetron and granisetron. Dexamethasone has also been reported to effective in reducing the incidence of postoperative nausea and vomiting (PONV) in paediatric patients undergoing strabismus repair, tonsillectomy, adenoidectomy, thyroidectomy, cholecystectomy, and women undergoing major gynecological surgery.
Dexamethasone when given as a prophylactic dose of 4mg intravenously is seen to effectively decrease nausea and vomiting following surgery (58)(59)(60). It is preferentially given after the induction of anaesthesia than at the end of surgery. A 4mg dose of dexamethasone IV is considered to be as efficacious as a 4mg IV dose of ondansetron or 1.25mg IV dose of droperidol.
Apart from addressing the problem of postoperative nausea and vomiting, dexamethasone also has a dose dependent effect on the quality of recovery. De Oliveira et al. compared a dose of 0.1mg/kg of dexamethasone with 0.05mg/kg and showed that with a higher dose there was less opioid requirement, less nausea, sore throat and muscle pain and thereby decreasing the time for discharge readiness(60).
double blind study. They concluded that dexamethasone was effective in the prevention of nausea and vomiting after laparoscopic cholecystectomy.(63)
Bianchin etal had similar results when he compared placebo vs 8 mg dexamethasone for prevention of PONV after laparoscopic cholecystectomy.
Dopamine Antagonists
Drugs belonging to this class, which exhibit antiemetic properties act on the D2 receptors. They are often used as rescue antiemetics. They may be classified into three-
• Phenothyazines (e.g. Promethazine) • Butyrophenones (e.g. Droperidol) • Benzamides (e.g. Metoclopromide)
Other Antiemetics
Neurokinin – 1 (NK-1) receptor antagonist
Aprepitant was found to have a greater antiemetic effect when compared with ondansetron. When used in combination with dexamethasone it was also found to be more effective in decreasing postoperative vomiting than the combination of ondansetron in a study done on patients undergoing craniotomy (64).
45
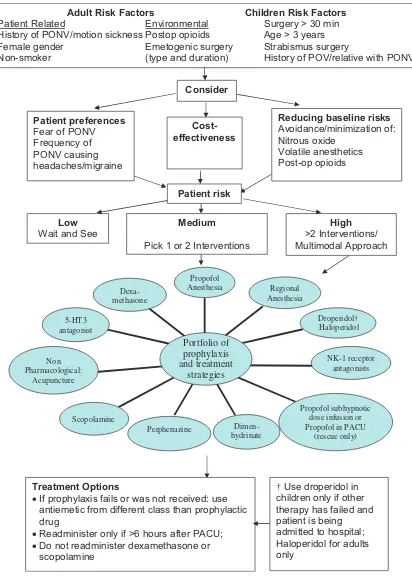
Consensus Guidelines for the Management of PONV
(5-HT3) receptor antagonists (ondansetron, dolasetron,
granisetron, tropisetron, ramosetron, and palonosetron), neurokinin-1 (NK-1) receptor antagonists (aprepitant, casopitant, and rolapitant), corticosteroids (dexametha-sone and methylprednisolone), butyrophenones (droperi-dol and haloperi(droperi-dol), antihistamines (dimenhydrinate and meclizine), and anticholinergics (transdermal scopolamine [TDS]). While PONV prevention is recommended in a
sub-prophylactic antiemetics to all patients who undergo surgi-cal procedures. However, with more inexpensive generics becoming available, properly conducted cost-effectiveness (C/E) studies need to be done to support the more uni-versal use of prophylactic antiemetics. Ondansetron 4 mg, droperidol 1.25 mg, and dexamethasone 4 mg were equally effective, and each independently reduced PONV risk by
approximately 25%.47 The recommended doses and timing
Adult Risk Factors Children Risk Factors
Patient Related Environmental Surgery > 30 min
History of PONV/motion sickness Postop opioids Age > 3 years
Female gender Emetogenic surgery Strabismus surgery
Non-smoker (type and duration) History of POV/relative with PONV
Cost-effectiveness
Consider
Low
Wait and See >2 Interventions/High
Multimodal Approach Dexa-methasone 5-HT3 antagonist Non Pharmacological: Acupuncture Scopolamine Perphenazine Dimen-hydrinate Propofol subhypnotic dose infusion or Propofol in PACU
(rescue only) NK-1 receptor antagonists Droperidol† Haloperidol Regional Anesthesia Propofol Anesthesia Portfolio of prophylaxis and treatment strategies Treatment Options
•If prophylaxis fails or was not received: use antiemetic from different class than prophylactic drug
•Readminister only if >6 hours after PACU;
•Do not readminister dexamethasone or scopolamine
†Use droperidol in children only if other therapy has failed and patient is being admitted to hospital; Haloperidol for adults only
Patient preferences
Fear of PONV Frequency of PONV causing headaches/migraine
Reducing baseline risks
Avoidance/minimization of: Nitrous oxide
Volatile anesthetics Post-op opioids
Medium
Pick 1 or 2 Interventions
[image:45.612.89.501.86.662.2]Patient risk
Ondansetron is generally considered as the gold standard in treating PONV (4). The efficacy of ondansetron in reducing PONV was proven with different anaesthetic agents as well (65). Since the early 1990s large randomized controlled studies and multi
centered studies have established the efficacy of 4mg of IV ondansetron in treating and preventing PONV. Eight mg of ondansetron has also been proven to also reduce the incidence of PONV in high-risk patients such as those with prior PONV history. However a metaanalysis done by Tramer et al highlighted some of the limitations of monotherapy for the management of PONV. Fifty-three trials studied proved the efficacy of ondansetron in reducing PONV was 20% at best. Therefore although ondansetron was the treating drug of choice newer drugs or combined therapy with other drugs needed to be explored (66).
With this in mind, literature emerged about the use of combination therapy of
ondansetron and dexamethasone for PONV prophylaxis and treatment. Many studies have shown the superiority of ondansetron plus dexamethasone.
Dexamethasone was found to have more effect than ondansetron in the late postoperative period (63). Combination antiemetic therapy has been seen to have better results in the prevention of PONV than using a single drug. Ondansetron has been compared with a combination of ondansetron and dexamethasone. Song et al outlined the superiority of the above-mentioned combination over ondansetron alone in a randomized control trial involving one hundred and thirty patients who received patient controlled analgesia with fentanyl. Four milligrams of ondansetron was administered to all patients undergoing thoracoscopic surgery and 12 mg was administered as boluses through patient controlled analgesic devices, the trial arm received 8 mg dexamethasone whereas the comparator arm did not receive dexamethasone. The combination of ondansetron with
dexamethasone was reported to be better in preventing and treating PONV over a 48-hour period (5). This was also confirmed by Ahsan et al in an RCT published in 2014. One hundred patients that underwent laparoscopic cholecystectomy were randomly assigned to receive ondansetron or ondansetron plus dexamethasone. The authors observed that the combination of dexamethasone and ondansetron was superior to ondansetron alone (6).Kumar et al compared a combination of ondansetron plus dexamethasone (4mg plus 8mg) vs either drug alone in the same dose and placebo in patients undergoing
laparoscopic cholecystectomy and found that the incidence was 85% in the placebo group 30% in dexamethasone group, 35% in ondansetron group and only 10% in the
combination group which was statistically significant.(67)
the efficacy of palonosetron with a placebo undergoing various surgeries. This double-blinded placebo controlled study divided patients into two arms, in one arm 0.075 mg of palonosetron was given and in the second arm normal saline was used. At 72 hours post operatively various parameters were assessed and the authors found PONV reduced significantly in the arm where palonosetron was used, 33% versus 47% at 0-24 hours and 6% versus 11% at 24 -72 hours. The study also proved that severity and intensity of nausea in the initial 24 hour period following the operation was reduced in the group that palonosetron was administered, however this difference although clinically significant did not show statistical significance (P value - 0.08) This has been corroborated by various other RCTs. Kovac et al in a study published in 2008 demonstrated the efficacy of palonosetron versus placebo in a randomized controlled trial involving over five hundred patients undergoing either gynaecological or breast surgery. Patients were randomly allocated to one of four groups – placebo vs. 0.025 mg of palonosetron vs. 0.050 mg palonosetron vs. 0.075 mg palonosetron. The primary end point analyzed was the ability to prevent vomiting with various doses. They concluded that a dose of 0.075mg was required for prevention of PONV(55).
study results confirmed that the incidence of PONV was significantly lower in the arm where palonosetron was given (42.2 percent versus 66.7 percentage). The severity of nausea as assessed by the visual analogue score was statistically equal between the two arms. The study suggested that palonosetron was better than ondansetron in preventing nausea and vomiting in the postoperative period (68).
The efficacy of a single dose palonosetron versus ondansetron was also studied by Kim et al. This study from Korea, randomly allotted one hundred patients to receive palonosetron as a single IV bolus of 0.075 mg versus ondansetron which was administered as a bolus dose of 8 mg intravenously following which 16 mg was added to the IV – PCA. The study revealed that a bolus of ondansetron followed by an infusion was equal in efficacy to a single dose of palonosetron in the prevention of PONV after laparoscopic gynaecological surgery (69). In a study done by Bhattacharjee et al,
consensus guidelines do not suggest the combination of palonosetron and dexamethasone, as both the drugs are long acting (24 hours) and it doesn’t offer any additional advantage.
The use of dexamethasone alone as PONV prophylaxis was also studied in patients undergoing laparoscopic cholecystectomies. Bianchin et al in 2007 published an RCT done on 80 patients that received either 8mh of dexamethasone or normal saline as placebo. The study showed that there was significant decrease in the incidence of PONV in the dexamethasone group with no side effects noted. (72)
METHODS
Design: A prospective blinded randomized controlled trial was designed. There were two arms in the study:
The patients were randomly allotted to either the ‘palonosetron group’ or ‘ondansetron plus dexamethasone group’.
Randomized groups
-Intervention: Palonosetron 0.075 mg IV
Comparator Agent: Dexamethasone 4 mg IV with Ondansetron 4mg IV
Inclusion and exclusion criteria
Inclusion Criteria:
-ASA grade I/II patients within the age group of 18 to 60 years scheduled to undergo laparoscopic cholecystectomy, under general anaesthesia.
Exclusion Criteria:
-Pregnant and lactating patients
-Patient with hypersensitivity to palonosetron, dexamethasone or ondansetron -Patients with history of motion sickness
Recruitment:
All patients who consented for the study and fulfilled the inclusion criteria were recruited by the principal investigator for the study.
Institutional review board clearance and ethics committee approval was obtained before the start of the study.
Method of randomization:
The patients were be randomized to one of two groups by a computer generated random assignment.
Method of allocation concealment:
Sequentially numbered, sealed, opaque envelopes opened in the operation theatre after the patient is anaesthetized.
Blinding and masking:
Allocation concealment opaque envelope
Intervention arm Comparator arm
Postoperative assessment for complaints of nausea or vomiting for 0-2 hours, 2-6 hours and 6-24 hours
Palonosetron 0.075 mg IV after induction of General Anaesthesia
Group A
Dexamethasone 4 mg IV after induction of General Anesthesia
+
Ondansetron 4mg IV 30 minutes prior to extubation
Group B
Inclusion criteria met -- >PISgiven- Consent and recruitment
Randomization - block randomization 2 or 4 or 6
In case of nausea or vomiting
Rescue antiemetics* are to be given and documented *Promethazine 6.25 to 12.5 mg IV
If it persists ondansetron 0.1mg/kg will be given with intension to treat
Sample size:
Proportion in the standard treatment 0.5 Proportion in the new treatment 0.5 Observed/Expected difference in proportions 0
Non-inferiority margin 0.2
Power (1- beta) % 80
Alpha Error % 5
Required sample size in each group 77
Statistical Analyses:
Statistical methods used for the primary outcome; include description of methods to estimate the strength of the effect (e.g.: Odds ratios, relative risks, etc.).
The comparison of the post-operative nausea and vomiting (primary outcome) were done using a chi-square test. The bootstrap 95% confidence interval was used to calculate the difference of proportions between the two methods. Risk estimates were calculated with 95% confidence interval.
All patients were explained about the study after giving them the information sheet (Annexure 2).
Informed consent (Annexure 3) was taken.
After informed consent, the details required as per the pro forma (Annexure 1) were collected.
They were all followed up during their stay in hospital. 67 patients were randomized into one of the 2 arms
Six patients had to be excluded due to logistical reasons such as lost randomization envelope and rescheduled surgeries.
Of the remaining 61 patients in the study, 2 were converted to open surgeries and 4 had protocol violation. Therefore55 patients were analyzed, 27 in the ‘palonosetron’ arm and 28 in the ‘ondansetron with dexamethasone’ arm.
Phase of trial: Phase 3
Duration of trial: 1 year
*Grp 1 = Palonosetron Grp 2 = Ondansetron with dexamethasone
Lost to follow-up (n= 0) Lost to follow-up (n=0)
Follow-‐Up
Analysed (n=27) Analysed (n=28)
Analysis
Excluded (n=6)
(n=2)envelope was misplaced due to logistical reasons and
n = 4 excluded as surgeries were rescheduled}
Randomized (n=61)
Enrollment
Assessed for eligibility (n=67))
Allocation
Allocated to intervention (Grp 1*) (n=30)
♦Received allocated intervention (n= 30) ♦Protocol violation (n = 3)
Allocated to intervention (Grp 2*) (n=31)
♦Received allocated intervention (n= 28) ♦Did not receive allocated intervention (n= 3)
♦Protocol violation (n = 1)
• Converted to open cholecystectomy
CATEGORICAL VARIABLES:
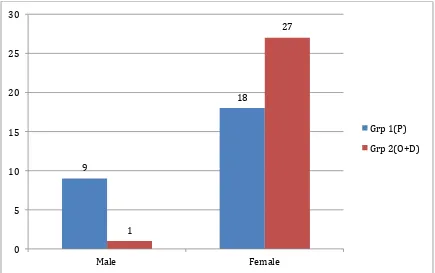
Sex distribution: Out of the total of 55 patients, 10 were male and 45 were females.
9
18
1
27
0 5 10 15 20 25 30
Male Female
Grp 1(P)
[image:60.612.71.506.229.502.2]Grp 2(O+D)
SEX DISTRIBUTION BETWEEN THE TWO ARMS:
In the intervention (Palonosetron) group 9 patients were males and 18 patients were females.
In the comparator (Ondansetron + Dexamethasone) group, 1 were males and 27 were females
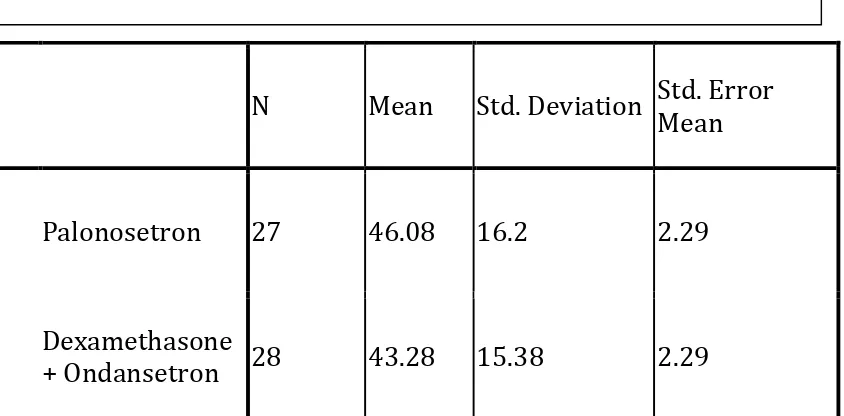
AGE DISTRIBUTION:
The mean age of patients undergoing laparoscopic cholecystectomy was 44.7
years
AGE DISTRIBUTION:
The mean age between the two arms was comparable.
N Mean Std. Deviation Std. Error
Mean
Palonosetron 27 46.08 16.2 2.29
Dexamethasone
+ Ondansetron 28 43.28 15.38 2.29
[image:62.612.74.496.347.555.2]
BODY MAS DISTRIBUTION:
The mean body mass index of patients having laparoscopic cholecystectomy was 20.4 kg/m2
BODY MASS INDEX DISTRIBUTION:
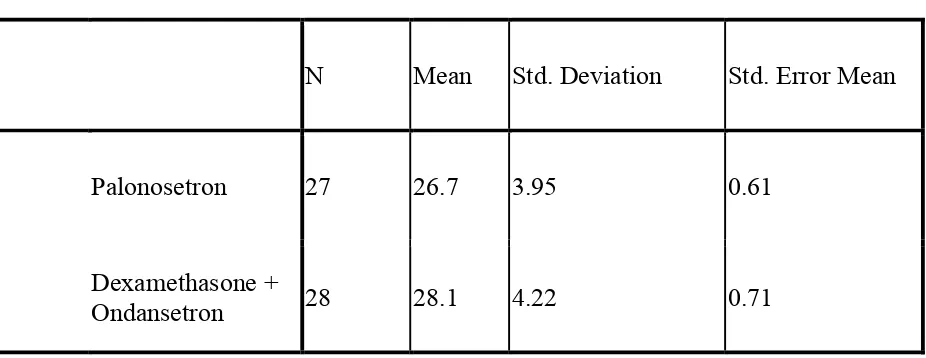
The body mass index in both groups was comparable.
N Mean Std. Deviation Std. Error Mean
Palonosetron 27 26.7 3.95 0.61
Dexamethasone +
[image:63.612.74.537.365.544.2]Ondansetron 28 28.1 4.22 0.71
DISTRIBUTION BETWEEN TWO ARMS ACCORDING TO THE PRESENCE OF COMORBID ILLNESSES
The following comorbidities were analyzed to ascertain the baseline characteristics in both groups
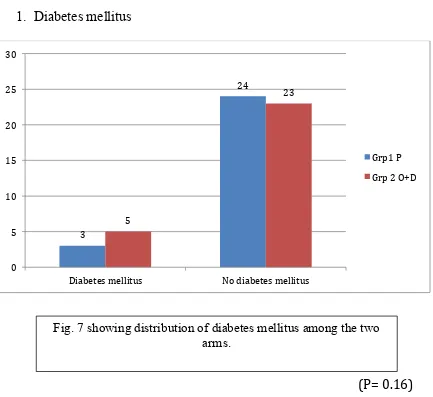
1. Diabetes mellitus
(P= 0.16)
3
24
5
23
0 5 10 15 20 25 30
Diabetes mellitus No diabetes mellitus
[image:64.612.74.507.243.640.2]Grp1 P Grp 2 O+D
2. Hypertension
(P= 0.9)
4
23
5
23
0 5 10 15 20 25
Hypertension No hypertension
Palonosetron
[image:65.612.71.491.93.357.2]Ondansetron +Dexamethasone
3. Smoking
P=0.093
3
24
1
27
0 5 10 15 20 25 30
Smoker Non smoker
Palonosetron
[image:66.612.72.506.136.424.2]Dexamethasone + Ondansetron
4. COPD or Asthma
1
26
2
26
0 5 10 15 20 25 30
COPD No COPD
Palonosetron
[image:67.612.70.507.135.390.2]Ondansetron + dexamethasone
5. Hypothyroidism
The baseline characteristics between the two groups were comparable.
3
24
1
27
0 5 10 15 20 25 30
Hypothyroidism No hypothyroidism
Palonosetron
[image:68.612.69.505.136.470.2]Ondansetron+Dexamethasone
The Apfel score comparison between the two groups
Palonosetron group
Score n(%)
1 3(11.1%)
2 6 (22.2%)
3 16 (59.3%)
4 2 (7.4%)
Ondansetron + dexamethasone group
Score n(%)
1 4(7.3%)
2 7(12.7%)
3 20(69.1%)
4 4(10.9%)
We did not analyze the relationship between Apfel score and the incidence of PONV. Since the data collection is not complete, our numbers are not big enough for a meaningful interpretation.
3
1 6
1 16
22
2
4
0 5 10 15 20 25
Palonosetron Ondansetron+Dexamethasone
N u m b er o
f p
at
ie
n
ts
1 2
[image:70.612.71.516.101.480.2]3 4
Nausea over a period of 24 hours
Nausea Palonosetron Dexamethasone+Ondansetron p value
n(%) n(%)
Yes 7(29) 10(42) 0.365
No 17(71) 14(58)
The above graph shows if a patient had nausea at any given point in time.
[image:71.612.88.493.229.574.2]
Fig.13 showing occurrence of nausea in the two arms
29
42 71
58
0 10 20 30 40 50 60 70 80
Palonosetron Dexamethasone+Ondansetron
P
er
ce
n
ta
ge
o
f c
ase
s
The occurrence of nausea at intervals of 0-2, 2 -6 and 6 – 24 hours
Palonosetron Dexamethasone+Ondansetron p value
n n
0-2hrs 0 5 0.182
2-6hrs 4 5
6-24hrs 4 5
0
4 4
5
5 5
0 1 2 3 4 5 6 7 8 9 10
0-‐2hrs 2-‐6hrs 6-‐24hrs
[image:72.612.71.510.112.597.2]Ondansetron +Dexamethasone Palonosetron
Vomiting over a period of 24 hours
Vomiting
Palonosetron Dexamethasone+Ondansetron
p value
n(%) n(%)
Yes 6(22.2) 10(35.7)
0.525
No 21(77.8) 18(64.3)
[image:73.612.60.537.118.663.2]
Fig.15 showing occurrence of vomiting in the two arms
22.2
35.7 77.8
64.3
0 10 20 30 40 50 60 70 80 90
Palonosetron Ondansetron+Dexamethasone
%
o
f C
ase
s
Vomiting
The occurrence of vomiting at intervals of 0-2, 2 -6 and 6 – 24 hours
Palonosetron Dexamethasone+Ondansetron
p value
n n
0-2hrs 1 0
0.2854
2-6hrs 3 3
6-12hrs 3 8
1
3 3 0
3
8
0 2 4 6 8 10 12
0-‐2hrs 2-‐6hrs 6-‐24hrs
N u m b er o
f p
at
ie
n
ts
Ondansetron+Dexamethasone
[image:74.612.51.522.97.549.2]Palonosetron
Occurrence of nausea in relation to opioid use
38.70%
29.40%
36.60%
28.60%
0.00% 5.00% 10.00% 15.00% 20.00% 25.00% 30.00% 35.00% 40.00% 45.00%
NAUSEA %
Title
FENTANYL <=2.5mg/kg
FENTANYL >2.5mg/kg MORPHINE <=.1mg/kg
MORPHINE >.1mg/kg NAUSEA
N(%) P VALUE
FENTANYL
.519
<=2.5mg/kg 12 (38.7%)
>2.5mg/kg 5(29.4%)
MORPHINE
>.999
<=.1mg/kg 15 (36.6%)
[image:75.612.91.532.187.699.2]>.1mg/kg 2 (28.6%)
The occurrence of vomiting in relation to opioid use
VOMITING
N (%) P VALUE
FENTANYL
>.999
<=2.5mg/kg 9 (29%)
>2.5mg/kg 5 (29.4%)
MORPHINE
>.999
<=.1mg/kg 12 (29.3%)
>.1mg/kg 2 (28.6%)
With increasing intra operative opioid dose no increase was seen in the incidence of vomiting postoperatively in both groups.
29%
29.40%
29.30%
28.60%
28% 28% 29% 29% 29% 29% 29% 30%
VOMITING %
P
er
ce
n
tga
e
of
c
ase
s
FENTANYL <=2.5mg/kg FENTANYL >2.5mg/kg
[image:76.612.70.518.100.645.2]MORPHINE <=.1mg/kg MORPHINE >.1mg/kg
Table 3: Correlation between Pain and Nausea at three time intervals 0 to 2hrs, 2 to 6 hrs and 6 to 24 hrs.
Time intervals n Correlation Coefficient p value
0 to 2 54 .279 .041
2 to 6 55 .126 .361
6 to 24 55 .241 .076
The Spearman’s Rank correlation coefficient was calculated here. The Pain and Nausea is correlated at 0 to 2hrs. The graphical representation is given below:
Table 4: Correlation between Pain and Vomiting at three time intervals 0 to 2hrs, 2 to hrs and 6 to 24 hrs.
Time intervals n Correlation
Coefficient p value
0 to 2 54 0.077 .582
2 to 6 55 -0.003 .980
6 to 24 55 -0.060 .663
RESULTS
As depicted by the consort diagram twenty-seven patients were randomized to the palonosetron arm and 28 patients were randomized to ondansetron and dexamethasone arm after the protocol violators were excluded.
Of the fifty-five patients, ten were male and forty-five were female. The mean age of patients assessed was 44.7 years.
The baseline demographics between the two groups were identical except for uneven gender distribution between the two groups (figure 6).
The distribution of patients based on comorbid illnesses such as diabetes mellitus, hypertension, COPD, smoking history and hypothyroidism were comparable.
The primary objective studied was to compare the efficacy of palonosetron versus ondansetron and dexamethasone in preventing PONV. There was no significant difference between the two groups studied(6 patients in the palonosetron arm versus 8 in the arm which received dexamethasone with ondansetron)(0.525).
The analysis also showed that the occurrence of nausea and vomiting was not significantly affected by the amount of fentanyl (nausea p = 0.519 and vomiting p>0.999) and morphine (nausea p >.0.999 and vomiting p= >0.99) used intraoperatively.
DISCUSSION
The problem of postoperative nausea and vomiting has troubled surgeon and anaesthetist alike since the advent of general anaesthesia in the early 1800s. With the advent of modern anaesthetic agents such as sevoflurane and desflurane; newer surgical techniques such as laparoscopy and robotic surgery and newer antiemetics, this wheel unfortunately needs to be re-invented and the question re-answered.
As more centers push towards doing laparoscopic cholecystectomy as a day care procedure with a same day discharge system the use of a efficacious anti-emetic agent may well answer most of the above mentioned questions.
Ondansetron has generally been considered as the gold standard in preventing and managing PONV. The addition of dexamethasone has further enhanced the efficacy of ondansetron. This has been recommended as a treatment recommendation in patients with moderately high risk of PONV.(4) Palonosetron because of the long t ½ has been suggested as the alternative choice by the consensus guideline published in 2014. We wanted to compare a combination of ondansetron plus dexamethasone vs single dose IV palonosetron for prevention of PONV after laparoscopic cholecystectomy.
emetic effects, 48 hours before surgery were also excluded, as they would have interfered with the actions of the study drugs. Patients with known hypersensitivity to palonosetron, ondansetron or dexamethasone and in whom the surgical procedure was converted to open cholecystectomy were also excluded due to obvious reasons. All the patients underwent laparoscopic cholecystectomy by the same team of anesthetists and surgeons. The anesthetic procedure, except for the test drug, was similar in all the three groups.
Our study was not designed to find out the incidence of PONV after laparoscopic cholecystectomy. We thought it was unethical to have a placebo arm as the side effect of PONV is quite distressing and the established guidance clearly recommends prophylactic anti emetic therapy.(73)
Even though a wide variety of doses of study drugs have been described in literature and various dose finding studies suggesting the optimal doses as 0.15 mg/kg of dexamethasone, 0.1 mg/kg of ondansetron and 0.075 mg of palonosetron, we followed the 2014 consensus guideline for PONV and used 4 mg ondansetron, 4 mg dexamethasone and 0.075mg of palonosetron.
Our study has shown that the incidence of PONV between the two drugs regimens is similar during the 1st 24 hours with no statistical difference. The use of rescue antiemetic was also similar between the two groups. There was no adverse event or drug side effect documented in either group. The sub group analysis shows similar incidence at 0-2 hours, 2-6 hours and 6-24 hours confirming that the faster onset of iv ondansetron combined with the longer duration of action of dexamethasone offers similar duration of anti emetic action of palonosetron.
Our results show good agreement with the consensus guidelines that palonosetron is an alternative to ondansetron and dexamethasone. The fact that palonosetron can be administered as a single dose during the surgery and that it can be administered to patients with endocrine abnormalities such as diabetes (dexamethasone may impair glycemic control) is an added benefit. It is also known to have a better safety profile than ondansetron as QT prolongation is not seen with this newer drug.
complication of dexamethasone. This indirect effect may be an added financial burden to the patient.
The analysis also showed weak correlation between PONV and pain. However as laparoscopic cholecystectomies are usually not very painful procedures it may not be possible to show accurate correlation as well
The BMI of patients in our study is lower than most western populations so we could not find a correlation between obesity and PONV.
Two patients in the palonosetron group and 4 patients in the ondansetron plus dexamethasone group had Apfel score of 4 which indicated high risk of PONV and the anesthetists should have adopted total intravenous anesthesia with propofol as per the recommendation of 2014 consensus guidelines. We agree with the statement in the recommendation that a major problem is the lack of compliance among anesthetists to follow the PONV guidelines.
LIMITATIONS
As this is a postgraduate dissertation and the study had to be done within a fixed time frame of one year, the target sample size was not reached. The sample size that was targeted prior to the start of the study was 77 patients in each arm. This number was not reached.
Deviation from protocol was another challenge due to pre-existing bias towards ondansetron from surgeon and anesthetist alike. Six patients were excluded from analysis as there was significant protocol violation. Protocol violation were for the following reasons – unwilling to give promethazine as rescue anti-emetic by the surgeon(1), the consultant anaesthetist preferring to use succinylcholine over rocuronium for anticipated difficult airway(1), conversion of laparoscopic cholecystectomies to open procedures (2) and surgical registrar accidentally writing round the clock ondansetron orders in the ward(2).
Blood glucose levels as well as delay in wound healing were not studied to see if dexamethasone had any negative effect on these.
We did not document the incidence of wound infection in the study groups.
CONCLUSION
Future direction and scope for further research
The study will be continued in the department of anesthesia of Christian Medical College till sample size will be reached.
BIBLIOGRAPHY
1. Islam S, Jain P. Post-operative nausea and vomiting (PONV). Indian J Anaesth. 2004 Jul 1;48(4):253–253.
2. Apfel CC, Heidrich FM, Jukar-Rao S, Jalota L, Hornuss C, Whelan RP, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012 Nov 1;109(5):742–53.
3. Fortier J, Chung F, Su J. Unanticipated admission after ambulatory surgery--a prospective study. Can J Anaesth J Can Anesth. 1998 Jul;45(7):612–9.
4. Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Consensus guidelines for the management of postoperative nausea and vomiting. AnesthAnalg. 2014 Jan;118(1):85–113.
5. Song JW, Park EY, Lee JG, Park YS, Kang BC, Shim YH. The effect of combining dexamethasone with ondansetron for nausea and vomiting associated with fentanyl-based intravenous patient-controlled analgesia. Anaesthesia. 2011 Apr;66(4):263–7.
6. Ahsan K, Abbas N, Naqvi SMN, Murtaza G, Tariq S. Comparison of efficacy of ondansetron and dexamethasone combination and ondansetron alone in preventing postoperative nausea and vomiting after laparoscopic cholecystectomy. JPMA J Pak Med Assoc. 2014 Mar;64(3):242–6.
7. Chun HR, Jeon IS, Park SY, Lee SJ, Kang SH, Kim SI. Efficacy of palonosetron for the prevention of postoperative nausea and vomiting: a randomized, double-blinded, placebo-controlled trial. Br J Anaesth. 2014 Mar;112(3):485–90.
8. Hasler WL, Chey WD. Nausea and vomiting. Gastroenterology. 2003 Dec;125(6):1860–7. 9. Singh P, Yoon SS, Kuo B. Nausea: a review of pathophysiology and therapeutics.
TherAdvGastroenterol. 2016 Jan;9(1):98–112.
10. Becker DE. Nausea, Vomiting, and Hiccups: A Review of Mechanisms and Treatment. AnesthProg. 2010;57(4):150–7.
11. Chepyala P, Olden KW. Nausea and vomiting. Curr Treat Options Gastroenterol. 2008 Apr;11(2):135–44.
12. Kowalski A, Rapps N, Enck P. Functional cortical imaging of nausea and vomiting: a possible approach. AutonNeurosci Basic Clin. 2006 Oct 30;129(1–2):28–35.
14. Kim J, Napadow V, Kuo B, Barbieri R. A combined HRV-fMRI approach to assess cortical control of cardiovagal modulation by motion sickness. ConfProcAnnuIntConf IEEE Eng Med BiolSoc IEEE Eng Med BiolSocAnnu Conf. 2011;2011:2825–8.
15. Napadow V, Sheehan JD, Kim J, LaCount LT, Park K, Kaptchuk TJ, et al. The Brain Circuitry Underlying the Temporal Evolution of Nausea in Humans. Cereb Cortex. 2013 Apr 1;23(4):806–13.
16. Horn CC. Why is the neurobiology of nausea and vomiting so important? Appetite. 2008 May;50(2–3):430–4.
17. LaCount LT, Barbieri R, Park K, Kim J, Brown EN, Kuo B, et al. Static and dynamic autonomic response with increasing nausea perception. Aviat Space Environ Med. 2011 Apr;82(4):424–33.
18. Muth ER. Motion and space sickness: intestinal and autonomic correlates. AutonNeurosci Basic Clin. 2006 Oct 30;129(1–2):58–66.
19. Koch KL. A noxious trio: nausea, gastric dysrhythmias and vasopressin. NeurogastroenterolMotil Off J EurGastrointestMotil Soc. 1997 Sep;9(3):141–2.
20. Page SR, Peterson DB, Crosby SR, Ang VT, White A, Jenkins JS, et al. The responses of arginine vasopressin and adrenocorticotrophin to nausea induced by ipecacuanha. ClinEndocrinol (Oxf). 1990 Dec;33(6):761–70.
21. Caras SD, Soykan I, Beverly V, Lin Z, McCallum RW. The effect of intravenous vasopressin on gastric myoelectrical activity in human subjects. NeurogastroenterolMotil Off J EurGastrointestMotil Soc. 1997 Sep;9(3):151–6.
22. Taché Y. Cyclic vomiting syndrome: the corticotropin-releasing-factor hypothesis. Dig Dis Sci. 1999 Aug;44(8 Suppl):79S–86S.
23. Koch KL. Gastric dysrhythmias: a potential objective measure of nausea. Exp Brain Res. 2014 Aug;232(8):2553–61.
24. Quigley EMM, Hasler WL, Parkman HP. AGA technical review on nausea and vomiting. Gastroenterology. 2001 Jan;120(1):263–86.
25. Kapur PA. Editorial: The Big “Little Problem”. AnesthAnalg [Internet]. 1991;73(3).
Available from:
http://journals.lww.com/anesthesia-analgesia/Fulltext/1991/09000/Editorial__The_Big__Little_Problem__.1.aspx
26. Smessaert A, Schehr CA, Artusio JF. Nausea and vomiting in the immediate postanes